The Ethanol Extracts of Osmanthus fragrans Leaves Ameliorate the Bone Loss via the Inhibition of Osteoclastogenesis in Osteoporosis
Abstract
:1. Introduction
2. Results
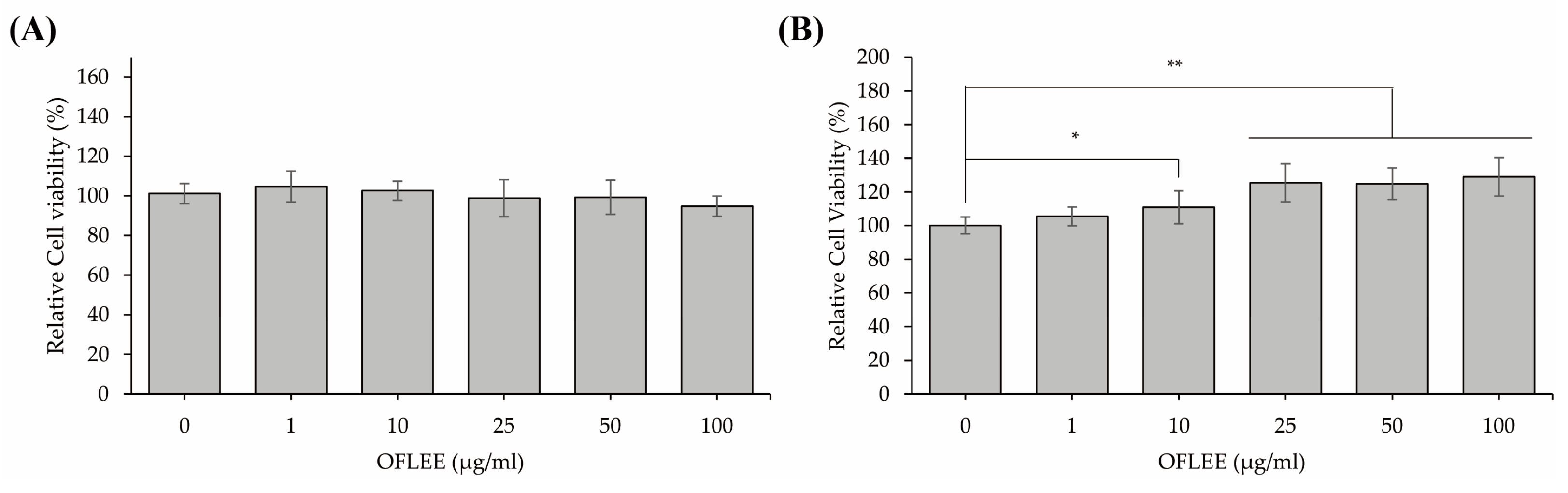
2.1. OFLEE Does Not Induce Cell Death in Either L929 Fibroblast Cells or BMMs
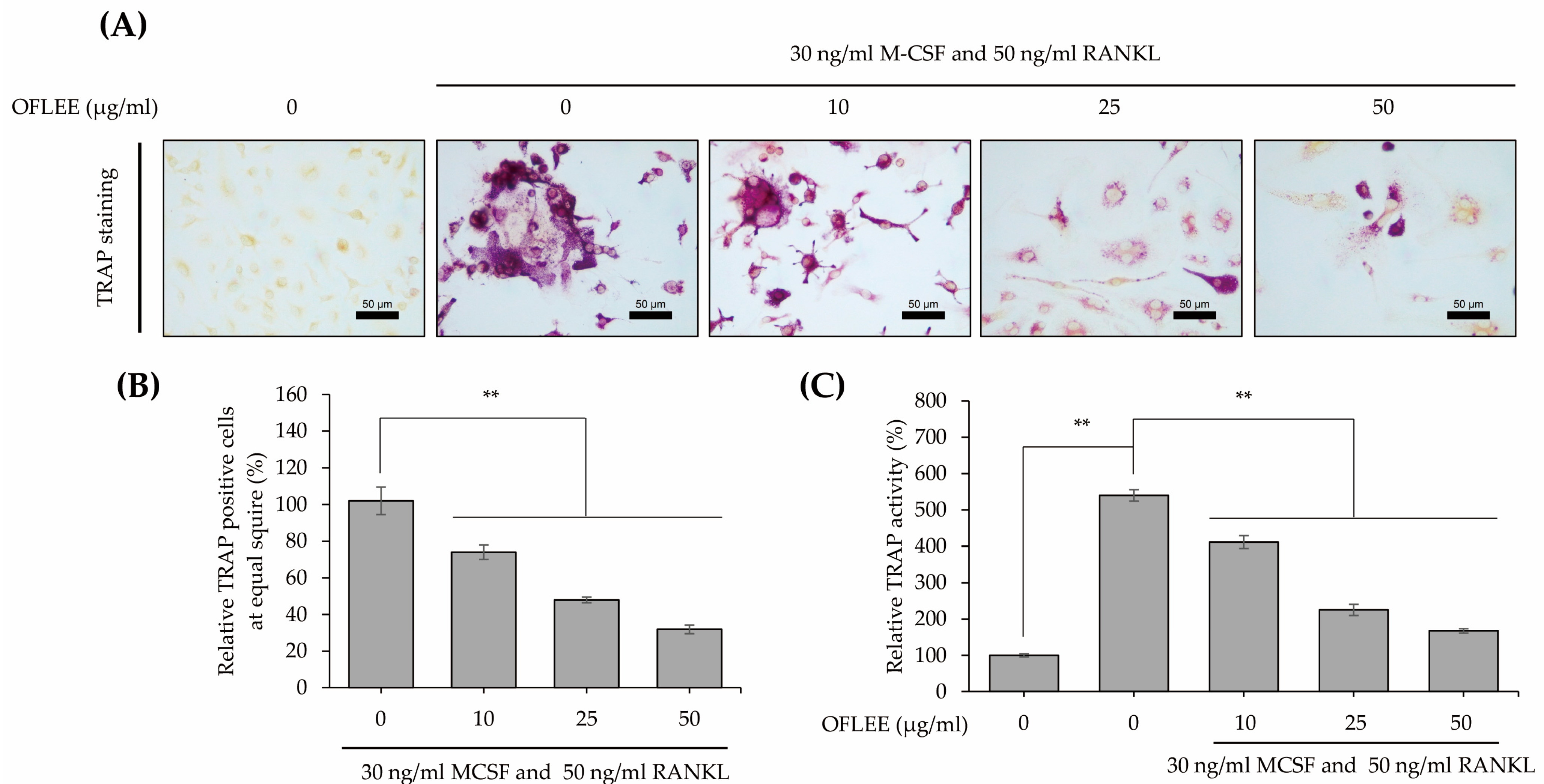
2.2. OFLEE Suppresses the Osteoclastic Differentiation of BMMs in the Presence of M-CSF and RANKL
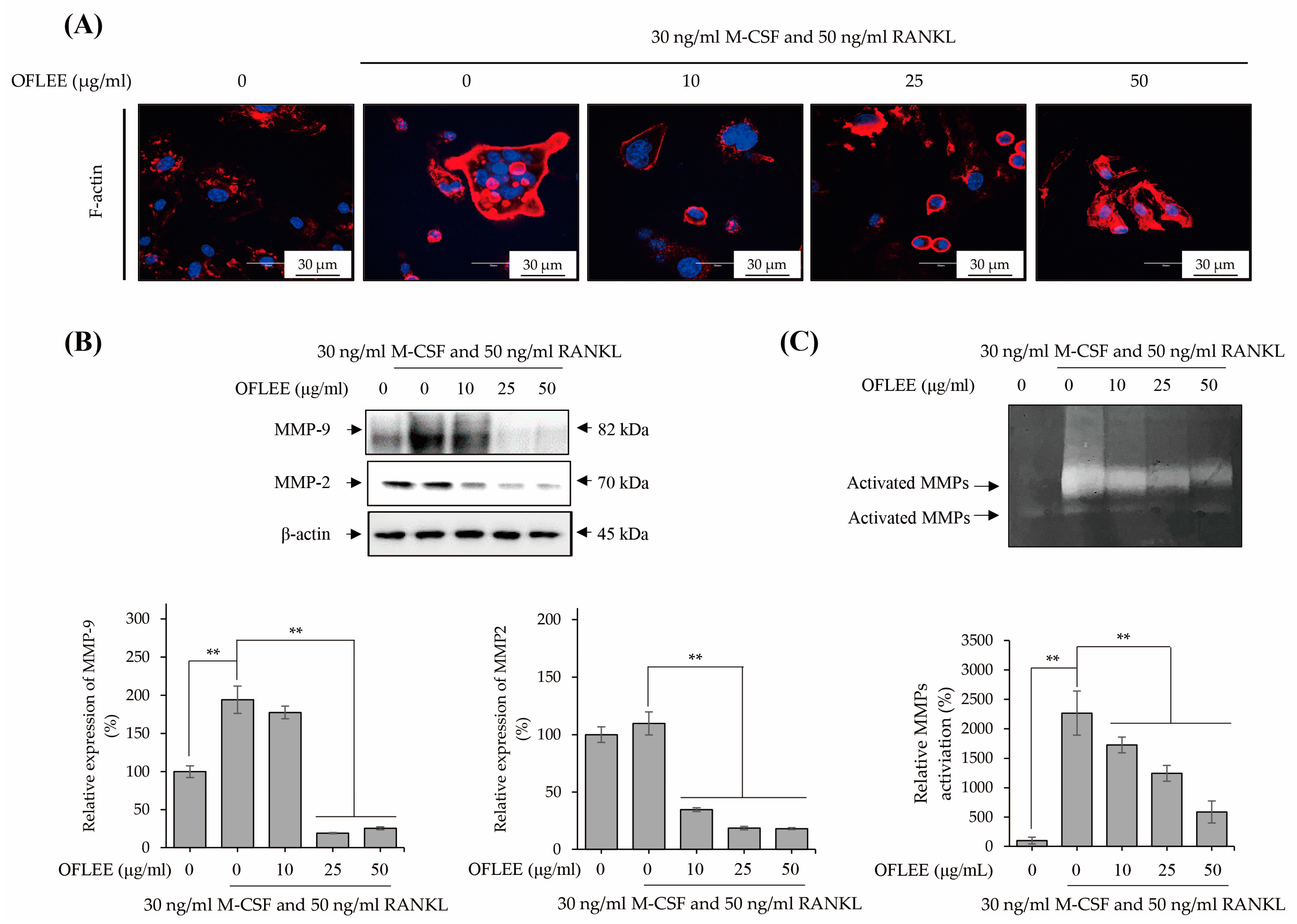
2.3. OFLEE Inhibits the Expression and Activation of Matrix Metalloproteinase via the Suppression of F-Actin Formation in the BMMs Treated with M-CSF and RANKL
2.4. OFLEE Suppresses the Osteoclastic Differentiation of BMMs through the Inhibition of Nucleus Factor-κB (NF-κB) Phosphorylation in the Presence of M-CSF and RANKL
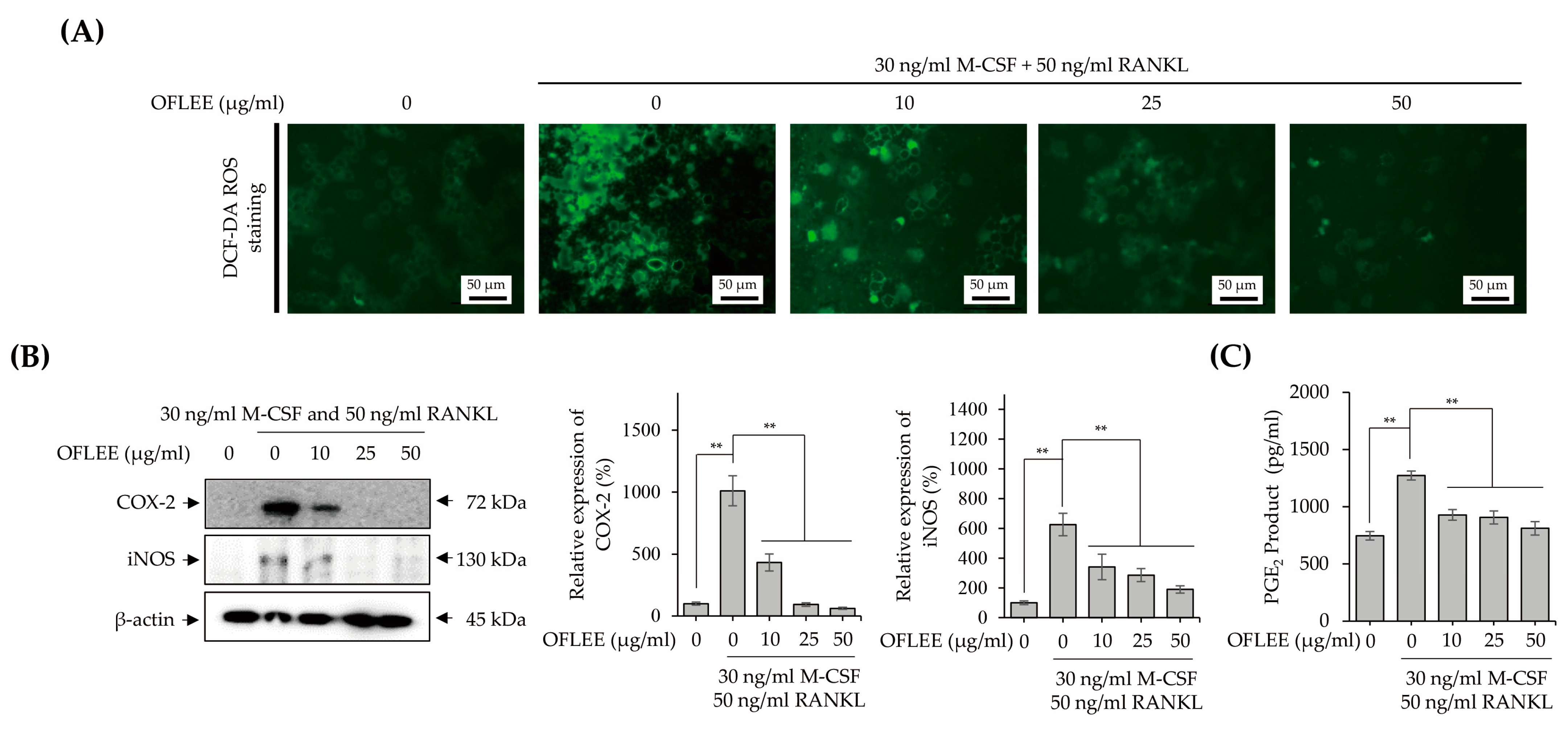
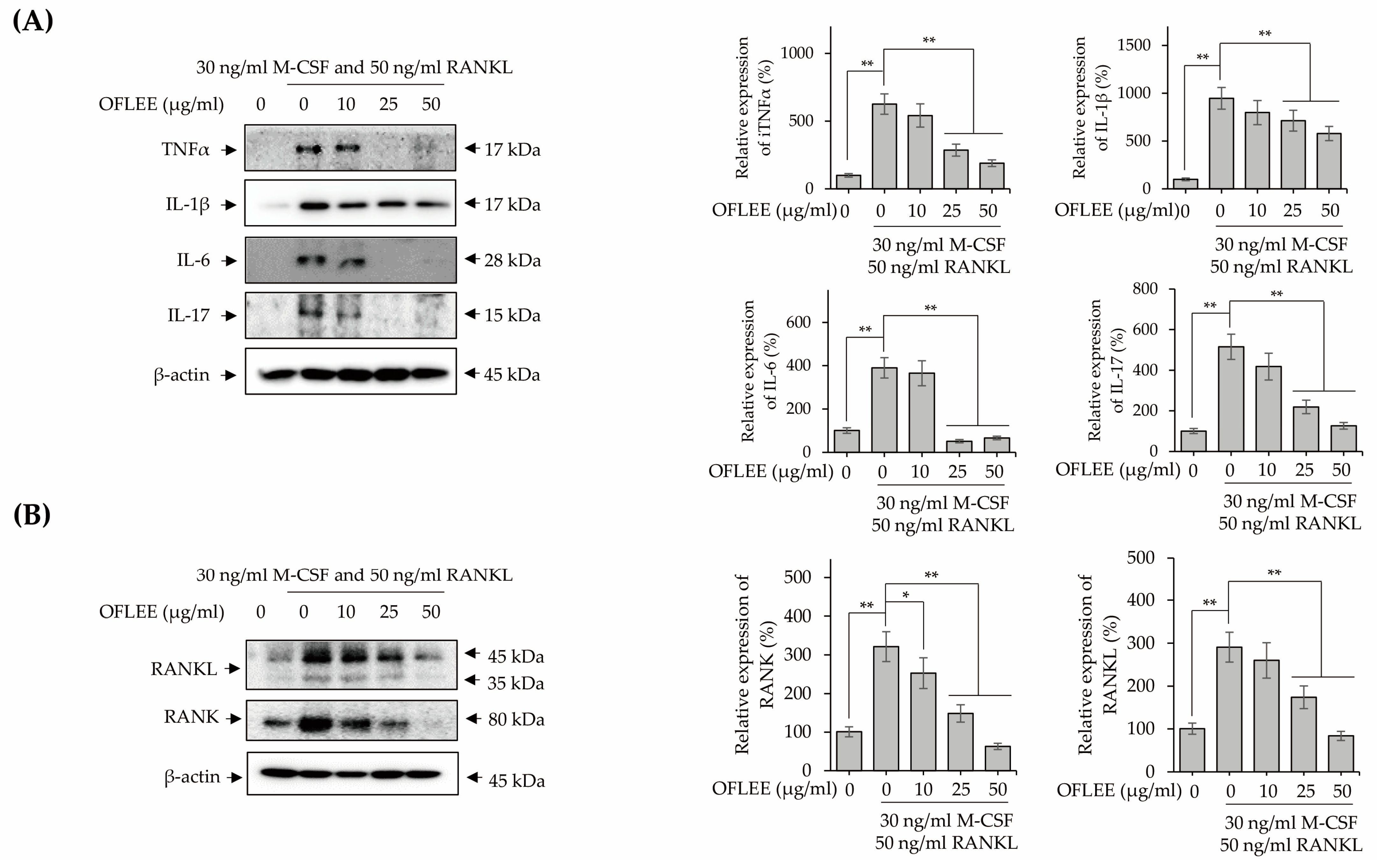
2.5. OFLEE-Mediated Anti-Osteoclastogenesis Is Mediated by the Suppression of Reactive Oxygen Species (ROS), Inflammatory Mediators, Pro-Inflammatory Cytokines, RANKL, and RANK in the BMMs Treated with M-CSF and RANKL
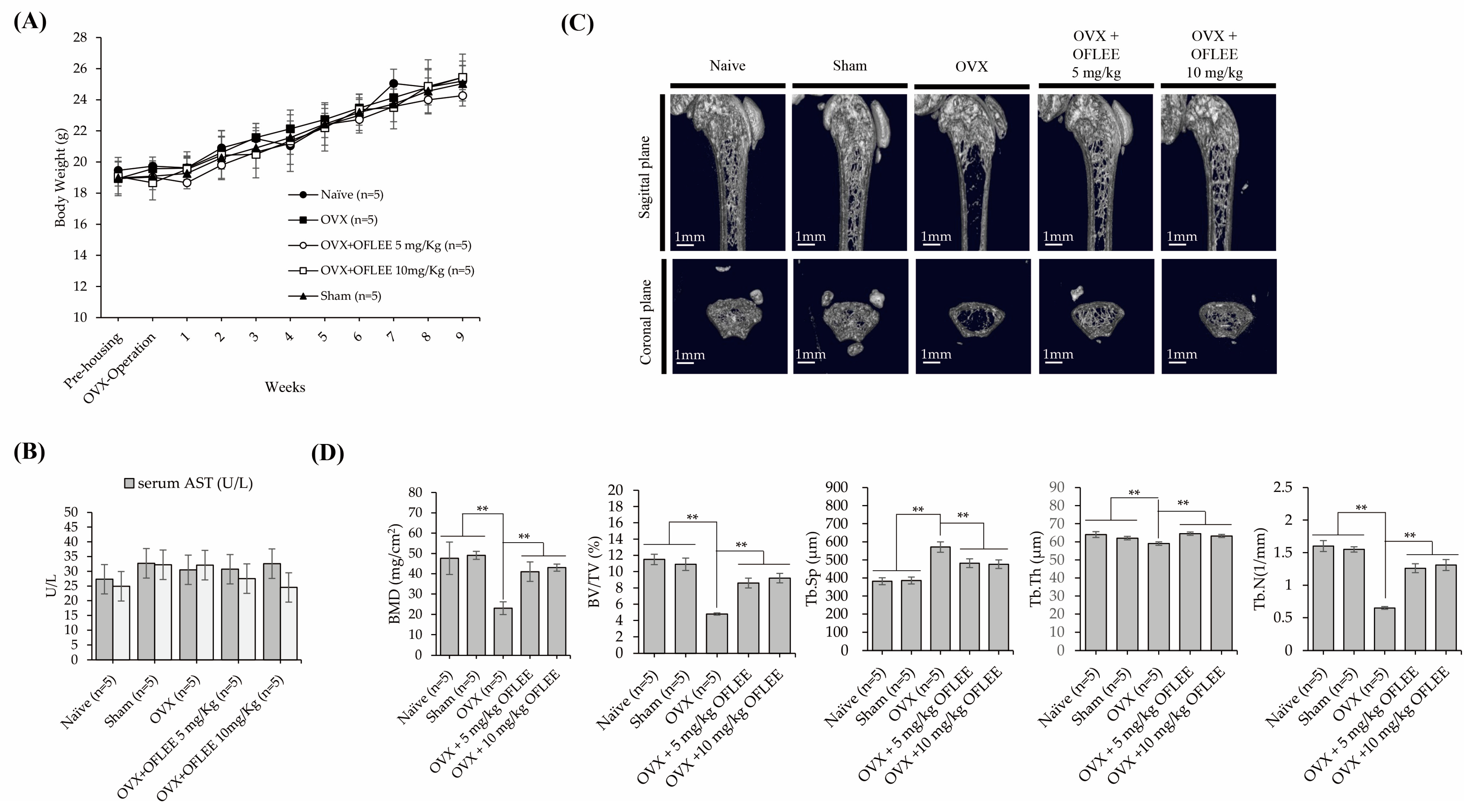
2.6. The Oral Administration of OFLEE Suppresses Osteoporotic Bone Loss without Hepatotoxicity in the Animals with Osteoporosis Generated by Ovariectomy
3. Discussion
4. Materials and Methods
4.1. Extraction of OFL
4.2. Isolation and Cultivation of BMMs
4.3. Cytotoxicity of OFLSS in BMMs
4.4. TRAP Staining and Activity Assay
4.5. F-Actin Staining
4.6. Western Blotting
4.7. Gelatin Zymography
4.8. ROS Detection
4.9. Measurement of PGE2
4.10. Animal Study
4.11. Histological Analysis
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lane, N.E. Epidemiology, etiology, and diagnosis of osteoporosis. Am. J. Obstet. Gynecol. 2006, 194 (Suppl. S2), S3–S11. [Google Scholar] [CrossRef] [PubMed]
- Arceo-Mendoza, R.M.; Camacho, P.M. Postmenopausal Osteoporosis: Latest Guidelines. Endocrinol. Metab. Clin. N. Am. 2021, 50, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xiao, Z.; Quarles, L.D.; Li, W. Osteoporosis: Mechanism, Molecular Target and Current Status on Drug Development. Curr. Med. Chem. 2021, 28, 1489–1507. [Google Scholar] [CrossRef]
- Kodama, J.; Kaito, T. Osteoclast Multinucleation: Review of Current Literature. Int. J. Mol. Sci. 2020, 21, 5685. [Google Scholar] [CrossRef]
- Maruotti, N.; Corrado, A.; Cantatore, F.P. Osteoblast role in osteoarthritis pathogenesis. J. Cell Physiol. 2017, 232, 2957–2963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raggatt, L.J.; Partridge, N.C. Cellular and molecular mechanisms of bone remodeling. J. Biol. Chem. 2010, 285, 25103–25108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mundy, G.R. Osteoporosis and inflammation. Nutr. Rev. 2007, 65 Pt 2, S147–S151. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Luan, F.; Bao, Y.; Peng, X.; Rao, Z.; Tang, Q.; Zeng, N. Traditional uses, phytochemical constituents and pharmacological properties of Osmanthus fragrans: A review. J. Ethnopharmacol. 2022, 293, 115273. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.E.; Shim, S.Y.; Lee, M. Anti-inflammatory activity of phenylpropyl triterpenoids from Osmanthus fragrans var. aurantiacus leaves. Int. Immunopharmacol. 2020, 86, 106576. [Google Scholar] [CrossRef]
- Wu, L.; Liu, J.; Huang, W.; Wang, Y.; Chen, Q.; Lu, B. Exploration of Osmanthus fragrans Lour.’s composition, nutraceutical functions and applications. Food. Chem. 2022, 377, 131853. [Google Scholar] [CrossRef]
- Song, H.Y.; Jeong, D.E.; Lee, M. Bioactivity-Guided Extract Optimization of Osmanthus fragrans var. aurantiacus Leaves and Anti-Inflammatory Activities of Phillyrin. Plants 2021, 10, 1545. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhang, Y.; Wang, C.; Zhou, C.; Yan, S. Phillyrin Prevents Ovariectomy-Induced Osteolysis by Inhibiting Osteoclast Differentiation. Evid. Based Complement. Alternat. Med. 2022, 2022, 6065494. [Google Scholar] [CrossRef] [PubMed]
- Biswas, R.S.; Baker, D.; Hruska, K.A.; Chellaiah, M.A. Polyphosphoinositides-dependent regulation of the osteoclast actin cytoskeleton and bone resorption. BMC Cell Biol 2004, 5, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, X.; Zhang, Y.; Guo, S.; Zhang, W.; Wang, J.; Lin, Y. Dynamic expression of matrix metalloproteinases 2, 9 and 13 in ovariectomy-induced osteoporosis rats. Exp. Ther. Med. 2018, 16, 1807–1813. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Zhai, Z.; Liu, G.; Tang, T.; Lin, Z.; Zheng, M.; Qin, A.; Dai, K. Sanguinarine inhibits osteoclast formation and bone resorption via suppressing RANKL-induced activation of NF-kappaB and ERK signaling pathways. Biochem. Biophys. Res. Commun. 2013, 430, 951–956. [Google Scholar] [CrossRef]
- Tong, X.; Yu, G.; Fu, X.; Song, R.; Gu, J.; Liu, Z. A Review of Signaling Transduction Mechanisms in Osteoclastogenesis Regulation by Autophagy, Inflammation, and Immunity. Int. J. Mol. Sci. 2022, 23, 9846. [Google Scholar] [CrossRef]
- Weitzmann, M.N. The Role of Inflammatory Cytokines, the RANKL/OPG Axis, and the Immunoskeletal Interface in Physiological Bone Turnover and Osteoporosis. Scientifica 2013, 2013, 125705. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.L.; Liu, C.; Shi, X.M.; Cheng, Y.T.; Zhou, Q.; Li, J.P.; Liao, J. Zoledronic acid inhibits osteoclastogenesis and bone resorptive function by suppressing RANKL-mediated NF-kappaB and JNK and their downstream signalling pathways. Mol. Med. Rep. 2022, 25, 59. [Google Scholar] [CrossRef]
- Jurdic, P.; Saltel, F.; Chabadel, A.; Destaing, O. Podosome and sealing zone: Specificity of the osteoclast model. Eur. J. Cell. Biol. 2006, 85, 195–202. [Google Scholar] [CrossRef]
- Matsubara, T.; Kinbara, M.; Maeda, T.; Yoshizawa, M.; Kokabu, S.; Takano Yamamoto, T. Regulation of osteoclast differentiation and actin ring formation by the cytolinker protein plectin. Biochem. Biophys. Res. Commun. 2017, 489, 472–476. [Google Scholar] [CrossRef]
- Montalcini, T.; Romeo, S.; Ferro, Y.; Migliaccio, V.; Gazzaruso, C.; Pujia, A. Osteoporosis in chronic inflammatory disease: The role of malnutrition. Endocrine 2013, 43, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.K.; Choi, Y.G.; Baik, J.Y.; Han, S.Y.; Jeong, D.W.; Bae, Y.S.; Kim, N.; Lee, S.Y. A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood 2005, 106, 852–859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashtar, M.; Tenshin, H.; Teramachi, J.; Bat-Erdene, A.; Hiasa, M.; Oda, A.; Tanimoto, K.; Shimizu, S.; Higa, Y.; Harada, T.; et al. The Roles of ROS Generation in RANKL-Induced Osteoclastogenesis: Suppressive Effects of Febuxostat. Cancers 2020, 12, 929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Callaway, D.A.; Jiang, J.X. Reactive oxygen species and oxidative stress in osteoclastogenesis, skeletal aging and bone diseases. J. Bone Miner. Metab. 2015, 33, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Zhong, M.; Huang, Y.; Zhu, J.; Tang, W.; Li, D.; Shi, J.; Lu, A.; Yang, H.; Geng, D.; et al. Puerarin alleviates osteoporosis in the ovariectomy-induced mice by suppressing osteoclastogenesis via inhibition of TRAF6/ROS-dependent MAPK/NF-kappaB signaling pathways. Aging 2020, 12, 21706–21729. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, C.; Wang, G.; Sun, Y.; Deng, Z.; Chen, L.; Chen, K.; Tickner, J.; Kenny, J.; Song, D.; et al. Loureirin B suppresses RANKL-induced osteoclastogenesis and ovariectomized osteoporosis via attenuating NFATc1 and ROS activities. Theranostics 2019, 9, 4648–4662. [Google Scholar] [CrossRef]
- Kondo, T.; Otsuka, Y.; Aoki, H.; Goto, Y.; Kawaguchi, Y.; Waguri-Nagaya, Y.; Miyazawa, K.; Goto, S.; Aoyama, M. The Inducible Nitric Oxide Synthase Pathway Promotes Osteoclastogenesis under Hypoxic Culture Conditions. Am. J. Pathol. 2021, 191, 2072–2079. [Google Scholar] [CrossRef]
- Cuzzocrea, S.; Mazzon, E.; Dugo, L.; Genovese, T.; Di Paola, R.; Ruggeri, Z.; Vegeto, E.; Caputi, A.P.; Van De Loo, F.A.; Puzzolo, D.; et al. Inducible nitric oxide synthase mediates bone loss in ovariectomized mice. Endocrinology 2003, 144, 1098–1107. [Google Scholar] [CrossRef] [Green Version]
- Han, S.Y.; Lee, N.K.; Kim, K.H.; Jang, I.W.; Yim, M.; Kim, J.H.; Lee, W.J.; Lee, S.Y. Transcriptional induction of cyclooxygenase-2 in osteoclast precursors is involved in RANKL-induced osteoclastogenesis. Blood 2005, 106, 1240–1245. [Google Scholar] [CrossRef] [Green Version]
- Boyce, B.F.; Schwarz, E.M.; Xing, L. Osteoclast precursors: Cytokine-stimulated immunomodulators of inflammatory bone disease. Curr. Opin. Rheumatol. 2006, 18, 427–432. [Google Scholar] [CrossRef]
- Yao, Z.; Getting, S.J.; Locke, I.C. Regulation of TNF-Induced Osteoclast Differentiation. Cells 2021, 11, 132. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Guo, Q.; Yang, J.; Ni, B. Tumor Necrosis Factor Alpha Promotes Osteoclast Formation Via PI3K/Akt Pathway-Mediated Blimp1 Expression Upregulation. J. Cell Biochem. 2017, 118, 1308–1315. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.; Takeshita, S.; Barker, J.E.; Kanagawa, O.; Ross, F.P.; Teitelbaum, S.L. TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J. Clin. Investig. 2000, 106, 1481–1488. [Google Scholar] [CrossRef] [PubMed]
- Hofbauer, L.C.; Lacey, D.L.; Dunstan, C.R.; Spelsberg, T.C.; Riggs, B.L.; Khosla, S. Interleukin-1beta and tumor necrosis factor-alpha, but not interleukin-6, stimulate osteoprotegerin ligand gene expression in human osteoblastic cells. Bone 1999, 25, 255–259. [Google Scholar] [CrossRef]
- Wei, S.; Kitaura, H.; Zhou, P.; Ross, F.P.; Teitelbaum, S.L. IL-1 mediates TNF-induced osteoclastogenesis. J. Clin. Investig. 2005, 115, 282–290. [Google Scholar] [CrossRef] [Green Version]
- Liao, R.; Feng, Z.; Li, W.; Liu, R.; Xu, X.; Yao, S.; Tian, J. Interleukin-1 induces receptor activator of nuclear factor-kappaB ligand-independent osteoclast differentiation in RAW264.7 cells. Exp. Ther. Med. 2021, 21, 640. [Google Scholar] [CrossRef]
- Kitazawa, R.; Kimble, R.B.; Vannice, J.L.; Kung, V.T.; Pacifici, R. Interleukin-1 receptor antagonist and tumor necrosis factor binding protein decrease osteoclast formation and bone resorption in ovariectomized mice. J. Clin. Investig. 1994, 94, 2397–2406. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Zhou, X.; Huang, D.; Ji, Y.; Kang, F. IL-6 Enhances Osteocyte-Mediated Osteoclastogenesis by Promoting JAK2 and RANKL Activity In Vitro. Cell Physiol. Biochem. 2017, 41, 1360–1369. [Google Scholar] [CrossRef]
- Kurihara, N.; Bertolini, D.; Suda, T.; Akiyama, Y.; Roodman, G.D. IL-6 stimulates osteoclast-like multinucleated cell formation in long term human marrow cultures by inducing IL-1 release. J. Immunol. 1990, 144, 4226–4230. [Google Scholar] [CrossRef]
- He, B.; Yin, X.; Hao, D.; Zhang, X.; Zhang, Z.; Zhang, K.; Yang, X. Blockade of IL-6 alleviates bone loss induced by modeled microgravity in mice. Can. J. Physiol. Pharmacol. 2020, 98, 678–683. [Google Scholar] [CrossRef]
- Yago, T.; Nanke, Y.; Ichikawa, N.; Kobashigawa, T.; Mogi, M.; Kamatani, N.; Kotake, S. IL-17 induces osteoclastogenesis from human monocytes alone in the absence of osteoblasts, which is potently inhibited by anti-TNF-alpha antibody: A novel mechanism of osteoclastogenesis by IL-17. J. Cell Biochem. 2009, 108, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Funaki, Y.; Hasegawa, Y.; Okazaki, R.; Yamasaki, A.; Sueda, Y.; Yamamoto, A.; Yanai, M.; Fukushima, T.; Harada, T.; Makino, H.; et al. Resolvin E1 Inhibits Osteoclastogenesis and Bone Resorption by Suppressing IL-17-induced RANKL Expression in Osteoblasts and RANKL-induced Osteoclast Differentiation. Yonago Acta. Med. 2018, 61, 8–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, D.; Li, L.; Sun, Y.; Wang, W.; Wang, X.; Ye, Y.; Chen, X.; Xu, Y. IL-17 regulates the expressions of RANKL and OPG in human periodontal ligament cells via TRAF6/TBK1-JNK/NF-kappaB pathways. Immunology 2014, 144, 472–485. [Google Scholar] [CrossRef]
- Khosla, S.; Hofbauer, L.C. Osteoporosis treatment: Recent developments and ongoing challenges. Lancet Diabetes Endocrinol. 2017, 5, 898–907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujita, H.; Ochi, M.; Ono, M.; Aoyama, E.; Ogino, T.; Kondo, Y.; Ohuchi, H. Glutathione accelerates osteoclast differentiation and inflammatory bone destruction. Free Radic Res 2019, 53, 226–236. [Google Scholar] [CrossRef]
- Hwang, Y.H.; Lee, A.; Kim, T.; Jang, S.A.; Ha, H. Anti-Osteoporotic Effects of Commiphora Myrrha and Its Poly-Saccharide via Osteoclastogenesis Inhibition. Plants 2021, 10, 945. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, Y.-S.; Lim, H.; Seo, J.-Y.; Kang, K.-R.; Kim, D.K.; Lee, H.-H.; Oh, D.-S.; Kim, J.-S. The Ethanol Extracts of Osmanthus fragrans Leaves Ameliorate the Bone Loss via the Inhibition of Osteoclastogenesis in Osteoporosis. Plants 2023, 12, 253. https://doi.org/10.3390/plants12020253
Seo Y-S, Lim H, Seo J-Y, Kang K-R, Kim DK, Lee H-H, Oh D-S, Kim J-S. The Ethanol Extracts of Osmanthus fragrans Leaves Ameliorate the Bone Loss via the Inhibition of Osteoclastogenesis in Osteoporosis. Plants. 2023; 12(2):253. https://doi.org/10.3390/plants12020253
Chicago/Turabian StyleSeo, Yo-Seob, HyangI Lim, Jeong-Yeon Seo, Kyeong-Rok Kang, Do Kyung Kim, Hyun-Hwa Lee, Deuk-Sil Oh, and Jae-Sung Kim. 2023. "The Ethanol Extracts of Osmanthus fragrans Leaves Ameliorate the Bone Loss via the Inhibition of Osteoclastogenesis in Osteoporosis" Plants 12, no. 2: 253. https://doi.org/10.3390/plants12020253





