How Tree Decline Varies the Anatomical Features in Quercus brantii
Abstract
1. Introduction
2. Results
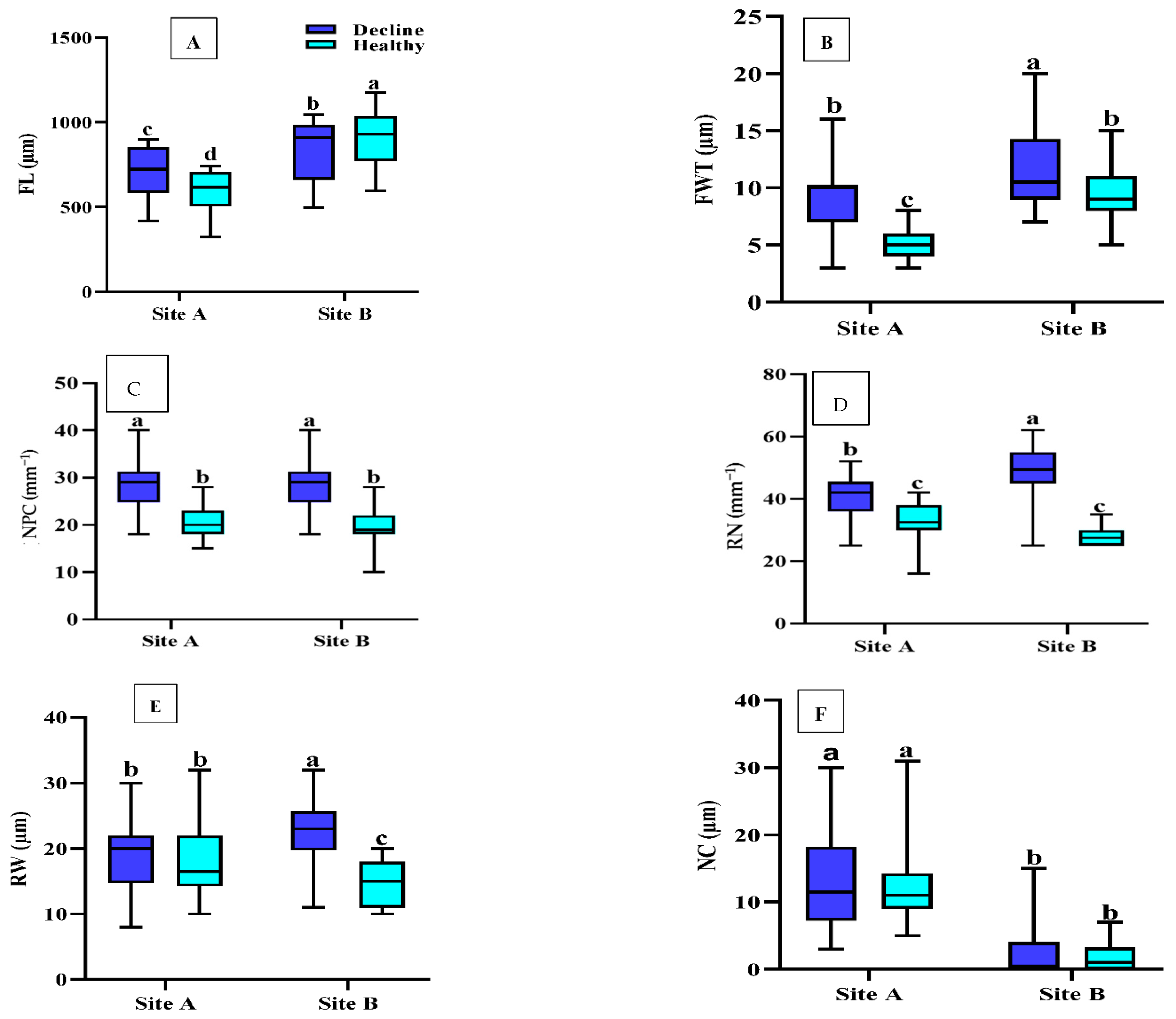
2.1. Fiber Biometric Characterization
2.1.1. Fiber Length (FL)
2.1.2. Fiber Wall Thickness (FWT)
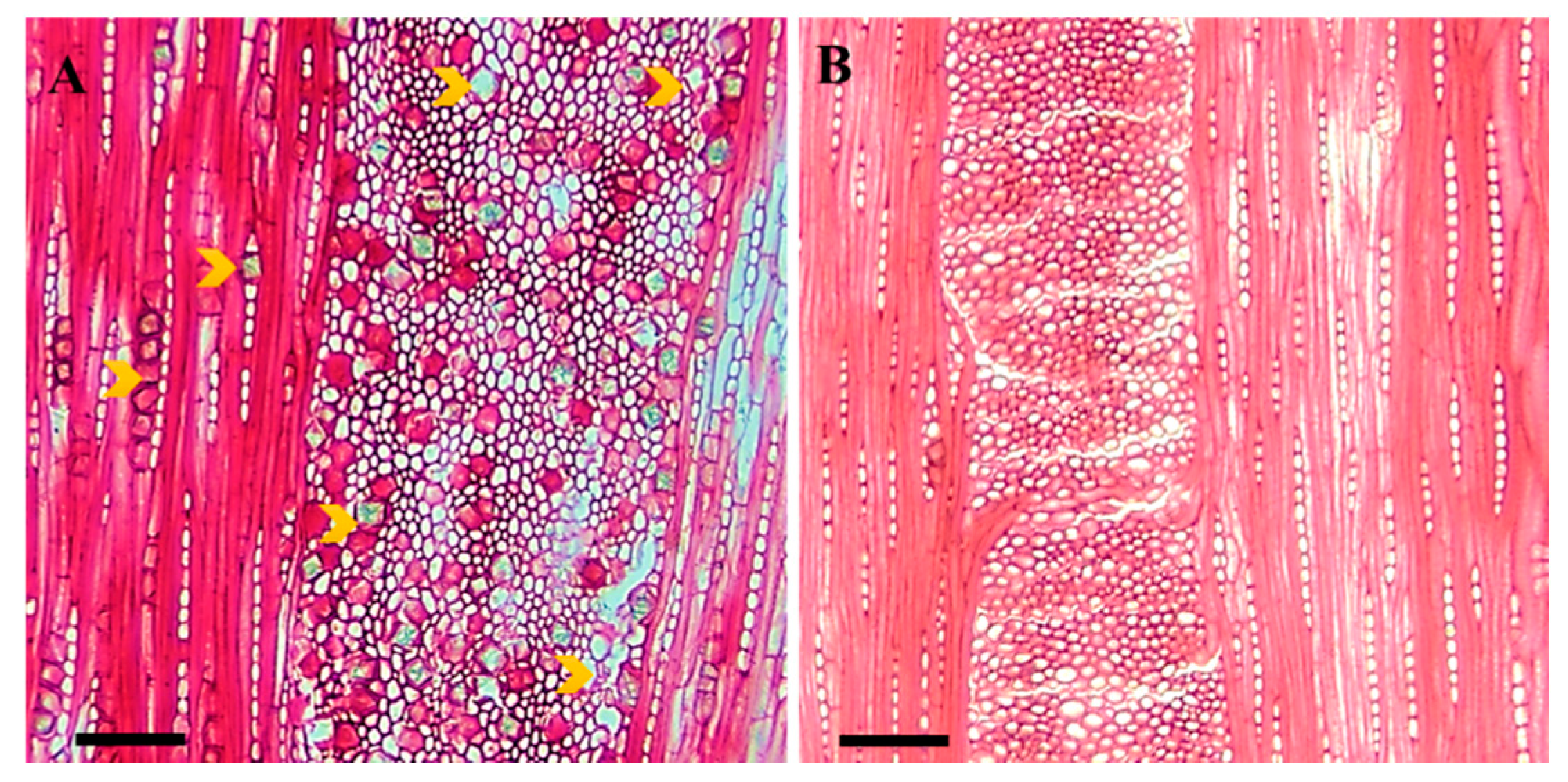
2.1.3. Number of Axial Parenchymal Cells (NPC)
2.1.4. Ray Number (RN)
2.1.5. Ray Width (RW)
2.1.6. Number of Crystals (NC)
2.1.7. Principal Component Analysis (PCA)
3. Discussion
4. Conclusions
5. Material and Methods
5.1. Study Areas
5.2. Sampling Method
5.3. Sample Preparation for Microscopic Investigation
5.4. Wood Sample Processing and Variable Measurement
5.5. Statistical Analysis
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IPCC. Summary for policymakers. In Climate Change: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.T.; et al. A global over-view of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Carnicer, J.; Coll, M.; Ninyerola, M.; Pons, X.; Sanchez, G.; Penuelas, J. Widespread crown condition decline, food web disruption, and amplified tree mortality with increased climate change-type drought. Proc. Natl. Acad. Sci. USA 2011, 108, 1474–1478. [Google Scholar] [CrossRef]
- Ramegowda, V.; Senthil-Kumar, M. The interactive effects of simultaneous biotic and abiotic stresses on plants: Mechanistic understanding from drought and pathogen combination. J. Plant Physiol. 2015, 176, 47–54. [Google Scholar] [CrossRef]
- Garrett, K.; Dendy, S.; Frank, E.; Rouse, M.; Travers, S. Climate change effects on plant disease: Genomes to ecosystems. Annu. Rev. Phytopathol. 2006, 44, 489–509. [Google Scholar] [CrossRef] [PubMed]
- Lindner, M.; Maroschek, M.; Netherer, S.; Kremer, A.; Barbati, A.; Garcia-Gonzalo, J.; Seidl, R.; Delzon, S.; Corona, P.; Kolströma, M.; et al. Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. For. Ecol. Manag. 2010, 259, 698–709. [Google Scholar] [CrossRef]
- McLaughlin, S.B.; Downing, D.J.; Blasing, T.J.; Cook, E.R.; Adams, H.S. An analysis of climate and competition as contributors to decline of red spruce in high elevation Appalachian forests of the eastern United States. Oecologia 1987, 72, 487–501. [Google Scholar] [CrossRef] [PubMed]
- Colangelo, M.; Camarero, J.J.; Borghetti, M.; Gentilesca, T.; Oliva, J.; Redondo, M.A.; Ripullone, F. Drought and Phytophthora are associated with the decline of oak species in southern Italy. Front Plant Sci. 2018, 9, 1595. [Google Scholar] [CrossRef]
- Desprez-Loustau, M.L.; Marçais, B.; Nageleisen, L.M.; Piou, D.; Vannini, A. Interactive effects of drought and pathogens in forest trees. Ann. For. Sci. 2006, 63, 597–612. [Google Scholar] [CrossRef]
- Sagheb-Talebi, K.; Sajedi, T.; Yazdian, F. Research Institute of Forests and Rangelands. For. Res. Div. 2004, 339, 28. [Google Scholar]
- Salehi, A.; Karltun, L.C.; So¨derberg, U.; Eriksson, L.O. Livelihood dependency on woodland resources in southern Zagros, Iran. Casp. J. Environ. Sci. 2010, 8, 181–194. [Google Scholar]
- Tongo, A.; Jalilvand, H.; Hosseininasr, M.; Naji, H.R. Leaf morphological and physiological variations in response to canopy dieback of Persian Oak (Quercus brantii Lindl.). For. Pathol. 2021, 51, 12671. [Google Scholar] [CrossRef]
- Aitken, S.N.; Yeaman, S.; Holliday, J.A.; Wang, T.; Curtis-McLane, S. Adaptation, migration or extirpation: Climate change out comes for tree populations. Evol. Appl. 2008, 1, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Sturrock, R.N.; Frankel, S.J.; Brown, A.V.; Hennon, P.E.; Kliejunas, J.T.; Lewis, K.T.; Worrall, J.J.; Woods, A.J. Climate change and forest diseases. Plant Pathol. 2011, 60, 133–149. [Google Scholar] [CrossRef]
- Baas, P.; Ewers, F.W.; Davis, S.D.; Wheeler, E. A. The evolution of xylem physiology. In The Evolution of Plant Physiology; Hemsley, A.R., Poole, I., Eds.; Academic Press: London, UK, 2004; pp. 273–295. [Google Scholar]
- Bradley, R.S. Natural archives, changing climates. Science 2011, 7, 21–25. [Google Scholar] [CrossRef]
- Pandey, S. Climatic infuence on tree wood anatomy: A review. Wood Sci. 2021, 67, 24. [Google Scholar] [CrossRef]
- Braun, H.J. The significance of the accessory tissues of the hydrosystem for water shifting as the second principle of water ascent, with some thoughts concerning the evolution of trees. IAWA Bull. 1984, 5, 275–294. [Google Scholar] [CrossRef]
- Trockenbrodt, M. Calcium oxalate crystals in the bark of Quercus robur, Ulmus glabra, Populus tremula and Betula pendula. Ann. Bot. 1995, 75, 281–284. [Google Scholar] [CrossRef]
- Pfautsch, S.; Renard, J.; Tjoelker, M.G.; Salih, A. Phloem as capacitor: Radial transfer of water into xylem of tree stems occurs via symplastic transport in ray parenchyma. Plant Physiol. 2015, 167, 963–971. [Google Scholar] [CrossRef]
- Plavcová, L.; Jansen, S. The role of xylem parenchyma in the storage and utilization of non-structural carbohydrates. In Functional and Ecological Xylem Anatomy; Hacke, U.G., Ed.; Springer: Basel, Switzerland, 2015; pp. 209–234. [Google Scholar] [CrossRef]
- Deflorio, G.; Johnson, C.; Fink, S.; Schwarze, F.M.W.R. Decay development in living sapwood of coniferous and deciduous trees inoculated with six wood decay fungi. For. Ecol. Manag. 2008, 255, 2373–2383. [Google Scholar] [CrossRef]
- Nawrot, M.; Pazdrowski, W.; Szymanski, M. Dynamics of heart-wood formation and axial and radial distribution of sapwood and heartwood in stems of European larch (Larix decidua Mill.). J. For. Sci. 2008, 54, 409–417. [Google Scholar] [CrossRef]
- Reiterer, A.; Burgert, I.; Sinn, G.; Tschegg, S. The radial reinforcement of the wood structure and its implication on mechanical and fracture mechanical properties—A comparison between two tree species. J. Mater. Sci. 2002, 37, 935–940. [Google Scholar] [CrossRef]
- Nakata, P.A. Plant calcium oxalate crystal formation, function, and its impact on human health. Front. Biol. 2012, 7, 254–266. [Google Scholar] [CrossRef]
- Franceschi, V.R.; Horner, H.T. Calcium oxalate crystals in plants. Bot. Rev. 1980, 46, 361–427. [Google Scholar] [CrossRef]
- Ruiz, N.; Ward, D.; Saltz, S. Responses of Pancratium sickenbergeri to simulated bulb herbivory: Combining defence and tolerance strategies. J. Ecol. 2002, 90, 472–479. [Google Scholar] [CrossRef]
- Volk, G.M.; Lynch-Holm, V.J.; Kostman, T.A.; Goss, L.J.; Franceschi, V.R. The role of druse and raphide calcium oxalate crystals in tissue calcium regulation in Pistia stratiotes leaves. Plant Biol. 2008, 4, 34–45. [Google Scholar] [CrossRef]
- Serdar, B.; Demiray, H. Calcium oxalate crystal types in three oak species (Quercus L.). Turkey. Turk. J. Biol. 2012, 36, 386–393. [Google Scholar]
- Morris, H.; Plavcová, L.; Cvecko, P.; Fichtler, E.; Gillingham, M.A.F.; Martinez-Cabrera, H.I.; McGlinn, D.J.; Wheeler, E.; Zheng, J.; Ziemińska, K.; et al. A global analysis of parenchyma tissue fractions in secondary xylem of seed plants. New Phytol. 2016, 209, 1553–1565. [Google Scholar] [CrossRef]
- Schwartz, F.W.M.R.; Fink, S.; Deflorio, G. Resistance of parenchyma cells in wood degraded by brown rot fungi. Mycol Prog. 2003, 2, 26–74. [Google Scholar] [CrossRef]
- Soheili, F.; Woodward, S.; Almasi, I.; Abdul-Hamid, H.; Naji, H.N. Variations in Wood Density, Annual Ring Width and Vessel Properties of Quercus brantii Affected by Crown Dieback. Forests 2021, 12, 642. [Google Scholar] [CrossRef]
- Lloret, F.; Martinez-Vilalta, J.; Serra-Diaz, J.M.; Ninyerola, M. Relationship between projected changes in future climatic suitability anddemographic and functional traits of forest tree species in Spain. Clim. Chang. 2013, 120, 449–462. [Google Scholar] [CrossRef]
- Polle, A.; Chen, S.L.; Eckert, C.; Harfouche, A. Engineering Drought Resistance in Forest Trees. Front. Plant Sci. 2019, 9, 1875. [Google Scholar] [CrossRef]
- Fischer, U.; Kucukoglu, M.; Helariutta, Y.; Bhalerao, R.P. The Dynamics of Cambial Stem Cell Activity. Annu. Rev. Plant Biol. 2019, 70, 293–319. [Google Scholar] [CrossRef] [PubMed]
- Fischer, U.; Polle, A. Populus responses to abiotic stress. In Genetics and Genomics of Populus; Jansson, S., Bhalerao, R., Groover, A., Eds.; Springer: Berlin, Germany, 2010; pp. 225–247. [Google Scholar] [CrossRef]
- Nola, P.; Bracco, F.; Assini, S.; von Arx, G.; Castagneri, D. Xylem anatomy of Robinia pseudoacacia L. and Quercus robur L. is diferently afected by climate in a temperate alluvial forest. Ann. For. Sci. 2020, 77, 8. [Google Scholar] [CrossRef]
- Seifert, G.J.; Blaukopf, C. Irritable walls: The plant extracellular matrix and signaling. Plant Physiol. 2010, 153, 467–478. [Google Scholar] [CrossRef]
- Kesten, C.; Menna, A.; Sánchez-Rodríguez, C. Regulation of cellulose synthesis in response to stress. Curr. Opin. Plant Biol. 2017, 40, 106–113. [Google Scholar] [CrossRef]
- Gaffal, K.H.; Mohnen, D. The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr. Res. 2009, 344, 1879–1900. [Google Scholar] [CrossRef]
- Zweifel, R.; Zimmermann, L.; Zeugin, F.; Newbery, D.M. Intra annual radial growth and water relations of trees: Implications towards a growth mechanism. J. Exp. Bot. 2006, 57, 1445–1459. [Google Scholar] [CrossRef] [PubMed]
- Steppe, K.; Lemeur, R. Effects of ring-porous and diffuse-porous stem wood anatomy on the hydraulic parameters used in a water flow and storage model. Tree Physiol. 2007, 27, 43–52. [Google Scholar] [CrossRef]
- Hacke, U. Functional and Ecological Xylem Anatomy; Springer: Berlin/Heidelberg, Germany, 2015; ISBN 978-3-319-15783-2. [Google Scholar]
- Wildhagen, H.; Paul, S.; Allwright, M.; Smith, H.K.; Malinowska, M.; Schnabel, S.K.; Paulo, M.J.; Cattonaro, F.; Vendramin, V.; Scalabrin, S.; et al. Genes and Gene Clusters Related to Genotype and Drought-Induced Variation in Saccharification Potential, Lignin Content and Wood Anatomical Traits in Populus Nigra. Tree Physiol. 2018, 38, 320–339. [Google Scholar] [CrossRef]
- Yu, D.; Janz, D.; Zienkiewicz, K.; Herrfurth, C.; Feussner, I.; Chen, S.; Polle, A. Wood Formation under Severe Drought Invokes Adjustment of the Hormonal and Transcriptional Landscape in Poplar. Int. J. Mol. Sci. 2021, 22, 9899. [Google Scholar] [CrossRef]
- Fonti, P.; García-González, I. Earlywood vessel size of oak as potential proxy for spring precipitation in mesic sites. J. Biogeogr. 2008, 35, 2249–2257. [Google Scholar] [CrossRef]
- Eilmann, B.; Weber, P.; Rigling, A.; Eckstein, D. Growth reactions of Pinus sylvestris L. and Quercus pubescens Willd. to drought years at a xeric site in Valais, Switzerland. Dendrochronologia 2006, 23, 121–132. [Google Scholar] [CrossRef]
- Roushani Nia, F.; Naji, H.R.; Bazgir, M.; Naderi, M. Effect of Simulated Dust Storm on some Bio-chemical features of Persian Oak (Quercus brantii Lindl.). Envirn. Eros. Res. 2018, 29, 59–73. (In Farsi) [Google Scholar]
- Naji, H.R.; Taher Pour, M. The effect of simulated dust storm on wood development and leaf stomata in Quercus brantii L. Desert 2019, 24, 43–49. [Google Scholar] [CrossRef]
- Aloni, R.; Zimmermann, M.H.Z. The control of vessel size and density along the plant axis. A new hypothesis. Differentiation 1983, 24, 203–208. [Google Scholar] [CrossRef]
- Evert, R.F.; Eichhorn, S.E. Esau’s Plant Anatomy: Meristems, Cells, and Tissues of the Plant Body: Their Structure, Function, and Development; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2006. [Google Scholar]
- Wheeler, E.A.; Bass, P.; Rodgers, S. Variations in dicot wood anatomy: A global analysis based on the Inside Wood database. IAWAJ. 2007, 28, 229–248. [Google Scholar] [CrossRef]
- Hölttä, T.; Vesala, T.; Perämäki, M.; Nikinmaa, E. Refilling of embolised conduits as a consequence of ‘Munch water’ circulation. Funct. Plant Biol. 2006, 33, 949–959. [Google Scholar] [CrossRef] [PubMed]
- von Arx, G.; Arzac, A.; Olano, J.M.; Fonti, P. Assessing Conifer Ray Parenchyma for Ecological Studies: Pitfalls and Guidelines. Front. Plant Sci. 2015, 6, 1016. [Google Scholar] [CrossRef]
- Safdari, V.; Golchinfar, M. Comparative wood anatomy of Wyche Elm, English Elm, Caucasian Elm and Hackberry. Iran. J. Wood Pap. Sci. Res. 2011, 26, 564–578. (In Farsi) [Google Scholar]
- Torkaman, J.; Ghodskhah Daryaee, M.; Zolghadry, S.H. The Role of Xylem Vessel Size and Ray Traits in Dutch Elm Disease Frequency in Ulmaceae. J. For. Wood Prod. 2014, 67, 453–462. (In Farsi) [Google Scholar]
- Martin, J.A.; Solla, A.; Esteban, L.G. Borderd pit and ray morphology involment in elm resistance to ophiostoma novo-ulmi. Can. J. For. Res. 2009, 39, 420–429. [Google Scholar] [CrossRef]
- Schenk, H.J.; Espino, S.; Goedhart, C.M.; Nordenstahl, M.; Martinez-Cabrera, H.I.; Jones, C.S. Hydraulic integration and shrub growth form linked across continental aridity gradients. Proc. Natl. Acad. Sci. USA 2008, 105, 11248–11253. [Google Scholar] [CrossRef]
- Knipfer, T.; Cuneo, I.F.; Brodersen, C.R.; McElrone, A.J. In-situ visualization of the dynamics in xylem embolism formation and removal in the absence of root pressure: A study on excised grapevine stems. Plant Physiol. 2016, 171, 1024–1036. [Google Scholar] [CrossRef]
- Jupa, R.; Plavcová, L.; Gloser, V.; Jansen, S. Linking xylem water storage with anatomical parameters in fivetemperate trees pecies. Tree Physiol. 2016, 36, 756–769. [Google Scholar] [CrossRef]
- Esau, K. Plant Anatomy; McGraw-Hill: New York, NY, USA, 1965; p. 567. [Google Scholar]
- Biggs, A.R. Occurrence and location of suberin in wound reaction zones in xylem of 17 tree species. Phytopathology 1987, 77, 718–725. [Google Scholar] [CrossRef]
- Schilling, J.S.; Jellison, J. Oxalate regulation by two brown rot fungi decaying oxalate-amended and non-amended wood. Holzforschung 2005, 59, 681–688. [Google Scholar] [CrossRef]
- Hatakka, A.; Hammel, K.E. Fungal Biodegradation of Lignocelluloses. In Industrial Applications. The Mycota; Hofrichter, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 10. [Google Scholar] [CrossRef]
- Penninckx, V.; Glineur, S.; Gruber, W.; Herbauts, J.; Meerts, P. Radial variations in wood mineral element concentrations: A comparison of beech and pedunculate oak from the Belgian Ardennes. Ann. For. Sci. 2001, 58, 253–260. [Google Scholar] [CrossRef]
- Ghazanfari, H.; Namiranian, M.; Sobhani, H.; Marvi Mohajer, R. Traditional forest management and its application to encourage public participation for sustainable forest management in the northern Zagros Mountains of Kurdistan Province, Iran. Scand J Forest Res. 2004, 19, 65–71. [Google Scholar] [CrossRef]
- Ilam Meteorological Bureau. Available online: http://www.ilammet.ir/ (accessed on 20 August 2018).
- Menati, T.; Bazgir, M.; Rostaminya, M.; Mahdavi, A. Chemical and physical characteristics of oak forest soils in different climates in Ilam Province. Iran. J. For. 2019, 10, 449–460. [Google Scholar]
- Ruzin, S.E. Plant Microtechnique and Microscopy; Oxford University Press: New York, NY, USA, 1999; 336p. [Google Scholar]
- Gartner, H.; Schweingruber, F.H. Microscopic Preparation Techniques for Plant Stem Analysis; Verlag Dr. Kessel: Remagen, Germany, 2013; p. 78. [Google Scholar]
- Camargo, M.A.B.; Marenco, R.A. Density, size and distribution of stomata in 35 rainforest tree species in Central Amazonia. Acta Amaz. 2011, 41, 205–212. [Google Scholar] [CrossRef]
- Meng, Q.; Fu, F.; Wang, J.; He, T.; Jiang, X.; Zhang, Y.; Yin, Y.; Li, N.; Guo, J. Ray Traits of Juvenile Wood and Mature Wood: Pinus massonia and Cunninghamia lanceolata. Forests 2021, 12, 1277. [Google Scholar] [CrossRef]


| Mean Square | |||||||
|---|---|---|---|---|---|---|---|
| Source of Variation | df | FL (µm) | FWT (µm) | NPC (mm−1) | RN (mm−1) | RW (μm) | NC (mm−2) |
| Decline | 1 | 0.206 ns | 37.899 ** | 113.733 ** | 142.474 ** | 21.338 ** | 0.500 ns |
| Sites | 1 | 67.690 ** | 43.954 ** | 0.308 ns | 2.882 ns | 1.125 ns | 135.380 ** |
| Decline × Sites | 1 | 11.384 ** | 4.686 * | 0.308 ns | 18.871 ** | 15.213 ** | 0.30 ns |
| Error | 116 | 23,817,771 | 7.283 | 18.318 | 46.643 | 25.795 | 22.830 |
| Trait | Component | |
|---|---|---|
| Axis 1 | Axis 2 | |
| FL (µm) | −0.746 ** | 0.167 ns |
| FWT (µm) | −0.644 ** | −0.315 * |
| NPC (mm−1) | −0.237 ns | 0.770 ** |
| RN (mm−1) | −0.280 ns | −0.748 ** |
| RW (µm) | −0.170 ns | −0.643 ** |
| NC (mm−2) | 0.804 ** | −0.291 ns |
| Eigenvalues | 2.431 | 1.962 |
| % of variance | 34.730 | 28.031 |
| Forest Site | Altitude (m) | Longitude | Latitude | A.M.P (mm) | A.M.T (°C) | Max. T | Min. T | Aridity I. | Soil Type |
|---|---|---|---|---|---|---|---|---|---|
| Dareh-shahr (A) | 933 | 33°3′30′′ N | 47°19′30′′ E | 465.1 | 19.5 | 44.7 | 2.6 | Semi-arid (16.48) | Sand-loamy |
| Ilam (B) | 1680 | 33°43′03′′ N | 46°14′36′′ E | 582.2 | 16.9 | 36.8 | 0.6 | Mediterranean (21.65) | Loam-clay-sandy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soheili, F.; Abdul-Hamid, H.; Almasi, I.; Heydari, M.; Tongo, A.; Woodward, S.; Naji, H.R. How Tree Decline Varies the Anatomical Features in Quercus brantii. Plants 2023, 12, 377. https://doi.org/10.3390/plants12020377
Soheili F, Abdul-Hamid H, Almasi I, Heydari M, Tongo A, Woodward S, Naji HR. How Tree Decline Varies the Anatomical Features in Quercus brantii. Plants. 2023; 12(2):377. https://doi.org/10.3390/plants12020377
Chicago/Turabian StyleSoheili, Forough, Hazandy Abdul-Hamid, Isaac Almasi, Mehdi Heydari, Afsaneh Tongo, Stephen Woodward, and Hamid Reza Naji. 2023. "How Tree Decline Varies the Anatomical Features in Quercus brantii" Plants 12, no. 2: 377. https://doi.org/10.3390/plants12020377
APA StyleSoheili, F., Abdul-Hamid, H., Almasi, I., Heydari, M., Tongo, A., Woodward, S., & Naji, H. R. (2023). How Tree Decline Varies the Anatomical Features in Quercus brantii. Plants, 12(2), 377. https://doi.org/10.3390/plants12020377






