Abstract
In this research, we have reported the valorization possibilities of Matricaria recutita white ray florets using supercritical fluid extraction (SFE) with CO2. Experiments were conducted at temperatures of 35–55 °C and separation pressures of 5–9 MPa to evaluate their impact on the chemical composition and biological activity of the extracts. The total obtained extraction yields varied from 9.76 to 18.21 g 100 g−1 DW input. The greatest extraction yield obtained was at 9 MPa separation pressure and 55 °C in the separation tank. In all obtained extracts, the contents of total phenols, flavonoids, tannins, and sugars were determined. The influence of the supercritical CO2 extraction conditions on the extract antioxidant capacity was evaluated using the quenching activity of 2,2-diphenyl-1-picrylhydrazyl (DPPH). The chemical composition of the extracts was identified using both gas and liquid chromatography–mass spectrometry methods, whereas analyses of major and minor elements as well as heavy metals by microwave plasma atomic emission spectrometer were provided. Moreover, extracts were compared with respect to their antimicrobial activity, as well as the cytotoxicity and phototoxicity of the extracts. The results revealed a considerable diversity in the phytochemical classes among all extracts investigated in the present study and showed that the Matricaria recutita white ray floret by-product possesses cytotoxic and proliferation-reducing activity in immortalized cell lines, as well as antimicrobial activity. To the best of our knowledge, this is the first paper presenting such comprehensive data on the chemical profile, antioxidant properties, and biological properties of SFE derived from Matricaria recutita white ray florets. For the first time, these effects have been studied in processing by-products, and the results generated in this study provide valuable preconditions for further studies in specific test systems to fully elucidate the mechanisms of action and potential applications, such as potential use in cosmetic formulations.
1. Introduction
Making industrial processes and products with the least amount of environmental impact is idea behind green chemistry. Anastas and Warner’s twelve principles of green chemistry are essentially the sustainability principles for chemistry and related businesses [1]. Management of herbal waste byproducts resulting from various medicinal plant industrial cycles is poorly defined. Generated byproducts and waste have a detrimental influence on the environment. Many of these streams are underutilized and end up in landfills, where they contribute to greenhouse gas emissions, can cause further problems owing to microbial decomposition and leachate formation, and contribute to greenhouse gas emissions [2]. Biomass is sometimes used in enterprises to create energy or to create compost [2,3]. Instead of creating new revenue streams, more expenditures are incurred to manage solid waste in landfills because resources are handled as waste from an economic standpoint. In addition, the handling of vast quantities of various degradable materials is a challenge [4]. Due to the antigerminative properties of some fragrant plants, which may also be present in the plant waste, recycling the byproduct to energy requires a significant financial investment, and recycling to composting is not always appropriate [5]. It is generally recognized that the plant’s aerial parts have the capacity to serve as natural antioxidants, scavenging free radicals with their rich polyphenol and terpene content. This suggests that byproducts from the industrial processing of medicinal plants may include bioactive compounds that may be used for a variety of applications. As the need for extractable compounds develops, so does interest in recovering valuable compounds from various industrial waste streams [6].
Innovative, health-promoting, plant-based additives and ingredients have become more popular in the cosmetics business over the past several years. Polyphenols and other bioactive chemicals are of interest to the cosmetic industry because they may be used to improve different aspects of skin health. This hugely diverse group of chemicals also possesses numerous biological activities, including UV protection, antioxidant and anti-inflammatory activity, regulation of the skin microbiome, and synthesis of the skin extracellular matrix [2,7,8]. Present findings indicate that residual sage (Salvia officinalis) contains considerable levels of flavonoids responsible for antioxidative, antibacterial, and antifungal activities, such as apigenin and luteolin [9]. Waste of lavandin (Lavandula x intermedia) and spike lavender (Lavandula latifolia) are rich sources of phenolic compounds, such as phenolic acids and flavonoids, many of which are strong antioxidants and antimicrobials [10]. Extracts of residual rosemary (Rosmarinus officinalis), savory, and oregano species are also a good source of natural antioxidants, especially phenolic acids such as caffeic and rosmarinic acids, and a phenolic diterpene called carnosic acid [11]. From the residual clary sage (Salvia sclarea), a diterpene sclareol can be extracted, which is an important precursor for the synthesis of ambrox, the crucial aromatic compound in ambergris and a staple in the contemporary perfume industry. Waste obtained after steam distillation of chamomile (Matricaria recutita) could be a source of water-soluble pectic polysaccharides [12], as well as processing waste that remains after chamomile processing in significant amounts and is considered a rich source of apigenin and ferulic acid derivatives, as well as coumarin derivatives, herniarin and umbelliferone. These compounds are often used in the cosmetic industry due to their strong absorption of UV light [13].
Knowledge of phytochemical constituents and their biological activities in by-products is valuable for authentication and is the first step towards studying the biological activities of the whole product. By-product extracts can contain various types of bioactive compounds with different polarities, which makes it hard to identify and characterize.
Bioactive compounds present in the medicinal plant by-products can be separated individually from the biological matrix using different physical or chemical extraction techniques. The need for more efficient and environmentally friendly extraction encourages the development of new methods and technologies. Extraction processes are affected by several factors, including the technique used, the raw material, and the organic solvent.
Conventional techniques such as maceration, percolation, and Soxhlet extraction generally require large amounts of organic solvents, a lot of energy, and a lot of time, which has sparked interest in new technologies known as clean or green technologies. Supercritical fluid extraction, pressurized liquid extraction, ultrasound- and microwave-assisted extraction, and pulse electric field extraction are examples of these technologies. These techniques have the potential to reduce or eliminate the use of toxic solvents, thereby protecting the natural environment and its resources [14]. Supercritical fluid extraction (SFE) with CO2 is considered a sustainable method for the extraction of plant materials as carbon dioxide is non-toxic, harmless to humans and the environment, and it can be recycled during the extraction process. SFE is mainly used in the food industry for the decaffeination of coffee and the deodorization of fish oils, and in the pharmaceutical industry for the extraction of bioactive compounds, but can also be used in other industries such as environmental engineering, which assists in soil remediation [15]. At a particular temperature and pressure point, gases become supercritical fluids; the gas and liquid phases are indistinguishable. Further changes in the supercritical fluid’s temperature and pressure alter its solvent properties for non-polar compound extraction. The SFE method is rapid, automatable, selective, and avoids the use of large amounts of toxic solvents. Due to the use of low temperature regimes, it can be considered suitable for thermosensitive compounds. The SFE method is incapable of extracting compounds with large molecular weights. The introduction of modifiers (co-solvents) into the system, such as ethanol, methanol, or water, enhances the solvating power of CO2. It may increase the selectivity and extraction yield of target compounds. The extraction process does not require further cleaning of the extract. No solvent residues are left in it as the CO2 evaporates completely. SFE equipment may also include several separation tanks with controlled temperature and pressure settings. Based on the reduction in pressure and change in temperature, different compounds will precipitate in each stage of separation. The separation may occur either because the supercritical fluid is no longer supercritical or because the solute is no longer soluble in the supercritical fluid. This step may concentrate and isolate compounds from the extract. The main advantage of the separation within the extraction process is time and resource savings. It can also be carried out on small amounts of biomass samples [16].
The aim of our study was to evaluate and compare the chemical composition, antimicrobial activity, cytotoxicity, and phototoxicity of supercritical fluid extracts of German chamomile processing waste. Matricaria recutita white ray florets were collected from a dry herb processing facility. Supercritical fluid extraction was carried out in a pilot-scale plant with carbon dioxide as a solvent and ethanol as a co-solvent. Phytochemical analyses of extracts were performed using high-throughput 96-well plate spectrophotometric methods, liquid chromatography–mass spectrometry, and gas chromatography–mass spectrometry methods. Microwave plasma atomic emission spectrometer analyses of major and minor elements, as well as heavy metals, were provided. Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) were examined to find out the antimicrobial properties of the extracts. For extract safety profiling, standard cytotoxicity and phototoxicity assessments following OECD (The Organization for Economic Co-operation and Development) guidelines were performed. Statistical analysis was carried out in order to determine the significant correlations between the different extracts and to create models that can meaningfully predict the targeted phytochemical recovery and the most promising chemical and biological activities.
2. Results and Discussion
2.1. Supercritical Fluid Extraction
The determination of the extraction yield is an important parameter to estimate the efficiency of SFE in the recovery of bioactive compounds. The extraction yields from the white ray florets of German chamomile are shown in Table 1 and Figure 1.

Table 1.
Extraction yields of Matricaria recutita white ray floret supercritical fluid extracts.
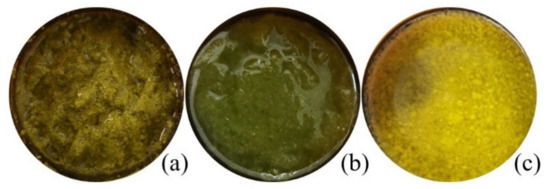
Figure 1.
Macroscopic appearance of Matricaria recucita white ray floret supercritical fluid extracts under different combinations of temperature and pressure: (a) sample FAF1, (b) sample FAF2, (c) sample FAF3.
The greatest extraction yield obtained was at 9 MPa separation pressure and 55 °C in the separation tank (sample FAF3). The highest overall extraction yields as obtained in the current research is higher than those previously reported. Extract yield between 0.23 and 3.64 g/100 g of different processed and unprocessed chamomile flowers has been reported by Molnar and team [13], whereas Kotnik [17] and Scalia [18] reported yields of 2.50–3.81 g/100 g and 9.2–9.7g/100 g, respectively, obtained from ground chamomile flower heads. The addition of ethanol as a co-solvent enhances the solvation power of supercritical carbon dioxide and improves the recovery of bioactive compounds, which will also appear in the following results.
2.2. Phytochemical Screening of Matricaria recutita White Ray Ffloret Supercritical Fluid Extracts
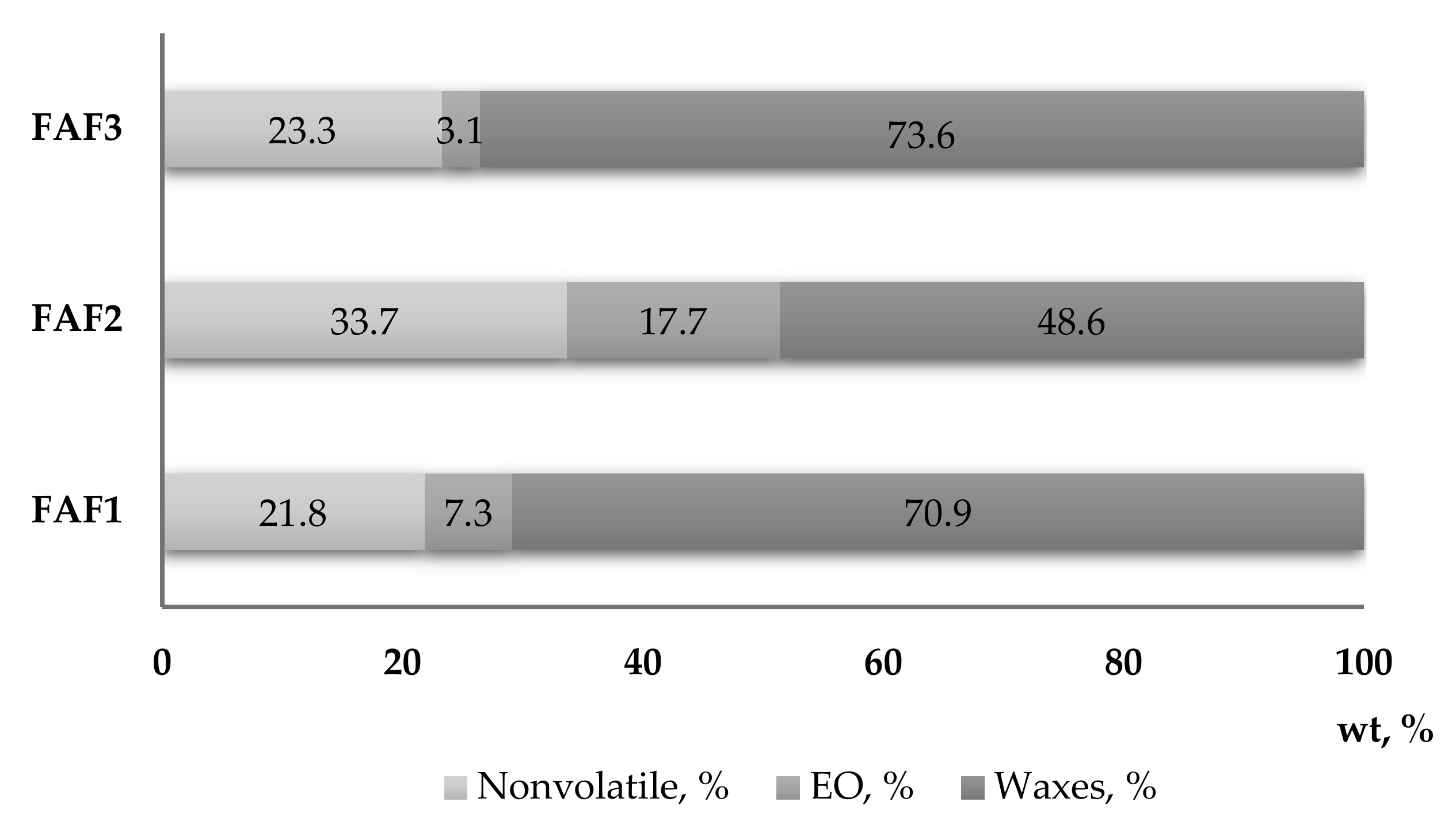
The solubility of obtained CO2 extracts in three different solvents at a concentration of 1:10 (w/v) was determined for testing purposes. It was observed that all CO2 extracts are partly soluble in water and ethanol, whereas they completely dissolve in cyclohexane. These observations are also confirmed by the gravimetrical analysis (Figure 2), which showed that the sum of essential oils (EO) and waxes in the tested CO2 extracts varied from 66.3% (FAF2), 76.72% (FAF1), and 78.17% (FAF3).
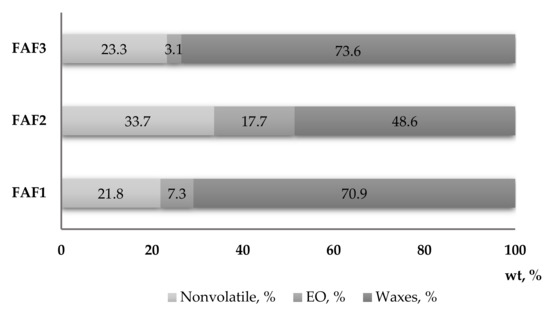
Figure 2.
Mass fraction content (wt, %) of essential oils, waxes, and nonvolatile compounds in Matricaria recutita white ray floret supercritical fluid extracts.
Based on the obtained data, it was decided that for phytochemical screening by high-throughput 96-well plate methods and LC-gTOF-MS, CO2 samples will be diluted in 70% ethanol (EtOH), whereas samples for testing by GC-MS should be diluted in cyclohexane.
Screening of phytochemical classes in CO2 ethanol extracts from chamomile herb processing by-products was performed using high-throughput 96-well plate spectrophotometric methods. Table 2 shows the data as the mean standard error of three independent experiments.

Table 2.
Content of total phenolics, flavonoids, tannins, sugars, and DPPH free radical scavenging activity of Matricaria recutita white ray floret supercritical fluid extracts in ethanol.
The results revealed a considerable diversity in the phytochemical classes among all three extracts investigated in the present study. Significant differences in total phenolic content (TPC) emerged among extracts, with the FAF2 extract showing the highest quantity (342.8 ± 17.3 mg of GAE/mL) and the FAF1 and FAF3 samples having the lowest content (98.1 ± 3.5 and 138.1 ± 3.7 mg of GAE/mL, respectively). Additionally, total flavonoid (TFC) content was rather uneven among all extracts, as it ranged from 45.6 ± 2.7 mg of APE/mL to 201.8 ± 11.3 mg of APE/mL. The extracts also differed in terms of total tannin content (TTC), although FAF1 and FAF3 contained almost the same content (46.4 ± 1.9 mg of TAE/mL and 49.8 ± 2.7 mg of TAE/mL, respectively) compared with FAF3 (128.8 ± 12.4 mg of TAE/mL). The highest amounts of TPC, TFC, and TTC were found in FAF2 extracts, accordingly showing the highest radical scavenging activity (66.5%). At the same time, the content of total sugars in the FAF2 extract in comparison with other extracts was the lowest (53.0 ± 2.2 mg of GLE/mL), suggesting that a concentration increase could negatively affect antiradical activity. Nevertheless, for a more correct interpretation of the antiradical activity, the groups of other active compounds shown in Figure 1 should also be considered. Among all studied samples, extract FAF2 contains not only the highest amount of non-volatile compounds (33.67%), but also the highest content of essential oils (17.70%). Many studies have shown a correlation between the phenolic content of plants and their antioxidant power, as well as the biological activities of essential oils [19,20,21]. A detailed discussion is presented in Section 2.7.
2.3. Qualitative Analysis of the Main Nonvolatile Plant Components in Supercritical Fluid Extracts of Matricaria recutita White Ray Florets
The untargeted screening and identification of nonvolatile phytocomponents from Matricaria recutita processing by-product CO2 extracts were conducted through LC-qTOF-MS in positive ESI+ mode. The obtained results show that the LC-qTOF-MS instrument is useful for determining and confirming the main extracted bioactive compounds. The most valuable aspect of the LC-qTOF-MS is its ability to provide accurate molecular mass information, allowing the empirical formulas to be matched against databases without the need for authentic standards. The tentative identification was based on high-resolution mass spectra, fragmentation, and databases such as Metlin and LipidMaps data. In total, ninety-six bioactive compounds were isolated from all three valorization extracts (Table 3). The results indicated that the major constituents of the investigated extracts are less polar prenol lipids, fatty acyls, lignans, and glycerophospahtes, followed by more polar flavonoids, phenolic acids, amino acids, and lactones. Significant amounts of Matricaria recutita waste are considered a rich source of apigenin and ferulic acid derivatives, as well as the coumarin derivatives herniarin and umbelliferone [13]. These compounds are often used in the cosmetics industry due to their strong absorption of UV light. Although the aim of this study was not to quantify the identified compounds, our observations confirm the significant presence of hydroxycoumarins such as umbelliferone, herniarin, and their derivatives, as well as a series of apigenin-, ferulic-acid-, and cinnamic-acid-derived flavonoids, for example.

Table 3.
Separated phytocomponents from the ethanol extracts of Matricaria recutita white ray floret supercritical fluid extracts.
2.4. Quantitative Analysis of the Main Volatile Plant Components in Supercritical Fluid Extracts of Matricaria recutita White Ray Florets
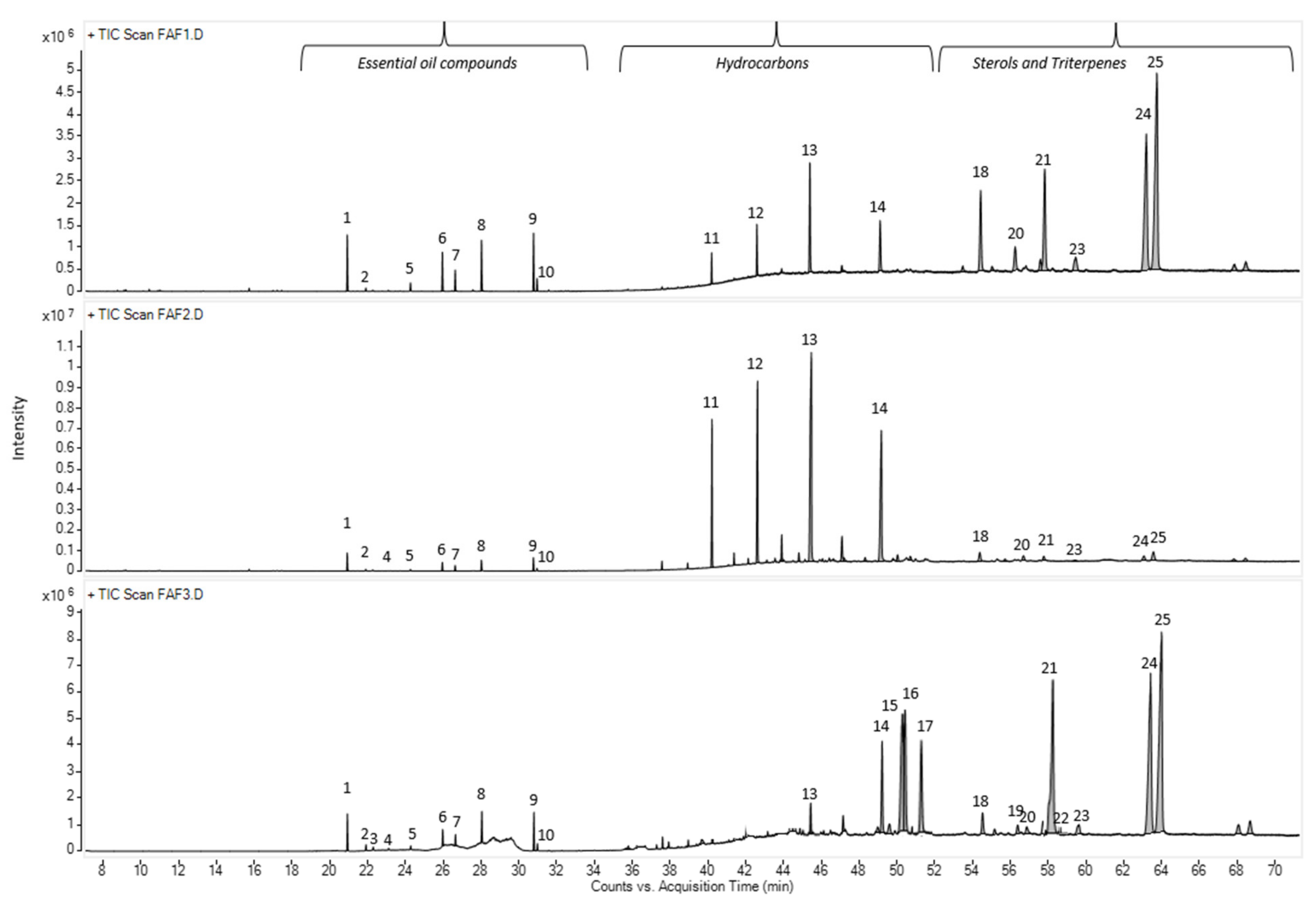
In total, forty-seven compounds were separated in the Matricaria recutita processing by-product CO2 cyclohexane extracts using gas chromatography–mass spectrometry. The main identified compounds depicted in Figure 3 and their relative amounts (%) are summarized in Table 4.
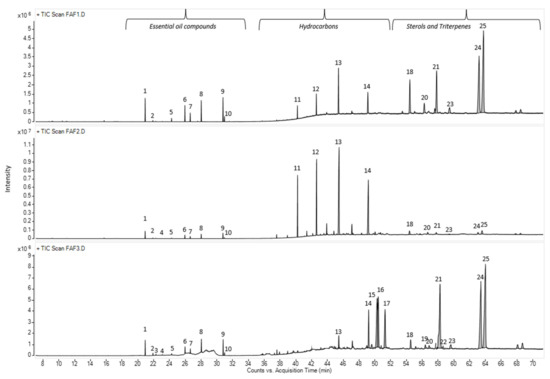
Figure 3.
Total ion current chromatogram (TIC) of main volatile compounds in Matricaria recutita white ray floret supercritical fluid extracts. The numbers above the peaks represent the main identified compounds in Table 4.

Table 4.
Chemical composition (%) of the cyclohexane extracts from Matricaria recutita white ray floret supercritical fluid extracts.
The chemical compositions of the CO2 extracts in cyclohexane were determined according to their retention times and spectrometric electronic library (NIST). The identity of the constituents was established using GC retention indices (RIs). Generally, the chemical composition of CO2 extracts showed the abundance of essential oil components (3.05 –17.7%), hydrocarbons (15.61–59.29%), pentacyclic triterpenes (11.35 –68.79%) and sterols (1.63–4.57%). The presence of essential oil compounds in CO2 extracts was already expected, whereas chamomile (Matricaria recutita) flowers themselves are a rich source of essential oils [22], even when obtained from dried white flower petals. The essential oil typically produced by steam distillation is characterized by its blue color because of the chamazulene present. Chamazulene is not present in the fresh flowers; therefore, in our study, the obtained CO2 extracts exhibited dark yellow (FAF1 and FAF3) and green (FAF2) hues (Table 1), which is consistent with prior findings [13,23,24]. Previous research on the essential oil amount in CO2 extracts showed that it varies between 0.5 and 4.5% [24]. In the framework of the same study, the author indicates the differences in both the amount and content of the essential oil at the different SFE times. Not only the yield but also the essential oil amount increases with pressure (100–120 bar), temperature (30 and 40 °C), and solvent flow rate.
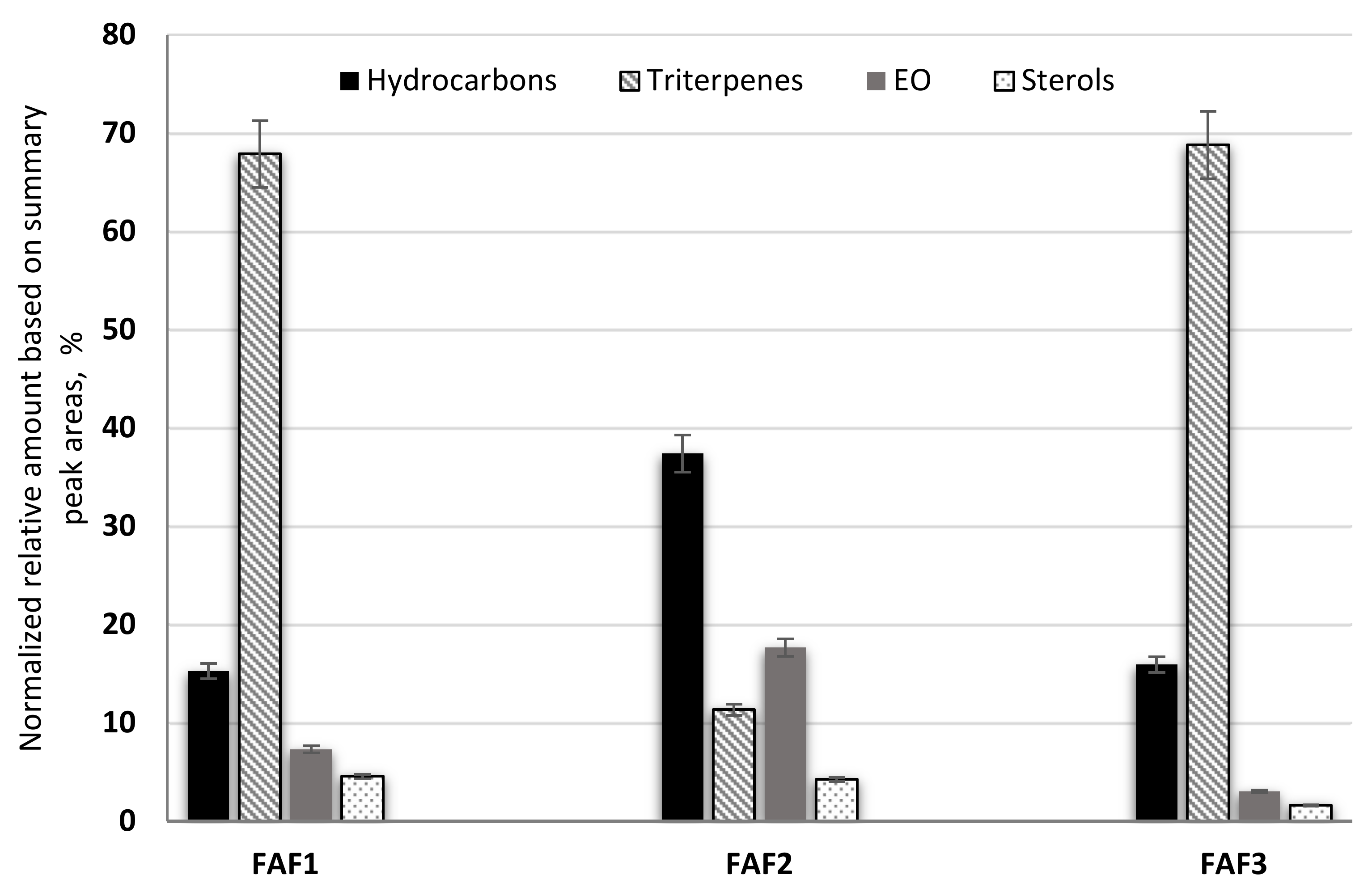
According to the obtained data, sample FAF2 showed the biggest differences within the analysis of volatile compounds (Figure 4). Samples FAF1 and FAF3 showed a higher content of pentacyclic triterpenes, whereas FAF2 contained more hydrocarbons. Based on GC-MS data, prenol lipids (pentacyclic triterpenes) were not only separated but also identified, whereas using LC-qTOF-MS was more challenging. It was found that these extracts contain valuable amounts of lupeol, α-amyrin and β-amyrin. The versatility of the pentacyclic triterpenes provides a promising approach not only for the food and cosmetics industries but for diabetes management as well. Among the identified aliphatic hydrocarbons, some of them are described as exhibiting antibacterial activity [24]. Essential oils obtained by steam distillation from areal parts of Matricaria recutita play an important role in a wide range of biological activities that allow their use to prevent or treat diseases, as well as being extensively used in the food industry (e.g., as anti-microbial and flavoring agents in beverages), perfumery, and cosmetics (e.g., as anti-inflammatory and calming agents in skin care products) [19]. Not only chamazulene, but also β-Farnesene, Bisabolol Oxide A, α-Bisabolone Oxide A, and α-Bisabolone Oxide B, as well as the spiroethers cis-ene-yne-Dicycloether and Tonghaosu, have been reported to influence the antioxidant effects of EO [19,20,21].
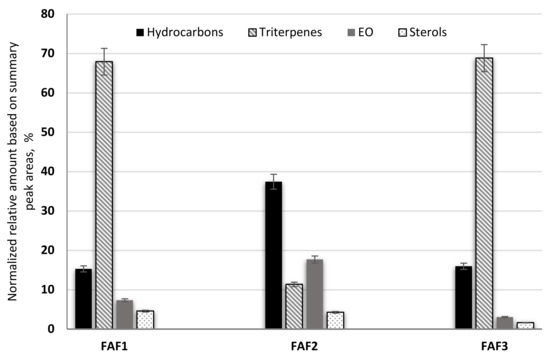
Figure 4.
The composition of volatile phytochemical classes in supercritical fluid extracts of Matricaria recutita white ray florets in cyclohexane.
2.5. Major and Minor Elements and Heavy Metal Screening in Supercritical Fluid Extracts of Matricaria recutita White Ray Florets
Elemental analysis of Matricaria recutita processing by-product white ray florets CO2 extracts appear to be an important part of the quality assurance system, assisting in determining the actual level of exposure to various elements. Essential and non-essential elements are well defined (e.g., Co, Cr, Fe, Mn, Mo, Ni, Se, Sn, V, and Zn are essential elements, whereas As, Cd, Pb, and Hg are unambiguously toxic elements) [25,26], and therefore it is important to have quality control of elemental analyses in by-product CO2 extracts in order to estimate concentration values that have no adverse influence on human health. The detection of major, minor elements, and heavy metals in Matricaria recutita processing by-product CO2 extracts was performed using a microwave plasma atomic emission spectrometer (MP-AES). The results are summarized in Table 5.

Table 5.
Detection of major, minor elements, and heavy metals in Matricaria recutita white ray floret supercritical fluid extracts.
No significant differences were observed between all extracts tested. The elemental screening revealed the presence of iron, calcium, sodium, and, in smaller amounts, potassium, magnesium, manganese, zinc, and copper. The highest contents of essential heavy metals were established for iron (1170–1189.3 mg/kg). The analyzed samples contained no heavy metals or the elements molybdenum (Mo) or cobalt (Co). So far, no data have been found on the elements present in CO2 extracts obtained from dried white flower petals of chamomile, whereas investigations of the content of micro- and macro-elements in different chamomile flower products are described. High levels of B, Ca, Cu, Fe, and P were found in chamomile raw materials and infusions, detected by inductively coupled plasma optical emission spectrometry (ICP-OES) and atomic absorption spectrometry (AAS) [26]. While testing five different tea samples produced by different manufacturers and purchased at a local market in Serbia, it was observed that the most present macro-element was potassium, followed by sodium, calcium, and magnesium. From the group of trace elements, the presence of iron, copper, zinc, and manganese was detected in all samples [27]. The mineral element content of the chamomile flower heads grown in Austria and their recovery in differently prepared infusions presented elemental concentrations of potassium, calcium, and magnesium [28]. Only data obtained in a study by Mihaljev [29] using atomic absorption and mass spectrometry with inductively coupled plasma revealed higher levels of iron, manganese, and zinc in raw chamomile flowerheads. The data obtained in our studies confirms that micro- and macro-elements and their concentration levels in samples obtained using CO2 extraction differ from other extraction techniques. These findings confirm those of Pohl [30] that elements such as Al, Ca, Cu, Ba, Fe, Mn, Sr, Ti, and V, for which the extraction rates in water are relatively small, appear to be much more strongly bound or immobilized to the organic matrix of medicinal plants. Therefore, using stronger extraction methods can help extract elements with lower solubility.
2.6. Antimicrobial Activity Screening of Ethanol Extracts from Matricaria recutita White Ray Floret Supercritical Fluid Extracts
CO2 extracts from Matricaria recutita processing by-products inhibited Gram-positive bacteria such as Staphylococcus aureus, Gram-negative bacteria such as Pseudomonas aeruginosa and Escherichia coli, and yeast Candida albicans. All tested extracts showed antimicrobial activity; however, no significant differences in MIC and MBC concentrations were observed between the tested extracts (Table 6). S. aureus and Candida albicans were inhibited, but higher extract concentrations were required to inhibit Gram-negative bacteria. This is in line with previously published data on the different antibacterial activities of natural compounds against Gram-negative and Gram-positive bacteria. Overall, Gram-positive bacteria are more susceptible to plant-derived antimicrobial compounds. Gram-negative bacteria are considered to be less susceptible, as the outer membrane of the cell wall hinders penetration of various compounds [31,32]. For all three extracts, MICs against P.aeruginosa were lower compared with E. coli, indicating species-specific differences in susceptibility of Gram-negative bacteria.

Table 6.
Minimum inhibitory concentrations (MIC), minimum bactericidal (MBC), and minimum fungicidal (MFC) concentrations, mg/mL of Matricaria recutita white ray floret supercritical fluid extracts.
The majority of compounds identified in the extracts have been reported in the literature to possess antimicrobial activity. Extracts FAF1 and FAF3 had the highest amyrin content, and amyrin-type triterpenoids have been shown to have inhibitory activity against bacteria and yeasts, including S. aureus and C. albicans [33]. Han and Lee [34] elucidated that amyrin’s antibacterial activity against E. coli is due to its ability to induce oxidative stress in bacteria. The strongest inhibitory and bactericidal activity of FAF1 against E. coli might be linked to the highest β-amyrin content among tested extracts. Another triterpenoid found in extracts, lupeol, is known to inhibit the growth of S. aureus and C. albicans [35]. It is hypothesized that it also contributes to the antifungal and antistaphylococcal activity of the FAF extracts. The effect of the total sugars in the extracts on antimicrobial, medicinal, and preservation properties also should be considered. Previous studies have also been conducted where concentrated sugar solutions have been shown to possess significant antimicrobial properties against pathogens such as Staphylococcus aureus, Bacillus subtilis, and Pseudomonas aeruginosa [36]. The results obtained by Mizzi [37] indicate that high sugar concentrations inhibit bacterial growth, whereas very low concentrations show the opposite effect, i.e., they stimulate bacterial growth, indicating that there is a threshold concentration upon which sugars cease to act as antimicrobial agents and become media instead.
2.7. Cytotoxicity and Phototoxicity Assessment of Matricaria recutita White Ray Floret Supercritical Fluid Extracts
It is important to show the safety profile of plant-derived extracts prior to testing for more specific biological activities. As a result, in vitro cytotoxicity and phototoxicity testing in the Balb/c 3T3 cell line were performed in accordance with OECD guidelines.
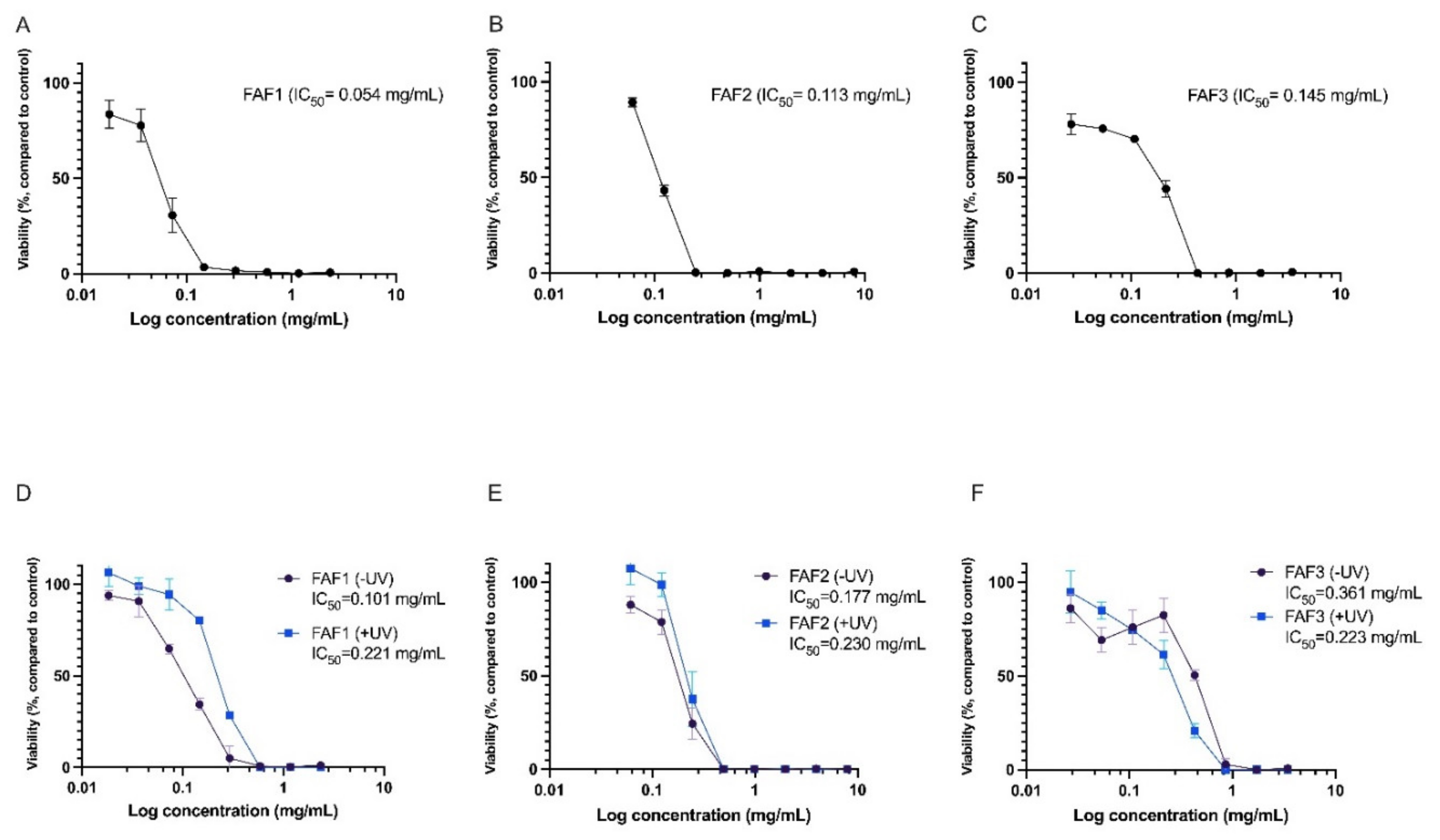
The cytotoxicity assay demonstrated that all three extracts have cell viability-decreasing activity. FAF1 extract reduced cell viability by more than 20% at concentrations exceeding 0.035 mg/mL. Concentrations above 0.14 mg/mL reduced cell viability by more than 90%. FAF2 was cytotoxic at concentrations above 0.062 mg/mL; at concentration levels of 0.24 mg/mL and higher, the reduction in cell viability exceeded 90%. Concentrations above 0.027 mg/mL were cytotoxic in case of the FAF3. The data are summarized in Figure 5A–C.
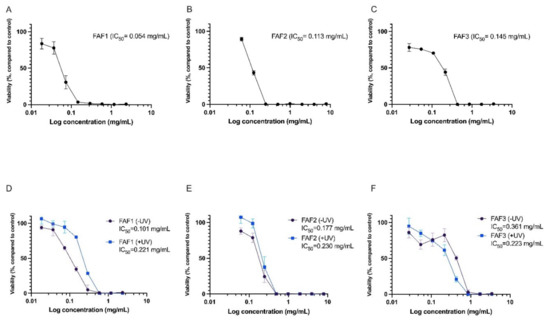
Figure 5.
Cytotoxicity (A–C) and phototoxicity (D–F) assessment of Matricaria recutita white ray floret supercritical fluid extracts. -UV cells incubated with extracts for 1h without UV irradiation, +UV cells preincubated for 1h with extracts and irradiated with 5 J/cm2 UVA; n = 3.
Negative effects on cell viability were also observed in the phototoxicity assay. Viability was rapidly reduced in cells incubated for 1h with extracts without UV irradiation (see Figure 5D–F, -UV samples). Interestingly, for FAF1 and FAF2, viability was slightly higher in UV-irradiated cell cultures compared with cells not exposed to UV. However, the differences were not statistically significant. An explanation for this might be the antioxidative activity of the extracts, which complement cellular antioxidative defense mechanisms induced by UV exposure.
Previously, the cytotoxic effects of various Chamomile extracts have been tested in different cell cultures. Sak [38] investigated the effects of Chamomile on melanocyte and oral epidermal cell lines, reporting a decrease in cell viability at concentrations less than 0.1 mg/mL. Other authors have used various cancerous cell lines for cell viability testing and observed toxic effects at low Chamomile extract concentrations [39]. The triterpenes lupeol and amyrins found in our Matricaria recutita processing by-product CO2 extracts are thought to contribute to the cytotoxic activity. Cytotoxicity and antiproliferative activity of lupeol have been shown in various cell cultures [40,41,42]. Similarly, Amyrin in vitro has demonstrated cytotoxicity and cell apoptosis-inducing activity [43,44].
Overall results show that Matricaria recutita processing by-product white ray floret CO2 extracts are rich in biologically active compounds that we have shown to have cytotoxic and proliferation-reducing activity in immortalized cell lines, as well as antimicrobial activity. For the first time, these effects have been studied in processing by-products, and the results generated in this study provide valuable preconditions for further studies in specific test systems to fully elucidate the mechanisms of action and potential applications.
2.8. Statistical Analysis
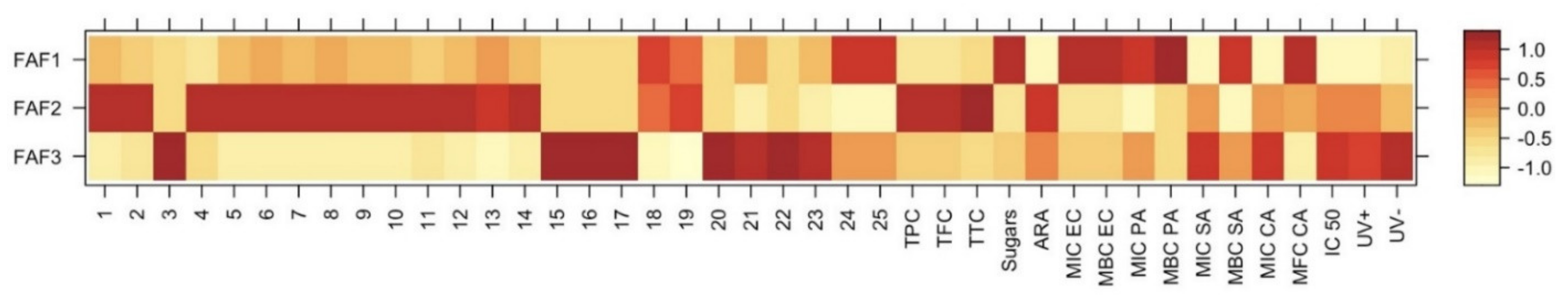
Statistical analysis using a heatmap dendrogram (Figure 6) and correlogram (Figure 7) was carried out to determine the significant correlations between the investigated extracts and to create models that can meaningfully predict the targeted phytochemical recovery and most promising chemical and biological activities. The heatmap in Figure 6 represents concentration levels of volatile compound content, average concentrations of total phenolic, total flavonoid, total tannin, and sugars, as well as cytotoxicity, phototoxicity, antiradical, and antibacterial activities among all Matricaria recutita processing by-product white ray floret CO2 extracts. The red color indicates higher concentrations and activities, whereas the orange-to-yellow color indicates lower corresponding values.
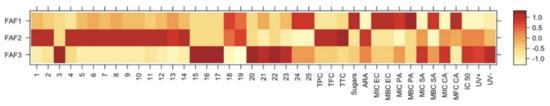
Figure 6.
Variation in chemical composition and biological activity of Matricaria recutita white ray floret supercritical fluid extracts. The legend denotes scaled values of the volatile chemical constituents (No 1-25, according to Table 4), total phenolics (TPC), flavonoids (TFC), tannins (TTC), sugars (according to Table 2), antiradical (ARA) and antimicrobial activity (according to Table 6), cytotoxicity, and phototoxicity (according to Figure 5).
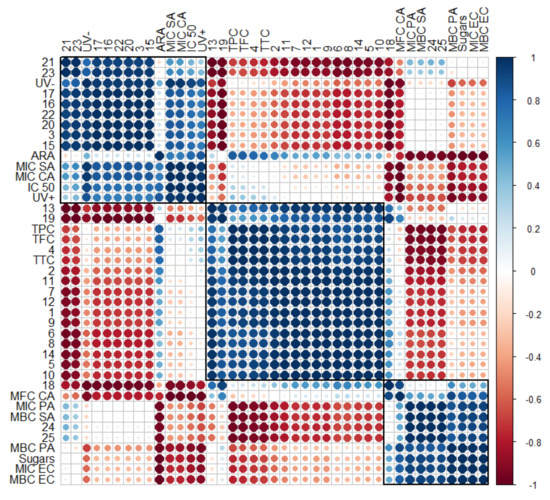
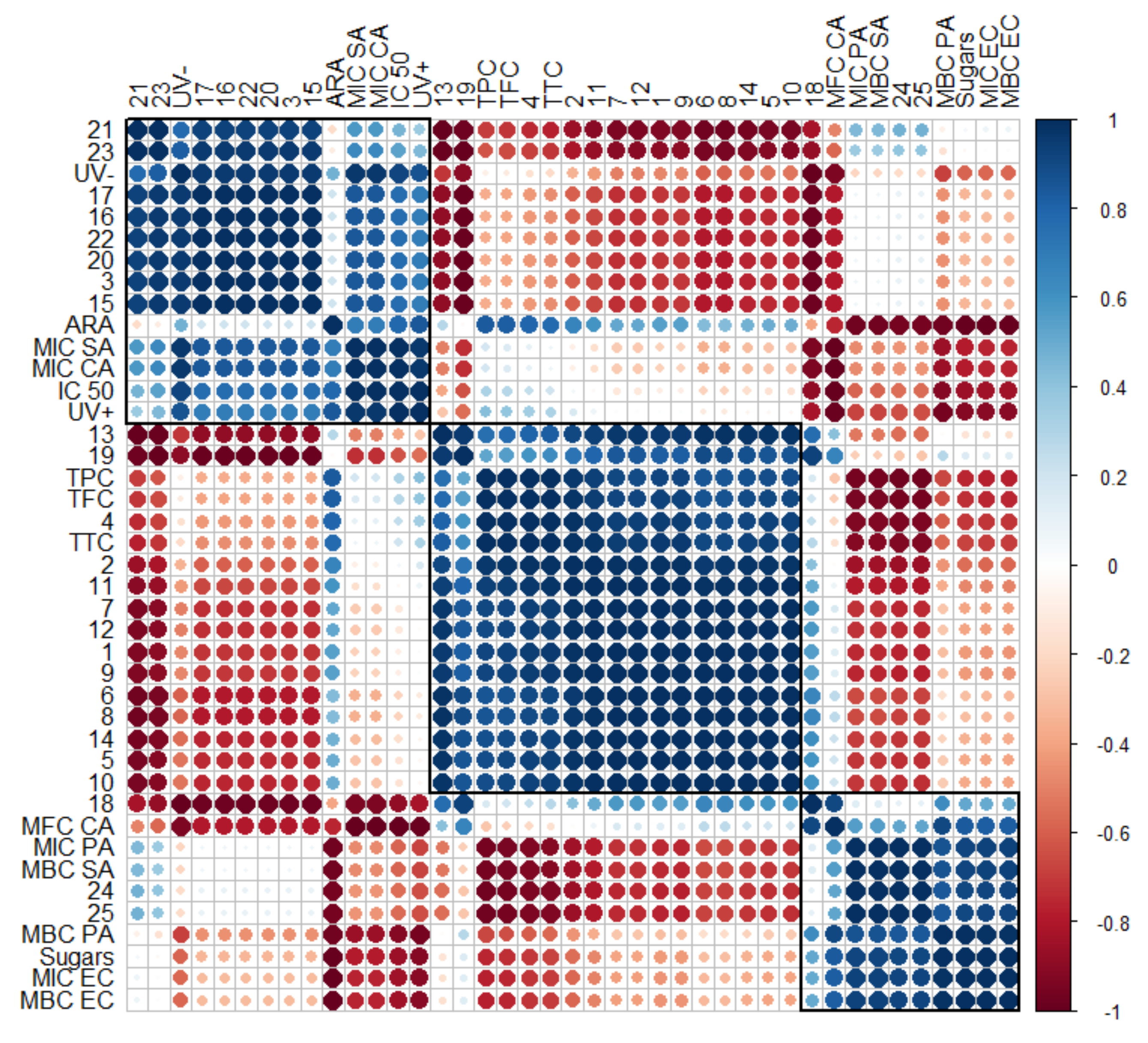
Figure 7.
Correlogram depicting the relationship between volatile compounds (No. 1–25, according to Table 4), phytochemical classes (total phenolics (TPC), flavonoids (TFC), tannins (TTC), and sugars, according to Table 2, and their antiradical (ARA) and antimicrobial activity (Table 6), as well as cytotoxicity and phototoxicity (Figure 4). Correlations with p > 0.05 are considered insignificant and are denoted by a white space with a blank. The color and size of the squares are proportional to the correlation coefficients, which are color-coded from deep red (−1) to deep blue (1).
The data obtained in Figure 6 illustrate obvious differences among all three investigated supercritical extracts and confirms the hypothesis that even small changes in the Matricaria recutita processing by-product white ray florets supercritical extraction parameters (such as temperature and pressure) can affect not only the yield but also the chemical composition, which in turn affects the further properties of the extracts. The extract with the smallest total extraction yield (FAF2) showed the highest concentration of total phenolics (TPC), flavonoids (TFC), and tannins (TTC), which in turn reflected on the increased antiradical activity (ARA) of the extract. However, the values of other parameters such as cytotoxicity and phototoxicity were higher for FAF3 extracts, suggesting that the values of these parameters are influenced by some individual hydrocarbons (15–17) and triterpenes (20–23). According to antimicrobial activity, the best results were extrapolated from extract FAF1. The highest inhibitory and bactericidal activity of FAF1 against E. coli (MIC EC and MBC EC) could be attributed to the highest β-amyrin (25) content among tested extracts, as well as the presence of high total sugar concentrations.
Although with the help of a heatmap it is possible to visually evaluate the changes quite accurately, as the number of extracts increases, data interpretation becomes more complicated and unwanted inaccuracies can occur. When correlograms are used, the correlations between all variables are determined uniformly, regardless of the number of extracts used. Moreover, it can help choose targeted supercritical extraction conditions to obtain meaningful phytochemical recovery and the most promising chemical and biological activities.
To verify the strength of the association between several variables, a correlogram was constructed (Figure 7). Previous descriptions of strong correlations of cytotoxicity (IC 50) and phototoxicity (UV− and UV+) among some individual hydrocarbons (15–17) and lupeol and taraxerol type-triterpenes (20–23), as well as inhibitory effects against S. aureus (MIC SA) and C. albicans (MIC CA), were confirmed by the obtained correlogram. The matrix also allows for the assessment of the relationships between detected α- and β-amyrin (24 and 25) and inhibitory against S. aureus (MBC SA) and P. aeruginosa (MIC PA). Total sugars were highly positively correlated with inhibitory against P. aeruginosa (MBC PA) and E. coli (MIC EC and MBC EC), with a weaker influence on inhibitory against S. aureus (MBC SA) and P. aeruginosa (MIC PA). Matrix correlation supports the role of sterols (18 and 19) on inhibitory activity against yeasts, including C. albicans (MFC CA). Strong negative correlations between essential oil compounds (1–10) and almost all tested variables, except antiradical activity (ARA), were also confirmed.
3. Materials and Methods
3.1. Plant Materials
Matricaria recutita white ray florets (Figure 8) were collected from the SIA Field and Forest dried herb processing facility located in Priekuli, Latvia. SIA Field and Forest grows, harvests, dries, and processes organic Matricaria recutita chamomile. White ray florets are a waste stream originating from broken flowerheads during processing of the German chamomile herbal biomass. The industrial separation of white ray florets was carried out with a cyclone separator, ensuring great separation efficiency and the purity of the separated waste flow. The waste flow contains more than 95% of white ray florets and less than 5% of yellow disc florets. The moisture content of white ray florets was 11.20 ± 0.34% wt%, determined with a Radwag MA 210.X2.IC.A.WH moisture analyzer (Radom, Poland). Prior to the extraction, white ray florets were pulverized using a universal dry product mill, the MLVS M500 (Mazeikiai, Lithuania). The average particle diameter was 0.5 mm.

Figure 8.
Matricaria recutita white ray florets before (a) and after (b) milling used for supercritical fluid extraction.
3.2. Supercritical Fluid Extraction
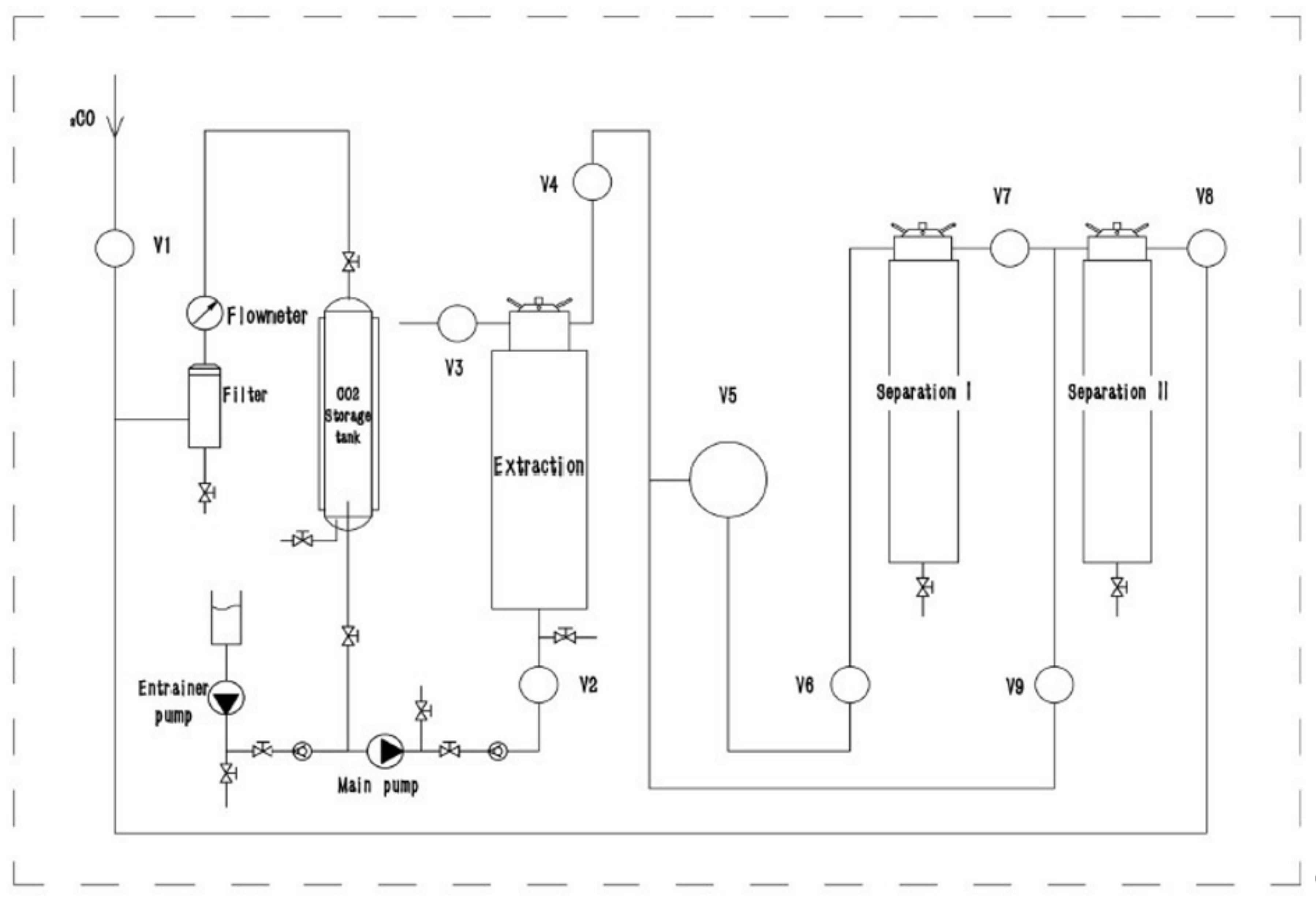
Supercritical fluid extraction was carried out with a pilot-scale supercritical fluid extractor, the CAREDDI SCFE-5L (UAB Analytical Solutions; Kaunas, Lithuania), using carbon dioxide (CO2) and ethanol (EtOH) as co-solvents. The supercritical extraction unit consisted of a high-pressure CO2 pump, a co-solvent pump, a cooling system, a heating system, a temperature control system, a CO2 storage tank, a 5 L extraction tank, and two-stage separation tanks (4 L and 2 L). The process diagram is depicted in Figure 9.
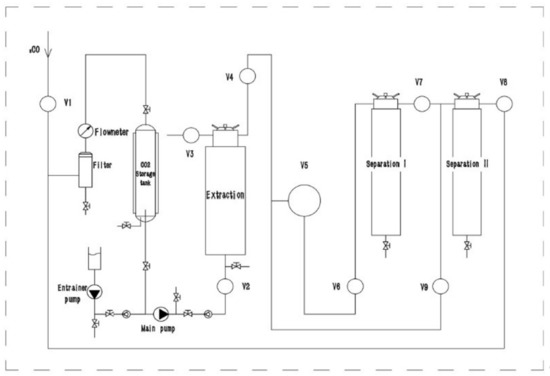
Figure 9.
Process diagram of pilot-scale supercritical fluid extractor CAREDDI SCFE-5L.
Flow rates and temperatures were automatically controlled. Manual back pressures were used to control the pressure in the extraction tank and both separation tanks. The maximum extraction pressure was 48 MPa, and the maximum separation pressure was 25 MPa. Dynamic extraction was used, with a CO2 flow rate of 100 L h−1 and an ethanol flow rate of 4 mL min−1. The optimal sample size of 600 g of pulverized white ray florets was determined in preliminary tests; sample sizes greater than 600 g showed decreased yield (−12.40% yield decrease with 700 g input; −19.65% yield decrease with 800 g input), most likely due to overpacking causing uneven solvent distribution in the extraction tank. The optimal extraction duration time of 2 h was determined in preliminary tests (30 min yields 4.32 ± 0.31 g 100 g−1 DW input, 60 min yields 5.86 ± 0.31 g 100 g−1 DW input, 90 min yields 9.52 ± 0.68 g 100 g−1 DW input, 120 min yields 12.91 ± 0.48 g 100 g−1 DW input, and 180 min yields 13.09 ± 0.57 g 100 g−1 DW input). The preliminary tests showed that optimal extraction conditions based on yield were using 25 MPa pressure and 55 °C temperature in the extraction tank with no interaction between the two parameters (p < 0.05); however, these tests did not take into account the variation of pressure and temperature in the separation tanks. As a result, three different separation temperature (35–55 °C) and separation pressure (5–9 MPa) variations were tested in this study (FAF1 at 45 °C and 7 MPa, FAF2 at 35 °C and 5 MPa, and FAF3 at 55 °C and 9 MPa, respectively). After extraction, the obtained extract was placed in dark glass vials, sealed, and stored at 4 °C to prevent possible degradation. The extraction yield was determined gravimetrically and expressed as g of extract per 100 g of dry white ray florets.
3.3. Chemicals and Reagents
The CO2 used in SCFE was food grade (purity >99.8%) and purchased from Elmemesser (Riga, Latvia). Ethanol (96%) used in SCFE was purchased from Kalsnavas elevators Ltd. (Jaunkalsnava, Latvia).
LC-MS grade acetonitrile, methanol, and formic acid were purchased from Fisher Scientific (Loughborough, UK), and water for LC-qTOF-MS analysis was purified using a Smart2Pure water purification system (Thermo Scientific, Germany). Gallic acid and AlCl3, cyclohexane, Trolox, ferulic acid, rutin, chlorogenic acid were purchased from Acros Organics (Geel, Belgium), Na2CO3 and NaNO2 were obtained from Honeywell (Charlotte, NC, USA), apigenin standard from Rotichrom, Carl Roth GmbH (Karlsruhe, Germany), tannic acid, 2,2-diphenyl-1-picrylhydrazyl (DPPH), phenol, coumarin from Alfa Aesar (Kandel, Germany). Folin–Ciocalteu reagent, H2SO4, HNO3, HCl, D-glucose, NaOH reagents, multi-element standard solution for ICP (33 elements) was purchased from Fisher Scientific (Loughborough, UK). A calibration mix with the major elements Ca, Fe, K, Na, and Mg was purchased from Agilent Technologies (Santa Clara, CA, USA).
Mueller–Hinton broth was purchased from Biolife (Milan, Italy), malt extract from Oxoid (Cheshire, UK), and Wilkins–Chalgren broth from Sigma (St Louis, MO, USA). DMEM medium was purchased from Sigma (Irvine, UK); penicillin–streptomycin as well as calf serum broth were purchased from Sigma (St Louis, MO, USA); and phosphate-buffered saline was purchased from Sigma (Irvine, UK). Neutral Red dye and glacial acetic acid were purchased from Sigma (Irvine, UK).
3.4. Quantification of Essential Oils and Waxes
A specific amount of the extract was placed on Petri plates and placed in a 160 °C oven with air circulation for 6 h. Afterwards, the Petri plates were allowed to cool down in a desiccator and weighed. This procedure was repeated until the mass of the extract was constant.
3.5. Determination of the Total Phenolic Content
The amount of total phenolic content (TPC) in the studied extracts was determined using a slightly modified Folin–Ciocalteu method [45]. In total, 25 μL of a known dilution of the extract was mixed with 75 μL H2O and 25 μL Folin–Ciocalteu reagent (previously diluted 10-fold with deionized water) and allowed to react for 6 min. Then, 100 μL of a 7% solution of sodium carbonate was added to the well. The plates were shaken for 30 s and allowed to stand for 90 min for color development in a dark place at room temperature. Absorbance was measured at 765 nm by using the Epoch 2 UV/VIS Microplate Spectrophotometer (BioTek, Agilent, Germany). The measurements were compared with a standard curve of prepared gallic acid solutions (0–0.2 mg/mL). The total phenolic content was expressed as gallic acid equivalent (GAE) mg. All measurements were performed in triplicates.
3.6. Determination of the Total Flavonoid Content
The determination of total flavonoid content (TFC) was performed according to the AlCl3 colorimetric method with slight modifications [46]. 20 μL of a known dilution of the extract was mixed with 15 μL of a 5% solution of sodium nitrite and allowed to react for 5 min in a dark place. Then, 15 µL of 10% aluminum (III) chloride was added to react for 6 min. 100 µL of 1M sodium hydroxide was added to the well after 6 min. The plates were shaken for 30 s and allowed to stand for 15 min for color development in a dark place at room temperature. The reaction forms orange or pink chromophore complexes. Absorbance was measured at 510 nm by using the Epoch 2 UV/VIS Microplate Spectrophotometer (BioTek, Agilent, Germany). The measurements were compared with a standard curve of prepared apigenin solutions (0.025–0.2 mg/mL). The total flavonoid content was expressed as apigenin equivalent (AE) mg. All measurements were performed in triplicates.
3.7. Determination of the Total Tannin Content
The number of total tannins (TTC) in the studied extracts was determined using a modified Folin–Ciocalteu method. In total, 50 μL of a known dilution of the extract was mixed with 50 μL H2O and 50 μL Folin–Ciocalteu reagent (previously diluted 1:1 with deionized water) and allowed to react for 5 min. Then, 100 μL of 35% sodium carbonate solution (35g Na2CO3/100 mL H2O) was added to the well. The plates were shaken for 30 s and allowed to stand for 30 min for color development in a dark place at the room temperature. The reaction forms a blue chromophore constituted by a phosphotungstic-phosphomolybdenum complex, where the maximum absorption of the chromophores depends on the alkaline solution and the concentration of tannins. Absorbance was measured at 700 nm by using the Epoch 2 UV/VIS Microplate Spectrophotometer (BioTek, Agilent, Germany). The measurements were compared to a standard curve of prepared tannic acid solutions (0.01–0.15 mg/mL). The total tannin content was expressed as tannic acid equivalent (TAE) mg. All measurements were performed in triplicates.
3.8. Determination of the Total Sugar Content
For total sugar estimation, the modified Phenol-Sulfuric acid colorimetric method was used. In total, 50 μL of sample, 150 μL of concentrated sulfuric acid (H2SO4), and 30 μL of 5% phenol (5 g C6H6O/100 mL H2O) reagent were mixed in well and kept in the oven for 5 min at 90 °C. After heating, plates were cooled, and absorbance was measured at 490 nm by using the Epoch 2 UV/VIS Microplate Spectrophotometer (BioTek, Agilent, Germany). The solution turns a yellow-orange color as a result of the interaction between the carbohydrates and the phenol. The absorbance at 490 nm is proportional to the carbohydrate concentration initially present in the sample. The measurements were compared to a standard curve of prepared glucose solutions (0.25–10 mM). The total sugar content was expressed as glucose equivalent (GLE) mg. All measurements were performed in triplicates.
3.9. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Assay
The free radical scavenging method DPPH was used to assess the antioxidant properties of tested extracts [45]. For the assay, 20 μL of a known dilution of the extract was mixed with 180 μL of 150 μM DPPH reagent in the wells. The plate was kept in the dark at room temperature for 60 min. When antioxidant samples are mixed with DPPH reagent solution, the color gradually changes from purple to yellow. Decreases in the absorbance at 517 nm were measured using the Epoch 2 UV/VIS Microplate Spectrophotometer (BioTek, Agilent, Germany). Trolox standard solutions in the concentration range of 0–800 μg/mL were used as a standard. All measurements were performed in triplicates. Antiradical activity (ARA) was expressed as trolox equivalent (TE) mg.
3.10. Determination of Major and Minor Elements and Heavy Metals by MP-AES Analysis
Approximately 1 g of extract was transferred to a porcelain crucible. Mineralization was conducted in a muffle furnace. The mineralized sample was then transferred to a 50 mL volumetric flask and diluted with deionized water with 37% HCl and 65% HNO3. If the concentration of elements was above the detection maximum, samples were diluted with a 5% HNO3 solution. Standards were prepared from stock solutions according to Table 7.

Table 7.
Concentration values of standard solutions prepared for MP-AES analysis.
Samples were microwave digested on a Berghof Speedwave XPERT DAK-100X (Berghof Products+ Instruments GmbH, Eningen unter Achalm, Germany). The speedwave XPERT Teflon vessels were used for digestion. The digestion program was used as follows: 1st step till 170 °C at a rate of 2 °C/min, hold for 10 min, pressure 80 bar, power 80%; 2nd step to 210 °C at a rate of 3 °C/min, hold for 10 min, power 90%; 3rd step from 210 to 50 °C at a rate of 1 °C/min, hold for 10 min, power 0%. Samples were dry mineralized in a muffle furnace (Nabertherm GmbH, Lilienthal, Germany) for 2h at 650 °C and analyzed on an Agilent 4210 MP-AES with an SPS 4 autosampler and a N2 generator (Agilent Technologies, Deutschland GmbH, Waldbronn, Germany). The Agilent MP Expert acquisition software was used to obtain and analyze the data. Instrument parameters were set as follows: automatic, system-adjusted nebulizer flow for each element (described in Table 8); a read time of 3 s; 3 replicates; pump speed of 15 rpm; an uptake time of 70 s; a rinse time of 45 s; a stabilization time of 25 s; and a correlation coefficient limit of 0.990. The used wavelengths for detected elements are described in Table 8.

Table 8.
Detection conditions for micro- and macro-elements by atomic emission spectrometer.
3.11. UHPLC-HRMS Analysis
Dilutions with ethanol (96%) were prepared to obtain a concentration of at least 10 mg/mL, the samples were filtered through a 0.45 µm filter and were afterwards injected into the chromatographic system. The obtained extracts were analyzed on an Agilent 1290 Infinity II series HPLC system combined with an Agilent 6530 qTOF MS system (Agilent Technologies, Deutschland GmbH, Waldbronn, Germany). Zorbax Eclipse Plus C18 Rapid Resolution HD (2.1 × 150 mm, 1.8 μm particle size) column was used at a flow rate of 0.3 mL/min. The column oven was set at 50 °C, and the sample injection volume was 1 μL with a 30 s needle wash (using 70% methanol). The mobile phase consisted of a combination of A (0.1% formic acid in water) and B (0.1% formic acid in acetonitrile). The gradient elution program was used as follows: Initial 2% B, 0–2 min 2% B, 2–10 min 40% B, 10–20 min 80% B, 20–27 min 95% B, 27–40 min 95% B, 40–42 min 1% B. UV/Vis spectra were recorded at 280 nm and 330 nm. The adjusted operating parameters of the mass spectrometer were set as follows: fragmentation: 70 V; gas temperature: 325 °C; drying gas flow: 10 L/min; nebulizer: 20 psi; sheath gas temperature: 400 °C; and sheath gas flow: 12 L/min. Electrospray ionization (ESI) was used as a source when operating in positive mode. Mass spectra in the m/z range 50 to 2000 were obtained. The internal reference masses of 121.050873 m/z and 922.009798 m/z (G1969-85001 ESI-TOF Reference Mass Solution Kit, Agilent Technologies and Supelco) were used for all analyses of the samples. The Agilent MassHunter Qualitative Analysis 10.0 data acquisition software was applied to analyze LCMS data. The Agilent MassHunter METLIN Metabolomics Database and LipidMaps Database were used for the identification of isolated compounds.
3.12. GC-MS Analysis
Samples were diluted in cyclohexane, and extracts were diluted using the mas by volume (1 mg/mL) method. Samples were mixed and filtered through a 0.45 µm filter before being injected into the chromatographic system. Analyses were performed on an Agilent Technologies 7820A gas chromatograph coupled to Agilent 5977B mass selective detector (MSD) equipment. A non-polar HP-5 capillary column (60 m × 0.25 mm, 0.25 µm film thickness) coated with 5% phenyl and 95% methyl polysiloxane. The carrier gas was helium (He) with a split ratio of 1:100 and a flow rate of 1.5 mL/min. The volume of the injection was 3 μL. The temperature program was started at 70 °C, then increased at a rate of 5 °C/min to 230 °C, after which the temperature was increased to 295 °C at a rate of 7 °C/min. Finally, 295 °C was maintained for 30 min. The injector temperature was 270 °C. The mass spectra were recorded at 70 eV. The mass range was 70–500 m/z. The ion source temperature was maintained at 230 °C. The components were identified based on their retention indices (determined with reference to homologous series of C5–C24 n-alkanes) by comparison of their mass spectra with those stored in the NIST (National Institute of Standards and Technology) MS Search 2.2 library. The Agilent MassHunter Qualitative Analysis 10.0 data acquisition software was applied to analyze GC-MS data. The content of separated compounds was calculated in peak areas using the normalization method without correction factors.
3.13. Antimicrobial Activity
Mueller–Hinton broth was used for susceptibility testing by a two-fold serial broth microdilution assay of Staphylococcus aureus ATCC 6538P, Pseudomonas aeruginosa ATCC 9027, and Escherichia coli ATCC 25922. A malt extract broth was used for the testing of Candida albicans ATCC 10261.
The inoculum of microorganisms was prepared in sterile water with a density of 0.08–0.10 at A625 and diluted 100-fold in an appropriate broth. Then, 96-well plates were incubated at 37 °C for 24 h. The MIC was determined as the lowest concentration of the studied material, which showed no visible growth of microorganisms. From wells where growth was not detected, 4 μL of media was seeded on appropriate solidified media for MBC/MFC determination.
3.14. Cytotoxicity
BALB/3T3 cells (ATCC) (passages 14–15) were seeded in 96-well microplates at a density of 4 × 103 cells/well. Cells were propagated in 100 μL S10 medium (DMEM medium supplemented with 1% penicillin (100 U/mL)–streptomycin (100 μg/mL) (P/S), and 10% calf serum (CS) and incubated overnight at 37 °C, 5% CO2. Cells were rinsed with phosphate-buffered saline (PBS), and 100 μL of S5 medium (DMEM medium supplemented with 1% P/S and 5% CS) and extract mix were added to the cells. Additionally, wells with vehicle (appropriate solvent), S5 medium, and sodium dodecyl sulfate (SDS) in S5 medium controls were prepared. After 48 h of incubation at 37 °C with 5% CO2, the cells were rinsed with PBS, and 250 μL of 25 μg/mL Neutral Red dye solution in S5 medium was added to all wells. The plate was incubated for 3h at 37 °C, 5% CO2, the cells were rinsed with PBS, and 100 μL of NR desorb solution (50% ethanol, 1% glacial acetic acid, 49% water) was added to all wells. The plate was covered and placed in a microplate shaker for 20–45 min, then removed for 5–10min before absorption at 540 nm was measured using a Tecan M200 Infinite Pro microplate reader (Tecan, Switzerland). Cell viability was calculated as a percent of the media control value. The IC50 values were also calculated.
3.15. Phototoxicity
The in vitro phototoxicology protocol was a modification of the procedure described in OECD Test Guideline 432 (TG 432). Balb/c 3T3 fibroblast cells were incubated with extracts in 96-well plates for 1 h and thereafter exposed to UVA light (5 J/cm2) using UVACUBE 400 (Honle UV Technology, Gilching, Germany). In parallel, cells were exposed to the extracts in the dark and evaluated in parallel. Neutral red dye uptake (NRU) was determined 24 h later, as described in Section 3.14 “Cytotoxicity”.
3.16. Statistical Analysis
The chemical data obtained by phytochemical screening using high-throughput 96-well plate methods (Table 2) as well as the composition of volatile phytochemical classes depicted in Figure 4 were expressed as the mean ± standard error of the mean (SEM) of three independent experiments and were analyzed using the computer software GraphPad Prism 8. Statistical analysis of cytotoxicity data was performed using GraphPad Prism 9 software (GraphPad Software, San Diego, California, US). Statistical analysis was performed using Student’s t-test (two tailed distribution, two sample equal variances). p < 0.05 was considered statistically significant. A heatmap and correlogram with scaled data were created in the R package pheatmap in R software version 4.1.4.(R Foundation Statutes: Vienna, Austria) [47,48,49].
4. Conclusions
Management of herbal waste byproducts resulting from various medicinal plant industrial cycles is poorly defined. It is generally recognized that the medicinal plant’s aerial parts have the capacity to serve as natural antioxidants, suggesting that by-products from the industrial processing of medicinal plants may include bioactive compounds that may be used for a variety of applications. This study showed that supercritical fluid extraction (SFE) with CO2 as the solvent and ethanol as the co-solvent is a fast, easy-to-automate, and selective way to valorize Matricaria recutita white ray florets. Differences in phytochemical content and biological activities of M. recutita white floret supercritical fluid extracts subjected to different extraction parameters suggest that their ultimate application potential is strongly dependent on the optimization technique used. Overall results show that Matricaria recutita processing by-product white ray floret CO2 extracts are rich in biologically active compounds and have cytotoxic and proliferation-reducing activity in immortalized cell lines, as well as antimicrobial activity. This study suggests that white floret extracts of M. recutita represent a promising product to produce in specific test systems to fully elucidate the mechanisms of action and possibilities for future applications, such as potential use in cosmetic formulations.
Author Contributions
Conceptualization, I.N., L.P., A.R.-S., M.B. (Marta Berga) and M.B. (Martins Boroduskis); methodology and investigation, I.N., L.P., L.K., A.R.-S., M.B. (Marta Berga), M.B. (Martins Boroduskis) and M.S.; data curation, I.N., L.P., A.R.-S. and M.B. (Martins Boroduskis); writing—original draft preparation, I.N., L.P, A.R.-S. and M.B. (Marta Berga); writing—review and editing, I.N., L.P., A.R.-S., M.B. (Marta Berga) and M.B. (Martins Boroduskis); visualization, I.N., M.B. (Marta Berga), L.P. and A.R.-S.; supervision, I.N.; project administration, I.N. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the European Regional Development Fund project “Biorefinery approach for the development of bioactive cosmetic ingredients from by-products of medicinal plant processing and plant cell cultivation” (No. 1.1.1.1/19/A/075).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Anastas, P.T.; Werner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
- Girotto, F.; Alibardi, L.; Cossu, R. Food waste generation and industrial uses: A review. Waste Manag. 2015, 45, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E. Production of Essential Oils. In Handbook of Essential Oils: Science, Technology and Applications; Hüsnü, K., Baser, C., Buchbauer, G., Eds.; CRC Press: Boca Raton, FL, USA, 2010; p. 111. [Google Scholar]
- Torres-Leon, C.; Ramirez-Guzman, N.; Londono-Hernandez, L.; Martinez-Medina, G.A.; Diaz-Herrera, R.; Navarro-Macias, V.; Alvarez-Perez, O.B.; Picazo, B.; Villarreal-Vazquez, M.; Ascacio-Valdes, J.; et al. Food Waste and Byproducts: An Opportunity to Minimize Malnutrition and Hunger in Developing Countries. Front. Sustain. Food Syst. 2018, 2, 52. [Google Scholar] [CrossRef]
- De Martino, L.; Mancini, E.; De Almeida, L.F. The antigerminative activity of twenty-seven monoterpenes. Molecules 2010, 15, 6630–6637. [Google Scholar] [CrossRef] [PubMed]
- Meyer, H.P.; Werbitzky, O. Biocatalysis of Green Chemistry and Chemical Process Development. In How Green Can the Industry Become With Biotechnology; John and Wiley and Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Nichols, J.A.; Katiyar, S.K. Skin protection by natural polyphenols. Anti-inflammatory, antioxidant and DNA repair mechanisms. Arch. Dermatol. Res. 2010, 302, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Zillich, O.V.; Schweiggert-Weisz, U.; Eisner, P.; Kerscher, M. Polyphenols as active ingredients for cosmetic products. Int. J. Cosmet. Sci. 2015, 37, 5. [Google Scholar] [CrossRef] [PubMed]
- Veličković, D.T.; Milenović, D.M.; Ristić, M.S.; Veljković, V.B. Ultrasonic extraction of waste solid residues from the Salvia sp. essential oil hydrodistillation. Biochem. Eng. J. 2008, 42, 97–104. [Google Scholar] [CrossRef]
- Méndez-Tovar, I.; Herrero, B.; Pérez-Magariño, S.; Pereira, J.A.; Manzanera, M.C.A.S. By-product of Lavandula latifolia essential oil distillation as source of antioxidants. J. Food Drug Anal. 2015, 23, 225–233. [Google Scholar] [CrossRef]
- Oreopoulou, A.; Papavassilopoulou, E.; Bardouki, H.; Vamvakias, M.; Bimpilas, A.; Oreopoulou, V. Antioxidant recovery from hydrodistillation residues of selected Lamiaceae species by alkaline extraction. J. Appl. Res. Med. Aromat. Plants 2018, 8, 83–89. [Google Scholar] [CrossRef]
- Slavov, A.; Yantcheva, N.; Vasileva, I. Chamomile Wastes (Matricaria recutita): New Source of Polysaccharides. Waste Biomass Valoriz. 2018, 10, 2583–2594. [Google Scholar] [CrossRef]
- Molnar, M.; Mendešević, N.; Šubarić, D.; Banjari, I.; Jokić, S. Comparison of various techniques for the extraction of umbelliferone and herniarin in Matricaria recutita processing fractions. Chem. Cent. J. 2017, 11, 78. [Google Scholar] [CrossRef]
- Soquetta, M.B.; Terra, L.; Bastos, C.P. Green technologies for the extraction of bioactive compounds in fruits and vegetables. J. Food 2018, 16, 1. [Google Scholar] [CrossRef]
- Hauthal, W.H. Advances with supercritical fluids Review. Chemosphere 2001, 43, 123–135. [Google Scholar] [CrossRef]
- Oroian, M.; Escriche, I. Antioxidants: Characterization, natural sources, extraction and analysis. Food Res. Int. 2015, 74, 10–36. [Google Scholar] [CrossRef]
- Kotnik, P.; Škerget, M.; Knez, Ž. Supercritical fluid extraction of chamomile flower heads: Comparison with conventional extraction, kinetics and scale-up. J. Supercrit. Fluids 2007, 43, 192–198. [Google Scholar] [CrossRef]
- Scalia, S.; Guiffreda, L.; Pallado, P. Analytical and preparative supercritical fluid extraction of Chamomile flowers and its comparison with conventional methods. J. Pharm. Biomed. Anal. 1999, 21, 549–558. [Google Scholar] [CrossRef]
- Formisano, C.; Delfine, S.; Oliviero, F.; Tenore, G.C.; Rigano, D.; Senatore, F. Correlation among environmental factors, chemical composition and antioxidative properties of essential oil and extracts of chamomile (Matricaria recutita L.) collected in Molise (South-central Italy). Ind. Crops. Prod. 2014, 63, 256–263. [Google Scholar] [CrossRef]
- Stanojevic, L.P.; Marjanovic-Balaban, Z.R.; Kalaba, V.D.; Stanojevic, J.S.; Cvetkovic, D.J. Chemical composition, antioxidant and antimicrobial activity of chamomile flowers essential oil (Matricaria recutita L.). J. Essent. Oil Bear. Plants 2016, 19, 2017–2028. [Google Scholar] [CrossRef]
- El Mihyaoui, A.; Esteves da Silva, J.C.G.; Charfi, S.; Candela Castillo, M.E.; Lamarti, A.; Arnao, M.B. Chamomile (Matricaria recutita L.): A Review of Ethnomedicinal Use, Phytochemistry and Pharmacological Uses. Life 2022, 12, 479. [Google Scholar] [CrossRef]
- Mežaka, I.; Kronberga, A.; Nakurte, I.; Taškova, I.; Jakovels, D.; Primavera, A. Genetic, chemical and morphological variability of chamomile (Chamomilla recutita L.) populations of Latvia. Ind. Crops Prod. 2020, 154, 112614. [Google Scholar] [CrossRef]
- Reverchon, E.; Senatore, F. Supercritical carbon dioxide extraction of chamomile essential oil and its analysis by gas chromatography–mass spectrometry. J. Agric. Food Chem. 1994, 42, 154–158. [Google Scholar] [CrossRef]
- Povh, N.P.; Marques, M.O.M.; Meireles, M.A.A. Supercritical CO2 extraction of essential oil and oleoresin from chamomile (Recutita recutita [L.] Rauschert). J. Supercrit. Fluids 2001, 21, 245–256. [Google Scholar] [CrossRef]
- WHO, G. Who Guidelines for Assessing Quality of Herbal Medicines with Reference to Contaminants and Residues, Geneva; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Malik, J.; Szakova, J.; Drabek, O.; Balik, J.; Kokoska, L. Determination of certain micro and macroelements in plant stimulants and their infusions. Food Chem. 2008, 111, 520–525. [Google Scholar] [CrossRef]
- Petrović, S.M.; Savić, S.R.; Dimitrijević, M.L.; Petronijević, Ž.B. The determination of macro and microelements in chamomile teas (Matricaria chammomilla L.). Adv. Technol. 2015, 4, 37–42. [Google Scholar] [CrossRef]
- Chizzola, R.; Michitsch, H.; Mitteregge, M.S. Extractability of selected mineral and trace elements in infusions of chamomile. Int. J. Food Sci. Nutr. 2008, 59, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Mihaljev, Ž.; Živkov-Baloš, M.; Ćupić, Ž.; Jakšić, S. Levels of some microelements and essential heavy metals in herbal teas in Serbia. Acta Pol. Pharm. 2014, 71, 385–391. [Google Scholar] [PubMed]
- Pohl, P.; Dzimitrowicz, A.; Jedryczko, D.; Szymczycha-Madeja, A.; Welna, M.; Jamroz, P. The determination of elements in herbal teas and medicinal plant formulations and their tisanes. J. Pharm. Biomed. Anal. 2016, 130, 326–335. [Google Scholar] [CrossRef]
- Inouye, S.; Yamaguchi, H.; Takizawa, T. Screening of the Antibacterial Effects of a Variety of Essential Oils on Respiratory Tract Pathogens, Using a Modified Dilution Assay Method. J. Infect. Chemother. 2001, 7, 251–254. [Google Scholar] [CrossRef]
- Patra, A.K. An Overview of Antimicrobial Properties of Different Classes of Phytochemicals. In Dietary Phytochemicals and Microbes; Springer: Dordrecht, The Netherlands, 2012; pp. 1–32. [Google Scholar]
- Bawazeer, S.; Rauf, A. In Vitro Antibacterial and Antifungal Potential of Amyrin-Type Triterpenoid Isolated from Datura metel Linnaeus. Bio. Med. Res. Int. 2021, 2021, 1543574. [Google Scholar] [CrossRef]
- Han, G.; Lee, D.G. Antibacterial Mode of Action of β-Amyrin Promotes Apoptosis-Like Death in Escherichia coli by Producing Reactive Oxygen Species. J. Microbiol. Biotechnol. 2022, 32, 1547–1552. [Google Scholar] [CrossRef]
- Shai, L.J.; McGaw, L.J.; Aderogba, M.A.; Mdee, L.K.; Eloff, J.N. Four pentacyclic triterpenoids with antifungal and antibacterial activity from Curtisia dentata (Burm. f) CA Sm. leaves. J. Ethnopharmacol. 2008, 119, 238–244. [Google Scholar] [CrossRef]
- Kwakman, P.H.S.; Te Velde, A.A.; De Boer, L.; Vandenbroucke-Grauls, C.M.J.; Zaat, S.A.J. Two major medicinal honeys have different mechanisms of bactericidal activit. PLoS ONE 2011, 6, e17709. [Google Scholar] [CrossRef]
- Mizzi, L.; Maniscalco, D.; Gaspari, S.; Chatzitzika, C.; Gatt, R.; Valdramidis, V.P. Assessing the individual microbial inhibitory capacity of different sugars against pathogens commonly found in food systems. Lett. Appl. Microbiol. 2020, 71, 251–258. [Google Scholar] [CrossRef]
- Sak, K.; Nguyen, T.H.; Ho, V.D.; Do, T.T.; Raal, A. Cytotoxic effect of chamomile (Matricaria recutita) and marigold (Calendula officinalis) extracts on human melanoma SK-MEL-2 and epidermoid carcinoma KB cells. Cogent Med. 2017, 4, 1333218. [Google Scholar] [CrossRef]
- Cvetanović, A.; Švarc-Gajić, J.; Mašković, P.; Savić, S.; Nikolić, L. Antioxidant and biological activity of chamomile extracts obtained by different techniques. Perspective of using superheated water for isolation of biologically active compounds. Ind. Crops. Prod. 2015, 65, 582–591. [Google Scholar] [CrossRef]
- Pitchai, D.; Roy, A.; Ignatius, C. In vitro evaluation of anticancer potentials of lupeol isolated from Elephantopus scaber L. on MCF-7 cell line. J. Adv. Pharm. Technol. Res. 2014, 5, 179–184. [Google Scholar] [CrossRef]
- Eldohaji, L.M.; Fayed, B.; Hamoda, A.M. Potential targeting of Hep3B liver cancer cells by lupeol isolated from Avicennia marina. Arch. Pharm. Weinh. 2021, 354, e2100120. [Google Scholar] [CrossRef]
- Malekinejad, F.; Kheradmand, F.; Khadem-Ansari, M.H.; Malekinejad, H. Lupeol synergizes with doxorubicin to induce anti-proliferative and apoptotic effects on breast cancer cells. Daru 2022, 30, 103–115. [Google Scholar] [CrossRef]
- Maiyo, F.; Moodley, R.; Singh, M. Phytochemistry, cytotoxicity and apoptosis studies of b-sitosterol-3-o-glucoside and β -amyrin from Prunus africana. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 105–112. [Google Scholar] [CrossRef]
- Neto, S.F.; Prada, A.L.; Achod, L.D.R.; Torquato, H.F.V.; Lima, C.S.; Paredes-Gamero, E.J.; Silva de Moraes, M.O.; Lima, E.S.; Sosa, E.H.; De Souza, T.P.; et al. α-Amyrin-loaded nanocapsules produce selective cytotoxic activity in leukemic cells. Biomed. Pharmacother. 2021, 139, 111656. [Google Scholar] [CrossRef]
- Herald, T.J.; Gadgil, P.; Tilley, M. High-throughput micro plate assays for screening flavonoid content and DPPH-scavenging activity in sorghum bran and flour. J. Sci. Food Agric. 2012, 92, 2326–2331. [Google Scholar] [CrossRef]
- Jing, L.; Ma, H.; Fan, P.; Gao, R.; Jia, Z. Antioxidant potential, total phenolic and total flavonoid contents of Rhododendron anthopogonoides and its protective effect on hypoxia-induced injury in PC12 cells. BMC Complement. Altern. Med. 2015, 15, 287. [Google Scholar] [CrossRef] [PubMed]
- Kolde, R. Pheatmap: Pretty Heatmaps. R Package, version 1.0.8; R Foundation Statutes: Vienna, Austria, 2015. [Google Scholar]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. Package “factoextra” for R: Extract and Visualize the Results of Multivariate Data Analyses. R Package, version 1.0.3; R Foundation Statutes: Vienna, Austria, 2017. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).