Application of Baltic Pine (Pinus sylvestris) Needle Extract as a Gut Microbiota-Modulating Feed Supplement for Domestic Chickens (Gallus gallus)
Abstract
:1. Introduction
2. Results
2.1. The Antimicrobial Activity of Baltic Pine (Pinus sylvestris) Extract
2.2. Summary of 16S rRNA Gene Sequencing
2.3. Diversity of Cecal Microbiota and the Trends Observed in Feeding Groups
3. Discussion
4. Materials and Methods
4.1. Baltic Pine (Pinus sylvestris) Needle Extract
4.2. Evaluation of the Antimicrobial Activity of the Pine Needle Extract against Bacterial Strains
4.3. Experimental and Sampling Procedures for the In Vivo Model
4.4. DNA Extraction and 16S rRNA Gene Sequencing Procedure for Cecal Samples
4.5. Bioinformatics Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kamarli Altun, H.; Karacil Ermumcu, M.S.; Seremet Kurklu, N. Evaluation of dietary supplement, functional food and herbal medicine use by dietitians during the COVID-19 pandemic. Public Health Nutr. 2021, 24, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Santos, P.; Zanuso, E.; Genisheva, Z.; Rocha, C.M.R.; Teixeira, J.A. Green and Sustainable Valorization of Bioactive Phenolic Compounds from Pinus By-Products. Molecules 2020, 25, 2931. [Google Scholar] [CrossRef] [PubMed]
- Lillehoj, H.; Liu, Y.; Calsamiglia, S.; Fernandez-Miyakawa, M.E.; Chi, F.; Cravens, R.L.; Oh, S.; Gay, C.G. Phytochemicals as antibiotic alternatives to promote growth and enhance host health. Vet. Res. 2018, 49, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diaz-Sanchez, S.; D’Souza, D.; Biswas, D.; Hanning, I. Botanical alternatives to antibiotics for use in organic poultry production. Poult. Sci. 2015, 94, 1419–1430. [Google Scholar] [CrossRef]
- Bhardwaj, K.; Silva, A.S.; Atanassova, M.; Sharma, R.; Nepovimova, E.; Musilek, K.; Sharma, R.; Alghuthaymi, M.A.; Dhanjal, D.S.; Nicoletti, M.; et al. Conifers Phytochemicals: A Valuable Forest with Therapeutic Potential. Molecules 2021, 26, 3005. [Google Scholar] [CrossRef]
- Guo, A.; Cheng, L.; Al-Mamun, M.; Xiong, C.; Yang, S. Effect of dietary pine needles powder supplementation on growth, organ weight and blood biochemical profiles in broilers. J. Appl. Anim. Res. 2018, 46, 518–522. [Google Scholar] [CrossRef] [Green Version]
- Zeng, W.C.; Jia, L.R.; Zhang, Y.; Cen, J.Q.; Chen, X.; Gao, H.; Feng, S.; Huang, Y.N. Antibrowning and antimicrobial activities of the water-soluble extract from pine needles of Cedrus deodara. J. Food Sci. 2011, 76, C318–C323. [Google Scholar] [CrossRef]
- Jeong, J.B.; Seo, E.W.; Jeong, H.J. Effect of extracts from pine needle against oxidative DNA damage and apoptosis induced by hydroxyl radical via antioxidant activity. Food Chem. Toxicol. 2009, 47, 2135–2141. [Google Scholar] [CrossRef]
- Hoai, N.T.; Duc, H.V.; Thao, D.T.; Orav, A.; Raal, A. Selectivity of Pinus sylvestris extract and essential oil to estrogen-insensitive breast cancer cells Pinus sylvestris against cancer cells. Pharmacogn. Mag. 2015, 11, S290–S295. [Google Scholar] [CrossRef] [Green Version]
- Haque, M.H.; Sarker, S.; Islam, M.S.; Islam, M.A.; Karim, M.R.; Kayesh, M.E.H.; Shiddiky, M.J.A.; Anwer, M.S. Sustainable Antibiotic-Free Broiler Meat Production: Current Trends, Challenges, and Possibilities in a Developing Country Perspective. Biology 2020, 9, 411. [Google Scholar] [CrossRef]
- Donoghue, D.J. Antibiotic residues in poultry tissues and eggs: Human health concerns? Poult. Sci. 2003, 82, 618–621. [Google Scholar] [CrossRef] [PubMed]
- Castanon, J.I.R. History of the Use of Antibiotics as Growth Promoters in European Poultry Feeds. Poult. Sci. 2007, 86, 2466–2471. [Google Scholar] [CrossRef]
- Chen, Y.N.; Li, H. Effect of green tea and mulberry leaf powders on the gut microbiota of chicken. BMC Vet. Res. 2019, 15, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apajalahti, J.; Vienola, K. Interaction between chicken intestinal microbiota and protein digestion. Anim. Feed Sci. Technol. 2016, 221, 323–330. [Google Scholar] [CrossRef] [Green Version]
- Rychlik, I. Composition and function of chicken gut microbiota. Animals 2020, 10, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tako, E.; Rutzke, M.A.; Glahn, R.P. Using the domestic chicken (Gallus gallus) as an in vivo model for iron bioavailability. Poult. Sci. 2010, 89, 514–521. [Google Scholar] [CrossRef]
- Buckle, J. Infection. In Clinical Aromatherapy, 3rd ed.; Chapter 7; Churchill Livingstone: London, UK, 2015; pp. 130–167. [Google Scholar] [CrossRef]
- McDowell, R.H.; Sands, E.M.; Friedman, H. Bacillus Cereus. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022; Available online: https://www.ncbi.nlm.nih.gov/books/NBK459121/ (accessed on 12 September 2022).
- Jajere, S.M. A review of Salmonella enterica with particular focus on the pathogenicity and virulence factors, host specificity and antimicrobial resistance including multidrug resistance. Vet. World 2019, 12, 504–521. [Google Scholar] [CrossRef] [Green Version]
- Morris, F.C.; Dexter, C.; Kostoulias, X.; Uddin, M.I.; Peleg, A.Y. The Mechanisms of Disease Caused by Acinetobacter baumannii. Front. Microbiol. 2019, 10, 1601. [Google Scholar] [CrossRef] [Green Version]
- Bartkiene, E.; Ruzauskas, M.; Lele, V.; Zavistanaviciute, P.; Bernatoniene, J.; Jakstas, V.; Ivanauskas, L.; Zadeike, D.; Klupsaite, D.; Viskelis, P.; et al. Development of antimicrobial gummy candies with addition of bovine colostrum, essential oils and probiotics. Int. J. Food Sci. 2018, 53, 1227–1235. [Google Scholar] [CrossRef]
- de Lacerda, J.R.M.; da Silva, T.F.; Vollú, R.E.; Marques, J.M.; Seldin, L. Generally recognized as safe (GRAS) Lactococcus lactis strains associated with Lippia sidoides Cham. are able to solubilize/mineralize phosphate. Springerplus 2016, 5, 828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Díaz-Sánchez, S.; Perrotta, A.R.; Rockafellow, I.; Alm, E.J.; Okimoto, R.; Hawken, R.; Hanning, I. Using fecal microbiota as biomarkers for predictions of performance in the selective breeding process of pedigree broiler breeders. PLoS ONE 2019, 14, e0216080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardenas, L.A.C.; Clavijo, V.; Vives, M.; Reyes, A. Bacterial meta-analysis of chicken cecal microbiota. PeerJ 2021, 9, e10571. [Google Scholar] [CrossRef] [PubMed]
- Clavijo, V.; Flórez, M.J.V. The gastrointestinal microbiome and its association with the control of pathogens in broiler chicken production: A review. Poult. Sci. 2018, 97, 1006–1021. [Google Scholar] [CrossRef]
- Kim, B.R.; Shin, J.; Guevarra, R.; Lee, J.H.; Kim, D.W.; Seol, K.H.; Lee, J.H.; Kim, H.B.; Isaascon, R. Deciphering Diversity Indices for a Better Understanding of Microbial Communities. J. Microbiol. Biotechnol. 2017, 12, 2089–2093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kollarcikova, M.; Faldynova, M.; Matiasovicova, J.; Jahodarova, E.; Kubasova, T.; Seidlerova, Z.; Babak, V.; Videnska, P.; Cizek, A.L.; Rychlik, I. Different Bacteroides Species Colonise Human and Chicken Intestinal Tract. Microorganisms 2020, 8, 1483. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, J.M.D.; Redondo, E.A.; Viso, N.D.P.; Redondo, L.M.; Farber, M.D.; Miyakawa, M.E.F. Tannins and bacitracin differentially modulate gut microbiota of broiler chickens. Biomed. Res. Int. 2018, 2018, 1879168. [Google Scholar] [CrossRef] [PubMed]
- Gustavo, A.R.; Richardson, E.; Clark, J.; Keshri, J.; Drechsler, Y.; Berrang, M.E.; Meinersmann, R.J.; Cox, N.A.; Oakley, B.B. Broiler chickens and early life programming: Microbiome transplant-induced cecal community dynamics and phenotypic effects. PLoS ONE 2020, 15, e0242108. [Google Scholar] [CrossRef]
- Ocejo, M.; Oporto, B.; Hurtado, A. 16S rRNA amplicon sequencing characterization of caecal microbiome composition of broilers and free-range slow-growing chickens throughout their productive lifespan. Sci. Rep. 2019, 9, 2506. [Google Scholar] [CrossRef] [Green Version]
- St-Pierre, B.; Cersosimo, L.M.; Ishaq, S.L.; Wright, A.-D.G. Toward the identification of methanogenic archaeal groups as 433 targets of methane mitigation in livestock animals. Front. Microbiol. 2015, 6, 776. [Google Scholar] [CrossRef] [PubMed]
- Richards, P.; Fothergill, J.; Bernardeau, M.; Wigley, P. Development of the Caecal Microbiota in Three Broiler Breeds. Front. Vet. Sci. 2019, 6, 201. [Google Scholar] [CrossRef]
- Meistere, I.; Ķibilds, J.; Eglīte, L.; Alksne, L.; Avsejenko, J.; Cibrovska, A.; Makarova, S.; Streikiša, M.; Grantiņa-Ieviņa, L.; Bērziņš, A. Campylobacter species prevalence, characterisation of antimicrobial resistance and analysis of whole-genome sequence of isolates from livestock and humans, Latvia. Euro Surveill. 2019, 24, 1800357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EFSA. European Food Safety Agency. EU Novel Food Catalogue. Available online: https://ec.europa.eu/food/safety/novel_food/catalogue/search/public/index.cfm?ascii=P (accessed on 10 October 2021).
- Roshchin, V.I.; Vasilev, S.N.; Pavlutskaja, I.S.; Kolodynskaja, L.A. Method for Processing Wood Green of Conifers. Russia 382 Patent No. RU 2 017 782 C1, 30 June 1994. Available online: https://yandex.ru/patents/doc/RU2017782C1_19940815 (accessed on 5 March 2022).
- Bespalov, V.G.; Alexandrov, V.A. Clinical Use of Conifer Green Needle Complex: A Review of Medical Applications. 2006. Available online: https://spbftu.ru/wp-content/uploads/2020/03/clinical.pdf. (accessed on 8 October 2021).
- Bartkiene, E.; Lele, V.; Sakiene, V.; Zavistanaviciute, P.; Ruzauskas, M.; Stankevicius, A.; Grigas, J.; Pautienius, A.; Bernatoniene, J.; Jakstas, V.; et al. Fermented, ultrasonicated, and dehydrated bovine colostrum: Changes in antimicrobial properties and immunoglobulin content. J. Dairy Sci. 2020, 103, 1315–1323. [Google Scholar] [CrossRef] [Green Version]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glockner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [Green Version]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahe, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 18, e2584. [Google Scholar] [CrossRef] [Green Version]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glocker, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Beule, L.; Karlovsky, P. Improved normalization of species count data in ecology by scaling with ranked subsampling (SRS): Application to microbial communities. PeerJ 2020, 8, e9593. [Google Scholar] [CrossRef]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. 1948, 27, 379–423. [Google Scholar] [CrossRef] [Green Version]
- Simpson, E.H. Measurement of Diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Sørensen, T.J. A Method of Establishing Groups of Equal Amplitude in Plant Sociology Based on Similarity of Species Content and Its Application to Analyses of the Vegetation on Danish Commons; Kongelige Danske Videnskabernes Selskab: Selskab, Ukraine, 1948. [Google Scholar]
- Chen, J.; Bittinger, K.; Charlson, E.S.; Hoffmann, C.; Lewis, J.; Wu, G.D.; Collman, R.G.; Bushman, F.D.; Li, H. Associating microbiome composition with environmental covariates using generalized UniFrac distances. Bioinformatics 2012, 28, 2106–2113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mallick, H.; Rahnavard, A.; McIver, L.J.; Ma, S.; Zhang, Y.; Nguyen, L.H.; Tickle, T.L.; Weingart, G.; Ren, B.; Schwager, E.H.; et al. Multivariable Association Discovery in Population-scale Meta-omics Studies. PLoS Comput. Biol. 2021, 17, e1009442. [Google Scholar] [CrossRef] [PubMed]
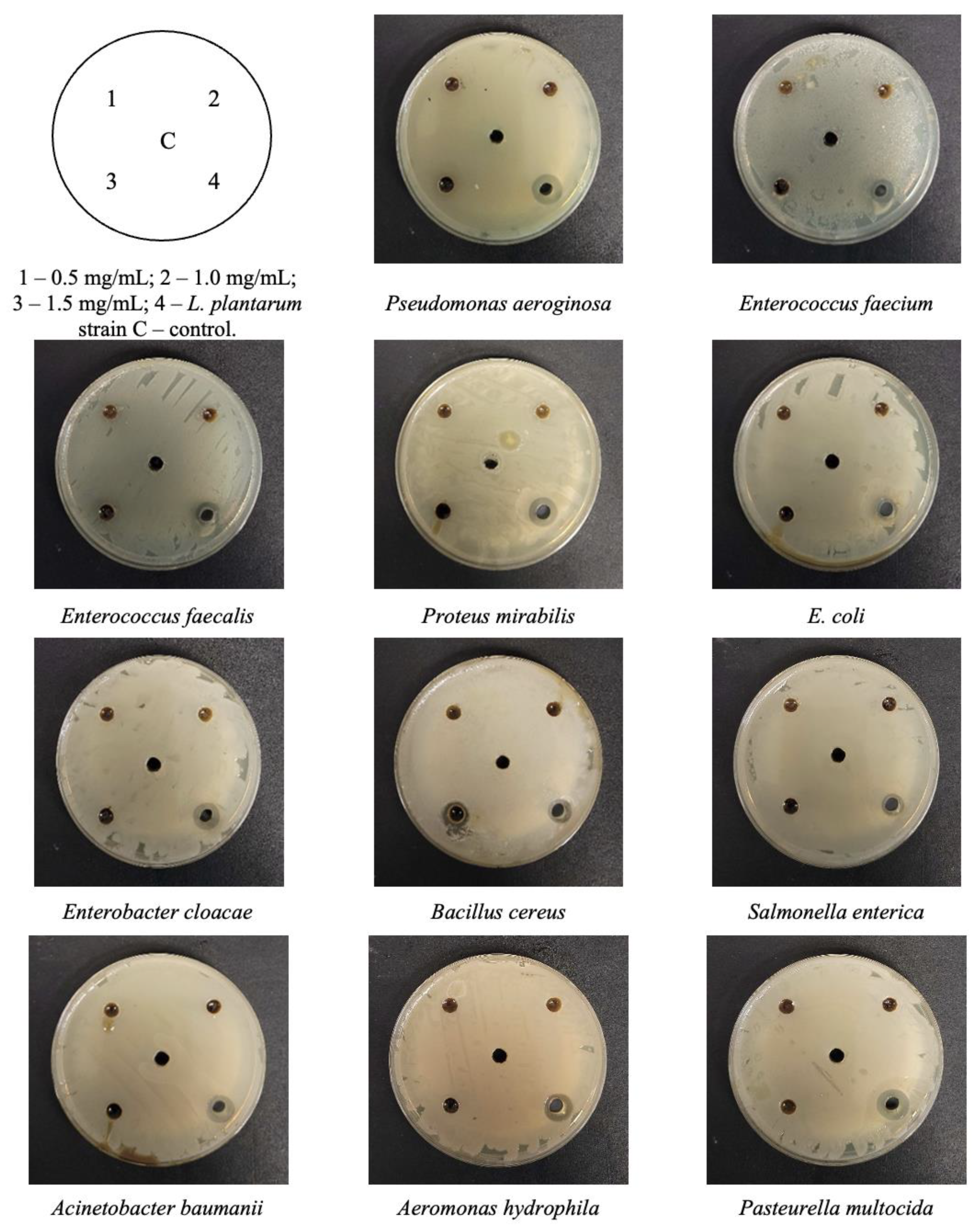

| Pine Extract Volume | Bacterial Strains | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | |
| 500 µL | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 1000 µL | + | + | + | + | + | + | + | + | + | + | - | + | + | + | + |
| 1500 µL | + | + | + | + | + | + | + | + | - | + | - | - | - | + | + |
| 0 µL | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Samples | Diameter of the Inhibition Zone, mm | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bacterial Strains | |||||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | |
| 0.5 mg/mL | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1.0 mg/mL | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1.5 mg/mL | - | - | - | - | - | - | - | - | - | - | 12.0 | - | - | - | - |
| L. plantarum | 13.0 | 10.0 | 10.0 | 12.0 | 9.0 | - | - | - | 11.0 | - | 10.0 | 9.0 | 11.0 | 12.0 | 13.0 |
| Phylum | Class | Order | Family | Genus | Coefficient from Linear Model | p Value | Q Value |
|---|---|---|---|---|---|---|---|
| Bacteroidota | Bacteroidia | Bacteroidales | Bacteroidaceae | Bacteroides | 0.0543 | 0.0126 | 0.1428 |
| Bacteroidota | Bacteroidia | Bacteroidales | Barnesiellaceae | −0.5771 | 0.0051 | 0.0956 | |
| Bacteroidota | Bacteroidia | Bacteroidales | Marinifilaceae | Odoribacter | 0.2604 | 0.0010 | 0.0520 |
| Bacteroidota | Bacteroidia | Bacteroidales | Prevotellaceae | 0.5776 | 0.0115 | 0.1428 | |
| Bacteroidota | Bacteroidia | Bacteroidales | Rikenellaceae | Alistipes | −0.1958 | 0.0251 | 0.1963 |
| Bacteroidota | Bacteroidia | Bacteroidales | Rikenellaceae | RikenellaceaeRC9 gut group | −0.5106 | 0.0273 | 0.1963 |
| Campilobacterota | Campylobacteria | Campylobacterales | Campylobacteraceae | Campylobacter | −0.6528 | 0.0040 | 0.0956 |
| Deferribacterota | Deferribactere | Deferribacterales | Deferribacteraceae | Mucispirillum | 0.2749 | 0.0013 | 0.0520 |
| Euryarchaeota | Methanobacteria | Methanobacteriales | Methanobacteriaceae | Methanobrevibacter | −0.1979 | 0.0261 | 0.1963 |
| Firmicutes | Clostridia | Lachnospirales | Lachnospiraceae | Eubacterium halliigroup | −0.1817 | 0.0060 | 0.0956 |
| Synergistota | Synergistia | Synergistales | Synergistaceae | Cloacibacillus | −0.2144 | 0.0171 | 0.1685 |
| Week 1 | Week 2 | Week 3 | Week 4 | |
|---|---|---|---|---|
| The average body weight per bird | 200 g | 270 g | 340 g | 410 g |
| The daily intake of feed per bird | 24 g | 29 g | 34 g | 38 g |
| The amount of feed for 20 birds for 7 days | 3360 g | 4060 g | 4760 g | 5320 g |
| Group A (non-supplement diet) | ||||
| % of supplement in feed | 0 | 0 | 0 | 0 |
| Group B (30 mg extract per kg b.w.) | ||||
| Amount of daily intake of extract per bird | 6.0 mg | 8.1 mg | 10.2 mg | 12.3 mg |
| Amount of extract for 20 chickens for 7 days | 840 mg | 1134 mg | 1428 mg | 1722 mg |
| % of supplement in feed | 0.025 | 0.027 | 0.030 | 0.032 |
| Group C (60 mg extract per kg b.w.) | ||||
| Amount of daily intake of extract per bird | 12.0 mg | 16.2 mg | 20.4 mg | 24.6 mg |
| Amount of extract for 20 chickens for 7 days | 1680 mg | 2268 mg | 2856 mg | 3444 mg |
| % of supplement in feed | 0.050 | 0.054 | 0.060 | 0.064 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubens, J.; Kibilds, J.; Jansons, M.; Piginka-Vjaceslavova, I.; Barene, I.; Daberte, I.; Liepa, L.; Malniece, A.; Rubens, A.; Starkute, V.; et al. Application of Baltic Pine (Pinus sylvestris) Needle Extract as a Gut Microbiota-Modulating Feed Supplement for Domestic Chickens (Gallus gallus). Plants 2023, 12, 297. https://doi.org/10.3390/plants12020297
Rubens J, Kibilds J, Jansons M, Piginka-Vjaceslavova I, Barene I, Daberte I, Liepa L, Malniece A, Rubens A, Starkute V, et al. Application of Baltic Pine (Pinus sylvestris) Needle Extract as a Gut Microbiota-Modulating Feed Supplement for Domestic Chickens (Gallus gallus). Plants. 2023; 12(2):297. https://doi.org/10.3390/plants12020297
Chicago/Turabian StyleRubens, Juris, Juris Kibilds, Martins Jansons, Inga Piginka-Vjaceslavova, Ilze Barene, Irena Daberte, Laima Liepa, Aija Malniece, Arturs Rubens, Vytaute Starkute, and et al. 2023. "Application of Baltic Pine (Pinus sylvestris) Needle Extract as a Gut Microbiota-Modulating Feed Supplement for Domestic Chickens (Gallus gallus)" Plants 12, no. 2: 297. https://doi.org/10.3390/plants12020297









