Phenotypic Diversity in Domesticated and Wild Timothy Grass, and Closely Related Species for Forage Breeding
Abstract
:1. Introduction
2. Results
2.1. Survival in the Field
2.2. Phenotypic Differences between Species
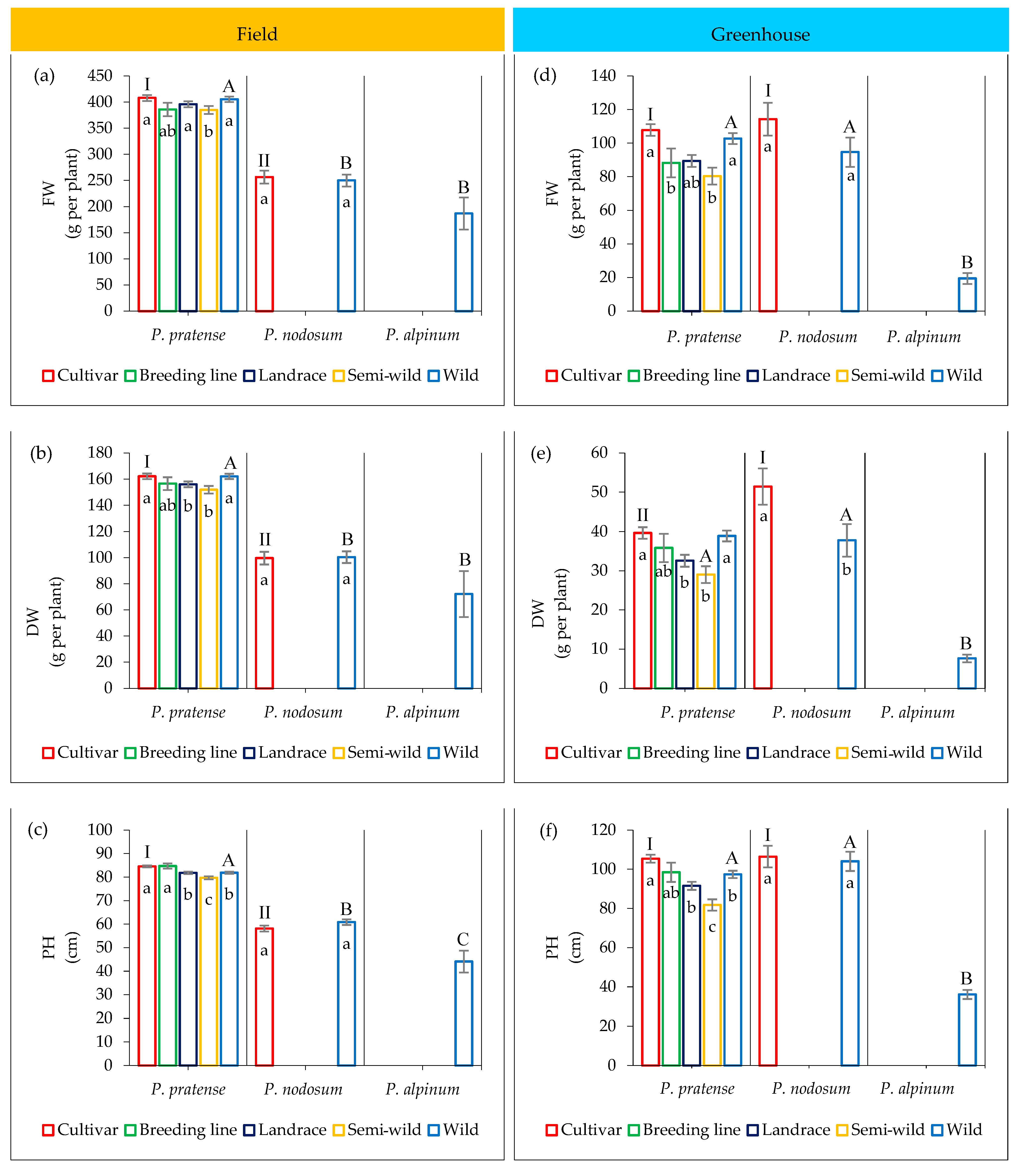
2.3. Variation in Growth Traits among Accessions
2.3.1. Field Trial
2.3.2. Greenhouse Trial
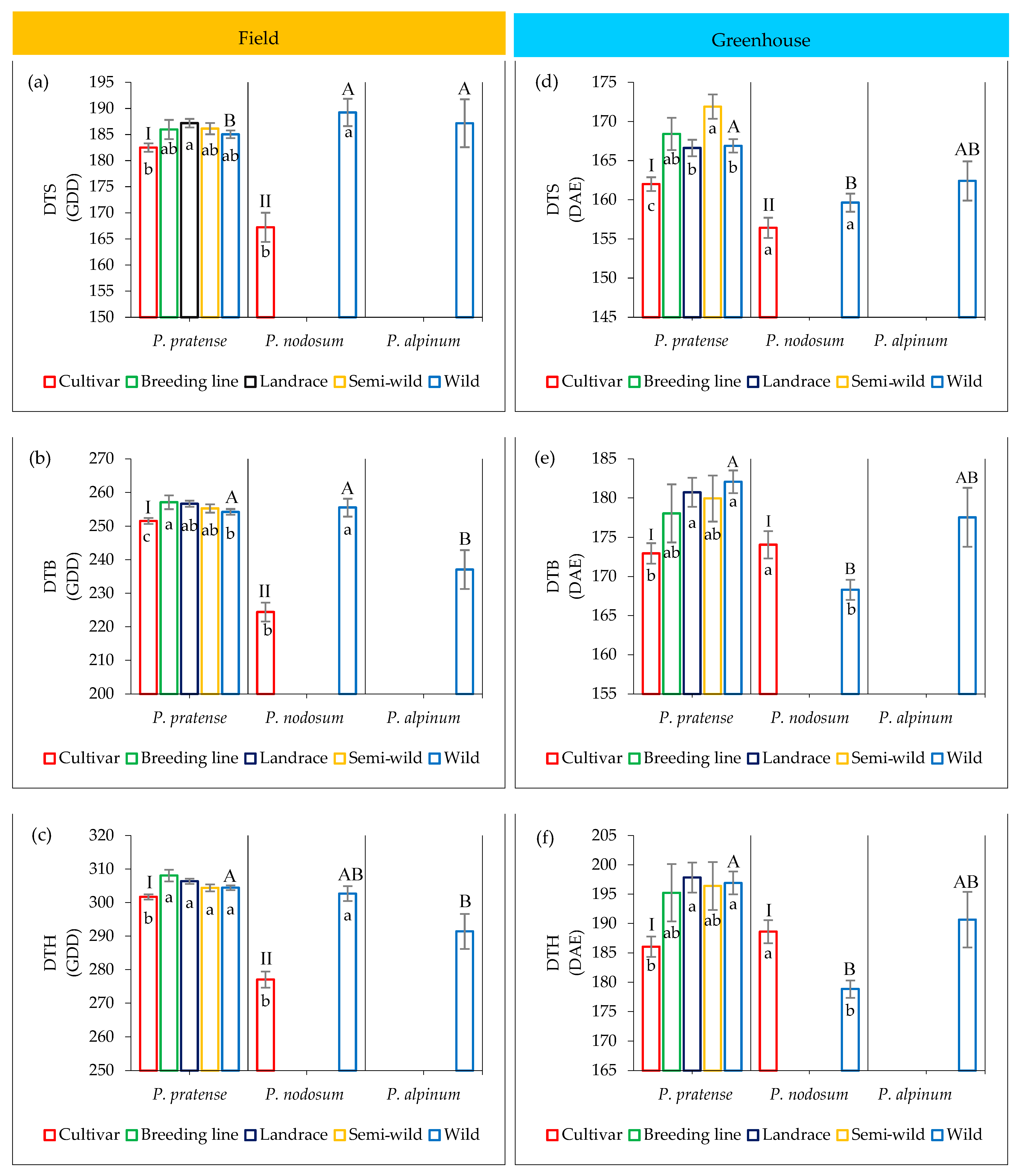
2.4. Variation in Development among Accessions
2.4.1. Field Trial
2.4.2. Greenhouse Trial
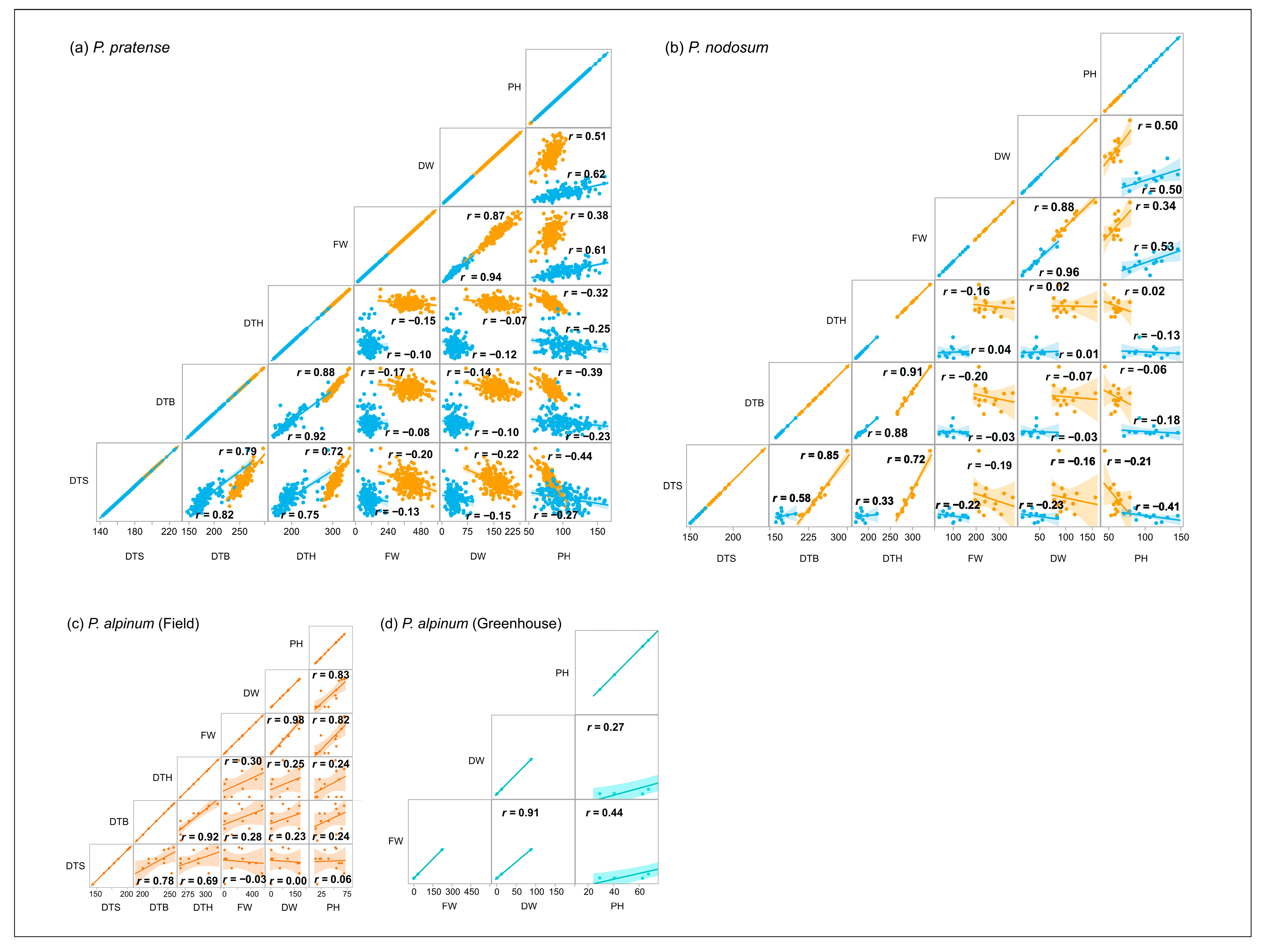
2.5. Correlation between Traits
2.5.1. Field Trial
2.5.2. Greenhouse Trial
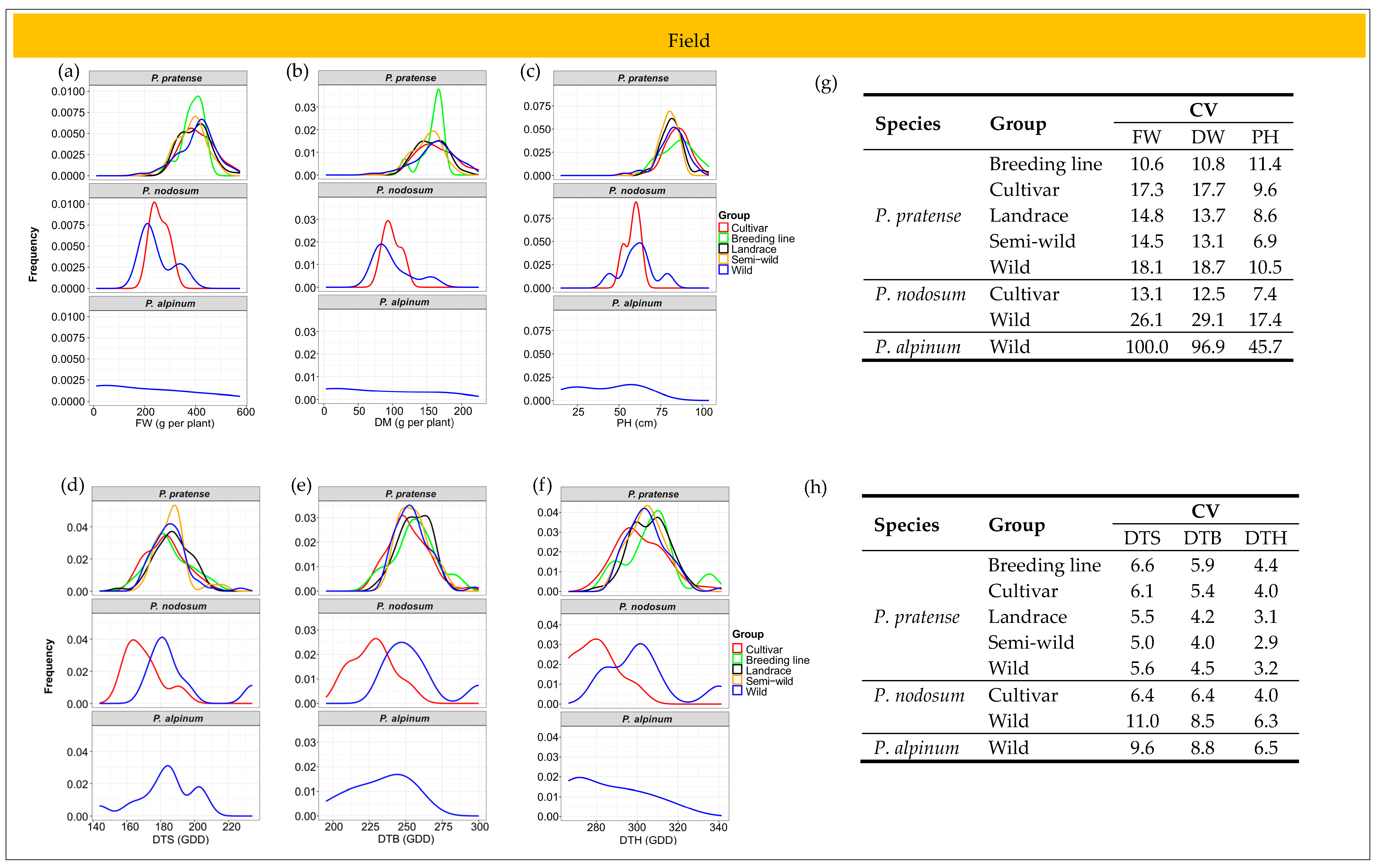
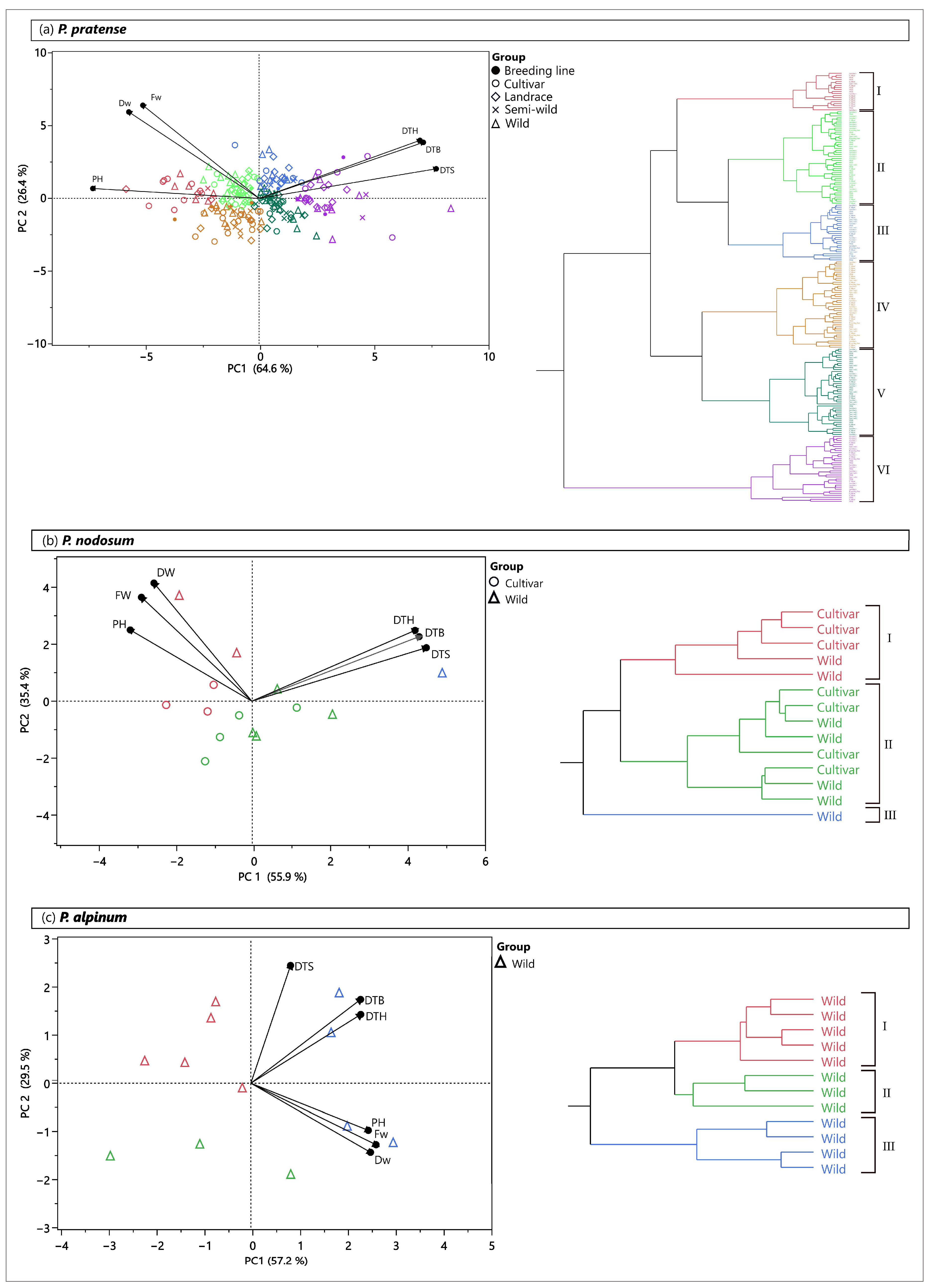
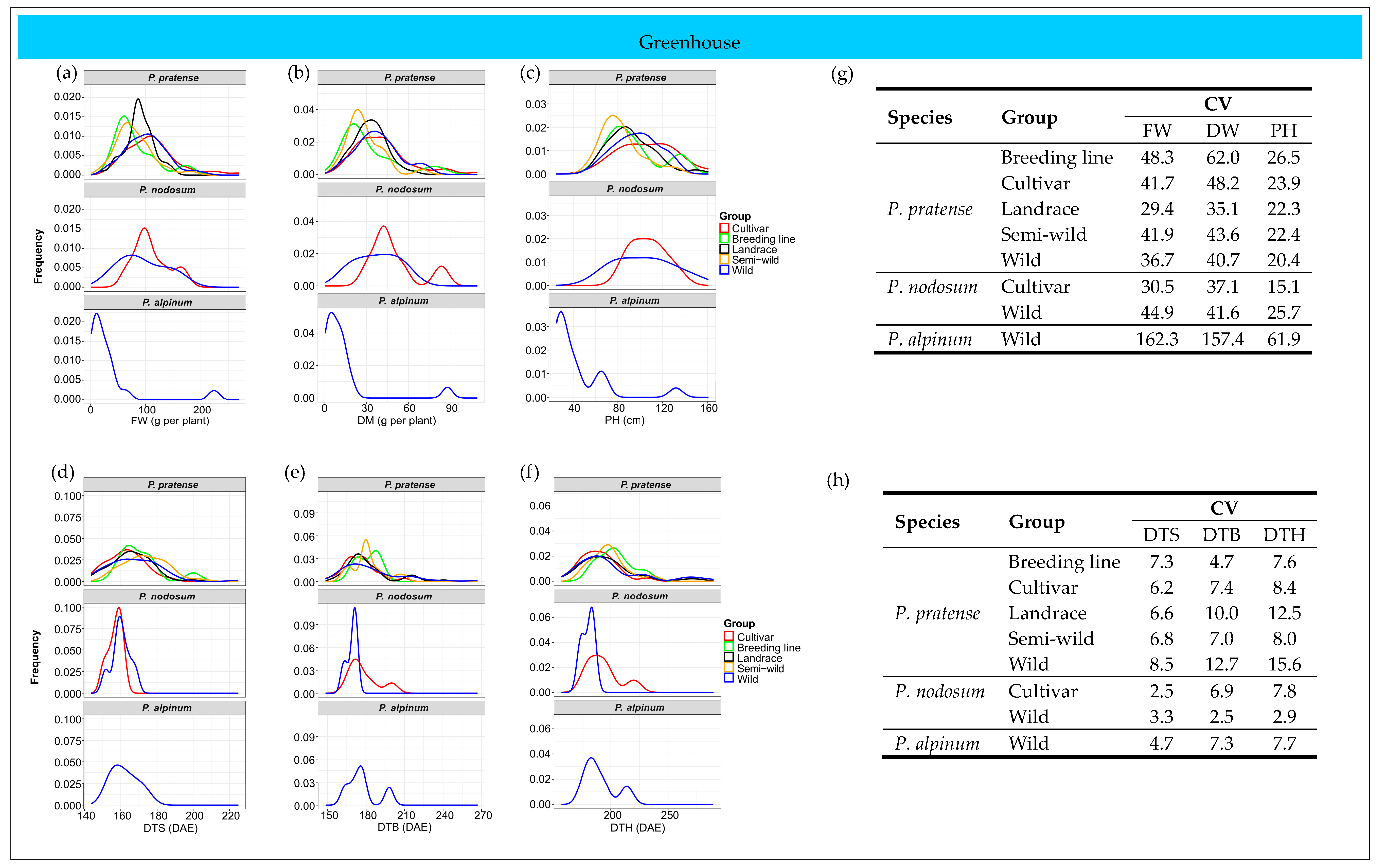
2.6. Patterns of Phenotypic Diversity
2.6.1. Field Trial
2.6.2. Greenhouse Trial
2.7. Heritability
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Pre-Cultivation and Cloning
4.3. Field Trial
4.4. Greenhouse Trial
4.5. Evaluated Traits
4.6. Data Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berg, C.C.; McElroy, A.R.; Kunelius, H.T. Timothy. In Cool-Season Forage Grasses; Moser, L.E., Buxton, D.R., Casler, M.D., Eds.; American Society of Agronomy: Madison, WI, USA, 1996; Volume 34, pp. 643–664. [Google Scholar]
- Helgadóttir, Á.; Frankow-Lindberg, B.; Seppänen, M.; Søegaard, K.; Østrem, L. European grasslands overview: Nordic region. Grassl. Sci. Eur. 2014, 19, 15–28. [Google Scholar]
- Bélanger, G.; Michaud, R.; Jefferson, P.; Tremblay, G.; Brégard, A. Improving the nutritive value of timothy through management and breeding. Can. J. Plant Sci. 2001, 81, 577–585. [Google Scholar] [CrossRef]
- Gustavsson, A.-M.; Martinsson, K. Seasonal variation in biochemical composition of cell walls, digestibility, morphology, growth and phenology in timothy. Eur. J. Agron. 2004, 20, 293–312. [Google Scholar] [CrossRef]
- Heide, O. Control of flowering and reproduction in temperate grasses. New Phytol. 1994, 128, 347–362. [Google Scholar] [CrossRef] [PubMed]
- Seppänen, M.M.; Pakarinen, K.; Jokela, V.; Andersen, J.R.; Fiil, A.; Santanen, A.; Virkajärvi, P. Vernalization response of Phleum pratense and its relationships to stem lignification and floral transition. Ann. Bot. 2010, 106, 697–707. [Google Scholar] [CrossRef]
- Fiil, A.; Jensen, L.B.; Fjellheim, S.; Lübberstedt, T.; Andersen, J.R. Variation in the vernalization response of a geographically diverse collection of timothy genotypes. Crop Sci. 2011, 51, 2689–2697. [Google Scholar] [CrossRef]
- Jokela, V.; Virkajärvi, P.; Tanskanen, J.; Seppänen, M.M. Vernalization, gibberellic acid and photo period are important signals of yield formation in timothy (Phleum pratense). Physiol. Plant. 2014, 152, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Jokela, V.; Trevaskis, B.; Seppänen, M.M. Genetic variation in the flowering and yield formation of timothy (Phleum pratense L.) accessions after different photoperiod and vernalization treatments. Front. Plant Sci. 2015, 6, 465. [Google Scholar] [CrossRef]
- Heide, O.M. Effects of photoperiod and temperature on growth and flowering in Norwegian and British timothy cultivars (Phleum pratense L.). Acta Agric. Scand. 1982, 32, 241–252. [Google Scholar] [CrossRef]
- Casler, M.D.; Undersander, D.J. Identification of temperate pasture grasses and legumes. In Horse Pasture Management; Sharpe, P., Ed.; Academic Press: London, UK, 2019; pp. 11–35. [Google Scholar]
- Medl, A.; Florineth, F.; Kikuta, S.B.; Mayr, S. Irrigation of ‘Green walls’ is necessary to avoid drought stress of grass vegetation (Phleum pratense L.). Ecol. Eng. 2018, 113, 21–26. [Google Scholar] [CrossRef]
- Stewart, A.V.; Joachimiak, A.; Ellison, N. Genomic and geographic origins of timothy (Phleum sp.) based on ITS and chloroplast sequences. In Molecular Breeding of Forage and Turf; Yamada, T., Spangenberg, G., Eds.; Springer: New York, NY, USA, 2009; pp. 71–82. [Google Scholar]
- Stewart, A.V.; Joachimiak, A.J.; Ellison, N.W. Phleum. In Wild Crop Relatives: Genomic and Breeding Resources. Millets and Grasses; Kole, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 257–274. [Google Scholar]
- Cai, Q.; Bullen, M. Analysis of genome-specific sequences in Phleum species: Identification and use for study of genomic relationships. Theor. Appl. Genet. 1994, 88, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Joachimiak, A. Heterochromatin and microevolution in Phleum. In Plant Genome: Biodiversity and Evolution, Part B: Phanerogams; Sharma, A.K., Sharma, A., Eds.; Science Publishers Inc.: Enfield, NH, USA; Plymouth, UK, 2005; Volume 1, pp. 89–117. [Google Scholar]
- Doebley, J.F.; Gaut, B.S.; Smith, B.D. The molecular genetics of crop domestication. Cell 2006, 127, 1309–1321. [Google Scholar] [CrossRef] [PubMed]
- Gepts, P. Crop domestication as a long-term selection experiment. Plant Breed. Rev. 2010, 24, 1–44. [Google Scholar]
- Olsen, K.M.; Wendel, J.F. A bountiful harvest: Genomic insights into crop domestication phenotypes. Annu. Rev. Plant Biol. 2013, 64, 47–70. [Google Scholar] [CrossRef] [PubMed]
- Dempewolf, H.; Baute, G.; Anderson, J.; Kilian, B.; Smith, C.; Guarino, L. Past and future use of wild relatives in crop breeding. Crop Sci. 2017, 57, 1070–1082. [Google Scholar] [CrossRef]
- Heide, O.; Solhaug, K. Growth and reproduction capacities of two bipolar Phleum alpinum populations from Norway and South Georgia. Arct. Antarct. Alp. Res. 2001, 33, 173–180. [Google Scholar] [CrossRef]
- Hultén, E.; Fries, M. Atlas of North European Vascular Plants: North of the Tropic of Cancer; Koeltz Scientific Books: Königstein, Germany, 1986. [Google Scholar]
- Sattler, M.C.; Carvalho, C.R.; Clarindo, W.R. The polyploidy and its key role in plant breeding. Planta 2016, 243, 281–296. [Google Scholar] [CrossRef] [PubMed]
- Rauf, S.; Ortiz, R.; Malinowski, D.P.; Clarindo, W.R.; Kainat, W.; Shehzad, M.; Waheed, U.; Hassan, S.W. Induced polyploidy: A tool for forage species improvement. Agriculture 2021, 11, 210. [Google Scholar] [CrossRef]
- Cheng, A.; Mohd Hanafiah, N.; Harikrishna, J.A.; Eem, L.P.; Baisakh, N.; Mispan, M.S. A reappraisal of polyploidy events in grasses (Poaceae) in a rapidly changing world. Biology 2022, 11, 636. [Google Scholar] [CrossRef]
- Gross, B.L.; Olsen, K.M. Genetic perspectives on crop domestication. Trends Plant Sci. 2010, 15, 529–537. [Google Scholar] [CrossRef]
- Casler, M. Breeding forage crops for increased nutritional value. Adv. Agron. 2001, 71, 51–107. [Google Scholar]
- Chand, S.; Indu; Singhal, R.K.; Govindasamy, P. Agronomical and breeding approaches to improve the nutritional status of forage crops for better livestock productivity. Grass Forage Sci. 2022, 77, 11–32. [Google Scholar] [CrossRef]
- Capstaff, N.M.; Miller, A.J. Improving the yield and nutritional quality of forage crops. Front. Plant Sci. 2018, 9, 535. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, M.O. Genetic improvement of forage crops—Past, present and future. J. Agric. Sci. 2005, 143, 441–448. [Google Scholar] [CrossRef]
- MacMillan, C.P.; Blundell, C.A.; King, R.W. Flowering of the grass Lolium perenne. Effects of vernalization and long days on gibberellin biosynthesis and signaling. Plant Physiol. 2005, 138, 1794–1806. [Google Scholar] [CrossRef]
- Fjellheim, S.; Boden, S.; Trevaskis, B. The role of seasonal flowering responses in adaptation of grasses to temperate climates. Front. Plant Sci. 2014, 5, 431. [Google Scholar] [CrossRef]
- Nordenskiöld, H. Intra- and interspecific hybrids of Phleum pratense and P. alpinum. Hereditas 1937, 23, 304–316. [Google Scholar] [CrossRef]
- Gustavsson, A.M. A developmental scale for perennial forage grasses based on the decimal code framework. Grass Forage Sci. 2011, 66, 93–108. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development for R. Rstudio; PBC: Boston, MA, USA, 2020; Available online: http://www.rstudio.com (accessed on 15 June 2023).

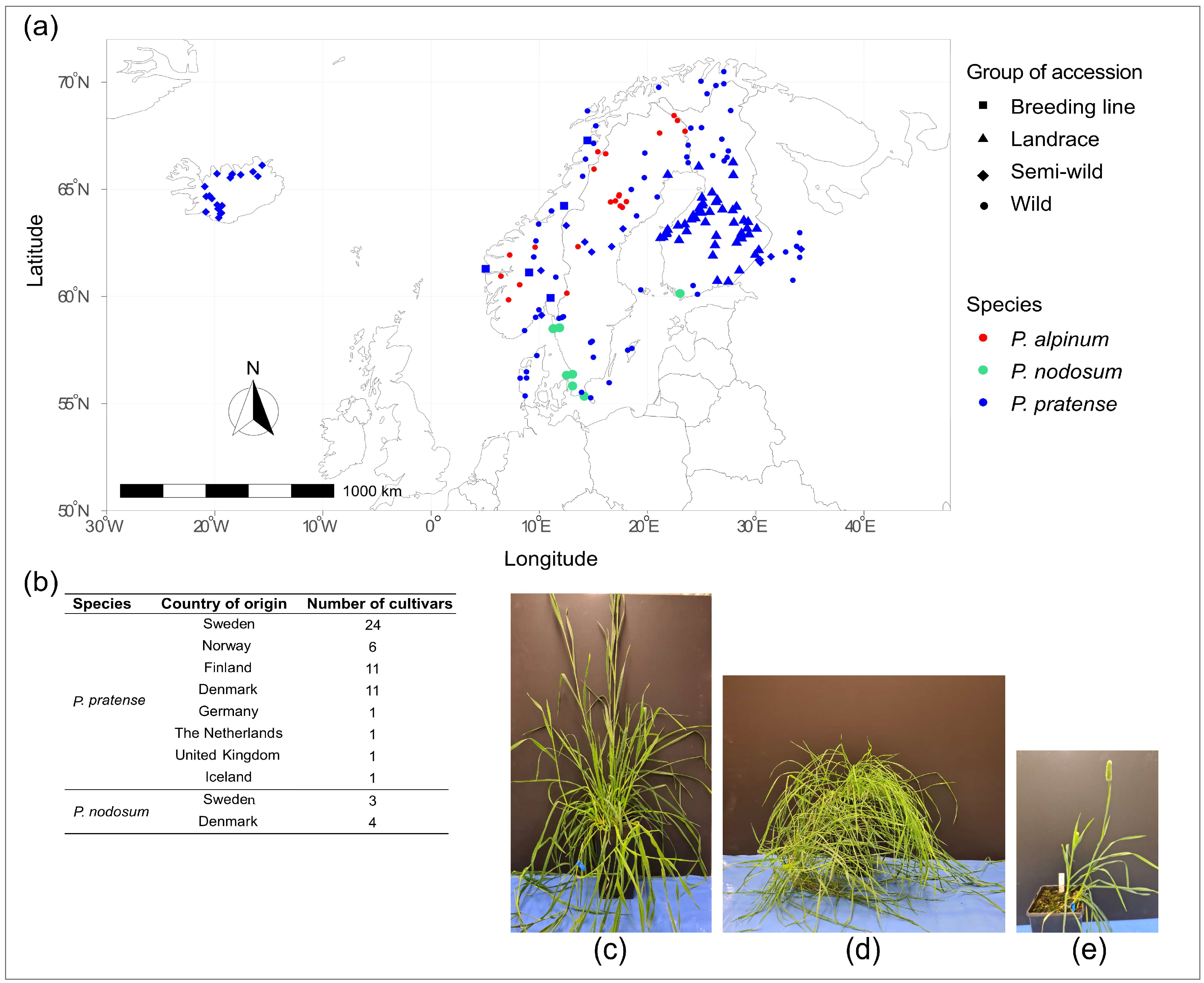
| Category | Species | Group | Planted (Number) | Survived (Number) | Survival Rate (%) |
|---|---|---|---|---|---|
| Accessions | P. pratense | Cultivar | 56 | 56 | 100.0 |
| Breeding line | 10 | 10 | 100.0 | ||
| Landrace | 55 | 55 | 100.0 | ||
| Wild | 61 | 61 | 100.0 | ||
| Semi-wild | 30 | 30 | 100.0 | ||
| P. nodosum | Cultivar | 7 | 7 | 100.0 | |
| Wild | 7 | 7 | 100.0 | ||
| P. alpinum | Wild | 18 | 12 | 66.6 | |
| Plants | P. pratense | Cultivar | 823 | 769 | 93.4 |
| Breeding line | 151 | 145 | 96.0 | ||
| Landrace | 779 | 741 | 95.1 | ||
| Wild | 928 | 876 | 94.4 | ||
| Semi-wild | 440 | 415 | 94.3 | ||
| P. nodosum | Cultivar | 90 | 86 | 95.6 | |
| Wild | 106 | 97 | 91.5 | ||
| P. alpinum | Wild | 29 | 19 | 65.5 |
| Species | Cluster | Number of Accessions | Trait | |||||
|---|---|---|---|---|---|---|---|---|
| DTS (GDD) | DTB (GDD) | DTH (GDD) | FW (g per Plant) | DW (g per Plant) | PH (cm) | |||
| P. pratense | I | 19 | 170.1 | 239.1 | 291.9 | 480.9 | 196.9 | 94.0 |
| II | 46 | 182.1 | 252.0 | 302.1 | 448.2 | 176.7 | 85.6 | |
| III | 28 | 190.8 | 266.0 | 314.4 | 442.9 | 173.7 | 81.8 | |
| IV | 43 | 176.7 | 243.1 | 293.9 | 364.8 | 148.4 | 86.7 | |
| V | 43 | 187.5 | 255.5 | 305.7 | 364.0 | 142.4 | 78.7 | |
| VI | 33 | 201.4 | 270.6 | 318.5 | 339.3 | 133.2 | 71.1 | |
| P. nodosum | I | 5 | 173.0 | 233.9 | 285.6 | 309.8 | 121.5 | 64.5 |
| II | 8 | 177.1 | 239.0 | 288.2 | 221.9 | 87.2 | 58.7 | |
| III | 1 | 234.1 | 300.1 | 340.0 | 212.3 | 86.6 | 44.2 | |
| P. alpinum | I | 5 | 188.9 | 232.4 | 279.5 | 69.0 | 25.4 | 27.0 |
| II | 3 | 164.2 | 205.9 | 268.5 | 168.3 | 80.7 | 46.0 | |
| III | 4 | 187.0 | 252.7 | 307.0 | 386.5 | 136.9 | 59.6 | |
| Species | Cluster | Number of Accessions | Trait | |||||
|---|---|---|---|---|---|---|---|---|
| DTS (DAE) | DTB (DAE) | DTH (DAE) | FW (g per Plant) | DW (g per Plant) | PH (cm) | |||
| P. pratense | I | 55 | 159.4 | 170.2 | 183.4 | 92.6 | 34.2 | 103.2 |
| II | 37 | 169.3 | 180.9 | 195.6 | 70.0 | 25.1 | 80.2 | |
| III | 12 | 183.7 | 224.2 | 252.3 | 71.4 | 24.9 | 87.6 | |
| IV | 17 | 175.1 | 196.1 | 217.1 | 123.1 | 46.0 | 96.6 | |
| V | 38 | 160.2 | 174.5 | 184.9 | 143.9 | 56.9 | 125.7 | |
| P. nodosum | I | 3 | 154.7 | 177.4 | 193.2 | 108.3 | 46.2 | 105.2 |
| II | 4 | 156.7 | 167.5 | 179.6 | 141.5 | 60.1 | 128.0 | |
| III | 1 | 161.3 | 200.0 | 220.0 | 100.7 | 43.8 | 92.1 | |
| IV | 5 | 161.9 | 170.0 | 179.9 | 65.4 | 27.4 | 88.8 | |
| P. alpinum | I | 3 | 161.2 | 174.7 | 187.2 | 3.1 | 1.6 | 44.1 |
| II | 1 | 173.0 | 198.0 | 214.0 | 33.8 | 12.8 | 67.1 | |
| III | 1 | 154.0 | 163.5 | 176.5 | 223.6 | 87.5 | 132.0 | |
| Trial | Trait | Vg | Ve | Vp | H2 |
|---|---|---|---|---|---|
| Field | DTS | 0.9 | 5.6 | 2.3 | 0.40 |
| DTB | 0.6 | 3.8 | 1.5 | 0.37 | |
| DTH | 0.5 | 3.2 | 1.3 | 0.37 | |
| FW | 2449.5 | 24,289.1 | 8521.7 | 0.29 | |
| DW | 401.0 | 3708.0 | 1328.0 | 0.30 | |
| PH | 48.7 | 160.6 | 88.9 | 0.55 | |
| Greenhouse | DTS | 99.2 | 111.6 | 127.1 | 0.78 |
| DTB | 286.4 | 60.1 | 301.5 | 0.95 | |
| DTH | 505.0 | 98.9 | 529.7 | 0.95 | |
| FW | 745.9 | 3204.1 | 1546.9 | 0.48 | |
| DW | 125.2 | 559.9 | 265.1 | 0.47 | |
| PH | 315.3 | 1092.8 | 588.4 | 0.54 |
| Trait | Abbreviation | Description |
|---|---|---|
| Fresh weight | FW | Fresh weight (g) of tillers cut at 3 cm above the soil surface |
| Dry weight | DW | Dry weight (g) of tillers cut at 3 cm above the soil surface |
| Plant height | PH | The average length of 5 tillers (cm) in the field and the average length of 9 tillers (cm) in the greenhouse |
| Days to stem elongation | DTS | Number of days from emergence of coleoptile until the first tiller internode started to elongate, and the inflorescence was palpable at least 1 cm above the stem base in about one-fourth of the total number of tillers |
| Days to booting | DTB | Number of days from emergence of coleoptile until the tip of the inflorescence was palpable in the flag leaf sheath below the flag leaf base in about one fourth of the total number of tillers |
| Days to heading | DTH | Number of days from emergence of coleoptile until the head was visible above the flag leaf base in about one-fourth of the total number of tillers |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahimi, Y.; Bedada, G.; Moreno, S.; Gustavsson, A.-M.; Ingvarsson, P.K.; Westerbergh, A. Phenotypic Diversity in Domesticated and Wild Timothy Grass, and Closely Related Species for Forage Breeding. Plants 2023, 12, 3494. https://doi.org/10.3390/plants12193494
Rahimi Y, Bedada G, Moreno S, Gustavsson A-M, Ingvarsson PK, Westerbergh A. Phenotypic Diversity in Domesticated and Wild Timothy Grass, and Closely Related Species for Forage Breeding. Plants. 2023; 12(19):3494. https://doi.org/10.3390/plants12193494
Chicago/Turabian StyleRahimi, Yousef, Girma Bedada, Silvana Moreno, Anne-Maj Gustavsson, Pär K. Ingvarsson, and Anna Westerbergh. 2023. "Phenotypic Diversity in Domesticated and Wild Timothy Grass, and Closely Related Species for Forage Breeding" Plants 12, no. 19: 3494. https://doi.org/10.3390/plants12193494
APA StyleRahimi, Y., Bedada, G., Moreno, S., Gustavsson, A.-M., Ingvarsson, P. K., & Westerbergh, A. (2023). Phenotypic Diversity in Domesticated and Wild Timothy Grass, and Closely Related Species for Forage Breeding. Plants, 12(19), 3494. https://doi.org/10.3390/plants12193494







