Density-Dependent Effects of Simultaneous Root and Floral Herbivory on Plant Fitness and Defense
Abstract
:1. Introduction
2. Results
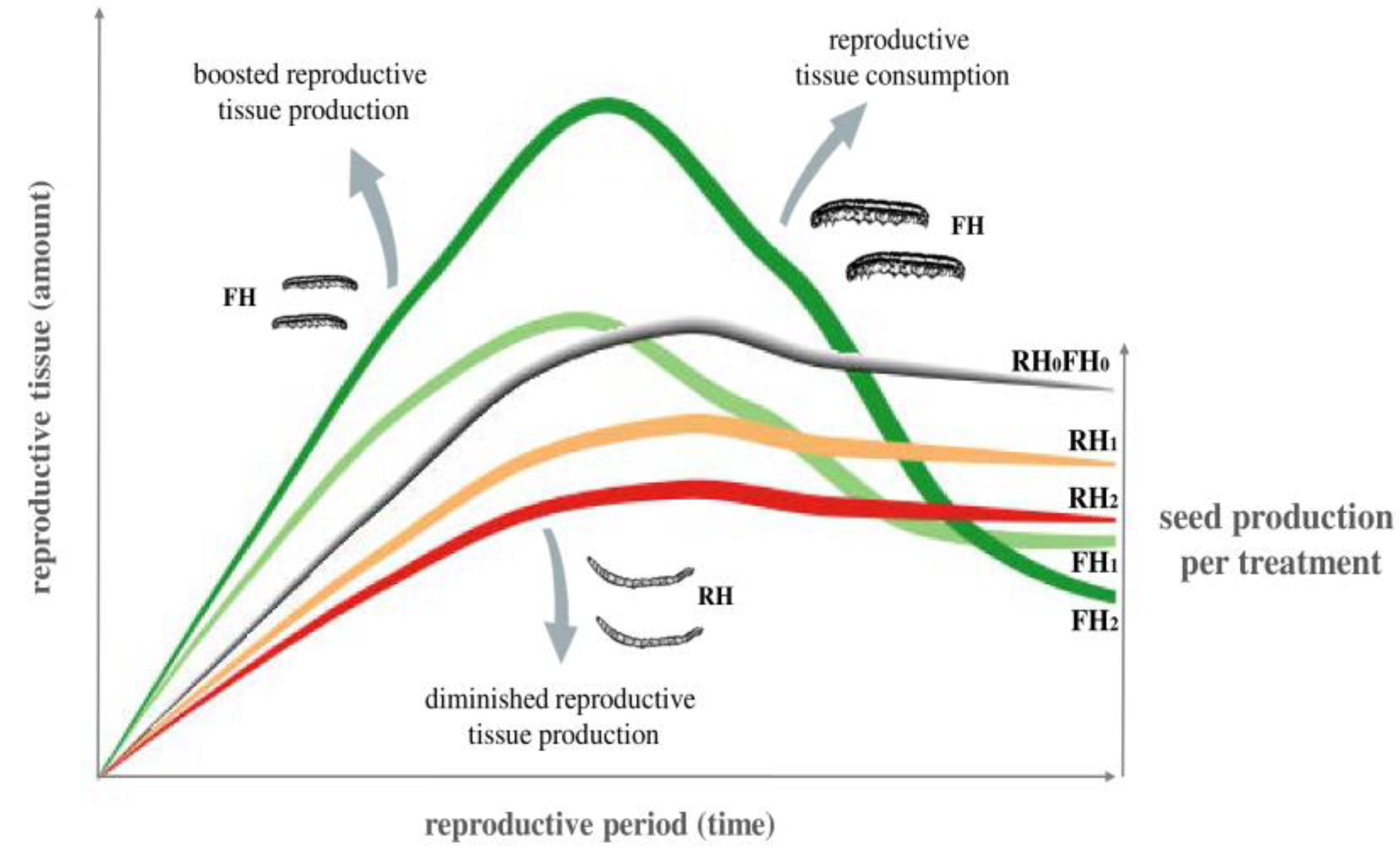
2.1. Development of Plant Reproduction over Time (Generalized Estimating Equations—GEEs)
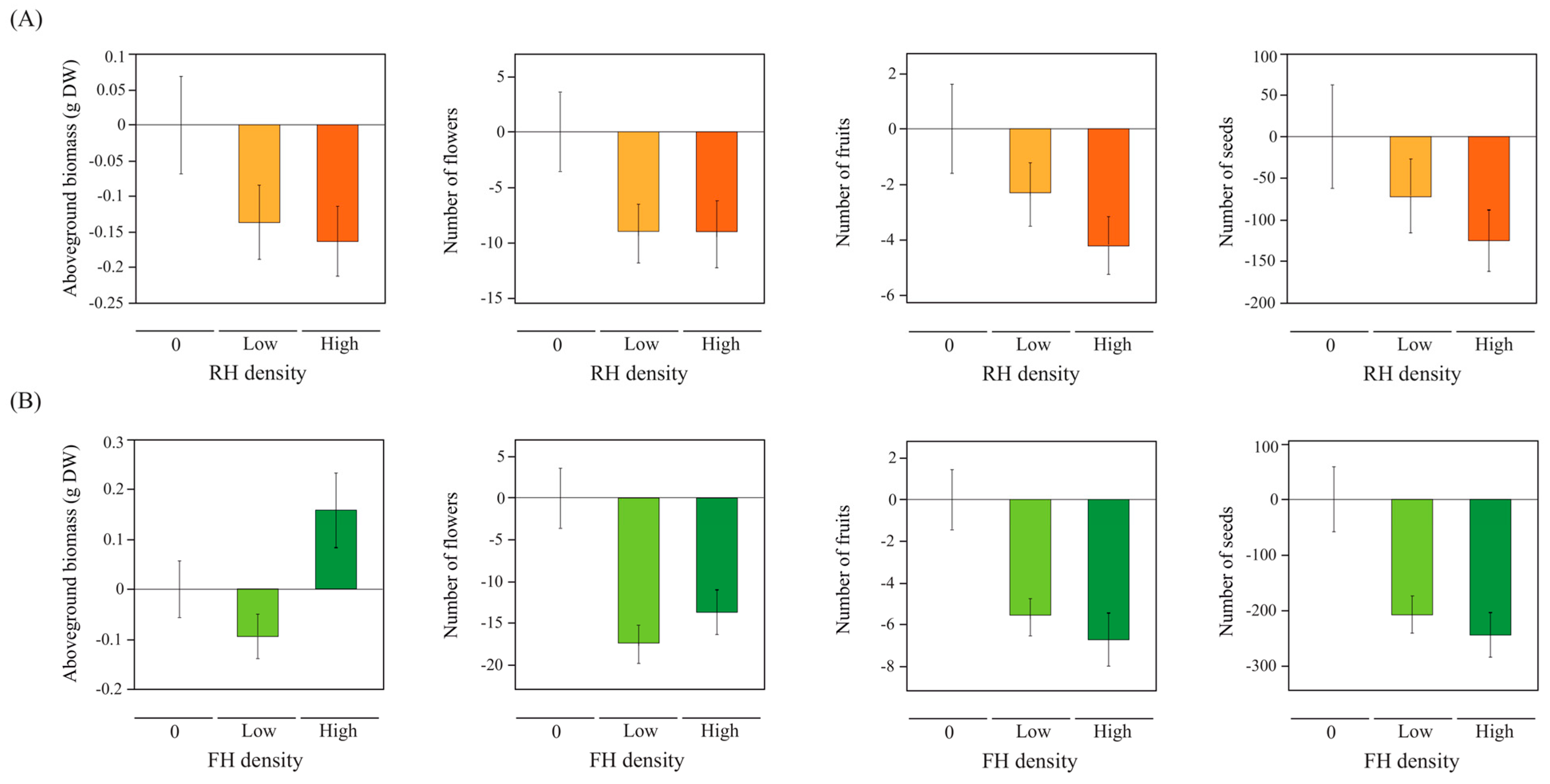
2.2. Linear and Non-Linear Effects of Herbviores on Plant Performance and Reproduction
2.3. Herbivore Effects on FH Caterpillar Development Time
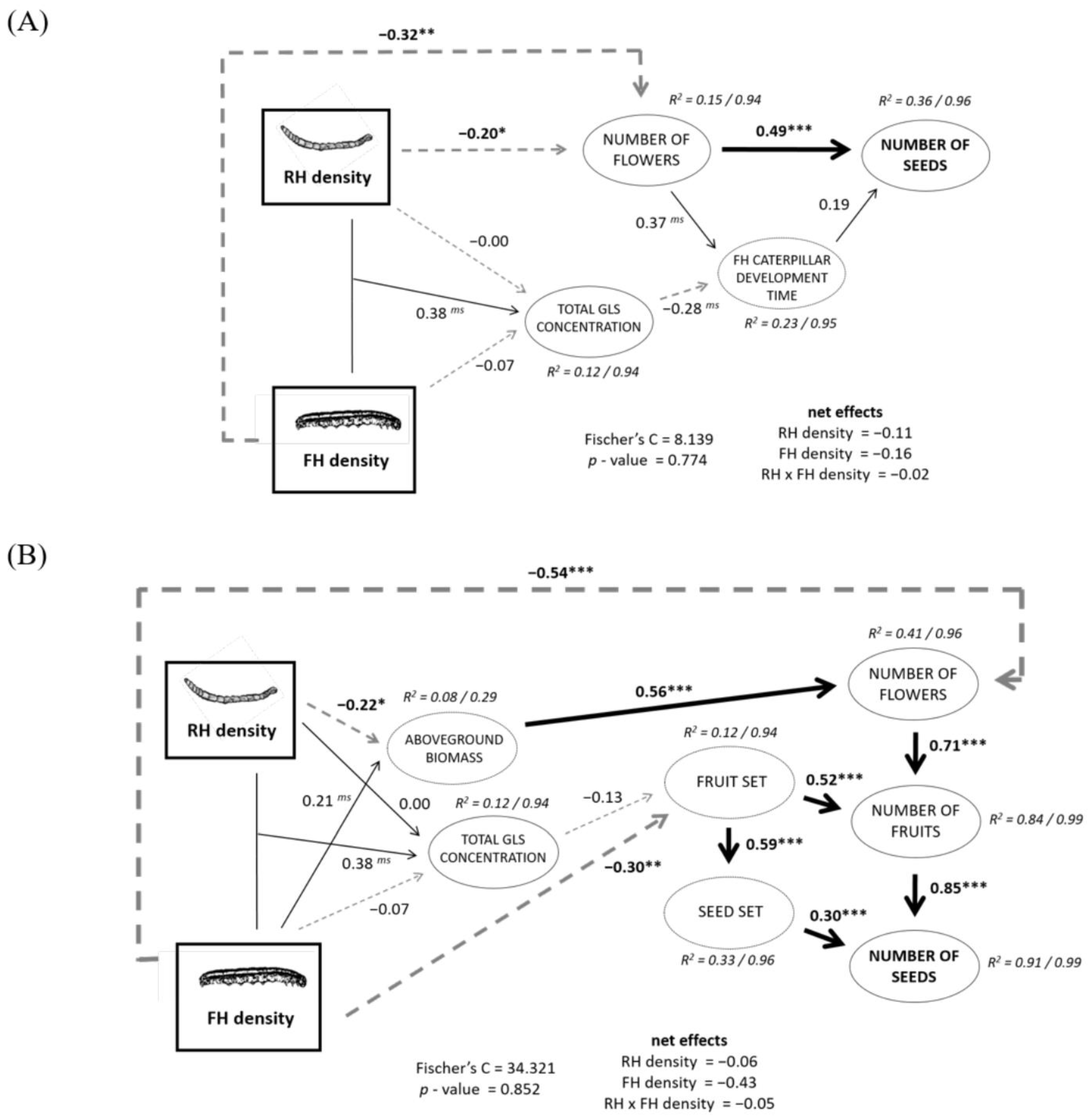
2.4. Structural Equation Models (SEMs) of RH and FH Density Effects on Plant Reproduction
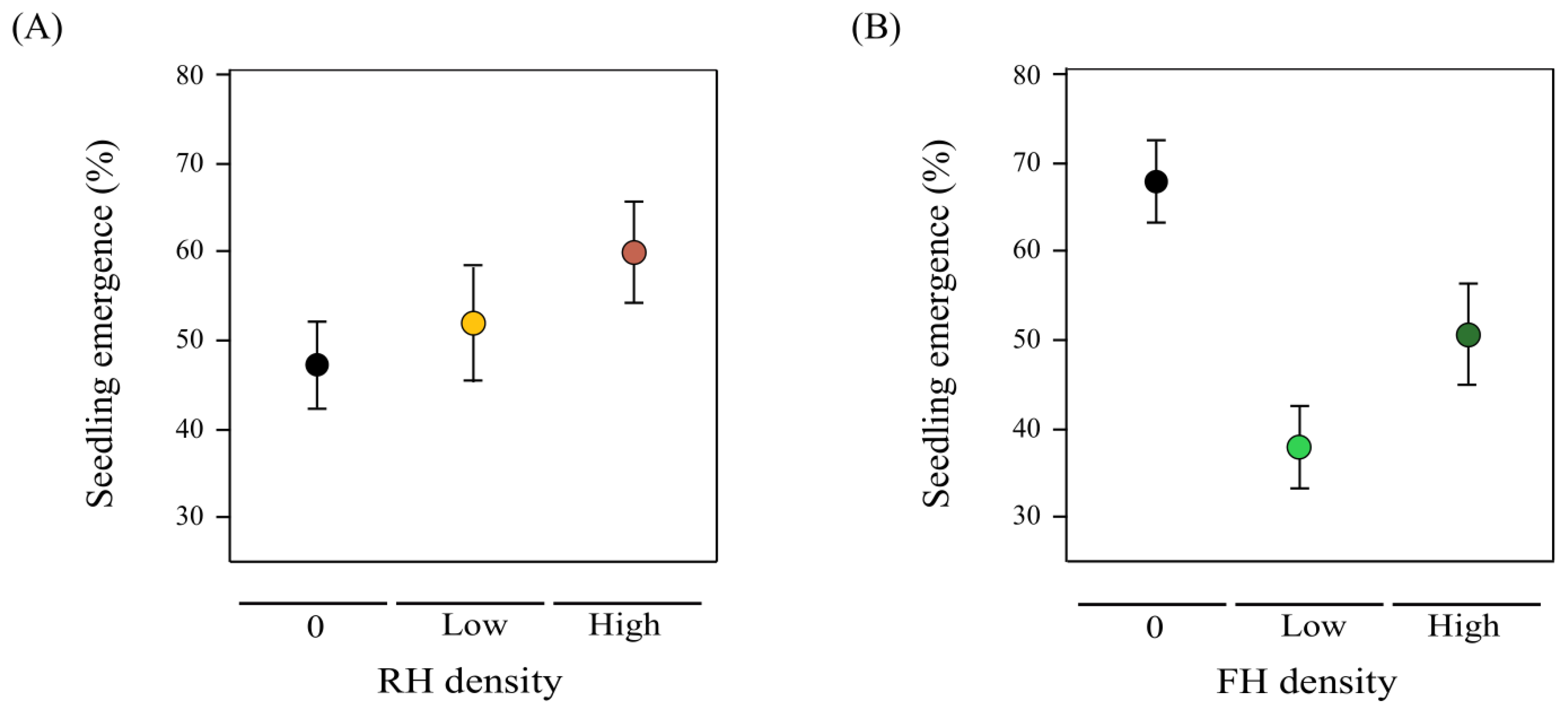
2.5. Transgenerational Herbivore Effects on Seedling Emergence: Lifetime Fitness Estimation
3. Discussion
3.1. Density-Dependent FH Damage
3.2. Density-Dependent RH Damage
3.3. RH Consequences for FH Caterpillar Performance
3.4. Additiveness and Non-Additiveness in Simultaneous Root and Floral Herbivory
4. Materials and Methods
4.1. Study System
4.2. Experimental Set-Up
4.3. Data Collection
4.4. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marquis, R.J. The selective impact of herbivores. In Plant Resistance to Herbivores and Pathogens: Ecology, Evolution, and Genetics; Fritz, R.S., Simms, E.L., Eds.; University of Chicago Press: Chicago, IL, USA, 1992; pp. 301–325. [Google Scholar]
- Mauricio, R.; Rausher, M.D. Experimental manipulation of putative selective agents provides evidence for the role of natural enemies in the evolution of plant defense. Evolution 1997, 51, 1435–1444. [Google Scholar] [CrossRef] [PubMed]
- Howe, G.A.; Jander, G. Plant immunity to insect herbivores. Annu. Rev. Plant Biol. 2008, 59, 41–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwachtje, J.; Baldwin, I.T. Why does herbivore attack reconfigure primary metabolism? Plant Physiol. 2008, 146, 845–851. [Google Scholar] [CrossRef] [Green Version]
- Mostafa, S.; Wang, Y.; Zeng, W.; Jin, B. Plant responses to herbivory, wounding, and infection. Int. J. Mol. Sci. 2022, 23, 7031. [Google Scholar] [CrossRef]
- Fornoni, J.; Núñez-Farfán, J.; Valverde, P.L.; Rausher, M.D. Evolution of mixed strategies of plant defense allocation against natural enemies. Evolution 2004, 58, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Farfán, J.; Fornoni, J.; Valverde, P.L. The evolution of resistance and tolerance to herbivores. Annu. Rev. Ecol. Evol. Syst. 2007, 38, 541–566. [Google Scholar] [CrossRef]
- Simms, E.L.; Rausher, M.D. Costs and benefits of plant resistance to herbivory. Am. Nat. 1987, 130, 570–581. [Google Scholar] [CrossRef]
- Herms, D.A.; Mattson, W.J. The dilemma of plants: To grow or defend. Q. Rev. Biol. 1992, 67, 283–335. [Google Scholar] [CrossRef] [Green Version]
- Strauss, S.Y.; Irwin, R.E. Ecological and evolutionary consequences of multispecies plant-animal interactions. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 435–466. [Google Scholar] [CrossRef]
- Morris, W.F.; Hufbauer, R.A.; Agrawal, A.A.; Bever, J.D.; Borowicz, V.A.; Gilbert, G.S.; Maron, J.L.; Mitchell, C.E.; Parker, I.M.; Power, A.G.; et al. Direct and interactive effects of enemies and mutualists on plant performance: A meta-analysis. Ecology 2007, 88, 1021–1029. [Google Scholar] [CrossRef]
- TerHorst, C.P.; Zee, P.C.; Heath, K.D.; Miller, T.E.; Pastore, A.I.; Patel, S.; Schreiber, S.J.; Wade, M.J.; Walsh, M.R. Evolution in a community context: Trait responses to multiple species interactions. Am. Nat. 2018, 191, 368–380. [Google Scholar] [CrossRef]
- Kaplan, I.; Denno, R.F. Interspecific interactions in phytophagous insects revisited: A quantitative assessment of competition theory. Ecol. Lett. 2007, 10, 977–994. [Google Scholar] [CrossRef] [PubMed]
- Strauss, S.Y. Ecological and evolutionary responses in complex communities: Implications for invasions and eco-evolutionary feedbacks. Oikos 2014, 123, 257–266. [Google Scholar] [CrossRef]
- Stephens, A.E.A.; Srivastava, D.S.; Myers, J.H. Strength in numbers? Effects of multiple natural enemy species on plant performance. Proc. R. Soc. B Biol. Sci. 2013, 280, 20122756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zvereva, E.L.; Kozlov, M.V. Sources of variation in plant responses to belowground insect herbivory: A meta-analysis. Oecologia 2012, 169, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Stamp, N. Out of the quagmire of plant defense hypotheses. Q. Rev. Biol. 2003, 78, 23–55. [Google Scholar] [CrossRef] [Green Version]
- Eschtruth, A.K.; Battles, J.J. Acceleration of exotic plant invasion in a forested ecosystem by a generalist herbivore. Conserv. Biol. 2009, 23, 388–399. [Google Scholar] [CrossRef]
- Koda, R.; Fujita, N. Is deer herbivory directly proportional to deer population density? Comparison of deer feeding frequencies among six forests with different deer density. For. Ecol. Manag. 2011, 262, 432–439. [Google Scholar] [CrossRef]
- Lesser, M.R.; Dovciak, M.; Wheat, R.; Curtis, P.; Smallidge, P.; Hurst, J.; Kramer, D.; Roberts, M.; Frair, J. Modelling white-tailed deer impacts on forest regeneration to inform deer management options at landscape scales. For. Ecol. Manag. 2019, 448, 395–408. [Google Scholar] [CrossRef]
- Vicari, M.; Puentes, A.; Granath, G.; Georgeff, J.; Strathdee, F.; Bazely, D.R. Unpacking multi-trophic herbivore-grass-endophyte interactions: Feedbacks across different scales in vegetation responses to Soay sheep herbivory. Sci. Nat. 2018, 105, 66. [Google Scholar] [CrossRef]
- Bonenfant, C.; Gaillard, J.M.; Coulson, T.; Festa-Bianchet, M.; Loison, A.; Garel, M.; Loe, L.E.; Blanchard, P.; Pettorelli, N.; Owen-Smith, N.; et al. Empirical evidence of density-dependence in populations of large herbivores. Adv. Ecol. Res. 2009, 41, 313–357. [Google Scholar] [CrossRef]
- Masters, G.J. The effect of herbivore density on host plant mediated interactions between two insects. Ecol. Res. 1995, 10, 125–133. [Google Scholar] [CrossRef]
- Kroes, A.; Van Loon, J.J.A.; Dicke, M. Density-dependent interference of aphids with caterpillar-induced defenses in Arabidopsis: Involvement of phytohormones and transcription factors. Plant Cell Physiol. 2015, 56, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, R.A.; Eubanks, M.D. Herbivore density mediates the indirect effect of herbivores on plants via induced resistance and apparent competition. Ecosphere 2016, 7, e01218. [Google Scholar] [CrossRef]
- Ponzio, C.; Papazian, S.; Albrectsen, B.R.; Dicke, M.; Gols, R. Dual herbivore attack and herbivore density affect metabolic profiles of Brassica nigra leaves. Plant Cell Environ. 2017, 40, 1356–1367. [Google Scholar] [CrossRef] [Green Version]
- McCall, A.C.; Irwin, R.E. Florivory: The intersection of pollination and herbivory. Ecol. Lett. 2006, 9, 1351–1365. [Google Scholar] [CrossRef]
- Brown, V.K.; Gange, A.C. Insect herbivory below ground. Adv. Ecol. Res. 1990, 20, 1–58. [Google Scholar] [CrossRef]
- Johnson, S.N.; Erb, M.; Hartley, S.E. Roots under attack: Contrasting plant responses to below- and aboveground insect herbivory. New Phytol. 2016, 210, 413–418. [Google Scholar] [CrossRef] [Green Version]
- Aguirrebengoa, M.; Menéndez, R.; Müller, C.; González-Megías, A. Altered rainfall patterns reduce plant fitness and disrupt interactions between below-and aboveground insect herbivores. Ecosphere 2020, 11, e03127. [Google Scholar] [CrossRef]
- Erb, M.; Lu, J. Soil abiotic factors influence interactions between belowground herbivores and plant roots. J. Exp. Bot. 2013, 64, 1295–1303. [Google Scholar] [CrossRef]
- Robert, C.; Ferrieri, R.; Schirmer, S.; Babst, B.; Schueller, M.; Machado, R.; Arce, C.; Hibbard, B.; Gershenzon, J.; Turlings, T.; et al. Induced carbon reallocation and compensatory growth as root herbivore tolerance mechanisms. Plant. Cell Environ. 2014, 37, 2613–2622. [Google Scholar] [CrossRef]
- Boaventura, M.G.; Villamil, N.; Teixido, A.L.; Tito, R.; Vasconcelos, H.L.; Silveira, F.A.O.; Cornelissen, T. Revisiting florivory: An integrative review and global patterns of a neglected interaction. New Phytol. 2022, 233, 132–144. [Google Scholar] [CrossRef]
- González-Browne, C.; Murúa, M.M.; Navarro, L.; Medel, R. Does plant origin influence the fitness impact of flower damage? PLoS ONE 2016, 11, e0146437. [Google Scholar] [CrossRef] [PubMed]
- Moreira, X.; Castagneyrol, B.; Abdala-Roberts, L.; Traveset, A. A meta-analysis of herbivore effects on plant attractiveness to pollinators. Ecology 2019, 100, e02707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehrlich, P.R.; Raven, P.H. Butterflies and plants: A study in coevolution. Evolution 1964, 18, 586–608. [Google Scholar] [CrossRef]
- Frame, D. Generalist flowers, biodiversity and florivory: Implications for angiosperm origins. Taxon 2003, 52, 681–685. [Google Scholar] [CrossRef]
- Xiao, L.; Labandeira, C.; Dilcher, D.; Ren, D. Florivory of Early Cretaceous flowers by functionally diverse insects: Implications for early angiosperm pollination. Proc. R. Soc. B 2021, 288, 20210320. [Google Scholar] [CrossRef] [PubMed]
- Roddy, A.B.; Martínez-Perez, C.; Teixido, A.L.; Cornelissen, T.G.; Olson, M.E.; Oliveira, R.S.; Silveira, F.A.O. Towards the flower economics spectrum. New Phytol. 2021, 229, 665–672. [Google Scholar] [CrossRef]
- Rusman, Q.; Lucas-Barbosa, D.; Poelman, E.H.; Dicke, M. Ecology of plastic flowers. Trends Plant Sci. 2019, 24, 725–740. [Google Scholar] [CrossRef]
- Caruso, C.M.; Eisen, K.E.; Martin, R.A.; Sletvold, N. A meta-analysis of the agents of selection on floral traits. Evolution 2019, 73, 4–14. [Google Scholar] [CrossRef]
- Siemens, D.H.; Mitchell-Olds, T. Glucosinolates and herbivory by specialists (Coleoptera: Chrysomelidae, Lepidoptera: Plutellidae): Consequences of concentration and induced resistance. Environ. Entomol. 1996, 25, 1344–1353. [Google Scholar] [CrossRef]
- Muola, A.; Mutikainen, P.; Laukkanen, L.; Lilley, M.; Leimu, R. Genetic variation in herbivore resistance and tolerance: The role of plant life-history stage and type of damage. J. Evol. Biol. 2010, 23, 2185–2196. [Google Scholar] [CrossRef] [PubMed]
- Olesen, J.M. Flower mining by moth larvae vs. pollination by beetles and bees in the Cauliflorous Sapranthus palanga (Annonaceae) in Costa Rica. Flora 1992, 187, 9–15. [Google Scholar] [CrossRef]
- Wise, M.J.; Cummins, J.J.; De Young, C. Compensation for floral herbivory in Solanum carolinense: Identifying mechanisms of tolerance. Evol. Ecol. 2008, 22, 19–37. [Google Scholar] [CrossRef]
- Erb, M.; Lenk, C.; Degenhardt, J.; Turlings, T.C.J. The underestimated role of roots in defense against leaf attackers. Trends Plant Sci. 2009, 14, 653–659. [Google Scholar] [CrossRef] [Green Version]
- Soler, R.; Van der Putten, W.H.; Harvey, J.A.; Vet, L.E.M.; Dicke, M.; Bezemer, T.M. Root herbivore effects on aboveground multitrophic interactions: Patterns, processes and mechanisms. J. Chem. Ecol. 2012, 38, 755–767. [Google Scholar] [CrossRef] [Green Version]
- Johnson, S.N.; Clark, K.E.; Hartley, S.E.; Jones, T.H.; McKenzie, S.W.; Koricheva, J. Aboveground-belowground herbivore interactions: A meta-analysis. Ecology 2012, 93, 2208–2215. [Google Scholar] [CrossRef]
- Rusman, Q.; Hooiveld-Knoppers, S.; Dijksterhuis, M.; Bloem, J.; Reichelt, M.; Dicke, M.; Poelman, E.H. Flowers prepare thyselves: Leaf and root herbivores induce specific changes in floral phytochemistry with consequences for plant interactions with florivores. New Phytol. 2022, 233, 2548–2560. [Google Scholar] [CrossRef]
- Masters, G.J.; Brown, V.K.; Gange, A.C. Plant mediated Interactions between above- and below-ground insect herbivores. Oikos 1993, 66, 148–151. [Google Scholar] [CrossRef]
- González-Megías, A.; Müller, C. Root herbivores and detritivores shape above-ground multitrophic assemblage through plant-mediated effects. J. Anim. Ecol. 2010, 79, 923–931. [Google Scholar] [CrossRef]
- González-Megías, A. Within- and trans-generational effects of herbivores and detritivores on plant performance and reproduction. J. Anim. Ecol. 2016, 85, 283–290. [Google Scholar] [CrossRef] [Green Version]
- Moles, A.T.; Leishman, M.R. The seedling as part of a plant’s life history strategy. In Seedling Ecology and Evolution; Leck, M.A., Parker, V.T., Simpson, R.L., Eds.; Cambridge University Press: Cambridge, UK, 2008; pp. 217–238. [Google Scholar]
- Herman, J.J.; Sultan, S.E. Adaptive transgenerational plasticity in plants: Case studies, mechanisms, and implications for natural populations. Front. Plant Sci. 2011, 2, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holeski, L.M.; Jander, G.; Agrawal, A.A. Transgenerational defense induction and epigenetic inheritance in plants. Trends Ecol. Evol. 2012, 27, 618–626. [Google Scholar] [CrossRef]
- Auge, G.A.; Leverett, L.D.; Edwards, B.R.; Donohue, K. Adjusting phenotypes via within- and across-generational plasticity. New Phytol. 2017, 216, 343–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balao, F.; Paun, O.; Alonso, C. Uncovering the contribution of epigenetics to plant phenotypic variation in Mediterranean ecosystems. Plant Biol. 2018, 20, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Zhou, M.; Lin, Z.; Li, Q.Q.; Zhang, Y. Transgenerational effects benefit offspring across diverse environments: A meta-analysis in plants and animals. Ecol. Lett. 2019, 22, 1976–1986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donohue, K.; Rubio de Casas, R.; Burghardt, L.; Kovach, K.; Willis, C.G. Germination, postgermination adaptation, and species ecological ranges. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 293–319. [Google Scholar] [CrossRef]
- Gutterman, Y. Maternal effects on seeds during development. In Seeds: The Ecology of Regeneration in Plant Communities; Fenner, M., Ed.; CABI Publishing: Wallingford, UK, 2000; pp. 59–84. [Google Scholar]
- Ehrlén, J. Fitness components versus total demographic effects: Evaluating herbivore impacts on a perennial herb. Am. Nat. 2003, 162, 796–810. [Google Scholar] [CrossRef]
- Aguirrebengoa, M.; García-Planas, M.; Müller, C.; González-Megías, A. Transgenerational effects of ungulates and pre-dispersal seed predators on offspring success and resistance to herbivory. PLoS ONE 2018, 13, e0207553. [Google Scholar] [CrossRef] [Green Version]
- Bukovinszky, T.; Poelman, E.H.; Gols, R.; Prekatsakis, G.; Vet, L.E.M.; Harvey, J.A.; Dicke, M. Consequences of constitutive and induced variation in plant nutritional quality for immune defence of a herbivore against parasitism. Oecologia 2009, 160, 299–308. [Google Scholar] [CrossRef]
- Pashalidou, F.G.; Fatouros, N.E.; van Loon, J.J.A.; Dicke, M.; Gols, R. Plant-mediated effects of butterfly egg deposition on subsequent caterpillar and pupal development, across different species of wild Brassicaceae. Ecol. Entomol. 2015, 40, 444–450. [Google Scholar] [CrossRef]
- Pashalidou, F.G.; Frago, E.; Griese, E.; Poelman, E.H.; van Loon, J.J.A.; Dicke, M.; Fatouros, N.E. Early herbivore alert matters: Plant-mediated effects of egg deposition on higher trophic levels benefit plant fitness. Ecol. Lett. 2015, 18, 927–936. [Google Scholar] [CrossRef]
- Veyrat, N.; Robert, C.A.M.; Turlings, T.C.J.; Erb, M. Herbivore intoxication as a potential primary function of an inducible volatile plant signal. J. Ecol. 2016, 104, 591–600. [Google Scholar] [CrossRef] [Green Version]
- Parry, D.; Herms, D.A.; Mattson, W.J. Responses of an insect folivore and its parasitoids to multiyear experimental defoliation of aspen. Ecology 2003, 84, 1768–1783. [Google Scholar] [CrossRef] [Green Version]
- Münzbergová, Z.; Skuhrovec, J. Data on herbivore performance and plant herbivore damage identify the same plant traits as the key drivers of plant-herbivore interaction. Insects 2020, 11, 865. [Google Scholar] [CrossRef]
- West, N.M.; Louda, S.M. Cumulative herbivory outpaces compensation for early floral damage on a monocarpic perennial thistle. Oecologia 2018, 186, 495–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mauricio, R.; Rausher, M.D.; Burdick, D.S. Variation in the defense strategies of plants: Are resistance and tolerance mutually exclusive? Ecology 1997, 78, 1301–1311. [Google Scholar] [CrossRef]
- Züst, T.; Agrawal, A.A. Trade-offs between plant growth and defense against insect herbivory: An emerging mechanistic synthesis. Annu. Rev. Plant Biol. 2017, 68, 513–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arendt, J.D. Adaptive intrinsic growth rates: An integration across taxa. Q. Rev. Biol. 1997, 72, 149–177. [Google Scholar] [CrossRef]
- Garcia, L.C.; Eubanks, M.D. Overcompensation for insect herbivory: A review and meta-analysis of the evidence. Ecology 2018, 100, e02585. [Google Scholar] [CrossRef]
- Aguirrebengoa, M.; Müller, C.; González-Megías, A. Pre-dispersal seed predators boost seed production in a short-lived plant. Oecologia 2021, 195, 971–982. [Google Scholar] [CrossRef]
- Poveda, K.; Steffan-Dewenter, I.; Scheu, S.; Tscharntke, T. Effects of below- and above-ground herbivores on plant growth, flower visitation and seed set. Oecologia 2003, 135, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Poveda, K.; Steffan-Dewenter, I.; Scheu, S.; Tscharntke, T. Effects of decomposers and herbivores on plant performance and aboveground plant-insect interactions. Oikos 2005, 108, 503–510. [Google Scholar] [CrossRef]
- Irwin, R.E.; Adler, L.S. Correlations among traits associated with herbivore resistance and pollination: Implications for pollination and nectar robbing in a distylous plant. Am. J. Bot. 2006, 93, 64–72. [Google Scholar] [CrossRef] [Green Version]
- Smallegange, R.C.; van Loon, J.J.A.; Blatt, S.E.; Harvey, J.A.; Agerbirk, N.; Dicke, M. Flower vs. leaf feeding by Pieris brassicae: Glucosinolate-rich flower tissues are preferred and sustain higher growth rate. J. Chem. Ecol. 2007, 33, 1831–1844. [Google Scholar] [CrossRef] [Green Version]
- Kessler, A.; Halitschke, R. Testing the potential for conflicting selection on floral chemical traits by pollinators and herbivores: Predictions and case study. Funct. Ecol. 2009, 23, 901–912. [Google Scholar] [CrossRef]
- Adler, L.S.; Seifert, M.G.; Wink, M.; Morse, G.E. Reliance on pollinators predicts defensive chemistry across tobacco species. Ecol. Lett. 2012, 15, 1140–1148. [Google Scholar] [CrossRef]
- McCall, A.C.; Fordyce, J.A. Can optimal defence theory be used to predict the distribution of plant chemical defences? J. Ecol. 2010, 98, 985–992. [Google Scholar] [CrossRef]
- Abdalsamee, M.K.; Müller, C. Uncovering different parameters influencing florivory in a specialist herbivore. Ecol. Entomol. 2015, 40, 258–268. [Google Scholar] [CrossRef]
- Strauss, S.Y.; Irwin, R.E.; Lambrix, V.M. Optimal defence theory and flower petal colour predict variation in the secondary chemistry of wild radish. J. Ecol. 2004, 92, 132–141. [Google Scholar] [CrossRef]
- Kant, M.R.; Jonckheere, W.; Knegt, B.; Lemos, F.; Liu, J.; Schimmel, B.C.J.; Villarroel, C.A.; Ataide, L.M.S.; Dermauw, W.; Glas, J.J.; et al. Mechanisms and ecological consequences of plant defence induction and suppression in herbivore communities. Ann. Bot. 2015, 115, 1015–1051. [Google Scholar] [CrossRef] [PubMed]
- Lucas-Barbosa, D.; Poelman, E.H.; Aartsma, Y.; Snoeren, T.A.L.; van Loon, J.J.A.; Dicke, M. Caught between parasitoids and predators-survival of a specialist herbivore on leaves and flowers of mustard plants. J. Chem. Ecol. 2014, 40, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Fei, M.; Gols, R.; Zhu, F.; Harvey, J.A. Plant quantity affects development and survival of a gregarious insect herbivore and its endoparasitoid wasp. PLoS ONE 2016, 11, e0149539. [Google Scholar] [CrossRef] [Green Version]
- Ohgushi, T. Herbivore-induced indirect interaction webs on terrestrial plants: The importance of non-trophic, indirect, and facilitative interactions. Entomol. Exp. Appl. 2008, 128, 217–229. [Google Scholar] [CrossRef]
- White, A.C.; Rogers, A.; Rees, M.; Osborne, C.P. How can we make plants grow faster? A source-sink perspective on growth rate. J. Exp. Bot. 2016, 67, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Huot, B.; Yao, J.; Montgomery, B.L.; He, S.Y. Growth-defense tradeoffs in plants: A balancing act to optimize fitness. Mol. Plant 2014, 7, 1267–1287. [Google Scholar] [CrossRef] [Green Version]
- Lozano-Durán, R.; Zipfel, C. Trade-off between growth and immunity: Role of brassinosteroids. Trends Plant Sci. 2015, 20, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Bekaert, M.; Edger, P.P.; Hudson, C.M.; Pires, J.C.; Conant, G.C. Metabolic and evolutionary costs of herbivory defense: Systems biology of glucosinolate synthesis. New Phytol. 2012, 196, 596–605. [Google Scholar] [CrossRef]
- Yang, D.-L.; Yao, J.; Mei, C.-S.; Tong, X.-H.; Zeng, L.-J.; Li, Q.; Xiao, L.-T.; Sun, T.-P.; Li, J.; Deng, X.-W.; et al. Plant hormone jasmonate prioritizes defense over growth by interfering with gibberellin signaling cascade. Proc. Natl. Acad. Sci. USA 2012, 109, E1192–E1200. [Google Scholar] [CrossRef] [Green Version]
- Campos, M.L.; Yoshida, Y.; Major, I.T.; de Oliveira Ferreira, D.; Weraduwage, S.M.; Froehlich, J.E.; Johnson, B.F.; Kramer, D.M.; Jander, G.; Sharkey, T.D.; et al. Rewiring of jasmonate and phytochrome B signalling uncouples plant growth-defense tradeoffs. Nat. Commun. 2016, 7, 12570. [Google Scholar] [CrossRef]
- González-Megías, A.; Menéndez, R. Climate change effects on above- and below-ground interactions in a dryland ecosystem. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 3115–3124. [Google Scholar] [CrossRef] [Green Version]
- Gómez, J.M. Predispersal reproductive ecology of an arid land crucifer, Moricandia moricandioides: Effect of mammal herbivory on seed production. J. Arid Environ. 1996, 33, 425–437. [Google Scholar] [CrossRef]
- Sobrino Vesperinas, E. Moricandia DC. In Flora iberica, Vol. IV, Cruciferae-Monotropaceae; Castroviejo, S., Aedo, C., Gómez Campo, C., Laínz, M., Montserrat, P., Morales, R., Muñoz Garmendia, F., Nieto Feliner, G., Rico, E., Talavera, S., Eds.; Real Jardín Botánico, C.S.I.C.: Madrid, Spain, 1993; pp. 337–344. [Google Scholar]
- Torices, R.; Gómez, J.M.; Pannell, J.R. Kin discrimination allows plants to modify investment towards pollinator attraction. Nat. Commun. 2018, 9, 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mithen, R.; Bennett, R.; Marquez, J. Glucosinolate biochemical diversity and innovation in the Brassicales. Phytochemistry 2010, 71, 2074–2086. [Google Scholar] [CrossRef]
- Breadmore, K.N.; Kirk, W.D.J. Factors affecting floral herbivory in a limestone grassland. Acta Oecologica 1998, 19, 501–506. [Google Scholar] [CrossRef]
- Halekoh, U.; Højsgaard, S.; Yan, J. The R package geepack for Generalized Estimating Equations. J. Stat. Softw. 2006, 15, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Pekár, S.; Brabec, M. Generalized estimating equations: A pragmatic and flexible approach to the marginal GLM modelling of correlated data in the behavioural sciences. Ethology 2018, 124, 86–93. [Google Scholar] [CrossRef] [Green Version]
- Pan, W. Akaike’s information criterion in generalized estimating equations. Biometrics 2001, 57, 120–125. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2017. [Google Scholar]
- Harrison, X.A. Using observation-level random effects to model overdispersion in count data in ecology and evolution. PeerJ 2014, 2, e616. [Google Scholar] [CrossRef] [Green Version]
- Grueber, C.E.; Nakagawa, S.; Laws, R.J.; Jamieson, I.G. Multimodel inference in ecology and evolution: Challenges and solutions. J. Evol. Biol. 2011, 24, 699–711. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Wood, S.N. Stable and efficient multiple smoothing parameter estimation for generalized additive models. J. Am. Stat. Assoc. 2004, 99, 673–686. [Google Scholar] [CrossRef] [Green Version]
- Lefcheck, J.S. piecewiseSEM: Piecewise structural equation modelling in r for ecology, evolution, and systematics. Methods Ecol. Evol. 2016, 7, 573–579. [Google Scholar] [CrossRef]
- Shipley, B. Cause and Correlation in Biology: A User’s Guide to Path Analysis Structural Equations and Causal Inference with R., 2nd ed.; Cambridge University Press: Cambridge, UK, 2016. [Google Scholar]


| RH | FH | RH × FH | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | df | χ² | z | p | df | χ² | z | p | df | χ² | z | p |
| Floral bud group production | 1,1704 | 4.14 | −2.05 | 0.04 | 1,1704 | 0.03 | 0.16 | 0.86 | 1,1704 | 0.12 | −0.34 | 0.73 |
| Flower production | 1,1389 | 0.50 | −0.70 | 0.47 | 1,1389 | 11.00 | 3.31 | <0.0001 | 1,1389 | 0.33 | 0.57 | 0.56 |
| Fruit production | 1,1202 | 8.15 | −2.85 | 0.004 | 1,1202 | 76.89 | 8.76 | <0.0001 | 1,1202 | 2.03 | 1.42 | 0.15 |
| RH | FH | RH × FH | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| GLMM/GAMM | F/χ² | df | Statistic | p | df | Statistic | p | df | Statistic | p | |
| Aboveground biomass and reproduction | |||||||||||
| Aboveground biomass | GLMM | χ² | 1,73 | 6.54 | 0.03 | 1,73 | 3.92 | 0.04 | 1,73 | 0.01 | 0.92 |
| Number of flowers | GAMM | χ² | 1,79 | 5.15 | 0.02 | 1,79 | 8.89 | 0.002 | 1,79 | 0.15 | 0.69 |
| Number of fruits | GAMM | χ² | 1,79 | 5.05 | 0.02 | 1,79 | 18.22 | <0.0001 | 1,79 | 0.03 | 0.85 |
| Number of seeds | GAMM | χ² | 1,79 | 2.17 | 0.14 | 1,79 | 12.05 | 0.0005 | 1,79 | 0.26 | 0.60 |
| Leaf and seed nutrient content | |||||||||||
| C/N ratio in leaves | GLMM | χ² | 1,76 | 1.10 | 0.29 | 1,76 | 0.43 | 0.51 | 1,76 | 0.00 | 0.92 |
| C/N ratio in seeds | GLMM | χ² | 1,59 | 0.04 | 0.82 | 1,59 | 2.04 | 0.15 | 1,59 | 0.44 | 0.50 |
| Leaf glucosinolates (GLSs) | |||||||||||
| Total GLSs | GLMM | F | 1,77 | 4.62 | 0.03 | 1,77 | 1.16 | 0.28 | 1,77 | 4.79 | 0.03 |
| Aliphatic GLSs | GLMM | F | 1,77 | 5.08 | 0.02 | 1,77 | 1.36 | 0.24 | 1,77 | 4.03 | 0.04 |
| Indolic GLSs | GLMM | F | 1,77 | 2.65 | 0.10 | 1,77 | 0.33 | 0.56 | 1,77 | 0.28 | 0.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguirrebengoa, M.; Müller, C.; Hambäck, P.A.; González-Megías, A. Density-Dependent Effects of Simultaneous Root and Floral Herbivory on Plant Fitness and Defense. Plants 2023, 12, 283. https://doi.org/10.3390/plants12020283
Aguirrebengoa M, Müller C, Hambäck PA, González-Megías A. Density-Dependent Effects of Simultaneous Root and Floral Herbivory on Plant Fitness and Defense. Plants. 2023; 12(2):283. https://doi.org/10.3390/plants12020283
Chicago/Turabian StyleAguirrebengoa, Martin, Caroline Müller, Peter A. Hambäck, and Adela González-Megías. 2023. "Density-Dependent Effects of Simultaneous Root and Floral Herbivory on Plant Fitness and Defense" Plants 12, no. 2: 283. https://doi.org/10.3390/plants12020283






