Abstract
The aim of the current research is to evaluate the allelopathic activity of fifty grass genotypes from different species and to identify phenolic compounds in the genotypes that have the highest allelopathic activity and inhibitory effect on Eruca sativa L. (Rocket). Aqueous extract was prepared from the leaves of grass genotypes in different concentrations and its effect on germination and growth of E. sativa L. was measured. According to the results, the type of genotype and the concentration of the extract significantly decreased the percentage of germination, hypocotyl length, radicle length, and dry weight of E. sativa L. seedlings. Increasing the concentration of the extract resulted in a decrease in germination and growth of seedlings. The genotypes of Festulolium (Festulolium) (GR 5009, GR 1692, GR 5004) had the most inhibitory effect on the growth of E. sativa L. Also, among the genotypes studied, two genotypes (DG-M) and (DG-P) of Dactylis glomerata L. (orchardgrass) species showed the least allelopathic activity. The results of HPLC-MS indicated nine phenolic compounds including caffeic acid, syringic acid, vanillic acid, p-coumaric acid, ferulic acid, apigenin acid, chlorogenic acid, 4-hydroxybenzoic acid, and gallic acid. The phenolic compound most present in the aqueous extract was caffeic acid. However, phenolic compounds derived from Festulolium genotypes showed the greatest allelopathic action on the growth parameters of E. sativa L. The aqueous extracts of the Festulolium genotypes can be considered valid systems of sustainable weed control thanks to the phytocomplex rich in phenols.
1. Introduction
Weed management is an important and challenging process in the face of exploding world population, loss of available resources, and increasing environmental stress [1]. Weeds can cause yield losses of about 40%, which can reach 100% without weed control measures [2]. Weed control is essential for global food security [3] and the use of herbicides is the most common method of weed control [4]. Herbicides account for nearly half (47.5%) of the pesticides used for weed control worldwide each year [5]. The widespread and inappropriate use of synthetic herbicides leads to environmental damage (soil and water pollution), herbicide residues in food [6], and the increase of herbicide-resistant weeds [7,8], which is a major threat to food safety and human health [9].
Allelopathy can be used as a sustainable and effective strategy to prevent environmental pollution and herbicide resistance in weed control [10]. Allelopathy is a process in which one plant species stimulates or inhibits the growth of another plant species through certain secondary metabolites [11]. These secondary metabolites, called allelochemicals, enter the environment mainly through evaporation, leaching, root secretion, or decomposition of plant residues and can affect seed germination and the growth of nearby seedlings [10]. These compounds exert their effects primarily on cell division, membrane permeability, phytohormone production, photosynthesis, respiration, and enzyme activity [12]. The chemical nature of allelochemicals is complex and diverse (organic acids, aldehydes, coumarins, quinones, flavonoids, alkaloids, terpenoids, etc.), but most are produced by three main biosynthetic pathways, the shikimic acid pathway (benzoic and cinnamic acids and their derivatives, coumarins, glycosides, alkaloids, etc.) and the acetic and mevalonic acid pathways (terpenoids, steroids, complex quinones). Allelopathic compounds exist in almost all plants and are found in many plant parts such as roots, seeds, leaves, fruits, and stems [13]. Most allelochemicals are phytotoxic and interfere with the physiological parameters of target plants when they encounter the plant cell wall [14]. These phytochemicals can be used as toxic compounds to introduce new herbicides [15,16].
Phenolic compounds are among the most abundant allelochemical groups in plants and are primarily synthesized through the shikimate pathway in plants [17]. Several phenolic compounds, including vanillic acid, syringic acid, p-coumaric acid, and ferulic acid, have been identified as allelochemicals in natural and controlled ecosystems that can act as natural herbicides; therefore, plants with allelopathic activity can be used for natural weed control [18].
Allelopathy studies have been conducted on different parts of the plant and it has been found that aqueous extracts of leaves show higher germination inhibition, probably due to the higher metabolic activity of leaves, which contain more allelochemical compounds than other tissues [19,20,21]. Allelopathic aqueous extracts are water-soluble allelochemicals extracted from plants that can be used as natural herbicides and are more environmentally friendly than synthetic herbicides [15,16]. Allelopathic compounds from plant extracts have inhibitory effects on weed growth due to their diverse structures and mechanisms of action [22].
The Poaceae family contains various allelochemicals that can suppress the population and growth of weeds [23]. These allelochemicals can prevent the germination and growth of various weeds, including herbicide-resistant weeds [24]. The allelopathic effect depends on the donor and recipient species, their growth stage, and the toxicity level of the released allelochemicals [1]. The study on grass species has demonstrated the existence of phenolic compounds such as vanillic acid, chlorogenic acid, caffeic acid, ferulic acid, and coumaric acid [25] and allelopathic activity in some genotypes and species of grasses, which can have an inhibitory effect on weed germination and growth [11,26,27].
Eruca sativa L. is a summer annual herbaceous plant of the Brassicaceae family that is widely distributed in temperate regions [28]. E. sativa is widely cultivated because of its importance in industry, agriculture, and medicine [29]. However, it has become an invasive weed in some areas, causing yield losses of 6–36% in some crops, including Sesamum indicum and Avena sativa L., due to its ability to grow rapidly and high seed production. E. sativa seeds have a wide germination temperature range and dormancy cycle, which creates a stable seed bank in the soil and allows it to better adapt to harsh climates and low temperatures [30].
Although Eruca sativa L. has been known as a crop plant for a long time, it can be a weed of cool-season crops. Therefore, its biological control through allelopathy is important. For this reason, E. sativa L. was investigated in this study.
The present study was conducted with the aim of (i) evaluating the aqueous extract of different grass genotypes (Table 1) on the inhibition of germination and growth of E. sativa L., and (ii) determining and identifying phenolic compounds in the genotypes that showed the highest allelopathy activity.

Table 1.
Information on 50 grass genotypes used for aqueous extract.
2. Results
2.1. Germination Percentage
The results of the experiment show that the two factors of genotype, the concentration of plant extract, and their interaction have a significant effect (at 1% level) on the percentage of germination Eruca sativa L. (Table 2).

Table 2.
Analysis of variance of the effect of the grass genotypes and extract concentrations on the germination indices of Eruca sativa L.
The highest decrease in the percentage of germination by (−76.5%) and (−76.13%) was observed in extract concentrations (100%) and (75%), respectively, compared to the control (Table 3), while there was no statistically significant difference at 1% level between these two treatments.

Table 3.
Mean comparison for seed germination indices of Eruca sativa L. affected by the extract concentrations of the grass genotypes.
The concentration of 12.5% did not show any inhibitory effect on germination. PCA analysis (Figure 1) showed that water extracts of genotypes GR 1692, GR 5004, GR 5009, and GR 5003 of X Festulolium species, FA-B, FA-F, 20L-HS, 23M-HS, 10E-P, 6L-HS genotypes of Festuca arundinacea Schreb. (tall fescue) species, LP-AR1 genotype of Lolium perenne L. (perennial ryegrass) species, LM-AL genotype of Lolium multiflorum Lam. (Italian ryegrass) species, BI-G25 genotype of Bromus inermis L. (smooth bromegrass) species, and LH-T genotype of Lolium×hybridum Hausskn species had the greatest inhibitory effect on E. sativa L. and caused a decrease in germination up to 46.1%. Among these genotypes, Festulolium genotypes showed the highest growth inhibition effect (Table 4).
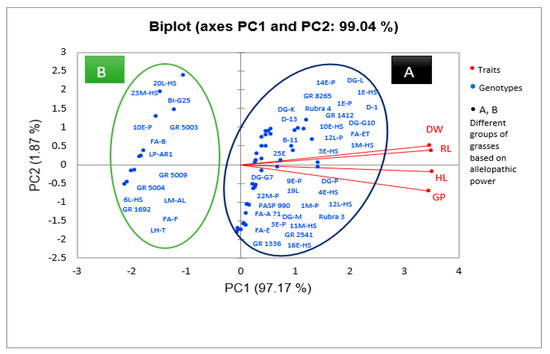
Figure 1.
Principal component analysis (PCA) of extracts of 50 grass genotypes of the family Poaceae with respect to their germination indices. According to the results obtained, two components explained 99% of the total variation. Therefore, the genotypes were divided into two groups. Group A comprises the genotypes that had the least inhibitory effect on the traits studied and showed high values of germination, rootlet length, shootlet length, and dry weight. Group B includes the genotypes that had the greatest inhibitory effect on the traits studied and caused a significant decrease in germination traits, indicating their greater allelopathic activity.

Table 4.
Mean comparison of the seed germination indices of E. sativa affected by the effects of the 50 grass genotypes.
2.2. Hypocotyl Length
The results showed that the factor of genotype type, extract concentration (12.5, 25, 50, 75, 100%), and also the interaction effect of these two factors had a significant effect (at 1% level) on the length of the hypocotyl of E. sativa L. (Table 2). The greatest decrease in hypocotyl length was observed by (79.36–78.86%) in the concentrations of (100%) and (75%), respectively, compared to the control (Table 3). Also, the concentration of 12.5% was not different from the control and showed no inhibitory effect on hypocotyl length. PCA analysis showed that water extracts from genotypes GR 1692, GR 5004, GR 5009, GR 5003 of X Festulolium species, FA-B, FA-F, 20L-HS, 23M-HS, 10E-P, 6L-HS genotypes of F. arundinacea Schreb. species, LP-AR1 genotype of L. perenne L. species, LM-AL genotype of L. multiflorum Lam. species, BI-G25 genotype of B. inermis Leyss species, and LH-T genotype of Lolium × hybridum Hausskn species had the greatest inhibitory effect on E. sativa L. and caused a decrease in hypocotyl length up to 43.9%. Among these genotypes, Festulolium genotypes showed the highest growth inhibition effect (Figure 1, Table 4).
2.3. Radicle Length
The results of our study showed that the effect of factors such as genotype, concentration of plant extract (12.5, 25, 50, 75, 100%), and their interaction on radicle length of E. sativa L. is significant at 1% level (Table 2). Application of the extract at the highest concentration caused the greatest reduction in radicle length by 81.4% compared to the control. Application of the extract at the lowest concentration (12.5%) did not show any inhibitory effect on radicle length (Table 3). PCA analysis showed that water extracts from genotypes GR 1692, GR 5004, GR 5009, GR 5003 of X Festulolium species, genotypes FA-B, FA-F, 20L-HS, 23M-HS, 10E- P, 6L-HS of F. arundinacea Schreb. Species, LP-AR1 genotype of L. perenne L. species, LM-AL genotype of L. multiflorum Lam. Species, BI-G25 genotype of B. inermis Leyss species, and LH-T genotype of Lolium×hybridum Hausskn species had the greatest inhibitory effect on radicle length and reduced radicle length by 50.7%. Among these genotypes, Festulolium genotypes showed the highest growth inhibition effect (Figure 1, Table 4).
2.4. Seedling Dry Weight
The results of the study showed that genotype factors, extract concentration (12.5, 25, 50, 75, 100%), and the interaction of these two factors had a significant effect (at 1% level) on the dry weight of E. sativa L. seedlings (Table 2). The maximum decrease in dry weight of seedlings was observed (−75.63%) in the presence of the highest extract concentration (100%). The 12.5% extract concentration did not show any inhibitory effect on seedling dry weight (Table 3). PCA analysis showed that water extracts of genotypes GR 1692, GR 5004, GR 5009, GR 5003 of X Festulolium species, FA-B, FA-F, 20L-HS, 23M-HS, 10E-P, 6L-HS genotypes of F. arundinacea Schreb. species, LP-AR1 genotype of L. perenne L. species, LM-AL genotype of L. multiflorum Lam. species, BI-G25 genotype of B. inermis Leyss species, and LH-T genotype of Lolium×hybridum Hausskn species had the greatest inhibitory effect on seedling dry weight of E. sativa L., reducing dry weight by 61%. Among these genotypes, Festulolium genotypes showed the highest growth inhibition effect (Figure 1, Table 4).
2.5. Total Phenol and Flavonoid
Among the fifty genotypes studied, three genotypes with the highest allelopathic activity and two genotypes with the lowest allelopathic activity were selected according to the results of the comparison of means and PCA for the study of phenolic and flavonoid compounds. The results showed the presence of phenolic and flavonoid compounds in the shoots of the grass genotypes (Table 5). The results of the comparison of means showed that genotypes X Festulolium sp. (GR 5009), X Festulolium sp. (GR 1692), and X Festulolium braunii (K. Richt.) (GR 5004) have more total phenolics and flavonoids (Table 5) and it seems that the high allelopathic activity of these genotypes could be due to their high phenolic compounds.

Table 5.
Total phenolic and flavonoid contents.
2.6. High-Performance Liquid Chromatography-MS (HPLC-MS) Analysis
The HPLC-MS results indicate the presence of phenolic compounds in the shoots of the grass genotypes (Table 6). Nine phenolic compounds were identified in the shoots of the grass genotypes, including caffeic acid, syringic acid, vanillic acid, p-coumaric acid, ferulic acid, apigenin acid, chlorogenic acid, 4-hydroxybenzoic acid, and gallic acid (Table 6). The HPLC-MS results showed that the shoot parts of the genotypes of Festulolium species have the highest amount of phenolic compounds. Among the nine identified phenolic compounds, the highest amount was related to caffeic acid, and it seems to be one of the most important phenolic compounds effective in allelopathy (Table 6). According to the HPLC-MS results, these compounds seemed to have the greatest effect in inhibiting the growth of E. sativa.

Table 6.
Identified phenolic compounds of studied grass genotypes.
3. Discussion
The results of our studies showed that the aqueous extract of the leaves of the grass genotypes decreased the percentage of germination, hypocotyl length, radicle length, and dry weight of Eruca sativa L. seedlings. It is also known that the amount and variety of allelochemicals in the leaves of grasses are high, which may have an inhibitory effect on the germination and growth of other plants [31]. The study conducted by Shi et al. (2023) showed that the aqueous extract of Abutilon theophrasti leaves at high concentrations had an inhibitory effect on the germination and growth of Glycine max L., Triticum aestivum L., Zea mays [32]. In another study on the allelopathic effects of aqueous leaf extracts of two grass species, Urochloa decumbens and Urochloa ruziziensis, it was shown that they had inhibitory effects on the weeds Chloris ventricosa, Bidens pilosa, Commelina benghalensis L., Conyza canadensis, and Digitaria insularis and caused a decrease in the percentage of germination, root growth, shoot growth, and biomass of the weeds [33].
It was determined in our study that as the concentration of the extract increased, a further decrease in the traits studied was observed (Table 2 and Table 3). The results of our study are consistent with previous studies that found that increasing the concentration of allelopathic plant extracts further reduced the germination and growth of target plants. In a study, the allelopathic effects of aqueous leaf extract of Cannabis sativa at concentrations of 25, 50, 75, and 100% on seed germination and seedling growth of Triticum durum and Hordeum vulgare were investigated, and it was found that the allelopathic effect of the extract was concentration-dependent and germination decreased with increasing concentration of the extract [34]. Hussain et al. (2020) investigated the allelopathic effects of Acacia melanoxylon R. Br shoot aqueous extract under laboratory conditions and at concentrations (0, 25%, 50%, 75%, and 100%) on the growth of Lactuca sativa seedlings and reported that germination, shoot length, root length, and dry weight of seedlings decreased after exposure to A. melanoxylon aerial extract, and the greatest decrease was observed at 75% and 100% concentrations. The allelopathic effects of A. melanoxylon extract may be due to the presence of phenolic and flavonoid allelochemical compounds, which often have inhibitory effects on the growth of target species [15].
Seed germination and the early stages of seedling growth are the most sensitive stages to environmental changes, so this stage is often used to study allelopathic effects [35,36]. It appears that the reduction in seed germination at higher concentrations of aqueous leaf extracts occurs because of reduced water uptake by seeds due to the presence of allelochemicals in the absorptive substrate. Seed uptake of allelochemicals leads to seed toxicity, which ultimately severely reduces water and nutrient uptake and arrests seedling growth and development [37]. Changes in gibberellic hormone activity, which regulates amylase production during germination, can also occur in the presence of allelochemicals (phenolic compounds) [38]. Susceptibility to allelopathic compounds may depend on small seed size. Small seeds and early-emerging species have been reported to be more susceptible to allelopathic effects than plants with larger seeds. Small seeds are more susceptible to allelopathy because of reduced carbohydrate storage [39].
Our study showed that the reduction in growth of E. sativa L. seedlings caused by the aqueous extract was observed more in the rootlets than in the shoot. Therefore, the rootlet is more sensitive to allelochemicals than the shoot, which may be because the rootlets are in direct contact with allelochemical compounds [36]. Also, the permeability of root tissue to allelopathic compounds is higher than that of the shoot, which makes the rootlet more sensitive to these compounds [40]. The reduction in root growth under the influence of allelopathic compounds may be due to the disruption of mitosis, resulting in a decrease in root length and a concomitant decrease in root volume. This effect on root growth may be responsible for the decrease in germination, shoot length, root length, and seedling dry weight due to the decrease in moisture and nutrient uptake [1]. Therefore, seedling growth, especially root growth, can be considered as a good indicator of plant sensitivity to allelopathy [41].
The reason for the decrease in dry weight is allelochemical toxicity, which causes a decrease in water uptake in tissues [42]. Allelochemical stress increases the concentration of reactive oxygen species (ROS) in plant cells [43]. As a result, ROS cause oxidative damage and increase lipid peroxidation in the membrane. Lipid peroxidation causes changes in the fluidity and permeability of lipid bilayer membranes, which can alter cell integrity and ultimately lead to cell death [44]. Allelopathic compounds can also reduce the activity of metabolic enzymes, proteins, carbohydrates, and nucleic acid content. This decrease in metabolite and enzyme activity is considered the mechanism of action of allelochemicals and provides the basis for further studies on the use of allelopathic plant extracts as biological herbicides for weed control [19].
Our study showed that different grass genotypes have different inhibitory effects on the growth of E. sativa L. (Figure 1, Table 4), such that the highest growth reduction was caused by the genotypes of Festulolium species including GR 1692, GR 5004, and GR 5009 (Figure 1, Table 4). The different allelopathic effects of plants on the target species may be due to the presence of different levels of allelochemicals in plants, which is caused by the different abilities of plants to synthesize allelopathic substances [32,45]. Previous studies have shown that there is variation among different grass species in their ability to suppress weeds, and the allelopathic effect varies among different species and genotypes. Lipinska et al. (2019) investigated the allelopathic potential of six cultivars of Festuca arundinacea, Festuca ovina, and reported that Festuca rubra had different inhibitory effects on the growth of grass weeds, and their allelopathic potential depended on the content of flavonoids and phenolic acids in their leaves [46]. In the study of Koo et al. (2022), the aqueous extract of the leaves of different cultivars of Lolium arundinaceum showed an inhibitory effect on the germination and growth of Poa annua L. in a petri dish, and there was a difference between different cultivars of L. arundinaceum in terms of allelopathic effects [47].
Advances in compound isolation techniques allow the identification of active compounds in allelopathy [48]. The results obtained from our study showed that genotypes of Festulolium species have high levels of phenolic compounds, which can have an inhibitory effect on the growth of the target species (Table 6). Based on HPLC-MS data, it was found that the shoot parts of Festulolium species genotypes (GR 5009, GR 1692) have phenolic compounds including caffeic acid, syringic acid, vanillic acid, p-coumaric acid, ferulic acid, apigenin acid, chlorogenic acid, 4-hydroxybenzoic acid, and gallic acid (Table 6). These nine compounds had the highest concentration, and it seems that they were effective compounds in the allelopathic activity of Festulolium species genotypes and caused an inhibitory effect on the germination and growth of E. sativa L. (Table 6). Phenolic compounds are among the most important secondary metabolites involved in allelopathy. These compounds can increase lipid peroxidation, ultimately leading to reduced growth or death of plant tissue [49]. In addition, phenolic allelochemicals prevent cell elongation and division by reducing nutrient uptake by plants, causing changes in plant cell structure, and reducing plant growth [18]. In other studies, the presence of phenolic compounds in allelochemicals has been proven. The presence of phenolic compounds in Helianthus annuus was shown and it was found that ferulic acid has the highest amount followed by vanillic acid, chlorogenic acid, and caffeic acid [50]. The aqueous extract of Lolium perenne L. leaves contains phenolic compounds, the highest amount of which is related to chlorogenic acid [51]. Allelopathic phenolic compounds of caffeic acid, p-coumaric acid, and ferulic acid in Lolium multiflorum reduced shoot and root length in rice cultivars [52]. These allelochemicals can disrupt plant physiological and biological processes, reducing or suppressing plant growth and development by reducing mineral uptake by the plant [53,54].
4. Materials and Methods
4.1. Plant Materials and Methods of Preparation
The grass genotypes (Table 1) at the research farm of Isfahan University of Technology in Lavark, Najaf Abad, Iran (40 km southwest of Isfahan, 32°32′, N 51°, 23′ E and 1630 m above sea level) were cultivated and used in 2015 [55]. Fresh samples of 50 grass genotypes from ten species were collected in late spring and at the flowering stage in 2019 and placed separately in paper bags. The samples were air-dried at room temperature, then powdered and stored in closed plastic bags at room temperature until use. The seeds of E. sativa L. were obtained from Pakan Bazr Co., Isfahan, Iran, and their germination percentage and dormancy were tested in petri dish conditions in the germinator.
4.2. Preparation of Leaf Extract of Grass Genotypes and Germination Experiments
Leaves of grass genotypes harvested at the flowering stage were used to prepare the extract. Samples were shade-dried. The method of Bali et al. (2016) was used to prepare the aqueous leaf extract [56]. For the extraction, the samples collected from the farm were first powdered and 12.5, 25, 50, 75, and 100 g of the powdered samples of each treatment were poured into 100 mL of deionized water and kept for 24 h at a temperature of 25 °C. Then, the prepared extracts were passed through Whatman #1 filter paper, and the extract of the prepared samples was stored at 4 °C until use. The seeds of E. sativa L. were first disinfected with 10% sodium hypochlorite solution for 10 min, then washed with normal water for 10 min and finally with deionized water for 5 min.
Fifty seeds of E. sativa L. were placed on filter paper in 9 cm petri dishes. The seeds were soaked with the prepared extracts (10 mL for each petri dish) and the petri dishes were placed in the growth chamber at 25°C and 12 h of light. Irrigation with distilled water was used as a control. After seed germination was determined, the percentage of germination and seedling growth were examined [34]. To calculate the dry weight of seedlings, samples were dried in an oven at 70 °C for 48 h and then weighed [31].
4.3. Measurement of Total Phenolic and Flavonoid Content in Leaves of Grass Genotypes
For this purpose, based on the results of comparison of means and PCA, three grass genotypes with the highest and two genotypes with the lowest allelopathic activity were selected among the genotypes for the measurement of total phenolics and flavonoids in them. The total concentrations of phenolics and flavonoids in the plant shoots were estimated using gallic acid as a standard and the Folin–Ciocalteu colorimetric method [57]. Results were expressed as gallic acid equivalents (GAE).
Briefly, 3 g of air-dried sample (leaf powder samples) was extracted with 10 mL of 80% methanol using an orbital shaker incubator (Jaltajhiz, Iran, Karaj, JTSL20) (110 rpm) at 25 °C for 24 h. A 0.5 mL aliquot of the methanol extract was then filtered and combined with 2.5 mL of Folin–Ciocalteu reagent (diluted with 1:10 volume of distilled water) and 2 mL of 7.5% (v/v) sodium carbonate. It was heated at 45 °C for 15 min and the absorbance was measured at 765 nm against a blank by spectrophotometry (HITAGHI-Japan model U-1800). The phenolic content of the shoots was recorded as gallic acid equivalents per 1 g of shoot dry matter.
4.4. Identification of Phenolic Compounds
For this purpose, five genotypes (three genotypes with high allelopathic activity and two genotypes with low allelopathic activity), whose total phenolics and flavonoids were studied in the previous step, were used. Leaf powder samples were extracted with 80% methanol [58]. A 100 g sample of each leaf powder was extracted with 300 mL of 80% methanol (HPLC grade, Merck) (stirring, 25 °C for 48 h, centrifugation, 1200× g for 15 min). The extracts were analyzed on an HPLC-MS system (model Agilent 1090). The instrument consisted of an Agilent 1100 HPLC, diode detector and mass spectrometer (MSD, SL mode) (Agilent Technologies, Palo Alto, CA, USA). The extracts were filtered through a 0.22 μm Acrodisc nylon filter. Injections on the analytical column were made from 20 μL of filtered extract. Standards were dissolved using HPLC grade methanol as the solvent. The stationary phase consisted of a 250 mm × 4.6 mm (5 μm) symmetrical C18 column (Waters Crop., Milford, MA, USA) (10 mm × 4 mm ID), and the mobile phase was formic acid (0.1%). Acetonitrile (99.8%) was used at a flow rate of 0.8 mL/min and the wavelength was set between 200 and 400 nm. The implementation of the gradient conditions was characterized by the following specifications: 10–26% solvent B (v/v) for 40 min, 65% solvent B for 70 min, and finally 100% solvent B for 75 min. Phenolic content was determined by setting the DAD at 350, 310, 270, and 520 nm, reading extreme peaks in real time, and continuously recording the entire spectrum (190–650 nm). Analysis of allelopathic compounds was repeated three times using extracts from each sample.
4.5. Statistical Analysis
The experiment was conducted as a completely randomized factorial design with three replications, and all statistical calculations were performed using SAS 9.4. The Kolmogorov–Smirnov test was used to check the normality of the data distribution before performing the analysis of variance (ANOVA). Principal component analysis (PCA) was performed on the correlation matrix of the traits using XLSTAT software version 2019.2.2.
5. Conclusions
According to this study, aqueous extracts of grass genotypes with concentration of 100% and 75% have significant allelopathic activity on germination and growth of E. sativa L. There was a difference between different grass genotypes in terms of allelopathic activity, so the studied genotypes were classified into two weak and strong groups in terms of inhibition of E. sativa L. growth. The genotypes of Festulolium including GR 1692, GR 5004, GR 5009 had the most inhibitory effect on the growth of E. sativa. It can be concluded from the present study that it is possible to use the extract of the tested genotypes as a natural alternative method for the control of E. sativa weeds. From the present study, it can be concluded that the tested genotypes of Festulolium can be used as a natural alternative method to control E. sativa weeds. Finally, it is suggested that further studies should be conducted to identify effective allelochemicals in the allelopathy of these genotypes.
Author Contributions
Conceptualization, M.M., H.K. and M.M.M.; methodology, H.K. and M.M.M.; formal analysis, M.M. and M.M.M.; data curation, H.K. and A.M.; writing—original draft preparation, M.M., H.K. and M.M.M.; writing—review and editing, H.K. and A.M.; supervision, A.M.; funding acquisition, A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are available from the corresponding authors upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Akter, P.; Ahmed, A.M.A.; Promie, F.K.; Haque, M.E. Root Exudates of Fifteen Common Weed Species: Phytochemical Screening and Allelopathic Effects on T. aestivum L. Agronomy 2023, 13, 381. [Google Scholar] [CrossRef]
- Chauhan, B.S. Grand Challenges in Weed Management. Front. Agron. 2020, 1, 3. [Google Scholar] [CrossRef]
- Jabran, K.; Mahajan, G.; Sardana, V.; Chauhan, B.S. Allelopathy for weed control in agricultural systems. Crop Prot. 2015, 72, 57–65. [Google Scholar] [CrossRef]
- Abbas, T.; Ahmad, A.; Kamal, A.; Nawaz, M.Y.; Jamil, M.A.; Saeed, T.; Abid, M.A.; Ali, H.H.; Ateeq, M. Ways to Use Allelopathic Potential for Weed Management: A Review. Int. J. Food Sci. Agric. 2021, 5, 492–498. [Google Scholar] [CrossRef]
- De, A.; Bose, R.; Kumar, A.; Mozumdar, S. Worldwide Pesticide Use; Springer: Berlin/Heidelberg, Germany, 2014; pp. 5–6. [Google Scholar] [CrossRef]
- Koehler-Cole, K.; Everhart, S.E.; Gu, Y.; Proctor, C.A.; Marroquin-Guzman, M.; Redfearn, D.D.; Elmore, R.W. Is allelopathy from winter cover crops affecting row crops? Agric. Environ. Lett. 2020, 5. [Google Scholar] [CrossRef]
- Ullah, H.; Khan, N.; Khan, I.A. Complementing cultural weed control with plant allelopathy: Implications for improved weed management in wheat crop. Acta Ecol. Sin. 2023, 43, 27–33. [Google Scholar] [CrossRef]
- Karimmojeni, H.; Rezaei, M.; Tseng, T.M.; Mastinu, A. Effects of metribuzin herbicide on some morpho-physiological characteristics of two Echinacea Species. Horticulturae 2022, 8, 169. [Google Scholar] [CrossRef]
- Li, J.; Chen, L.; Chen, Q.; Miao, Y.; Peng, Z.; Huang, B.; Guo, L.; Liu, D.; Du, H. Allelopathic effect of Artemisia argyi on the germination and growth of various weeds. Sci. Rep. 2021, 11, 4303. [Google Scholar] [CrossRef]
- Choudhary, C.S.; Behera, B.; Raza, M.B.; Mrunalini, K.; Bhoi, T.K.; Lal, M.K.; Nongmaithem, D.; Pradhan, S.; Song, B.; Das, T.K. Mechanisms of allelopathic interactions for sustainable weed management. Rhizosphere 2023, 25, 100667. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, X.; Ding, L.; Kong, C.H. Allelopathy and allelochemicals in grasslands and forests. Forests 2023, 14, 562. [Google Scholar] [CrossRef]
- Novakoski, A.D.; Coelho, E.M.P.; Ravagnani, G.T.; da Costa, A.C.P.R.; Rocha, S.A.; Zucareli, V.; Lopes, A.D. Allelopathic potential of plant aqueous mixtures on Euphorbia heterophylla. Agriculture 2020, 10, 449. [Google Scholar] [CrossRef]
- Kato-Noguchi, H. Involvement of allelopathy in the invasive potential of Tithonia diversifolia. Plants 2020, 9, 766. [Google Scholar] [CrossRef] [PubMed]
- Rob, M.M.; Hossen, K.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Phytotoxic activity and identification of phytotoxic substances from Schumannianthus dichotomus. Plants 2020, 9, 102. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.I.; El-Sheikh, M.A.; Reigosa, M.J. Allelopathic potential of aqueous extract from Acacia melanoxylon R. Br. on Lactuca sativa. Plants 2020, 9, 1228. [Google Scholar] [CrossRef]
- Hussain, M.I.; Shackleton, R.T.; El-Keblawy, A.; Del Mar Trigo Pérez, M.; González, L. Invasive Mesquite (Prosopis juliflora), an allergy and health challenge. Plants 2020, 9, 141. [Google Scholar] [CrossRef]
- Favaretto, A.; Scheffer-Basso, S.M.; Perez, N.B. Allelopathy in Poaceae species present in Brazil: A review. Agron. Sustain. Dev. 2018, 38, 22. [Google Scholar] [CrossRef]
- Li, Z.-H.; Wang, Q.; Ruan, X.; Pan, C.-D.; Jiang, D.-A. Phenolics and plant allelopathy. Molecules 2010, 15, 8933–8952. [Google Scholar] [CrossRef]
- El-Shora, H.M.; Alharbi, M.M.; Darwish, D.B.; Gad, D. Allelopathic potential of aqueous leaf extract of Rumex dentatus L. on metabolites and enzyme activities of common purslane leaves. J. Plant Interact. 2022, 17, 267–276. [Google Scholar] [CrossRef]
- Liu, H.M.; Huang, J.G.; Yang, S.F.; Amist, N.; Zhou, L.J. Plant autotoxicity: A review (Part IV). families: Poaceae to Zingiberaceae. Allelopath. J. 2020, 50, 1–22. [Google Scholar] [CrossRef]
- Gharibvandi, A.; Karimmojeni, H.; Ehsanzadeh, P.; Rahimmalek, M.; Mastinu, A. Weed management by allelopathic activity of Foeniculum vulgare essential oil. Plant Biosyst. 2022, 156, 1298–1306. [Google Scholar] [CrossRef]
- Hasan, M.; Ahmad-Hamdani, M.S.; Rosli, A.M.; Hamdan, H. Bioherbicides: An eco-friendly tool for sustainable weed management. Plants 2021, 10, 1212. [Google Scholar] [CrossRef] [PubMed]
- Masum, S.M.; Hossain, M.A.; Akamine, H.; Sakagami, J.-I.; Bhowmik, P.C. Allelopathic potential of indigenous Bangladeshi rice varieties. Weed Biol. Manag. 2016, 16, 119–131. [Google Scholar] [CrossRef]
- Khamare, Y.; Chen, J.J.; Marble, S.C. Allelopathy and its application as a weed management tool: A review. Front. Plant Sci. 2022, 13, 1034649. [Google Scholar] [CrossRef] [PubMed]
- Gebashe, F.; Aremu, A.O.; Gruz, J.; Finnie, J.F.; Van Staden, J. Phytochemical profiles and antioxidant activity of grasses used in South African traditional medicine. Plants 2020, 9, 371. [Google Scholar] [CrossRef] [PubMed]
- Scrivanti, L.R.; Anton, A.M. Germination inhibitory activity of aqueous extracts of native grasses from South America. Rodriguésia 2021, 72, e01672019. [Google Scholar] [CrossRef]
- Sheldon, K.; Purdom, S.; Shekoofa, A.; Steckel, L.; Sykes, V. Allelopathic impact of cover crop species on soybean and goosegrass seedling germination and early growth. Agriculture 2021, 11, 965. [Google Scholar] [CrossRef]
- Kanya, T.C.S.; Urs, M.K. Studies on taramira (Eruca Sativa) Seed Oil and Meal. J. Am. Oil Chem. Soc. 1989, 66, 139–140. [Google Scholar]
- Piragine, E.; Flori, L.; Mannelli, L.D.; Ghelardini, C.; Pagnotta, E.; Matteo, R.; Lazzeri, L.; Martelli, A.; Miragliotta, V.; Pirone, A.; et al. Eruca sativa Mill. seed extract promotes anti-obesity and hypoglycemic effects in mice fed with a high-fat diet. Phytother. Res. 2021, 35, 1983–1990. [Google Scholar] [CrossRef]
- Jia, C.-Z.; Wang, J.-J.; Chen, D.-L.; Hu, X.-W. Seed germination and seed bank dynamics of Eruca sativa (Brassicaceae): A weed on the Northeastern edge of Tibetan Plateau. Front. Plant Sci. 2022, 13, 820925. [Google Scholar] [CrossRef]
- Fragasso, M.; Platani, C.; Miullo, V.; Papa, R.; Iannucci, A. A bioassay to evaluate plant responses to the allelopathic potential of rhizosphere soil of wild oat (Avena fatua L.). Agrochimica 2012, 56, 120–128. [Google Scholar]
- Shi, S.; Cheng, J.B.; Ahmad, N.; Zhao, W.Y.; Tian, M.F.; Yuan, Z.Y.; Li, C.Y.; Zhao, C.J. Effects of potential allelochemicals in a water extract of Abutilon theophrasti Medik. on germination and growth of Glycine max L., Triticum aestivum L., and Zea mays L. J. Sci. Food Agric. 2023, 103, 2155–2165. [Google Scholar] [CrossRef] [PubMed]
- Villela, A.L.G.; Martinelli, R.; Zenatti, T.F.; Rufino-Jr, L.R.; Monquero, P.A.; Conceição, P.M.d.; Azevedo, F.A.d. Potential of two cover crops, signal grass and ruzi grass: Suggested allelopathic effect on some important weeds. Aust. J. Crop Sci. 2021, 15, 260–270. [Google Scholar] [CrossRef]
- Patane, C.; Pellegrino, A.; Cosentino, S.L.; Testa, G. Allelopathic effects of Cannabis sativa L. aqueous leaf extracts on seed germination and seedling growth in durum wheat and barley. Agronomy 2023, 13, 454. [Google Scholar] [CrossRef]
- Karimmojeni, H.; Taab, A.; Rashidi, B.; Bazrafshan, A.H. Dormancy breaking and seed germination of the annual weeds Thlaspi arvense, Descurainia sophia and Malcolmia africana (Brassicaceae). J. Plant Prot. Res. 2014, 54, 179–187. [Google Scholar] [CrossRef]
- Wang, K.L.; Wang, T.; Ren, C.; Dou, P.P.; Miao, Z.Z.; Liu, X.Q.; Huang, D.; Wang, K. Aqueous extracts of three herbs allelopathically inhibit lettuce germination but promote seedling growth at low concentrations. Plants 2022, 11, 486. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Wang, S.; Wei, M.; Yu, Y.; Wang, C. Effect of leaf water extracts of four Asteraceae alien invasive plants on germination performance of Lactuca sativa L. under acid deposition. Plant Ecol. 2021, 222, 433–443. [Google Scholar] [CrossRef]
- Wang, D.; Chen, J.; Xiong, X.; Wang, S.; Liu, J. Allelopathic effects of Cinnamomum migao on seed germination and seedling growth of its associated species Liquidambar formosana. Forests 2019, 10, 535. [Google Scholar] [CrossRef]
- Haramoto, E.R.; Gallandt, E.R. Brassica cover cropping: II. Effects on growth and interference of green bean (Phaseolus vulgaris) and redroot pigweed (Amaranthus retroflexus). Weed Sci. 2017, 53, 702–708. [Google Scholar] [CrossRef]
- Nishida, N.; Tamotsu, S.; Nagata, N.; Saito, C.; Sakai, A. Allelopathic effects of volatile monoterpenoids produced by Salvia leucophylla: Inhibition of cell proliferation and DNA synthesis in the root apical meristem of Brassica campestris seedlings. J. Chem. Ecol. 2005, 31, 1187–1203. [Google Scholar] [CrossRef]
- Cheng, F.; Cheng, Z.H. Research progress on the use of plant allelopathy in agriculture and the physiological and ecological mechanisms of allelopathy. Front. Plant Sci. 2016, 7, 1697. [Google Scholar] [CrossRef]
- Ziaebrahim, L.; Khavari-Ne, R.A.; Fahimi, H.; Nejadsatar, T. Effects of aqueous Eucalyptus extracts on seed germination, seedling growth and activities of peroxidase and polyphenoloxidase in three wheat cultivar seedlings (Triticum aestivum L.). Pak. J. Biol. Sci. 2007, 10, 3415–3419. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Goraya, G.K.; Asthir, B. Magnificant role of intracellular reactive oxygen species production and its scavenging encompasses downstream processes. J. Plant Biol. 2016, 59, 215–222. [Google Scholar] [CrossRef]
- Razavifar, Z.; Karimmojeni, H.; Sini, F.G. Effects of wheat-canola intercropping on Phelipanche aegyptiaca parasitism. J. Plant Prot. Res. 2017, 57, 268–274. [Google Scholar] [CrossRef][Green Version]
- Lipińska, H.; Kępkowicz, A.; Sykut, M.; Jackowska, I. Effects of decomposing biomass of Festuca arundinacea, Festuca ovina and Festuca rubra lawn cultivars on growth of other lawn grasses. Allelopath. J. 2019, 46, 107–120. [Google Scholar] [CrossRef]
- Koo, D.; Gonçalves, C.G.; Askew, S.D. Lolium arundinaceum leaf and root developmental temperatures influence its allelopathic potency on Poa annua. Int. Turfgrass Soc. Res. J. 2022, 14, 787–790. [Google Scholar] [CrossRef]
- Scavo, A.; Pandino, G.; Restuccia, A.; Lombardo, S.; Pesce, G.R.; Mauromicale, G. Allelopathic potential of leaf aqueous extracts from Cynara cardunculus L. on the seedling growth of two cosmopolitan weed species. Ital. J. Agron. 2019, 14, 78–83. [Google Scholar] [CrossRef]
- Inderjit. Plant phenolics in allelopathy. Bot. Rev. 1996, 62, 186–202. [Google Scholar] [CrossRef]
- Sahoo, T.R.; Behera, B.; Paikaray, R.K.; Garnayak, L.M.; Sethi, D.; Jena, S.; Raza, M.B.; Panda, R.K.; Song, B.Q.; Lal, M.K.; et al. Effects of sunflower residue management options on productivity and profitability of succeeding rice under different crop establishment methods. Field Crops Res. 2023, 290, 108763. [Google Scholar] [CrossRef]
- Kagan, I.A. Soluble phenolic compounds of perennial ryegrass (Lolium perenne L.): Potential effects on animal performance, and challenges in determining profiles and concentrations. Anim. Feed Sci. Technol. 2021, 277, 114960. [Google Scholar] [CrossRef]
- Jang, S.J.; Kim, K.R.; Yun, Y.B.; Kim, S.S.; Kuk, Y.I. Inhibitory effects of Italian ryegrass (Lolium multiflorum Lam.) seedlings of rice (Oryza sativa L.). Allelopath. J. 2018, 44, 219–232. [Google Scholar] [CrossRef]
- Djurdjević, L.; Gajić, G.; Kostić, O.; Jarić, S.; Pavlović, M.; Mitrović, M.; Pavlović, P. Seasonal dynamics of allelopathically significant phenolic compounds in globally successful invader Conyza canadensis L. plants and associated sandy soil. Flora Morphol. Distrib. Funct. Ecol. Plants 2012, 207, 812–820. [Google Scholar] [CrossRef]
- Khaleghnezhad, V.; Yousefi, A.R.; Tavakoli, A.; Farajmand, B.; Mastinu, A. Concentrations dependent effect of exogenous abscisic acid on photosynthesis, growth and phenolic content of Dracocephalum moldavica L. under drought stress. Planta 2021, 253, 127. [Google Scholar] [CrossRef] [PubMed]
- Majidi, M.M.; Mirlohi, A.; Amini, F. Genetic variation, heritability and correlations of agro-morphological traits in tall fescue (Festuca arundinacea Schreb.). Euphytica 2009, 167, 323–331. [Google Scholar] [CrossRef]
- Bali, A.S.; Batish, D.R.; Singh, H.P.; Kaur, S.; Kohli, R.K. Phytotoxicity and weed management potential of leaf extracts of Callistemon viminalis against the weeds of rice. Acta Physiol. Plant 2016, 39, 25. [Google Scholar] [CrossRef]
- Alinian, S.; Razmjoo, J.; Zeinali, H. Flavonoids, anthocynins, phenolics and essential oil produced in cumin (Cuminum cyminum L.) accessions under different irrigation regimes. Ind. Crops Prod. 2016, 81, 49–55. [Google Scholar] [CrossRef]
- Proestos, C.; Sereli, D.; Komaitis, M. Determination of phenolic compounds in aromatic plants by RP-HPLC and GC-MS. Food Chemistry 2006, 95, 44–52. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).