Effects and Mechanism Analysis of Non-Bagging and Bagging Cultivation on the Growth and Content Change of Specific Substances of Fuji Apple Fruit
Abstract
:1. Introduction
2. Results
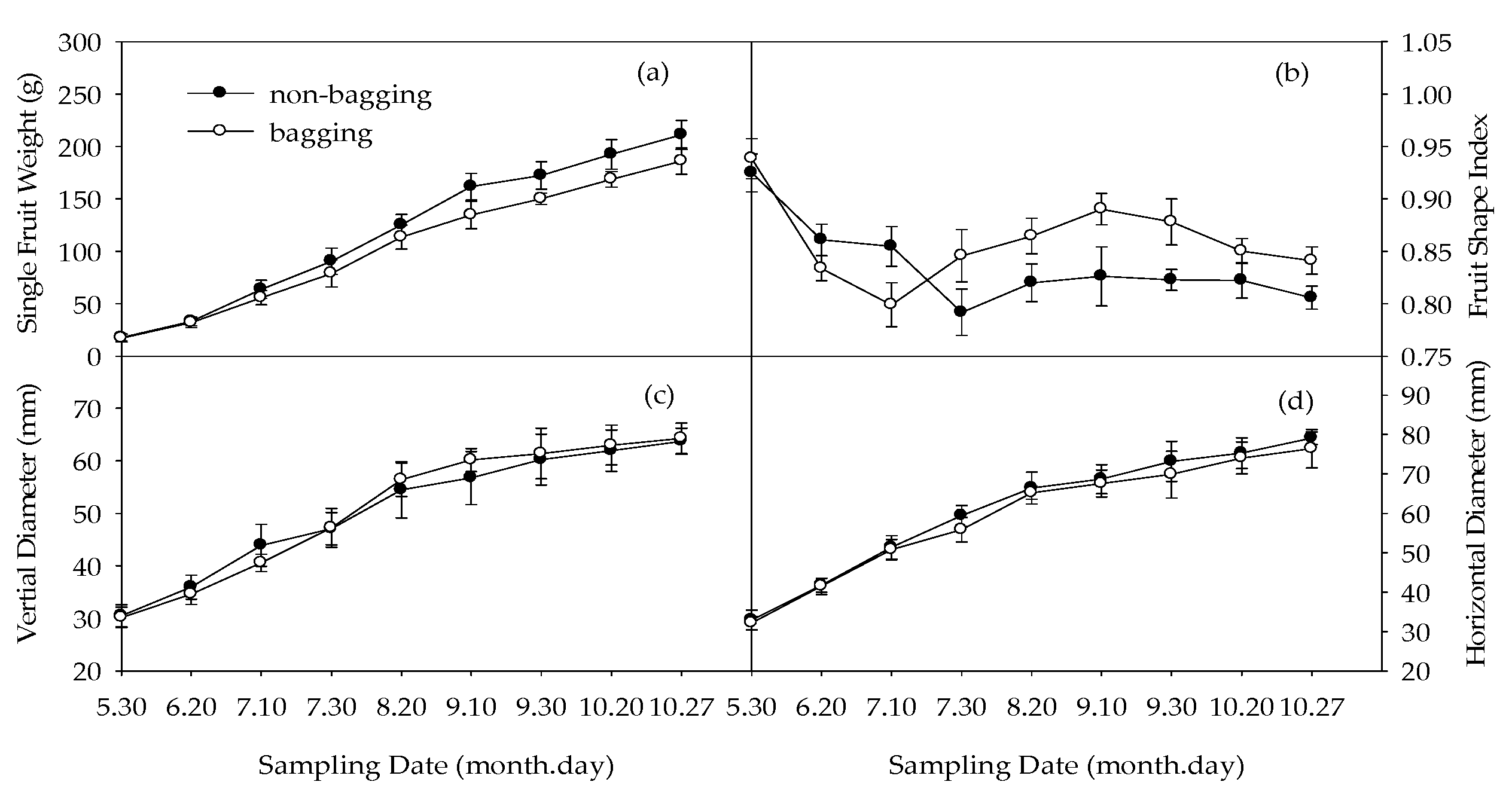
2.1. Effects of Non-Bagging and Bagging on Changes in Single Fruit Weight, Fruit Shape Index, and Diameter of Fuji Apple
2.2. Effects of Non-Bagging and Bagging on the Content of Hormones Related to Fuji Apple Fruit Growth and Development
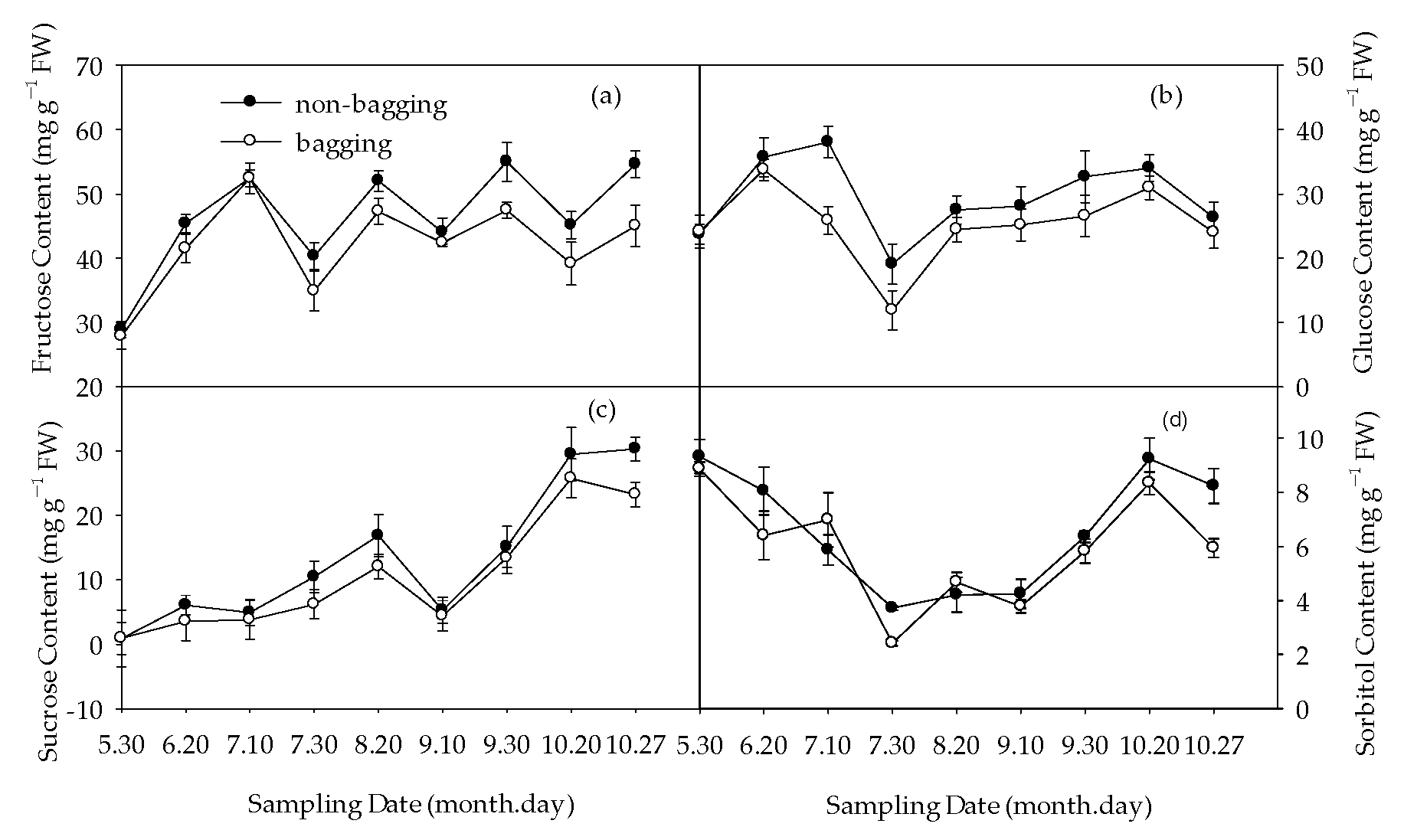
2.3. Effects of Non-Bagging and Bagging on Sugar and Acid Accumulation of Fuji Apple Fruit
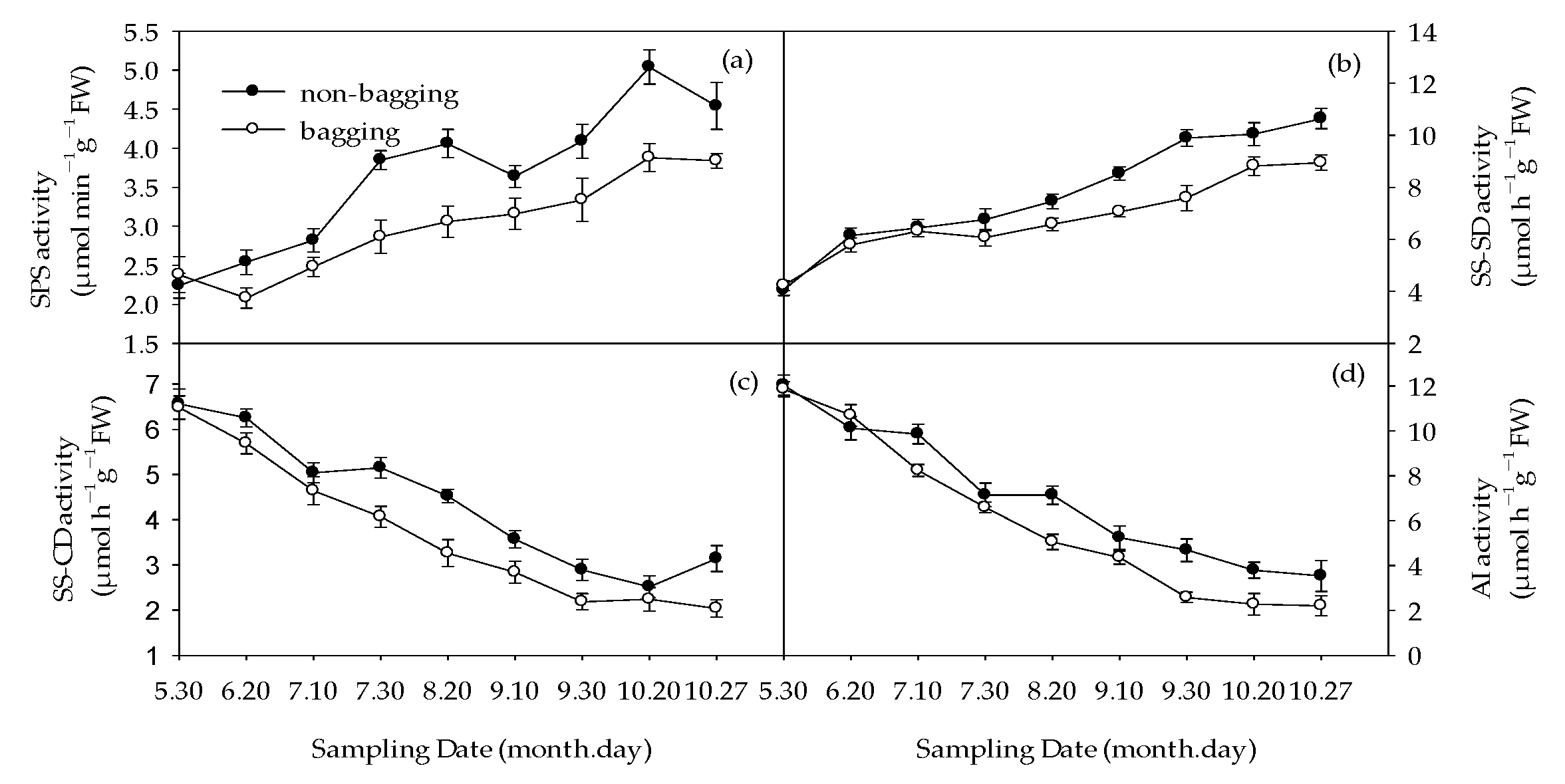
2.4. Effects of Non-Bagging and Bagging on the Activity of Sugar and Acid Metabolism Enzymes in Fuji Fruits
2.4.1. Activity of Enzymes Related to Sugar Metabolism
2.4.2. Acid-Metabolism-Related Enzyme Activity
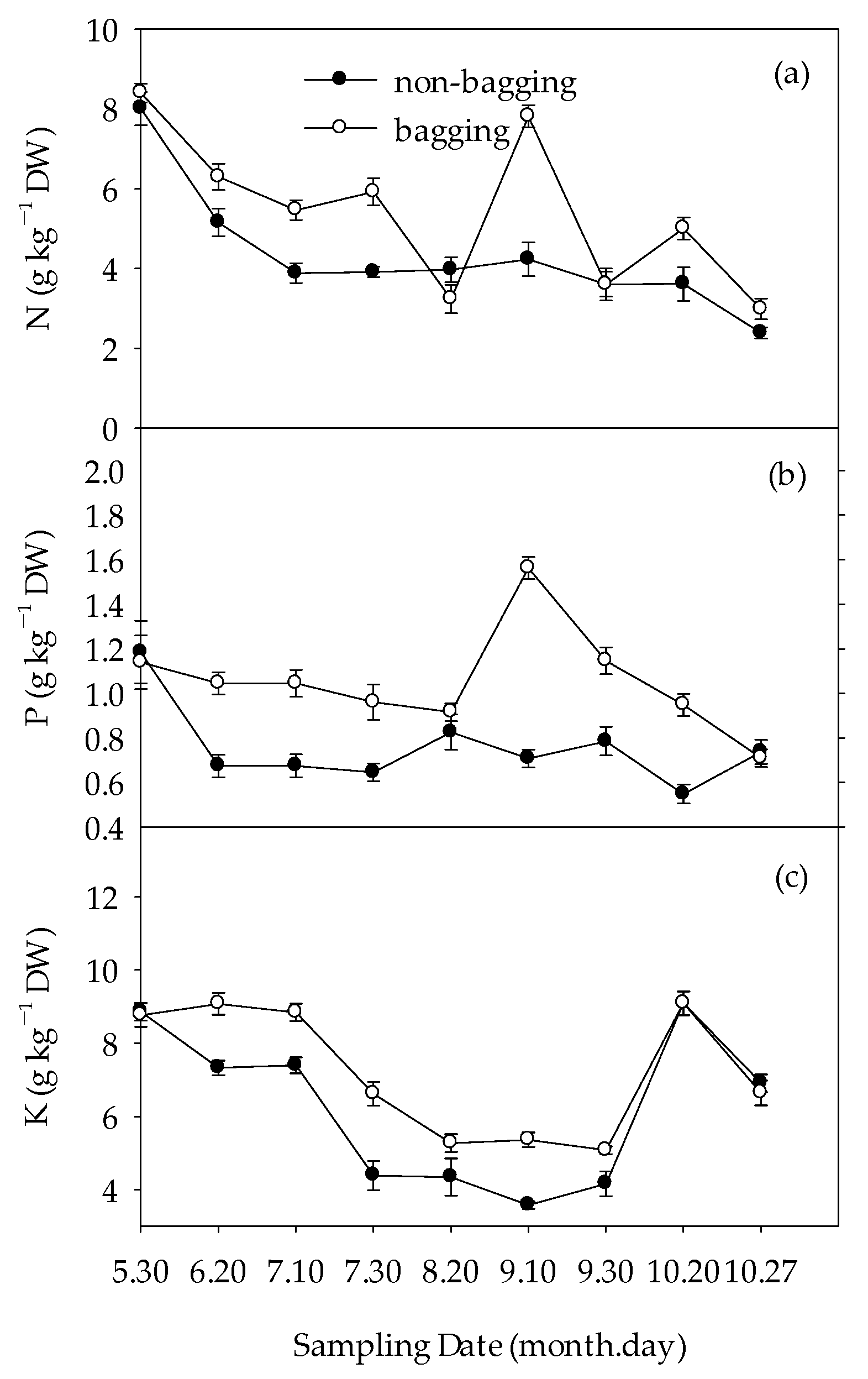
2.5. Effects of Non-Bagging and Bagging on Mineral Element Content of Fuji Apple Fruits
2.5.1. Major Element Contents in Apple Fruits
2.5.2. Medium and Trace Element Contents in Fruits of Apple
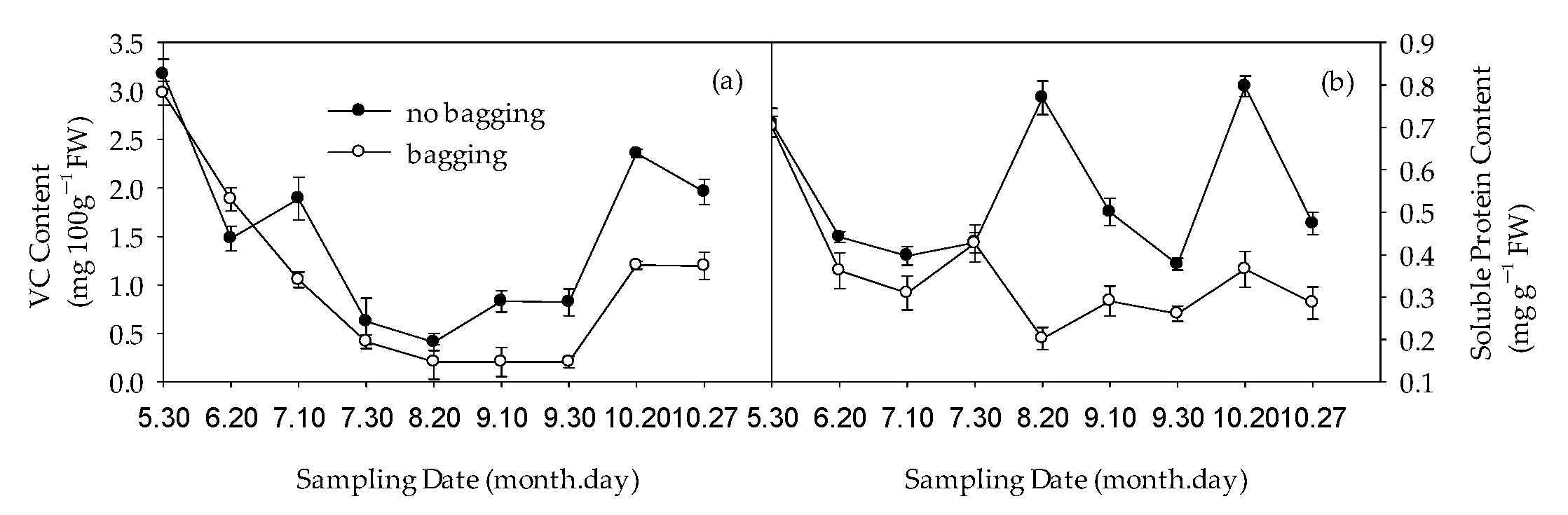
2.6. Effects of Non-Bagging and Bagging on Changes in Vitamin C (VC) and Soluble Protein Content of Fruits
2.7. Effects of Non-Bagging and Bagging on the Aroma Substances of Fuji Apple Fruits during Harvesting Period
2.8. Effects of Non-Bagging and Bagging on Malondialdehyde (MDA) Content and Antioxidant Enzyme Activity in Fuji Apple Fruits
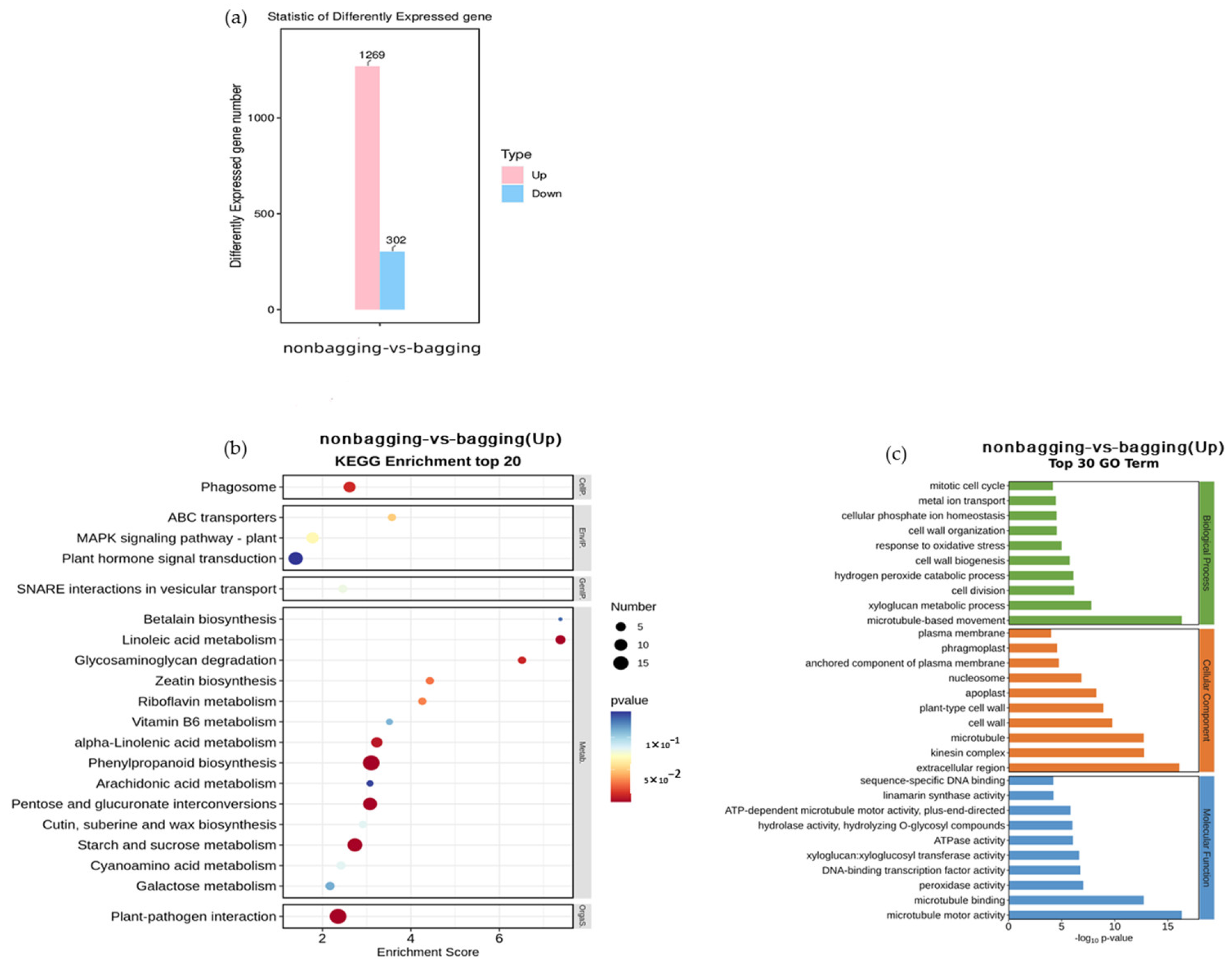
2.9. Effects of Non-Bagging and Bagging on Gene Transcription and Expression in Fuji Apple Fruits
3. Discussion
3.1. Improving Single Fruit Weight and Reducing Fruit Shape Index of Fuji Apple by Non-Bagging Cultivation
3.2. Non-Bagging Cultivation to Increase Sugar Content, Reduce Acid Content, and Improve Fruit Taste of Fuji Apples
3.3. Cultivation without Bagging to Improve the Nutritional Composition and Aroma Substances of Fuji Apple Fruit
3.4. Different Effects of Non-Bagging and Bagging Cultivation on the Absorption and Accumulation of Mineral Nutrition in Fuji Apple Fruits
3.5. Improving the Antioxidant Capacity of Fuji Apple Fruits by Non-Bagging Cultivation
3.6. The Gene Expression of Fuji Apple Fruit between Non-Bagging and Bagging Cultivation
4. Materials and Methods
4.1. Basic Information of Test Site
4.2. Test Design
4.3. Measuring Methods
4.3.1. Fruit Size and Shape Index
4.3.2. Determination of Hormone Level
4.3.3. Determination of Sugar and Acid Content of Fruit
4.3.4. Determination of Sugar-Metabolism-Related Enzyme Activity in Fruit
4.3.5. Determination of Enzyme Activity Related to Acid Metabolism in Fruit
4.3.6. Determination of Mineral Element Content of Fruit
4.3.7. Determination of Other Main Substances Contents of Fruit
4.3.8. Determination of Volatile Matter Content in Apple Fruits
4.3.9. Determination of Sugar-Metabolism-Related Enzyme Activity in Fruit
4.3.10. Gene Expression Analysis
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ge, Y.Q.; Li, H.F.; Wang, C.Y.; Wei, X.X.; Su, J.; Dong, S.S. Status and sustainable development proposals of apple industry in China. Xian Dai Shi Pin. 2021, 11, 4–6. [Google Scholar] [CrossRef]
- Wang, X.; Liu, J.D.; Shao, L.Q.; Yan, Z.Y.; Han, M.Y.; Huo, X.X. Annual development status, trends and suggestions of China’s apple industry. China Fruits 2018, 3, 101–104+108. [Google Scholar] [CrossRef]
- Liu, Q. Should apples be bagged or not? Farmers Dail 2022, 8, 1–5. [Google Scholar] [CrossRef]
- Wang, G.P.; Xue, X.M.; Wang, J.Z. Research progress and development trend of apple bagging technology in China. J. Hebei Agric. Sci. 2021, 25, 44–48. [Google Scholar]
- Zhu, W.M. The central government has established a demonstration subsidy project for key technologies of apple bagging [N]. China Foods Ltd. Qual. Rep. 2005, 4, 12. [Google Scholar]
- Miqueloto, A.; do Amarante, C.V.T.; Steffens, C.A.; dos Santos, A.; Heinzen, A.S.; Miqueloto, T.; Strauss, R.; Finger, F.L.; Picoli, E.A.T.; Souza, G.A. Mechanisms regulating fruit calcium content and susceptibility to bitter pit in cultivars of apple. Acta Hortic. 2018, 1194, 467–474. [Google Scholar] [CrossRef]
- Hou, Y.M.; Zhang, X.; Zhang, N.N.; Naklumpa, W.; Zhao, W.Y.; Liang, X.F.; Zhang, R.; Sun, G.Y.; Gleason, M.L. Genera acremonium and sarocladium cause brown spot on bagged apple fruit in China. Plant Dis. 2019, 103, 1889–1901. [Google Scholar] [CrossRef]
- Sun, H.Y.; Li, L.; Li, M.S.; Wang, D.F.; Wang, D.X. Reflection on baggless cultivation of apple. J. Fruit Resour. 2020, 1, 63–65. [Google Scholar] [CrossRef]
- The ministry of agriculture and rural affairs released the top 10 leading technologies for 2020. Agric. Eng. 2020, 10, 3–4.
- Li, P.P.; Li, J.M.; Li, G.L.; Li, Y.F.; He, Y.; Li, Z.X. Preliminary report on the cultivation experiment of 12 apple varieties in Jingning, Gansu province, with low rootstock and close planting without bagging. China Fruits 2023, 78–82. [Google Scholar] [CrossRef]
- Sun, Y.X.; Song, L.Q.; Zhao, L.L.; Zhang, X.Y.; Liu, D.L.; Zhao, J.; Liu, X.Q.; Tang, Y. Breeding of a new early-ripening, yellow and bagging-free apple cultivar Yanxiangyu. J. Fruit Sci. 2022, 39, 499–501. [Google Scholar] [CrossRef]
- He, X.X. Quality Evaluation of Apples Cultivated without Bagging in Weibei Area of Shaanxi Province. Master’s Dissertation, Northwest A&F University, Xianyang, China, 2022. [Google Scholar] [CrossRef]
- Zhai, H.; Wang, J.Z.; Xue, X.M.; Wang, L.P.; Chen, R.; Nie, P.X.; Zhang, Y. Population dynamics of Grapholitha molesta (Lepidoptera:Tortrididae) and control effects by sexual pheromone in apple orchards with non-bagged-fruit cultivation pattern. Sci. Silvae Sin. 2019, 55, 111–118. [Google Scholar] [CrossRef]
- Zhai, H.; Wang, J.Z.; Wang, D.; Li, X.J.; Xue, X.M. Fungal pathogens identification of postharvest apples in bagging-free cul tivation pattern and antifungal activity of essential oil. J. Fruit Sci. 2021, 38, 549–559. [Google Scholar] [CrossRef]
- Zeng, X. Study on Green Prevention and Control Technology of Non-Bagging Apple Diseases and Screening of Biocontrol Bacteria and Antifungal and Growth-Promoting Effects. Master’s Dissertation, Northwest A&F University, Xianyang, China, 2022. [Google Scholar] [CrossRef]
- Geng, J.; Wang, S.H.; Li, C. Comparison of fruit quality between non-bagged and bagged apples and comparison of pesticide residues. Northern Fruits 2018, 13–15. [Google Scholar] [CrossRef]
- Ma, Q.R.; Zhang, K.X.; Xu, F.L. Comparative analysis of apple bagging and bagging free cultivation. Anhui Agri. Sci. Bull. 2021, 27, 69–70+95. [Google Scholar] [CrossRef]
- Yang, W.Y.; Gao, M.N.; Yin, B.Y.; Liang, B.W.; Li, Z.Y.; Xu, J.Z. Effects of unbagging cultivation on fruit quality of red Fuji apple. Northern Hortic. 2021, 41–47. [Google Scholar]
- Wang, G.P.; Han, X.P.; Nie, P.X.; Wang, J.Z. Effects of unbagging and bagging density on canopy photometric characteristics of “red Fuji” apple tree. Acta Agric. Jiangxi 2019, 31, 36–38+60. [Google Scholar] [CrossRef]
- Wang, G.P.; Xue, X.X.; Zhao, H.Q.; Chen, R.; Han, X.P.; Wang, J.Z. Effects of no-bagging and bagging density on photosynthetic characteristics of ‘Fuji’ apple trees. Chin. Agri. Sci. Bull. 2022, 38, 54–59. [Google Scholar] [CrossRef]
- Ahmed, T.; Hasan, M.M.; Hassan, K.D.; Ahmed, J.D.; Ahmed, K.S.D.; Azam, A.; Mondal, M.F. Fruit bagging of custard apple (Annona reticulata) as an eco-friendly protection approach against mealybug (Phenacoccus solenopsis) infestation in the north-eastern Bangladesh. Int. J. Trop. Insect Sci. 2022, 42, 723–732. [Google Scholar] [CrossRef]
- Yue, Z.Y. The Effect of Non Bagging Cultivation on the Quality and Storage Tolerance of Apple Fruits. Master’s Dissertation, Northwest A&F University, Xianyang, China, 2021. [Google Scholar] [CrossRef]
- Mao, N.N.; Su, X.Y.; Wang, Z.J.; Ren, J.P.; Ji, M.X.; Guo, J.; Liu, Z.T. Effects of microenvironment changed inside different bags on grape fruit quality. Jiangsu J. Agr. Sci. 2021, 37, 1270–1277. [Google Scholar] [CrossRef]
- Du, Y. Study on the Formation Mechanism and Regulation of Fruit Shape of Fuji Apple in Akes. Ph.D. Thesis, Xinjiang Agricultural University, Urumqi, China, 2013. [Google Scholar] [CrossRef]
- Huang, B.N.; Chen, B.H.; Mao, J.; Liu, Y.L.; Wang, Y.X.; Yang, L.P.; Zhang, T. Effects of bagging and harvest timing on fruit quality of ‘Starkrimson’ apple. J. Fruit Sci. 2015, 32, 824–834. [Google Scholar] [CrossRef]
- Wang, S.M.; Wang, Z.Y.; Zhao, H.J.; Hu, W.J.; Zhang, X.Y.; Li, X. Comparative experiment on bagging techniques for short branch red Fuji apple fruits. Shandong J. Agr. Sci. 1998, 3, 28–30. [Google Scholar] [CrossRef]
- Boyaci, S. Effects of “bagging” treatment on some pomological and quality feature of red species. Fresen. Environ. Bull. 2019, 28, 150–155. [Google Scholar]
- Jing, C.J.; Feng, D.P.; Zhao, Z.Y.; Wu, X.H.; Chen, X.F. Effect of environmental factors on skin pigmentation and taste in three apple cultivars. Acta Physiol. Plant. 2020, 42, 69–80. [Google Scholar] [CrossRef]
- Liu, M.Y.; Zhao, M.; Zhao, L.L.; Tang, Y.; Sun, Y.X.; Liu, D.L.; Zhang, X.Y.; Jiang, Z.W. Bagged and non-bagged fruits of 4 dwarfing rootstocks short-shoot Fuji cultivars: Quality comparison. Chin. Agric. Sci. Bull. 2020, 36, 38–43. [Google Scholar]
- Sun, S.; Zhang, L.T.; Gao, H.Y.; Shu, H.R. Photosynthetic characteristics of green apple fruits. Sci. Silvae Sin. 2011, 47, 33–37. [Google Scholar] [CrossRef]
- Xue, X.X.; Wang, J.Z.; Lu, C.; Zhang, S.J. Effect of bagging on the quality and storage characteristics of Huaniu apple fruit. Jiangsu J. Agr. Sci. 2011, 39, 231–233. [Google Scholar] [CrossRef]
- Wang, S.Y.; Chen, D.Y.; Wang, F.; Wu, T.G.; Zeng, M.; Qiu, J.H.; Liu, W. Storageproperties of ‘Hongyang’Kiwifruit. Acta Agric. Jiangxi 2020, 32, 41–46. [Google Scholar] [CrossRef]
- Abelenda, J.A.; Prat, S. Cytokinins: Determinants of sink storage ability. Curr. Biol. 2013, 23, 561–563. [Google Scholar] [CrossRef] [PubMed]
- Rottmann, T.; Zierer, W.; Subert, C.; Sauer, N.; Stadler, R. STP10 encodes a high affinity monosaccharide transporter and is induced under low-glucose conditions in pollen tubes of Arabidopsis. J. Exp. Bot. 2016, 67, 2387–2399. [Google Scholar] [CrossRef]
- Boyacioglu, M.; Sekkin, S.; Kum, C.; Korkmaz, D.; Kiral, F.; Yalinkilinc, H.S.; Ak, M.O.; Akar, F. The protective effects of vitamin C on the DNA damage, antioxidant defenses and aorta histopathology in chronic hyperhomocysteinemia induced rats. Exp. Toxicol. Pathol. 2014, 66, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.; GE, R.; Yu, W.Z.; Mao, Y.F.; Liu, Q.; Mao, Z.Q.; Chen, X.S.; Shen, X. Effects of different pollination combinations on ASA content, activity of antioxidant coordinating enzymes and dynamic changes of sugars and organic acid compositions in apple fruits. J. Fruit Sci. 2017, 34, 670–681. [Google Scholar] [CrossRef]
- Jing, C.J.; Ma, C.Q.; Zhang, J.; Jing, S.J.; Jiang, X.B.; Yang, Y.Z.; Zhao, Z.Y. Effect of debagging time on pigment patterns in the peel and sugar and organic acid contents in the pulp of ‘Golden Delicious’ and ‘Qinguan’ apple fruit at mid and late stages of development. PLoS ONE 2016, 11, e0165050. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.S.; Yan, C.T.; Zhang, T.H.; Zhang, L.; Ji, M.M.; Wang, F.; Gao, H. Effect of bagging on aroma volatiles and related gene expression in ‘Ruixue’ Apple Fruit. Food Sci. 2020, 41, 185–192. [Google Scholar] [CrossRef]
- Zhang, L.P.; Ji, S.J. Effects of different treatments on the content of esters and the key enzymes for ethylene synthesis in refrigerated Nanguo Pear pretreated with 1-MCP. Food Sci. 2013, 34, 285–288. [Google Scholar] [CrossRef]
- Brian, F.; Mario, D.G.; Iuliia, K.; Luca, C.; Franco, B.; Riccardo, V.; Fabrizio, C. Genome-wide association study unravels the genetic control of the apple volatilome and its interplay with fruit texture. J. Exp. Bot. 2017, 68, 1467–1478. [Google Scholar] [CrossRef]
- He, J.; Cheng, X.T.; Gao, Y.; Zhang, X.N.; Jia, X.H. Comparison of quality and storability among bagged and non-bagged fruits of three apple varieties. China Fruits 2022, 6–9. [Google Scholar] [CrossRef]
- Niu, X.L.; Ma, W.F.; Chen, G.M.; Liang, J.; Zhang, H.Y. Effects of foliar spraying calcium fertilizer on mineral elements contents in gold silk jujube. Plant Sci. J. 2018, 36, 141–146. [Google Scholar] [CrossRef]
- Yosef, A.S.; Jacqueline, F.N.; Tara, A.B.; Richard, P.M.; Christopher, B.W. Bitter pit and soft scald development during storage of unconditioned and conditioned ‘Honeycrisp’apples in relation to mineral contents and harvest indices. Postharvest Biol. Technol. 2020, 160, 111044. [Google Scholar] [CrossRef]
- Torres, E.; Recasens, I.; Lordan, J.; Alegre, S. Combination of strategies to supply calcium and reduce bitter pit in ‘Golden Delicious’ apples. Sci. Hortic. 2017, 217, 179–188. [Google Scholar] [CrossRef]
- Ma, D.H.; Pang, J.A.; Li, S.J.; Huo, Z.R. Effects of Temperature Stress Acclimation on some Physiological Characters in Leaves of Cucumber Seedlings. Acta Hortic. Sin. 1998, 25, 350–355. [Google Scholar]
- Ou, Z.F.; Liu, L.; Jiang, Y.M.; Wei, S.C.; Li, H. Effects of preharvest calcium sprays on quality and storage properties of Red Fuji apple (Malus pumila Mill.). Food Ferment. Ind. 2013, 39, 192–196. [Google Scholar] [CrossRef]
- José, A.Y.; Neira, A.; Fuentes, M.; Razmilic, I.; Lepe, V.; González, M.F.; Erwerbs-Obstbau. Bagging cv. Fuji, Raku Raku apple fruit affects their phenolic profile and antioxidant capacity. Erwerbsobstbau 2020, 62, 221–229. [Google Scholar] [CrossRef]
- Wang, H.; Wang, S.; Fan, M.M.; Zhang, S.H.; Sun, L.L.; Zhao, Z.Y. Metabolomic insights into the browning of the peel of bagging ‘Rui Xue’ apple fruit. BMC Plant Biol. 2021, 21, 209. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.F. The Effect of un-bagged on apple quality and analysis of main factors of fruit russet. Master’s Dissertation, Northwest A&F University, Xianyang, China, 2019. [Google Scholar]
- Almeida Trapp, M.; De Souza, G.D.; Rodrigues-Filho, E.; Boland, W.; Mithöfer, A. Validated method for phytohormone quantification in plants. Front. Plant Sci. 2014, 5, 417. [Google Scholar] [CrossRef]
- Hu, G.J.; Liu, J.F.; Liu, J.M. Determination of Nutritional Components of fruit Trees, 1st ed.; Tai’an Press and Publication Bureau: Tai’an, China, 1997; p. 5. [Google Scholar]
- Tong, Y.A.; Zhou, H.J. Nutritional Diagnosis of Fruit Trees, 1st ed.; Agricultural Press: Beijing, China, 1982; pp. 113–115. [Google Scholar]
- Hu, Z.Q.; Wang, H.C.; Hu, G.B. Measurement of sugars, organic acids and vitamin C in litchi fruit by high performance liquid chromatography. J. Fruit Sci. 2005, 5, 582–585. [Google Scholar] [CrossRef]
- Li, Y.N.; Yan, L.Y.; Zhang, B.; Yang, S.B.; Zhao, Z.Y. A study on sugar and organic acid components in different apple cultivars. J. Fruit Sci. 2021, 38, 1877–1889. [Google Scholar] [CrossRef]
- Lowell, C.; Tomlinson, P.T.; Koch, K.E. Sucrose-metabolizing enzymes in transport tissues and adjacent sink structures in developing citrus fruit. Plant Physiol. 1989, 90, 1394–1402. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, J.; Han, X.; Chen, R.; Xue, X. Effects of spraying calcium fertilizer on photosynthesis, mineral content, sugar–acid metabolism and fruit quality of Fuji apples. Agronomy 2022, 12, 2563. [Google Scholar] [CrossRef]
- Zhu, Y.J.; Komor, E.; Moore, P.H. Sucrose accumulation in the sugarcane stem is regulated by the difference between the activities of soluble acid invertase and sucrose phosphate synthase. Plant Physiol. 1997, 115, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Huber, S.C. Role of sucrose-phosphate synthase in partitioning of carbon in leaves. Plant Physiol. 1983, 71, 818–821. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, N.L.; Huber, S.C.; Pharr, D.M. Sucrose phosphate synthase and acid invertase as determinants of sucrose concentration in developing muskmelon (Cucumis melo L.) fruits. Plant Physiol. 1989, 91, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
- Hirai, M.; Ueno, I. Development of citrus fruits: Fruit development and enzymatic changes in juice vesicle tissue. Plant Cell Physiol. 1977, 18, 791–799. [Google Scholar] [CrossRef]
- Luo, A.C.; Yang, X.H.; Deng, Y.Y.; Li, C.F.; Xiang, K.S.; Li, D.G. Organic acid concentrations and the relative enzymatic changes during the development of citrus fruits. Sci. Agric. Sin. 2003, 36, 941–944. [Google Scholar] [CrossRef]
- Lu, Y.Q.; Liuh, P.; Wang, Y.; Zhang, X.Z.; Han, Z.H. Synergistic roles of leaf boron and calcium during the growing season in affecting sugar and starch accumulation in ripening apple fruit. Acta Physiol. Plant. 2013, 35, 2483–2492. [Google Scholar] [CrossRef]
- National Standard of the People’s Republic of China. Determination of Vitamin C Content in Fruits and Vegetables; Agricultural Press: Beijing, China, 1986. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantification of microgram quantities of protein using the Principal of protein–dye binding. Anal Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.R.; Ni, W.R.; He, X.Y.; Xu, J.; Wang, J.Z.; Mao, Z.Q.; Shen, X. Effects of vitamin B6 application time and doses on fruit quality of Fuji. J. Plant Nutr. Fertil. 2016, 22, 1348–1355. [Google Scholar]
- Quan, R.D.; Shang, M.; Zhang, H.; Zhao, Y.X.; Zhang, J.R. Improved chilling tolerance by transformation with betA gene for the enhancement of glycinebetaine synthesis in maize. Plant Sci. 2004, 166, 141–149. [Google Scholar] [CrossRef]
- Prochazkova, D.; Sairam, R.K.; Srivastava, G.C.; Singh, D.V. Oxidative stress and antioxidant activity as the basis of senescence in maize leaves. Plant Sci. 2001, 161, 765–771. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357. [Google Scholar] [CrossRef]
- Nicolas, D.; Jean-Marc, C.; Gareth, L.; Claude, B.; Nathalie, C.; lio, S.; van de Henri, G.; Luca, B.; Diego, M.; Riccardo, V.; et al. High-quality de novo assembly of the apple genome and methylome dynamics of early fruit development. Nat. Genet. 2017, 49, 1099–1106. [Google Scholar] [CrossRef]






| Sampling Date (Month.Day) | Treatment | Hormone Content (μg g−1 FW) | |||
|---|---|---|---|---|---|
| IAA | ZR | ABA | SA | ||
| 5.30 | non-bagging | 23.00 ± 1.6 a | 27.18 ± 1.01 a | 0.18 ± 0.005 a | - |
| bagging | 22.89 ± 1.1 a | 26.46 ± 2.03 a | 0.17 ± 0.004 a | - | |
| 6.20 | non-bagging | 13.07 ± 0.61 a | 7.27 ± 1.02 a | 0.06 ± 0.005 b | - |
| bagging | 5.47 ± 0.64 b | 6.73 ± 1.56 ab | 0.09 ± 0.005 a | - | |
| 7.30 | non-bagging | 0.32 ± 0.07 a | 0.22 ± 0.09 a | 0.02 ± 0.007 ab | 1.02 ± 0.05 a |
| bagging | 0.23 ± 0.03 ab | 0.00 ±0.00 b | 0.03 ± 0.003 a | 0.84 ± 0.04 b | |
| 8.20 | non-bagging | 0.14 ± 0.05 a | 0.35 ± 0.05 a | 0.04 ± 0.004 b | 0.68 ± 0.03 a |
| bagging | 0.03 ± 0.02 b | 0.09 ± 0.01 b | 0.11 ± 0.006 a | 0.61 ± 0.03 a | |
| 9.10 | non-bagging | 0.09 ± 0.05 a | 1.18 ± 0.05 a | 0.04 ± 0.006 b | 1.00 ± 0.04 a |
| bagging | 0.04 ± 0.03 ab | 0.18 ± 0.03 b | 0.07 ± 0.004 a | 0.86 ± 0.05 b | |
| 9.30 | non-bagging | 0.76 ± 0.07 a | 0.17 ± 0.06 a | 0.03 ± 0.002 a | 0.99 ± 0.06 a |
| bagging | 0.38 ± 0.04 b | 0.15 ± 0.04 ab | 0.03 ± 0.006 a | 0.43 ± 0.07 b | |
| 10.27 | non-bagging | 1.40 ± 0.09 a | 0.17 ± 0.05 a | 0.05 ± 0.003 b | 0.79 ± 0.09 a |
| bagging | 0.48 ± 0.07 b | 0.12 ± 0.02 b | 0.10 ± 0.004 a | 0.44 ± 0.04 b | |
| Categories | Compounds | Relative Content (%) | |
|---|---|---|---|
| Non-Bagging | Bagging | ||
| Alcohols | 1-Propanol, 2-methyl- | 0.44 ± 0.05 b | 1.02 ± 0.02 a |
| 1-Butanol | 3.13 ± 0.1 a | 0.05 ± 0.01 b | |
| 1-Penten-3-ol | 0.09 ± 0.01 a | 0.05 ± 0.01 b | |
| 2-Methyl-1-butanol | 5.77 ± 1.01 a | 6.45 ± 0.92 a | |
| 1-Pentanol | 0.3 ± 0.02 a | 0.31 ± 0.01 a | |
| 3-Methylpenta-1,3-diene-5-ol, (E)- | 25.18 ±± 1.12 b | 29.44 ± 1.08 a | |
| 1-Hexanol | 1.06 ± 0.51 b | 10.05 ± 1.04 a | |
| Aldehydes | Butanal, 3-methyl- | - b | 0.09 ± 0.04 a |
| Pentanal | 0.35 ± 0.01 a | -b | |
| Hexanal | 37.48 ± 0.88 a | 36.49 ± 1.12 b | |
| 2-Hexenal, (E)- | 6.73 ± 0.86 b | 9.66 ± 1.03 a | |
| 2-hexenal | 3.28 ± 0.05 a | 0.45 ± 0.02 b | |
| (2E)-2-Ethyl-2-butenal | 0.17 ± 0.01 a | - b | |
| Ketones | 1-Penten-3-one | 0.08 ± 0.02 a | 0.09 ± 0.02 a |
| trans-3-Ethylidene-1-vinyl-2-pyrrolidone | 1.23 ± 0.10 a | - b | |
| 5-Hepten-2-one, 6-methyl- | 0.06 ± 0.02 a | - b | |
| Esters | Acetic acid, propyl ester | - b | 0.13 ± 0.02 a |
| Acetic acid, 2-methylpropyl ester | 0.23 ± 0.01 a | 0.42 ± 0.05 a | |
| Acetic acid, butyl ester | 1.58 ± 0.51 a | 1.29 ± 0.44 a | |
| 1-Butanol, 2-methyl-, acetate | 0.44 ± 0.01 a | - b | |
| 1-Butanol, 3-methyl-, acetate | 9.68 ± 1.04 a | - b | |
| Butanoic acid, propyl ester | 0.08 ± 0.02 a | - b | |
| Propanoic acid, butyl ester | 0.1 ± 0.02 a | 0.07 ± 0.02 a | |
| Acetic acid, pentyl ester | 0.07 ± 0.02 a | - b | |
| Butanoic acid, 2-methyl-, propyl ester | 0.04 ± 0.01 a | - b | |
| 2-Pentanol, propanoate | 0.04 ± 0.01 a | - b | |
| Butanoic acid, butyl ester | 0.07 ± 0.01 a | 0.08 ± 0.01 a | |
| Acetic acid, hexyl ester | 0.14 ± 0.01 a | 0.15 ± 0.01 a | |
| Butyl 2-methylbutanoate | 0.3 ± 0.02 a | 0.15 ± 0.01 b | |
| Butanoic acid, 2-methyl-, hexyl ester | 1.22 ± 0.19 a | 0.39 ± 0.02 b | |
| Hydrocarbons | 7-Oxabicyclo [4.1.0]heptane | 0.54 ± 0.10 a | - b |
| Cyclohexene, 1-methyl-4-(1-methylethenyl)-, (S)- | 0.04 ± 0.01 a | - b | |
| alpha.-Farnesene | 0.13 ± 0.05 a | - b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, G.; Chen, R.; Han, X.; Xue, X. Effects and Mechanism Analysis of Non-Bagging and Bagging Cultivation on the Growth and Content Change of Specific Substances of Fuji Apple Fruit. Plants 2023, 12, 3309. https://doi.org/10.3390/plants12183309
Wang G, Chen R, Han X, Xue X. Effects and Mechanism Analysis of Non-Bagging and Bagging Cultivation on the Growth and Content Change of Specific Substances of Fuji Apple Fruit. Plants. 2023; 12(18):3309. https://doi.org/10.3390/plants12183309
Chicago/Turabian StyleWang, Guiping, Ru Chen, Xueping Han, and Xiaomin Xue. 2023. "Effects and Mechanism Analysis of Non-Bagging and Bagging Cultivation on the Growth and Content Change of Specific Substances of Fuji Apple Fruit" Plants 12, no. 18: 3309. https://doi.org/10.3390/plants12183309
APA StyleWang, G., Chen, R., Han, X., & Xue, X. (2023). Effects and Mechanism Analysis of Non-Bagging and Bagging Cultivation on the Growth and Content Change of Specific Substances of Fuji Apple Fruit. Plants, 12(18), 3309. https://doi.org/10.3390/plants12183309






