Morphoanatomical Changes in Eucalyptus grandis Leaves Associated with Resistance to Austropuccinia psidii in Plants of Two Ages
Abstract
1. Introduction
2. Materials and Methods
2.1. Morphological Characteristics
2.2. Anatomical Characteristics
2.3. Leaf Differentiation over Time
2.4. Statistical Analysis
3. Results
3.1. Morphological Characteristics
3.2. Anatomical Characteristics
3.3. Period of Leaf Differentiation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alfenas, A.C.; Zauza, E.A.V.; Mafia, R.G.; Assis, T.F. Clonagem e Doenças do Eucalipto, 2nd ed; Editora UFV: Viçosa, Brazil, 2009. [Google Scholar]
- De Moraes Goncalves, J.L.; Alvares, C.A.; Higa, A.R.; Silva, L.D.; Alfenas, A.C.; Stahl, J.; Ferraz, S.F.d.B.; Lima, W.d.P.; Brancalion, P.H.S.; Hubner, A.; et al. Integrating genetic and silvicultural strategies to minimize abiotic and biotic constraints in Brazilian eucalypt plantations. For. Ecol. Manag. 2013, 301, 6–27. [Google Scholar] [CrossRef]
- Stape, J.L.; Binkley, D.; Ryan, M.G. Production and carbon allocation in a clonal Eucalyptus plantation with water and nutrient manipulations. For. Ecol. Manag. 2008, 255, 920–930. [Google Scholar] [CrossRef]
- Silva, P.H.M.; Miranda, A.C.; Moraes, M.L.T.; Furtado, E.L.; Stape, J.L.; Alvares, C.A.; Sentelhas, P.C.; Mori, E.S.; Sebbenn, A.M. Selecting for rust (Puccinia psidii) resistance in Eucalyptus grandis in São Paulo State, Brazil. For. Ecol. Manag. 2013, 303, 91–97. [Google Scholar] [CrossRef]
- Junghans, D.T.; Alfenas, A.C.; Brommonschenkel, S.H.; Oda, S.; Mello, E.J.; Grattapaglia, D. Resistance to rust (Puccinia psidii Winter) in Eucalyptus: Mode of inheritance and mapping of a major gene with RAPD markers. Theor. Appl. Genet. 2003, 108, 175–180. [Google Scholar] [CrossRef]
- Pegg, G.S.; Brawner, J.T.; Lee, D.J. Screening Corymbia populations for resistance to Puccinia psidii. Plant Pathol. 2014, 63, 425–436. [Google Scholar] [CrossRef]
- Harwood, C. New introductions—Doing it right. In Developing a Eucalypt Resource; Walker, J., Ed.; Learning from Australia and elsewhere Wood Technology Research Centre; University of Canterbury: Christchurch, New Zealand, 2011; pp. 125–136. [Google Scholar]
- Zanuncio, A.J.V.; Carvalho, A.G.; Carneiro, A.D.C.O.; Filho, M.T.; Valenzuela, P.; Gacitúa, W.; Colodette, J.L. Anatomical, ultrastructural, physical and mechanical wood properties of two-year-old Eucalyptus grandis × Eucalyptus urophylla clones. Rev. Árvore 2018, 42, e420201. [Google Scholar] [CrossRef]
- Furtado, Q.G.; Castro, H.A.; Pozza, E.A. Variabilidade fisiológica de Puccinia psidii Winter em Eucalyptus grandis e no híbrido urograndis. Summa Phytopathol. 2005, 31, 227–231. [Google Scholar]
- Coutinho, T.A.; Wingfield, M.J.; Alfenas, A.C.; Crous, P.W. Eucalyptus Rust: A disease with the potential for serious international implications. Plant Dis. 1998, 82, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Pérez, C.A.; Wingfield, M.J.; Altier, N.A.; Simeto, S.; Blanchette, R.A. Puccinia psidii infecting cultivated Eucalyptus and native Myrtaceae in Uruguay. Mycol. Prog. 2011, 10, 273–282. [Google Scholar] [CrossRef][Green Version]
- Miranda, A.C.; de Moraes, M.L.T.; Tambarussi, E.V.; Furtado, E.L.; Mori, E.S.; Da Silva, P.H.M.; Sebbenn, A.M. Heritability for resistance to Puccinia psidii Winter rust in Eucalyptus grandis Hill ex Maiden in Southwestern Brazil. Tree Genet. Genomes 2012, 9, 321–329. [Google Scholar] [CrossRef]
- Alves, A.A.; Guimarães, L.M.S.; Chaves, A.R.M.; DaMatta, F.M.; Alfenas, A.C. Leaf gas exchange and chlorophyll a fluorescence of Eucalyptus urophylla in response to Puccinia psidii infection. Acta Physiol. Plant. 2011, 33, 1831–1839. [Google Scholar] [CrossRef]
- Ferreira, F.A. Ferrugem do Eucalipto. Rev. Arvore 1983, 7, 91–109. [Google Scholar]
- Silva-Souza, R.R.; Silva, A.C.; Rodella, R.A.; Serrão, J.E.; Zanuncio, J.C.; Furtado, E.L. Pre-infection stages of Austropuccinia psidii in the epidermis of Eucalyptus hybrid leaves with different resistance levels. Forests 2017, 8, 362. [Google Scholar] [CrossRef]
- Xavier, A.A.; Da Silva, A.C.; Guimarães, L.M.D.S.; Matsuoka, K.; Hodges, C.S.; Alfenas, A.C. Infection process of Puccinia psidii in Eucalyptus grandis leaves of different ages. Trop. Plant Pathol. 2015, 40, 318–325. [Google Scholar] [CrossRef]
- Xavier, A.A.; Alfenas, A.C.; Matsuoka, K.; Hodges, C.S. Infection of resistant and susceptible Eucalyptus grandis genotypes by urediniospores of Puccinia psidii. Australas. Plant Pathol. 2001, 30, 277–281. [Google Scholar] [CrossRef]
- Beresford, R.M.; Shuey, L.S.; Pegg, G.S. Symptom development and latent period of Austropuccinia psidii (myrtle rust) in relation to host species, temperature, and ontogenic resistance. Plant Pathol. 2020, 69, 484–494. [Google Scholar] [CrossRef]
- Develey-Rivière, M.; Galiana, E. Resistance to pathogens and host developmental stage: A multifaceted relationship within the plant kingdom. New Phytol. 2007, 175, 405–416. [Google Scholar] [CrossRef]
- Furtado, G.Q.; Alves, S.A.M.; Carneiro, L.C.; Godoy, C.V.; Massola, N.S., Jr. Influência do estádio fenológico e da idade dos trifólios de soja na infecção de Phakopsora pachyrhizi. Trop. Plant Pathol. 2009, 34, 118–122. [Google Scholar] [CrossRef]
- Boava, L.P.; Kuhn, O.J.; Pascholati, S.F.; Di Piero, R.M.; Furtado, E.L. Atividade de quitinases e peroxidases em folhas de eucalipto em diferentes estágios de desenvolvimento após tratamento com acibenzolar-S-metil (ASM) e inoculação com Puccinia psidii. Trop. Plant Pathol. 2010, 35, 124–128. [Google Scholar] [CrossRef]
- Silva, R.R.; Silva, A.C.; Rodella, R.A.; Marques, M.O.M.; Zanuncio, A.J.V.; Soares, M.A.; Serrão, J.E.; Zanuncio, J.C.; Furtado, E.L. Limonene, a chemical compound related to the resistance of Eucalyptus to Austropuccinia psidii. Plant Dis. 2020, 104, 414–422. [Google Scholar] [CrossRef]
- Bonora, F.S.; Nahrung, H.F.; Hayes, R.A.; Scharaschkin, T.; Pegg, G.; Lee, D.J. Changes in leaf chemistry and anatomy of Corymbia citriodora subsp. variegata (Myrtaceae) in response to native and exotic pathogens. Australas. Plant Pathol. 2020, 49, 641–653. [Google Scholar] [CrossRef]
- Hickey, L.J. Classification of architecture of dicotyledonous leaves. Am. J. Bot. 1973, 60, 17–33. [Google Scholar] [CrossRef]
- Graf, A.B. Exotical horticultural color guide. In Exotica 3: Pictorial Cyclopedia of Exotic Plants; Graf, A.B., Ed.; Roehrs: Rutherford, NJ, USA, 1970; pp. 37–38. [Google Scholar]
- Gerrits, P.O. The Application of Glycolmetacrylate in Histotechnology; Some Fundamental Principles; LeicaGmbH: Wetzlar, Germany, 1991; 80p. [Google Scholar]
- O’Brien, T.P.; Feder, N.E.; Mccully, M.E. Polychromatic staining of plant cell walls by Toluidine Blue O. Protoplasma 1964, 59, 367–373. [Google Scholar] [CrossRef]
- Sneath, P.H.A.E.; Sokal, R.R. Numerical Taxonomy; W.H. Freeman: San Francisco, CA, USA, 1973; 573p. [Google Scholar]
- Hallé, F.; Oldeman, R.A.; Tomlinson, P.B. Tropical Trees and Forest; Springer-Verlag: Berlin/Heidelberg, Germany, 1978; 441p. [Google Scholar]
- Sambugaro, R.; Furtado, E.L.; Rodella, R.A.; Mattos, C.R.R. Anatomia foliar de seringueira (Hevea spp.) e desenvolvimento da infecção por Microcyclus ulei. Summa Phytopathol. 2004, 30, 51–56. [Google Scholar]
- Gasparotto, L.; Pereira, J.C.R. Doenças da Seringueira No Brasil; Embrapa: Brasilia, Brazil, 2012; 255p. [Google Scholar]
- Banniza, S.; Hashemi, P.; Warkentin, T.D.; Vandenberg, A.; Davis, A.R. The relationships among lodging, stem anatomy, degree of lignification, and resistance to mycosphaerella blight in field pea (Pisum sativum). Can. J. Bot. 2005, 83, 954–967. [Google Scholar] [CrossRef]
- Li, W.; Wang, K.; Chern, M.; Liu, Y.; Zhu, Z.; Liu, J.; Zhu, X.; Yin, J.; Ran, L.; Xiong, J.; et al. Sclerenchyma cell thickening through enhanced lignification induced by OsMYB30 prevents fungal penetration of rice leaves. New Phytol. 2020, 226, 1850–1863. [Google Scholar] [CrossRef]
- Crang, R.; Lyons-Sobaski, S.; Wise, R. Parenchyma, collenchyma, and sclerenchyma. In Plant Anatomy (A Concept-Based Approach to the Structure of Seed Plants); Crang, R., Lyons-Sobaski, S., Eds.; Springer-Verlag Press: Berlin/Heidelberg, Germany, 2018; pp. 181–212. [Google Scholar]
- Nyadanu, D.; Akromah, R.; Adomako, B.; Kwoseh, C.; Lowor, S.T.; Dzahini-Obiatey, H.; Akrofi, A.Y.; Owusu Ansah, F.; Assuah, M.K. Histological mechanisms of resistance to black pod disease in cacao (Theobroma cacao L.). J. Plant Sci. 2012, 7, 39–54. [Google Scholar] [CrossRef][Green Version]
- Jerba, V.F.; Rodella, R.A.; Furtado, E.L. Características anatômicas quantitativas e histopatológica foliar de feijoeiro infectado por Glomella cingulata f. sp. phaseoli. Summa Phytopathol. 2005, 31, 334–342. [Google Scholar]
- Metcalfe, C.R.; Chalk, L. Anatomy of Dicotyledons; Clarendon Press: Oxford, UK, 1979; pp. 620–631. [Google Scholar]
- Sarwar, A.; Ali, M. Studies on the leaf epidermis of rice (Oryza sativa L.). Indian J. Agric. Res. 2002, 1, 24–28. [Google Scholar]
- Gui, J.; Shen, J.; Li, L. Functional characterization of evolutionarily divergent 4-coumarate: Coenzyme a ligases in rice. Plant Physiol. 2011, 157, 574–586. [Google Scholar] [CrossRef]
- Santos, I.B.; Lopes, M.D.S.; Bini, A.P.; Tschoeke, B.A.P.; Verssani, B.A.W.; Figueredo, E.F.; Cataldi, T.R.; Marques, J.P.R.; Silva, L.D.; Labate, C.A.; et al. The Eucalyptus cuticular waxes contribute in preformed defense against Austropuccinia psidii. Front. Plant Sci. 2019, 9, 1978. [Google Scholar] [CrossRef] [PubMed]
- Batish, D.R.; Singh, H.P.; Kohli, R.K.; Kaur, S. Eucalyptus essential oil as a natural pesticide. For. Ecol. Manag. 2008, 256, 2166–2174. [Google Scholar] [CrossRef]
- Amorim, E.P.D.R.; De Andrade, F.W.R.; Moraes, E.M.D.S.; Da Silva, J.C.; Lima, R.D.S.; De Lemos, E.E.P. Antibacterial activity of essential oils and extracts on the development of Ralstonia solanacearum in banana seedlings. Rev. Bras. De Frutic. 2011, 33, 392–398. [Google Scholar] [CrossRef]
- Pereira, R.B.; Lucas, G.C.; Perina, F.J.; De Resende, M.L.V.; Alves, E. Potential of essential oils for the control of brown eye spot in coffee plants. Ciência E Agrotecnologia 2011, 35, 115–123. [Google Scholar] [CrossRef]
- Vilela, G.R.; Almeida, G.S.; D’Arce, M.A.B.R.; Moraes, M.H.D.; Brito, J.O.; Silva, M.F.G.F.; Silva, S.C.; Piedade, S.M.S.; Calori-Domingues, M.A.; Gloria, E.M. Activity of essential oil and its major compound, 1,8-cineole, from Eucalyptus globulus Labill., against the storage fungi Aspergillus flavus Link and Aspergillus parasiticus Speare. J. Stored Prod. Res. 2009, 45, 108–111. [Google Scholar] [CrossRef]
- Passador, M.M.; Marubayashi, J.M.; Uzzo, R.P.; Marques, M.O.M.; Conceição, D.M.; da Silva Marques, A.P.; Furtado, E.L. Influence of Mycosphaerella and Teratosphaeria leaf diseases on chemical composition of essential oils of Eucalyptus globulus and effect of these essential oils on ascospores germination. Arch. Microbiol. 2021, 203, 3415–3423. [Google Scholar] [CrossRef] [PubMed]


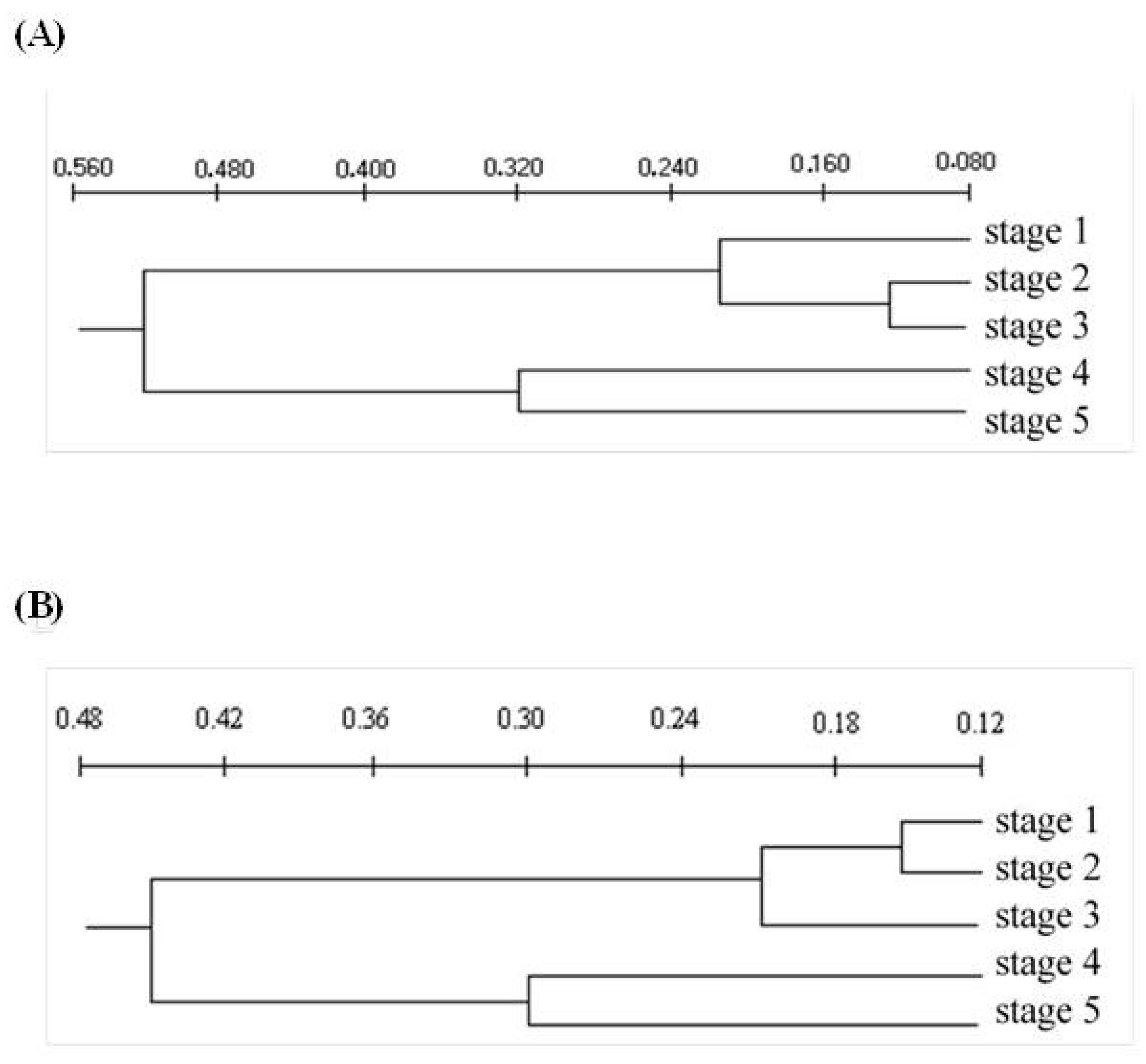

| Leaf Growth Stage | |||||
|---|---|---|---|---|---|
| Characteristic | 1° | 2° | 3° | 4° | 5° |
| Leaf blade length (cm) | 2.0(1.7–2.4) * a *** | 3.8(3.5–4.3) b | 5.7(5.0–6.2) c | 9.0(8.3–9.5) d | 10.2(10.1–0.3) e |
| Leaf blade width (cm) | 0.6(0.5–0.7) * a *** | 1.3(1.2–1.5) b | 2.3(2.2–2.5) c | 4.2(3.7–4.7) d | 5.1(4.8–5.7) e |
| Leaf area (cm2) | 0.5(0.3–0.6) * a *** | 2.9(2.3–3.8) b | 8.0(7.2–8.9) c | 23.5(19.2–27.5) d | 31.8(30.4–33.2) e |
| Diameter of petiole (cm) | 0.6(0.5–0.7) * a *** | 0.9(0.8–1.0) b | 0.96(0.9–1.0) c | 1.31(1.2–1.38) c | 1.5(1.4–1.6) d |
| Leaf shape 1 | Acute 73% ** | Obtuse 73% | Obtuse 73% | Obtuse 60% | Obtuse 73% |
| Leaf color 2 | Ch (7) 71% ** | OG (84) 53% | OG (84) 51% | OG (84) 84% | IG (70) 98% |
| Midrib-Percentage | Six Months | Twenty Months | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Of Tissues (%) | 1° | 2° | 3° | 4° | 5° | 1° | 2° | 3° | 4° | 5° |
| Upper epidermis | 5.5 | 5.0 | 4.0 | 3.0 | 2.2 | 4.9 | 3.8 | 3.0 | 2.6 | 1.9 |
| Lower epidermis | 4.1 | 3.8 | 3.0 | 2.4 | 2.1 | 4.1 | 3.2 | 2.6 | 2.4 | 1.6 |
| Vascular bundles | 28.2 | 33.8 | 35.6 | 39.9 | 36.2 | 31.2 | 40.6 | 43.7 | 49.9 | 47.5 |
| Collenchyma | 0.0 | 9.1 | 9.2 | 16.2 | 12.8 | 6.4 | 7.0 | 7.4 | 12.0 | 7.9 |
| Sclerenchyma-like tissue | 0.0 | 0.0 | 0.0 | 0.0 | 2.3 | 0.0 | 0.0 | 0.0 | 0.0 | 2.3 |
| Parenchyma | 62.1 | 48.3 | 48.2 | 38.5 | 40.5 | 52.8 | 46.1 | 43.4 | 34.3 | 38.1 |
| Total area (×103 µm2) | 207.7 | 274.3 | 413.1 | 479.9 | 756.0 | 220.8 | 287.2 | 382.5 | 505.3 | 881.9 |
| Internerval Region | Six Months | Twenty Months | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Leaf Stage | ||||||||||
| Percentage of Tissues (%) | 1° | 2° | 3° | 4° | 5° | 1° | 2° | 3° | 4° | 5° |
| Upper epidermis | 11.6 | 11.0 | 10.3 | 7.3 | 5.0 | 8.6 | 9.8 | 6.5 | 5.8 | 6.8 |
| Upper palisade parenchyma | 28.2 | 29.5 | 34.4 | 27.2 | 20.5 | 24.7 | 28.8 | 34.3 | 24.4 | 22.6 |
| Spongy parenchyma | 26.8 | 23.4 | 20.3 | 51.6 | 69.7 | 28.0 | 26.8 | 30.3 | 65.0 | 65.2 |
| Lower palisade parenchyma | 25.9 | 28.7 | 28.5 | 7.4 | 0.0 | 30.4 | 28.6 | 19.0 | 0.0 | 0.0 |
| Lower epidermis | 7.4 | 7.3 | 6.3 | 6.6 | 4.8 | 8.5 | 6.1 | 9.9 | 4.4 | 5.5 |
| Leaf thickness (µm) | 179.5 | 187.1 | 227.1 | 232.4 | 297.1 | 170.0 | 207.0 | 215.0 | 243.0 | 248.0 |
| Area of oil cavities (×103 µm2) | 1.7 | 2.1 | 2.9 | 4.5 | 8.0 | 2.1 | 2.2 | 2.4 | 11.0 | 12.9 |
| Total area (×103 µm2) | 95.0 | 97.0 | 115.6 | 122.1 | 153.0 | 83.9 | 101.4 | 118.7 | 122.4 | 131.6 |
| Anatomical Characteristics | Six Months | Twenty Months | ||||||
|---|---|---|---|---|---|---|---|---|
| Y1 | Or. z | Y2 | Or. | Y1 | Or. | Y2 | Or. | |
| Upper epidermis (Mi) | −0.9496 | 5 | 0.2338 | 7 | −0.9210 | 4 | −0.1511 | 9 |
| Lower epidermis (Mi) | −0.9148 | 6 | 0.3071 | 6 | −0.7052 | 9 | −0.5045 | 3 |
| Vascular bundles (Mi) | 0.6240 | 13 | −0.7686 | 1 | 0.8386 | 7 | 0.3474 | 6 |
| Collenchyma (Mi) | 0.7045 | 11 | −0.6859 | 3 | 0.6611 | 12 | 0.6805 | 2 |
| Sclerenchyma-like tissue (Mi) | 0.8494 | 9 | 0.4774 | 4 | 0.6939 | 11 | −0.7090 | 1 |
| Parenchyma (Mi) | −0.6537 | 12 | 0.7302 | 2 | −0.8941 | 6 | −0.3756 | 5 |
| Upper epidermis (IR) | −0.9962 | 1 | 0.0712 | 12 | −0.7053 | 8 | −0.4157 | 4 |
| Palisade parenchyma upper (IR) | −0.8153 | 10 | −0.4040 | 5 | −0.5466 | 13 | 0.3013 | 7 |
| Spongy parenchyma (IR) | 0.9722 | 3 | 0.0792 | 11 | 0.9812 | 2 | 0.0835 | 12 |
| Palisade parenchyma lower (IR) | −0.9649 | 4 | 0.0196 | 13 | −0.9823 | 1 | −0.1490 | 10 |
| Lower epidermis (IR) | −0.8978 | 7 | −0.1351 | 10 | −0.7044 | 10 | −0.1031 | 11 |
| Leaf thickness (IR) | 0.8848 | 8 | 0.1840 | 8 | 0.9147 | 5 | 0.1537 | 8 |
| Area of oil cavities (IR) | 0.9892 | 2 | 0.1354 | 9 | 0.9760 | 3 | 0.0761 | 13 |
| Retained information (%) | 86.44 | 13.56 | 80.50 | 19.50 | ||||
| Accumulated information | 86.44 | 100.00 | 80.50 | 100.00 | ||||
| Plant Age (Months) | Leaf Stage of Change (Days) * | |
|---|---|---|
| 1–3 | 1–5 | |
| 6 | 34 aB | 53 aA |
| 20 | 24 bB | 43 bA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furtado, E.L.; Silva, A.C.d.; Silva, É.A.R.; Rodella, R.A.; Soares, M.A.; Serrão, J.E.; de Pieri, C.; Zanuncio, J.C. Morphoanatomical Changes in Eucalyptus grandis Leaves Associated with Resistance to Austropuccinia psidii in Plants of Two Ages. Plants 2023, 12, 353. https://doi.org/10.3390/plants12020353
Furtado EL, Silva ACd, Silva ÉAR, Rodella RA, Soares MA, Serrão JE, de Pieri C, Zanuncio JC. Morphoanatomical Changes in Eucalyptus grandis Leaves Associated with Resistance to Austropuccinia psidii in Plants of Two Ages. Plants. 2023; 12(2):353. https://doi.org/10.3390/plants12020353
Chicago/Turabian StyleFurtado, Edson Luiz, André Costa da Silva, Érica Araújo Rodrigues Silva, Roberto Antônio Rodella, Marcus Alvarenga Soares, José Eduardo Serrão, Cristiane de Pieri, and José Cola Zanuncio. 2023. "Morphoanatomical Changes in Eucalyptus grandis Leaves Associated with Resistance to Austropuccinia psidii in Plants of Two Ages" Plants 12, no. 2: 353. https://doi.org/10.3390/plants12020353
APA StyleFurtado, E. L., Silva, A. C. d., Silva, É. A. R., Rodella, R. A., Soares, M. A., Serrão, J. E., de Pieri, C., & Zanuncio, J. C. (2023). Morphoanatomical Changes in Eucalyptus grandis Leaves Associated with Resistance to Austropuccinia psidii in Plants of Two Ages. Plants, 12(2), 353. https://doi.org/10.3390/plants12020353








