Comparison of Salt Stress Tolerance among Two Leaf and Six Grain Cultivars of Amaranthus cruentus L.
Abstract
:1. Introduction
2. Results
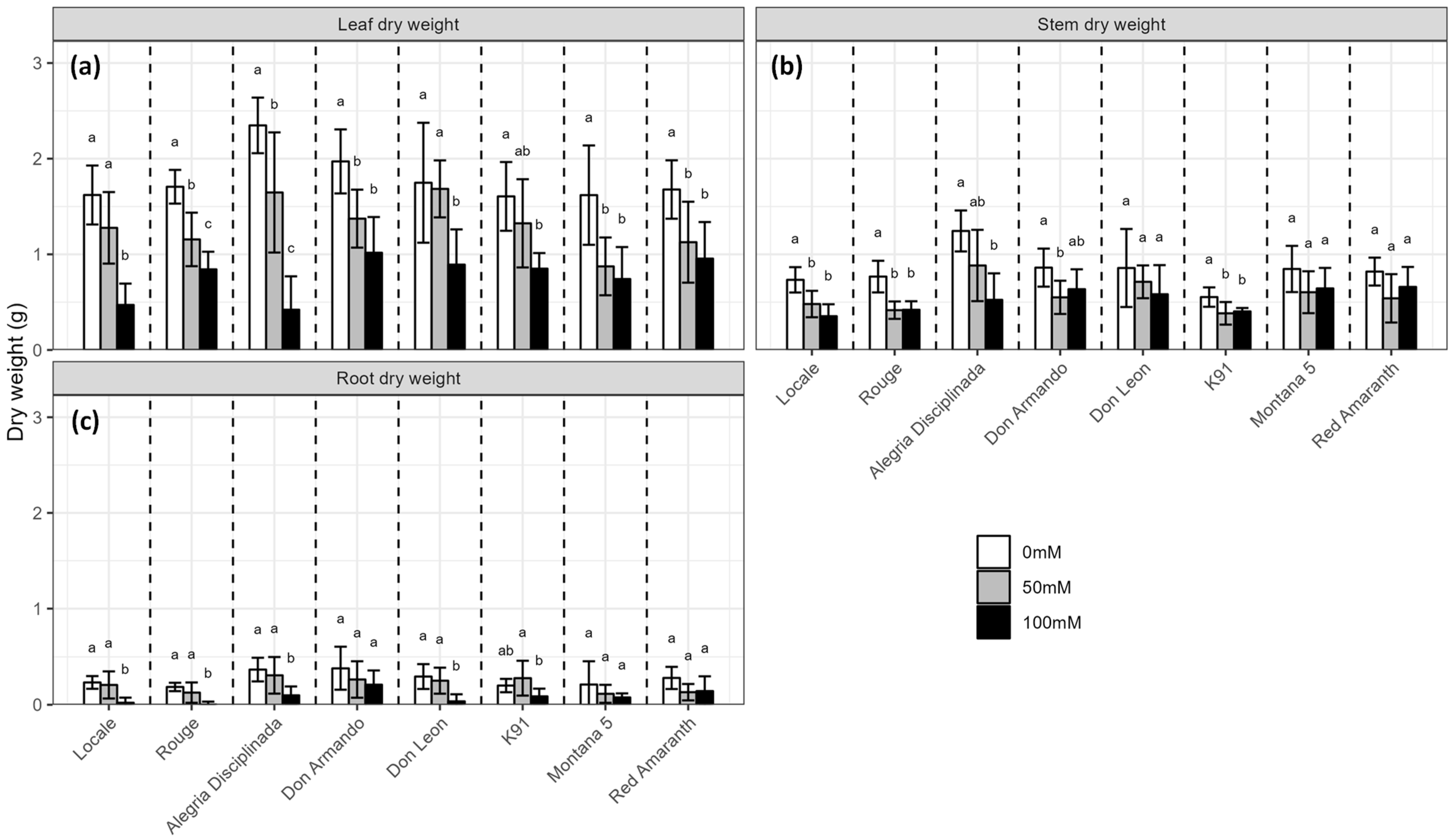
2.1. Biomass Production
2.2. Sodium Distribution in the Plant Organs
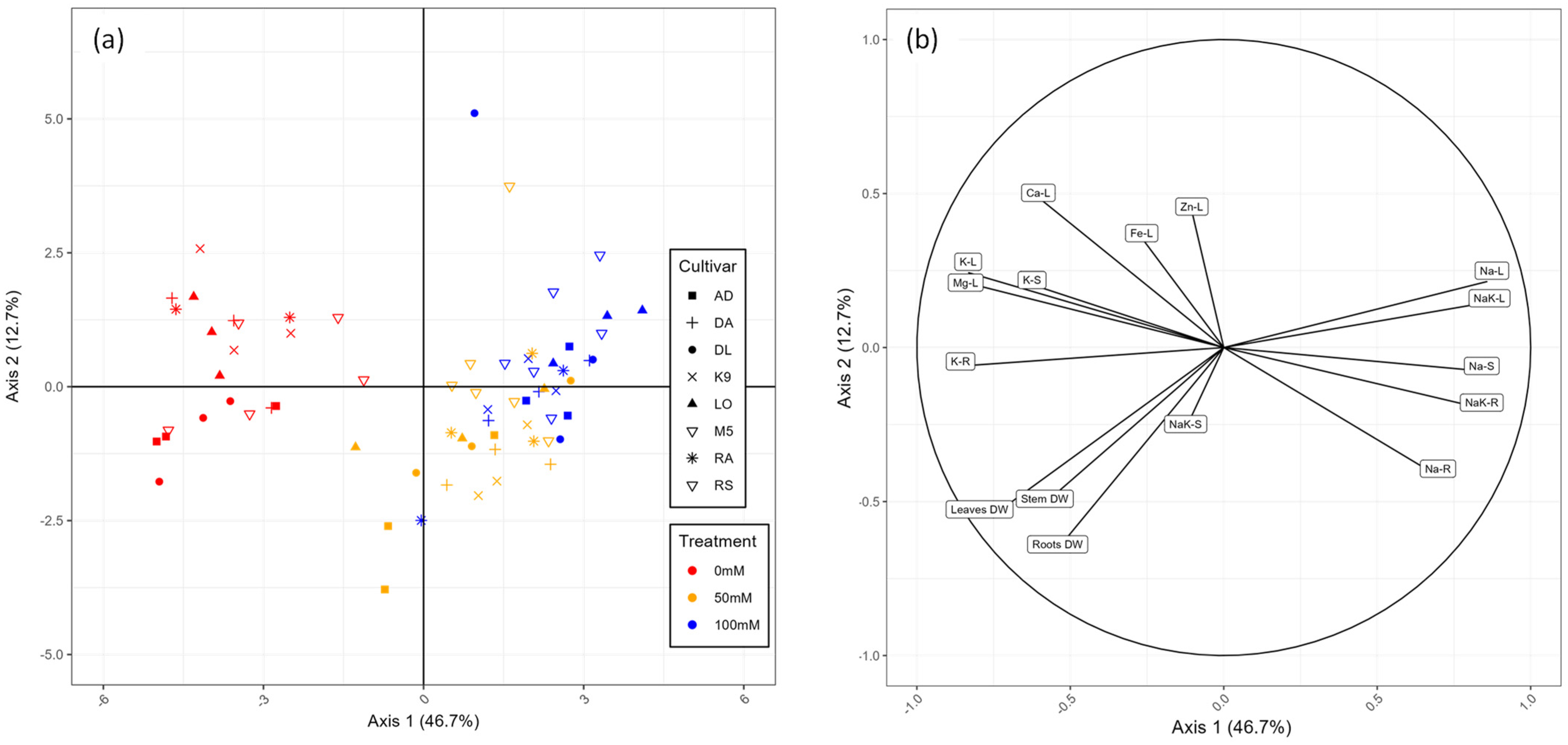
2.3. Overview of the Physiological Response of the Cultivars to Salt Stress
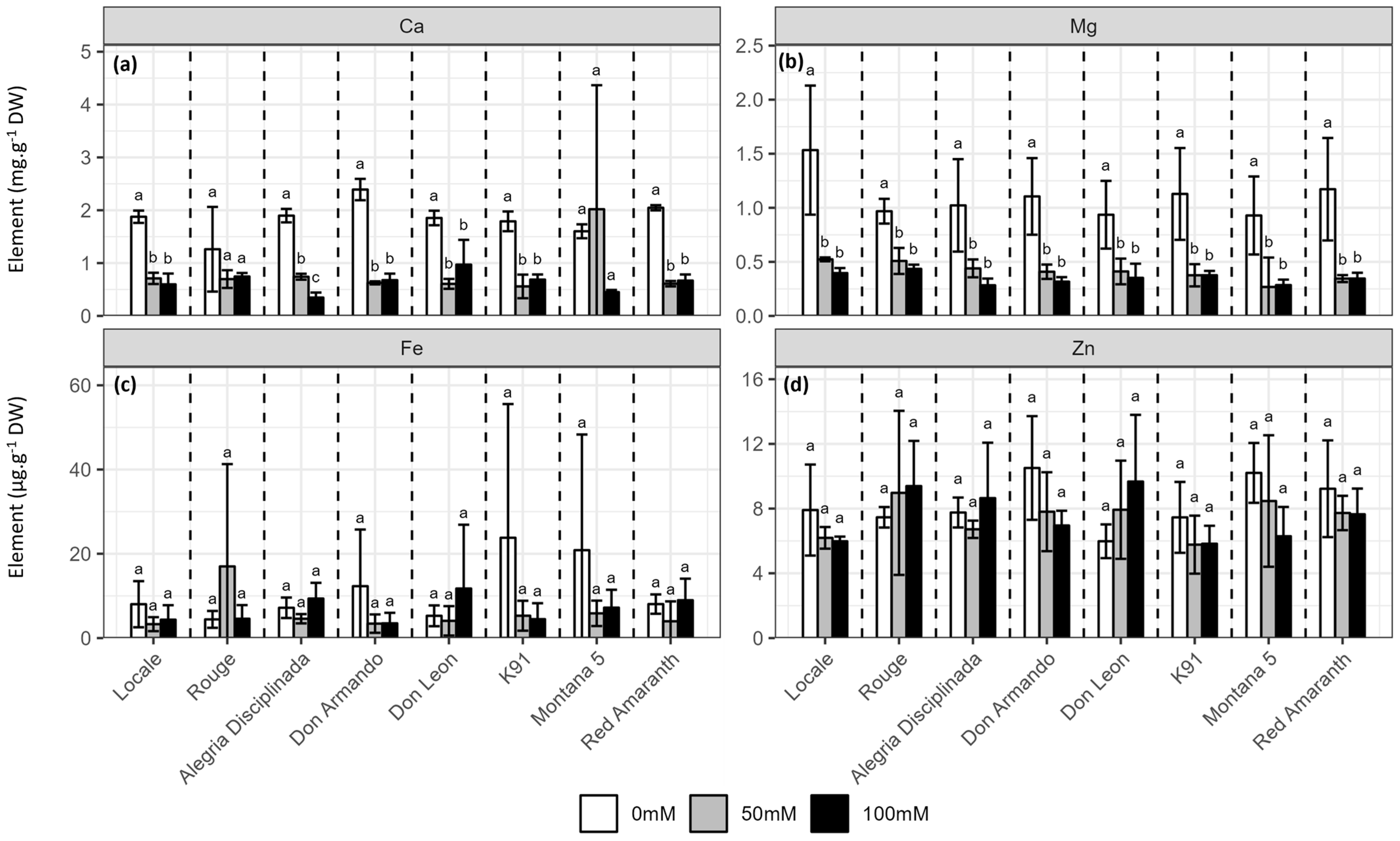
2.4. Mineral Content
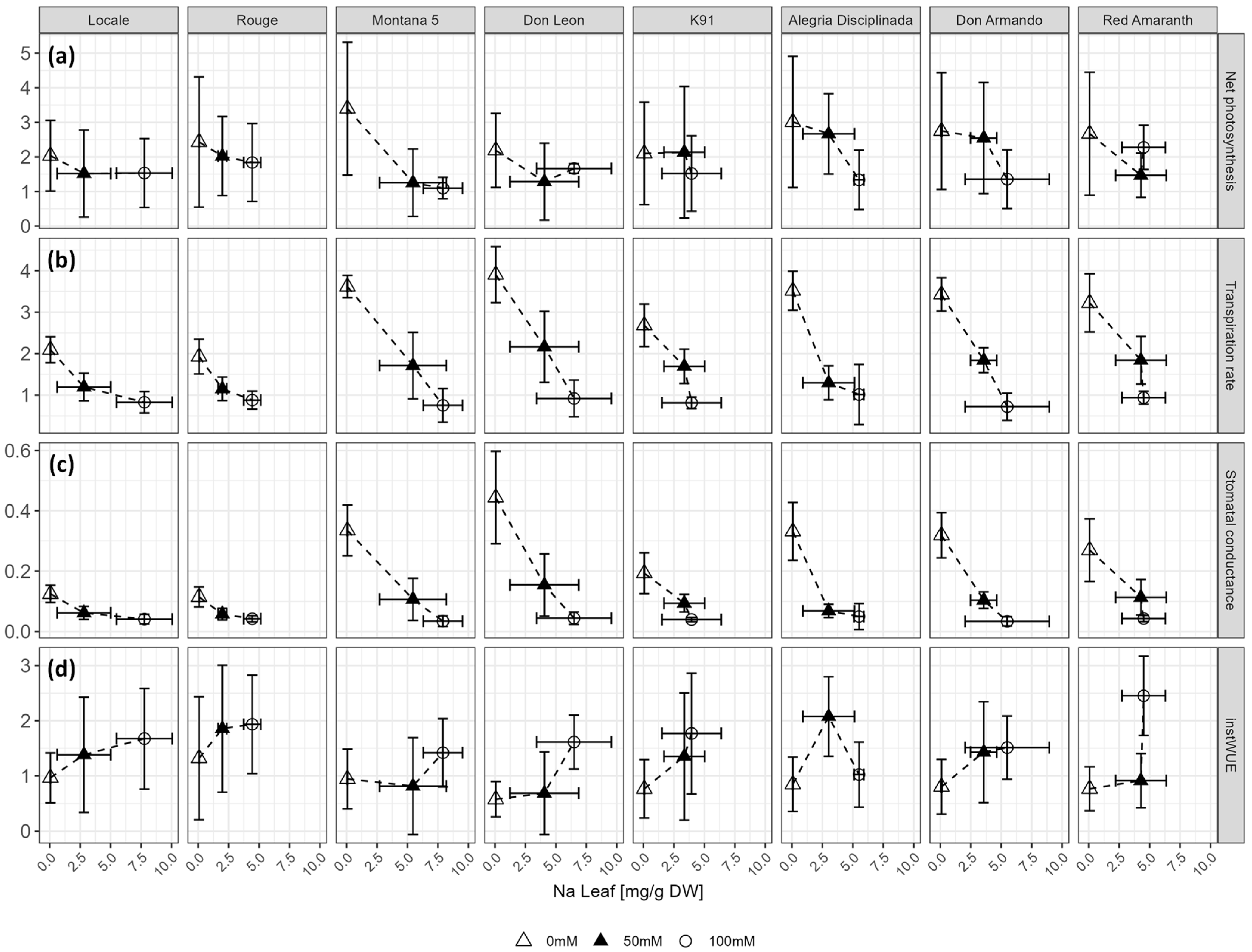
2.5. Photosynthetic Activity in Relation to Sodium Accumulation in Leaves
2.6. Biochemical Compound Contents in Relation to Sodium Accumulation in Leaves
3. Discussion
3.1. Variability in Salt Tolerance among Leaf and Seed Cultivars of A. cruentus
3.2. Putative Physiological Role of Sodium in Amaranth
3.3. Impact of Sodium Accumulation on Photosynthetic Activity
3.4. Foliar Biochemical Activity Response to Salt Stress
3.5. Differences between Leaf and Seed Cultivars
3.6. Effect of Salt of the Nutritional Quality of Amaranth
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Biomass and Harvest
4.3. Biochemical Analyses
4.3.1. Pigments
4.3.2. Phenolics
4.3.3. Malondialdehyde
4.3.4. Ascorbate
4.3.5. Mineral Content
4.4. Photosynthetic Activity
4.5. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mukhopadhyay, R.; Sarkar, B.; Jat, H.S.; Sharma, P.C.; Bolan, N.S. Soil Salinity under Climate Change: Challenges for Sustainable Agriculture and Food Security. J. Environ. Manag. 2021, 280, 111736. [Google Scholar] [CrossRef]
- Butcher, K.; Wick, A.F.; DeSutter, T.; Chatterjee, A.; Harmon, J. Soil Salinity: A Threat to Global Food Security. Agron. J. 2016, 108, 2189–2200. [Google Scholar] [CrossRef]
- Montanarella, L.; Badraoui, M.; Chude, V.; Baptista Costa, I.D.S.; Mamo, T.; Yemefack, M.; Singh Aulakh, M.; Yagi, K.; Young Hong, S.; Vijarnsorn, P.; et al. Status of the World’s Soil Resources Main Report; FAO: Rome, Italy, 2015; ISBN 978-92-5-109004-6. [Google Scholar]
- Hassani, A.; Azapagic, A.; Shokri, N. Predicting Long-Term Dynamics of Soil Salinity and Sodicity on a Global Scale. Proc. Natl. Acad. Sci. USA 2020, 117, 33017–33027. [Google Scholar] [CrossRef] [PubMed]
- Eswar, D.; Karuppusamy, R.; Chellamuthu, S. Drivers of Soil Salinity and Their Correlation with Climate Change. Curr. Opin. Environ. Sustain. 2021, 50, 310–318. [Google Scholar] [CrossRef]
- FAO. Global Map of Salt-Affected Soils: GSASmap v1.0; FAO: Rome, Italy, 2021. [Google Scholar]
- Hopmans, J.W.; Qureshi, A.S.; Kisekka, I.; Munns, R.; Grattan, S.R.; Rengasamy, P.; Ben-Gal, A.; Assouline, S.; Javaux, M.; Minhas, P.S.; et al. Critical Knowledge Gaps and Research Priorities in Global Soil Salinity. In Advances in Agronomy; Academic Press: Cambridge, MA, USA, 2021. [Google Scholar]
- Munns, R.; Tester, M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Passioura, J.B.; Colmer, T.D.; Byrt, C.S. Osmotic Adjustment and Energy Limitations to Plant Growth in Saline Soil. New Phytol. 2020, 225, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- Maathuis, F.J.M. Sodium in Plants: Perception, Signalling, and Regulation of Sodium Fluxes. J. Exp. Bot. 2014, 65, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Day, D.A.; Fricke, W.; Watt, M.; Arsova, B.; Barkla, B.J.; Bose, J.; Byrt, C.S.; Chen, Z.-H.; Foster, K.J.; et al. Energy Costs of Salt Tolerance in Crop Plants. New Phytol. 2019, 225, 1072–1090. [Google Scholar] [CrossRef]
- Chourasia, K.N.; Lal, M.K.; Tiwari, R.K.; Dev, D.; Kardile, H.B.; Patil, V.U.; Kumar, A.; Vanishree, G.; Kumar, D.; Bhardwaj, V.; et al. Salinity Stress in Potato: Understanding Physiological, Biochemical and Molecular Responses. Life 2021, 11, 545. [Google Scholar] [CrossRef]
- EL Sabagh, A.; Islam, M.S.; Skalicky, M.; Ali Raza, M.; Singh, K.; Anwar Hossain, M.; Hossain, A.; Mahboob, W.; Iqbal, M.A.; Ratnasekera, D.; et al. Salinity Stress in Wheat (Triticum aestivum L.) in the Changing Climate: Adaptation and Management Strategies. Front. Agron. 2021, 3, 661932. [Google Scholar] [CrossRef]
- Cao, D.; Li, Y.; Liu, B.; Kong, F.; Tran, L.-S.P. Adaptive Mechanisms of Soybean Grown on Salt-Affected Soils. Land Degrad. Dev. 2018, 29, 1054–1064. [Google Scholar] [CrossRef]
- Zaidi, S.S.-A.; Vanderschuren, H.; Qaim, M.; Mahfouz, M.M.; Kohli, A.; Mansoor, S.; Tester, M. New Plant Breeding Technologies for Food Security. Science 2019, 363, 1390–1391. [Google Scholar] [CrossRef] [PubMed]
- Dawson, I.K.; Powell, W.; Hendre, P.; Bančič, J.; Hickey, J.M.; Kindt, R.; Hoad, S.; Hale, I.; Jamnadass, R. The Role of Genetics in Mainstreaming the Production of New and Orphan Crops to Diversify Food Systems and Support Human Nutrition. New Phytol. 2019, 224, 37–54. [Google Scholar] [CrossRef] [PubMed]
- Curtin, S.; Qi, Y.; Peres, L.E.P.; Fernie, A.R.; Zsögön, A. Pathways to de Novo Domestication of Crop Wild Relatives. Plant Physiol. 2022, 188, 1746–1756. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, W.; Ibe, C.N.; Rhee, S.Y. Renaming Indigenous Crops and Addressing Colonial Bias in Scientific Language. Trends Plant Sci. 2022, 27, 1189–1192. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Dubey, P.K.; Chaurasia, R.; Dubey, R.K.; Pandey, K.K.; Singh, G.S.; Abhilash, P.C. Domesticating the Undomesticated for Global Food and Nutritional Security: Four Steps. Agronomy 2019, 9, 491. [Google Scholar] [CrossRef]
- Ye, C.-Y.; Fan, L. Orphan Crops and Their Wild Relatives in the Genomic Era. Mol. Plant 2021, 14, 27–39. [Google Scholar] [CrossRef] [PubMed]
- van Zonneveld, M.; Kindt, R.; McMullin, S.; Achigan-Dako, E.G.; N’Danikou, S.; Hsieh, W.; Lin, Y.; Dawson, I.K. Forgotten Food Crops in Sub-Saharan Africa for Healthy Diets in a Changing Climate. Proc. Natl. Acad. Sci. USA 2023, 120, e2205794120. [Google Scholar] [CrossRef]
- Razzaq, A.; Saleem, F.; Wani, S.H.; Abdelmohsen, S.A.M.; Alyousef, H.A.; Abdelbacki, A.M.M.; Alkallas, F.H.; Tamam, N.; Elansary, H.O. De-Novo Domestication for Improving Salt Tolerance in Crops. Front. Plant Sci. 2021, 12, 1623. [Google Scholar] [CrossRef]
- Massel, K.; Lam, Y.; Wong, A.C.S.; Hickey, L.T.; Borrell, A.K.; Godwin, I.D. Hotter, Drier, CRISPR: The Latest Edit on Climate Change. Theor. Appl. Genet. 2021, 134, 1691–1709. [Google Scholar] [CrossRef]
- Stetter, M.G.; Schmid, K.J. Analysis of Phylogenetic Relationships and Genome Size Evolution of the Amaranthus Genus Using GBS Indicates the Ancestors of an Ancient Crop. Mol. Phylogenet. Evol. 2017, 109, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Marx, J.L. Speaking of Science: Amaranth: A Comeback for the Food of the Aztecs? Sci. New Ser. 1977, 198, 40. [Google Scholar]
- Kauffman, C.S. Realizing the Potential of Grain Amaranth. Food Rev. Int. 1992, 8, 5–21. [Google Scholar] [CrossRef]
- Stallknecht, G.F.; Schulz-Schaeffer, J.R. Amaranth Rediscovered; Wiley: New York, NY, USA, 1993; pp. 211–218. [Google Scholar]
- Wouyou, A.; Gandonou, C.; Komlan, F.; Montcho, D.; Zanklan, A.; Lutts, S.; Gnancadja, S. Salinity Resistance of Five Amaranth (Amaranthus cruentus) Cultivars at Young Plants Stage. Int. J. Plant Soil Sci. 2017, 14, 1–11. [Google Scholar] [CrossRef]
- Gandonou, C.B.; Prodjinoto, H.; Ahissou Zanklan, S.; Dossou Wouyou, A.; Lutts, S.; Hambada Montcho, D.; Assogba Komlan, F.; Clément Goudjo Mensah, A. Effects of Salinity Stress on Growth in Relation to Gas Exchanges Parameters and Water Status in Amaranth (Amaranthus cruentus). Int. J. Plant Physiol. Biochem. 2018, 10, 19–27. [Google Scholar] [CrossRef]
- Omami, E.N.; Hammes, P.S.; Robbertse, P.J. Differences in Salinity Tolerance for Growth and Water-use Efficiency in Some Amaranth (Amaranthus spp.) Genotypes. N. Z. J. Crop Hortic. Sci. 2006, 34, 11–22. [Google Scholar] [CrossRef]
- Huerta-Ocampo, J.A.; Barrera-Pacheco, A.; Mendoza-Hernández, C.S.; Espitia-Rangel, E.; Mock, H.-P.; Barba de la Rosa, A.P. Salt Stress-Induced Alterations in the Root Proteome of Amaranthus cruentus L. J. Proteome Res. 2014, 13, 3607–3627. [Google Scholar] [CrossRef]
- Wouyou, A.; Prodjinoto, H.; Zanklan, A.S.; Vanpee, B.; Lutts, S.; Gandonou, C.B. Implication of Ions and Organic Solutes Accumulation in Amaranth (Amaranthus cruentus L.) Salinity Resistance. Am. J. Plant Sci. 2019, 10, 2335–2353. [Google Scholar] [CrossRef]
- Barba de la Rosa, A.P.; Huerta-Ocampo, J.A.; González-Escobar, J.L.; Aguilar-Hernández, H.S.; Salcedo-Barrientos, G.; Espitia-Rangel, E. Differential Expression of Iron Transporters in Amaranthus cruentus Roots When Are Subjected to Salt Stress: The Influence of Root Endophytes. Rhizosphere 2022, 24, 100620. [Google Scholar] [CrossRef]
- Luyckx, A.; Beghin, C.; Quinet, M.; Achadé, B.; Prodjinoto, H.; Gandonou, C.B.; Lutts, S. Salinity Differently Affects Antioxidant Content and Amino Acid Profile in Two Cultivars of Amaranthus cruentus Differing in Salinity Tolerance. J. Sci. Food Agric. 2021, 101, 6211–6219. [Google Scholar] [CrossRef]
- Mlakar, S.G.; Bavec, M.; Jakop, M.; Bavec, F. The Effect of Drought Occurring at Different Growth Stages on Productivity of Grain Amaranth Amaranthus cruentus G6. J. Life Sci. 2012, 6, 283–286. [Google Scholar]
- Babayev, H.; Mehvaliyeva, U.; Aliyeva, M.; Feyziyev, Y.; Guliyev, N. The Study of NAD-Malic Enzyme in Amaranthus cruentus L. under Drought. Plant Physiol. Biochem. 2014, 81, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Tebini, M.; Luu, D.T.; Mguis, K.; Ben Ahmed, H.; Meddich, A.; Zribi, F.; Chalh, A. Physiological Exploration of Intra-Specific Variability in Salinity Tolerance of Amaranth. Russ. J. Plant Physiol. 2022, 69, 59. [Google Scholar] [CrossRef]
- Tebini, M.; Rabaoui, G.; M’Rah, S.; Luu, D.-T.; Ben Ahmed, H.; Chalh, A. Effects of Salinity on Germination Dynamics and Seedling Development in Two Amaranth Genotypes. Physiol. Mol. Biol. Plants 2022, 28, 1489–1500. [Google Scholar] [CrossRef] [PubMed]
- Pulvento, C.; Sellami, M.H.; Lavini, A. Yield and Quality of Amaranthus hypochondriacus Grain Amaranth under Drought and Salinity at Various Phenological Stages in Southern Italy. J. Sci. Food Agric. 2022, 102, 5022–5033. [Google Scholar] [CrossRef] [PubMed]
- Lavini, A.; Pulvento, C.; d’Andria, R.; Riccardi, M.; Jacobsen, S.E. Effects of Saline Irrigation on Yield and Qualitative Characterization of Seed of an Amaranth Accession Grown under Mediterranean Conditions. J. Agric. Sci. 2016, 154, 858–869. [Google Scholar] [CrossRef]
- Sarker, U.; Islam, M.T.; Oba, S. Salinity Stress Accelerates Nutrients, Dietary Fiber, Minerals, Phytochemicals and Antioxidant Activity in Amaranthus tricolor Leaves. PLoS ONE 2018, 13, e0206388. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Salinity Stress Enhances Color Parameters, Bioactive Leaf Pigments, Vitamins, Polyphenols, Flavonoids and Antioxidant Activity in Selected Amaranthus Leafy Vegetables. J. Sci. Food Agric. 2019, 99, 2275–2284. [Google Scholar] [CrossRef]
- Guo, S.-H.; Hu, N.; Li, Q.-S.; Yang, P.; Wang, L.-L.; Xu, Z.-M.; Chen, H.-J.; He, B.-Y.; Zeng, E.Y. Response of Edible Amaranth Cultivar to Salt Stress Led to Cd Mobilization in Rhizosphere Soil: A Metabolomic Analysis. Environ. Pollut. 2018, 241, 422–431. [Google Scholar] [CrossRef]
- Bellache, M.; Allal Benfekih, L.; Torres-Pagan, N.; Mir, R.; Verdeguer, M.; Vicente, O.; Boscaiu, M. Effects of Four-Week Exposure to Salt Treatments on Germination and Growth of Two Amaranthus Species. Soil. Syst. 2022, 6, 57. [Google Scholar] [CrossRef]
- Sage, R.F.; Sage, T.L.; Pearcy, R.W.; Borsch, T. The Taxonomic Distribution of C4 Photosynthesis in Amaranthaceae Sensu Stricto. Am. J. Bot. 2007, 94, 1992–2003. [Google Scholar] [CrossRef] [PubMed]
- Mapes, C.; Basurto, F.; Bye, R. Ethnobotany of Quintonil: Knowledge, Use and Management of Edible Greens Amaranthus spp. (Amaranthaceae) in the Sierra Norte de Puebla, México. Econ. Bot. 1997, 51, 293–306. [Google Scholar] [CrossRef]
- Ogwu, M.C. Value of Amaranthus [L.] Species in Nigeria. In Nutritional Value of Amaranth; IntechOpen: London, UK, 2020; ISBN 978-1-83880-084-0. [Google Scholar]
- Kietlinski, K.D.; Jimenez, F.; Jellen, E.N.; Maughan, P.J.; Smith, S.M.; Pratt, D.B. Relationships between the Weedy Amaranthus hybridus (Amaranthaceae) and the Grain Amaranths. Crop Sci. 2014, 54, 220–228. [Google Scholar] [CrossRef]
- Stetter, M.G.; Müller, T.; Schmid, K.J. Genomic and Phenotypic Evidence for an Incomplete Domestication of South American Grain Amaranth (Amaranthus caudatus). Mol. Ecol. 2017, 26, 871–886. [Google Scholar] [CrossRef] [PubMed]
- Stetter, M.G.; Vidal-Villarejo, M.; Schmid, K.J. Parallel Seed Color Adaptation during Multiple Domestication Attempts of an Ancient New World Grain. Mol. Biol. Evol. 2019, 37, 1407–1419. [Google Scholar] [CrossRef]
- Dinssa, F.F.; Yang, R.-Y.; Ledesma, D.R.; Mbwambo, O.; Hanson, P. Effect of Leaf Harvest on Grain Yield and Nutrient Content of Diverse Amaranth Entries. Sci. Hortic. 2018, 236, 146–157. [Google Scholar] [CrossRef]
- Hoidal, N.; Díaz Gallardo, M.; Jacobsen, S.-E.; Alandia, G. Amaranth as a Dual-Use Crop for Leafy Greens and Seeds: Stable Responses to Leaf Harvest Across Genotypes and Environments. Front. Plant Sci. 2019, 10, 817. [Google Scholar] [CrossRef]
- Hoidal, N.; Jacobsen, S.-E.; Odone, A.; Alandia, G. Defoliation Timing for Optimal Leaf Nutrition in Dual-Use Amaranth Production Systems. J. Sci. Food Agric. 2020, 100, 4745–4755. [Google Scholar] [CrossRef]
- Martinez-Lopez, A.; Millan-Linares, M.C.; Rodriguez-Martin, N.M.; Millan, F.; Montserrat-de la Paz, S. Nutraceutical Value of Kiwicha (Amaranthus caudatus L.). J. Funct. Foods 2020, 65, 103735. [Google Scholar] [CrossRef]
- José Rodríguez Gómez, M.; Maestro-Gaitán, I.; Calvo Magro, P.; Cruz Sobrado, V.; Reguera Blázquez, M.; Matías Prieto, J. Unique Nutritional Features That Distinguish Amaranthus cruentus L. and Chenopodium Quinoa Willd Seeds. Food Res. Int. 2023, 164, 112160. [Google Scholar] [CrossRef]
- Villarreal, M.; Iturriaga, L.B. Amaranth: An Andean Crop with History, Its Feeding Reassessment in America. In Traditional Foods; Kristbergsson, K., Oliveira, J., Eds.; Springer: Boston, MA, USA, 2016; pp. 217–232. ISBN 978-1-4899-7646-8. [Google Scholar]
- Ngugi, C.C.; Oyoo-Okoth, E.; Manyala, J.O.; Fitzsimmons, K.; Kimotho, A. Characterization of the Nutritional Quality of Amaranth Leaf Protein Concentrates and Suitability of Fish Meal Replacement in Nile Tilapia Feeds. Aquac. Rep. 2017, 5, 62–69. [Google Scholar] [CrossRef]
- Tang, Y.; Li, X.; Chen, P.X.; Zhang, B.; Hernandez, M.; Zhang, H.; Marcone, M.F.; Liu, R.; Tsao, R. Lipids, Tocopherols, and Carotenoids in Leaves of Amaranth and Quinoa Cultivars and a New Approach to Overall Evaluation of Nutritional Quality Traits. J. Agric. Food Chem. 2014, 62, 12610–12619. [Google Scholar] [CrossRef] [PubMed]
- Gimplinger, D.M.; Dobos, G.; Schönlechner, R.; Kaul, H.-P. Yield and Quality of Grain Amaranth (Amaranthus Sp.) in Eastern Austria. Plant Soil Environ. 2008, 53, 105–112. [Google Scholar] [CrossRef]
- Dinssa, F.F.; Hanson, P.; Ledesma, D.R.; Minja, R.; Mbwambo, O.; Tilya, M.S.; Stoilova, T. Yield of Vegetable Amaranth in Diverse Tanzanian Production Environments. HortTechnology 2019, 29, 516–527. [Google Scholar] [CrossRef]
- Bigot, S.; Fuksová, M.; Martínez, J.-P.; Lutts, S.; Quinet, M. Sodium and Chloride Accumulation and Repartition Differed between the Cultivated Tomato (Solanum lycopersicum) and Its Wild Halophyte Relative Solanum Chilense under Salt Stress. Sci. Hortic. 2023, 321, 112324. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Raihan, M.R.H.; Masud, A.A.C.; Rahman, K.; Nowroz, F.; Rahman, M.; Nahar, K.; Fujita, M. Regulation of Reactive Oxygen Species and Antioxidant Defense in Plants under Salinity. Int. J. Mol. Sci. 2021, 22, 9326. [Google Scholar] [CrossRef] [PubMed]
- Prodjinoto, H.; Gandonou, C.; Lutts, S. Screening for Salinity Tolerance of Oryza glaberrima Steud. Seedlings. Afr. J. Agric. Res. 2018, 13, 561–573. [Google Scholar] [CrossRef]
- Aubert, L.; Quinet, M. Comparison of Heat and Drought Stress Responses among Twelve Tartary Buckwheat (Fagopyrum tataricum) Varieties. Plants 2022, 11, 1517. [Google Scholar] [CrossRef]
- Munns, R.; Hare, R.A.; James, R.A.; Rebetzke, G.J. Genetic Variation for Improving the Salt Tolerance of Durum Wheat. Aust. J. Agric. Res. 2000, 51, 69–74. [Google Scholar] [CrossRef]
- Subbarao, G.V.; Ito, O.; Berry, W.L.; Wheeler, R.M. Sodium—A Functional Plant Nutrient. Crit. Rev. Plant Sci. 2003, 22, 391–416. [Google Scholar] [CrossRef]
- Rao, X.; Dixon, R.A. The Differences between NAD-ME and NADP-ME Subtypes of C4 Photosynthesis: More than Decarboxylating Enzymes. Front. Plant Sci. 2016, 7, 1525. [Google Scholar] [CrossRef] [PubMed]
- Johnston, M.; Grof, C.P.L.; Brownell, P.F. The Effect of Sodium Nutrition on the Pool Sizes of Intermediates of the C4 Photosynthetic Pathway. Funct. Plant Biol. 1988, 15, 749–760. [Google Scholar] [CrossRef]
- Murata, S.; Kobayashi, M.; Matoh, T.; Sekiya, J. Sodium Stimulates Regeneration of Phosphoenolpyruvate in Mesophyll Chloroplasts of Amaranthus tricolor. Plant Cell Physiol. 1992, 33, 1247–1250. [Google Scholar] [CrossRef]
- Ohta, D.; Yasuoka, S.; Matoh, T.; Takahashi, E. Sodium Stimulates Growth of Amaranthus tricolor L. Plants through Enhanced Nitrate Assimilation. Plant Physiol. 1989, 89, 1102–1105. [Google Scholar] [CrossRef] [PubMed]
- Ohta, D.; Matoh, T.; Takahashi, E. Early Responses of Sodium-Deficient Amaranthus tricolor L. Plants to Sodium Application. Plant Physiol. 1987, 84, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Ohta, D.; Matoh, T.; Takahashi, E. Sodium-Stimulated NO3− Uptake in Amaranthus tricolor L. Plants. Plant Physiol. 1988, 87, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Matoh, T.; Ohta, D.; Takahashi, E. Effect of Sodium Application on Growth of Amaranthus tricolor L. Plant Cell Physiol. 1986, 27, 187–192. [Google Scholar] [CrossRef]
- Yan, J.; Zhu, C.; Liu, W.; Luo, F.; Mi, J.; Ren, Y.; Li, J.; Sang, T. High Photosynthetic Rate and Water Use Efficiency of Miscanthus lutarioriparius Characterize an Energy Crop in the Semiarid Temperate Region. GCB Bioenergy 2015, 7, 207–218. [Google Scholar] [CrossRef]
- Tarin, T.; Nolan, R.H.; Medlyn, B.E.; Cleverly, J.; Eamus, D. Water-Use Efficiency in a Semi-Arid Woodland with High Rainfall Variability. Glob. Chang. Biol. 2020, 26, 496–508. [Google Scholar] [CrossRef]
- Liao, Q.; Gu, S.; Kang, S.; Du, T.; Tong, L.; Wood, J.D.; Ding, R. Mild Water and Salt Stress Improve Water Use Efficiency by Decreasing Stomatal Conductance via Osmotic Adjustment in Field Maize. Sci. Total Environ. 2022, 805, 150364. [Google Scholar] [CrossRef]
- Bromham, L.; Bennett, T.H. Salt Tolerance Evolves More Frequently in C4 Grass Lineages. J. Evol. Biol. 2014, 27, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Tao, R.; Ding, J.; Li, C.; Zhu, X.; Guo, W.; Zhu, M. Evaluating and Screening of Agro-Physiological Indices for Salinity Stress Tolerance in Wheat at the Seedling Stage. Front. Plant Sci. 2021, 12, 646175. [Google Scholar] [CrossRef] [PubMed]
- Nieves-Cordones, M.; Al Shiblawi, F.R.; Sentenac, H. Roles and Transport of Sodium and Potassium in Plants. In The Alkali Metal Ions: Their Role for Life; Sigel, A., Sigel, H., Sigel, R.K.O., Eds.; Metal Ions in Life Sciences; Springer International Publishing: Cham, Switzerland, 2016; pp. 291–324. ISBN 978-3-319-21756-7. [Google Scholar]
- Yalcinkaya, T.; Uzilday, B.; Ozgur, R.; Turkan, I.; Mano, J. Lipid Peroxidation-Derived Reactive Carbonyl Species (RCS): Their Interaction with ROS and Cellular Redox during Environmental Stresses. Environ. Exp. Bot. 2019, 165, 139–149. [Google Scholar] [CrossRef]
- Alché, J.d.D. A Concise Appraisal of Lipid Oxidation and Lipoxidation in Higher Plants. Redox Biol. 2019, 23, 101136. [Google Scholar] [CrossRef]
- Singh, A.; Roychoudhury, A. Role of Phenolic Acids and Flavonoids in the Mitigation of Environmental Stress in Plants. In Biology and Biotechnology of Environmental Stress Tolerance in Plants: Volume 1: Secondary Metabolites in Environmental Stress Tolerance; Apple Academic Press: Palm Bay, FL, USA, 2023; pp. 227–248. ISBN 978-1-00-334617-3. [Google Scholar]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive Oxygen Species and Antioxidant Machinery in Abiotic Stress Tolerance in Crop Plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Duarte, B.; Santos, D.; Marques, J.C.; Caçador, I. Ecophysiological Adaptations of Two Halophytes to Salt Stress: Photosynthesis, PS II Photochemistry and Anti-Oxidant Feedback—Implications for Resilience in Climate Change. Plant Physiol. Biochem. 2013, 67, 178–188. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Augmentation of Leaf Color Parameters, Pigments, Vitamins, Phenolic Acids, Flavonoids and Antioxidant Activity in Selected Amaranthus tricolor under Salinity Stress. Sci. Rep. 2018, 8, 12349. [Google Scholar] [CrossRef]
- Nakashima, T.; Araki, T.; Ueno, O. Photoprotective Function of Betacyanin in Leaves of Amaranthus cruentus L. under Water Stress. Photosynthetica 2011, 49, 497–506. [Google Scholar] [CrossRef]
- Vargas-Ortiz, E.; Délano-Frier, J.P.; Tiessen, A. The Tolerance of Grain Amaranth (Amaranthus cruentus L.) to Defoliation during Vegetative Growth Is Compromised during Flowering. Plant Physiol. Biochem. 2015, 91, 36–40. [Google Scholar] [CrossRef]
- Vargas-Ortiz, E.; Espitia-Rangel, E.; Tiessen, A.; Délano-Frier, J.P. Grain Amaranths Are Defoliation Tolerant Crop Species Capable of Utilizing Stem and Root Carbohydrate Reserves to Sustain Vegetative and Reproductive Growth after Leaf Loss. PloS ONE 2013, 8, e67879. [Google Scholar] [CrossRef] [PubMed]
- Castrillón-Arbeláez, P.A.; Martínez-Gallardo, N.; Arnaut, H.A.; Tiessen, A.; Délano-Frier, J.P. Metabolic and Enzymatic Changes Associated with Carbon Mobilization, Utilization and Replenishment Triggered in Grain Amaranth (Amaranthus cruentus) in Response to Partial Defoliation by Mechanical Injury or Insect Herbivory. BMC Plant Biol. 2012, 12, 163. [Google Scholar] [CrossRef] [PubMed]
- Achigan-Dako, E.G.; Sogbohossou, O.E.D.; Maundu, P. Current Knowledge on Amaranthus Spp.: Research Avenues for Improved Nutritional Value and Yield in Leafy Amaranths in Sub-Saharan Africa. Euphytica 2014, 197, 303–317. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Response of Nutrients, Minerals, Antioxidant Leaf Pigments, Vitamins, Polyphenol, Flavonoid and Antioxidant Activity in Selected Vegetable Amaranth under Four Soil Water Content. Food Chem. 2018, 252, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Escudero, N.L.; Albarracín, G.J.; Lucero López, R.V.; Giménez, M.S. Antioxidant Activity and Phenolic Content of Flour and Protein Concentrate of Amaranthus cruentus Seeds: Antioxidant Activity of Amaranthus cruentus. J. Food Biochem. 2011, 35, 1327–1341. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Drought Stress Enhances Nutritional and Bioactive Compounds, Phenolic Acids and Antioxidant Capacity of Amaranthus Leafy Vegetable. BMC Plant Biol. 2018, 18, 258. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. In Methods in Enzymology; Plant Cell Membranes; Academic Press: Cambridge, MA, USA, 1987; Volume 148, pp. 350–382. [Google Scholar]
- Howard, J.E.; Villamil, M.B.; Riggins, C.W. Amaranth as a Natural Food Colorant Source: Survey of Germplasm and Optimization of Extraction Methods for Betalain Pigments. Front. Plant Sci. 2022, 13, 932440. [Google Scholar] [CrossRef] [PubMed]
- Sarker, U.; Hossain, M.M.; Oba, S. Nutritional and Antioxidant Components and Antioxidant Capacity in Green Morph Amaranthus Leafy Vegetable. Sci. Rep. 2020, 10, 1336. [Google Scholar] [CrossRef]
- Woisky, R.G.; Salatino, A. Analysis of Propolis: Some Parameters and Procedures for Chemical Quality Control. J. Apic. Res. 1998, 37, 99–105. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of Total Phenolic Content and Other Oxidation Substrates in Plant Tissues Using Folin–Ciocalteu Reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Tsikas, D. Assessment of Lipid Peroxidation by Measuring Malondialdehyde (MDA) and Relatives in Biological Samples: Analytical and Biological Challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in Isolated Chloroplasts: I. Kinetics and Stoichiometry of Fatty Acid Peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Kampfenkel, K.; Vanmontagu, M.; Inze, D. Extraction and Determination of Ascorbate and Dehydroascorbate from Plant Tissue. Anal. Biochem. 1995, 225, 165–167. [Google Scholar] [CrossRef]
- Bigot, S.; Leclef, C.; Rosales, C.; Martínez, J.-P.; Lutts, S.; Quinet, M. Comparison of the Salt Resistance of Solanum lycopersicum × Solanum chilense Hybrids and Their Parents. Front. Hortic. 2023, 2, 1130702. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]





| Cultivar | STI50mM | STI100mM |
|---|---|---|
| Locale | 0.759 | 0.327 |
| Rouge | 0.639 | 0.476 |
| Montana 5 | 0.595 | 0.546 |
| Don Leon | 0.913 | 0.521 |
| K91 | 0.841 | 0.569 |
| Alegria Disciplinada | 0.717 | 0.264 |
| Don Armando | 0.681 | 0.580 |
| Red Amaranth | 0.631 | 0.617 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luyckx, A.; Lutts, S.; Quinet, M. Comparison of Salt Stress Tolerance among Two Leaf and Six Grain Cultivars of Amaranthus cruentus L. Plants 2023, 12, 3310. https://doi.org/10.3390/plants12183310
Luyckx A, Lutts S, Quinet M. Comparison of Salt Stress Tolerance among Two Leaf and Six Grain Cultivars of Amaranthus cruentus L. Plants. 2023; 12(18):3310. https://doi.org/10.3390/plants12183310
Chicago/Turabian StyleLuyckx, Adrien, Stanley Lutts, and Muriel Quinet. 2023. "Comparison of Salt Stress Tolerance among Two Leaf and Six Grain Cultivars of Amaranthus cruentus L." Plants 12, no. 18: 3310. https://doi.org/10.3390/plants12183310






