Application of Phytosociological Information in the Evaluation of the Management of Protected Areas
Abstract
:1. Introduction
2. Results
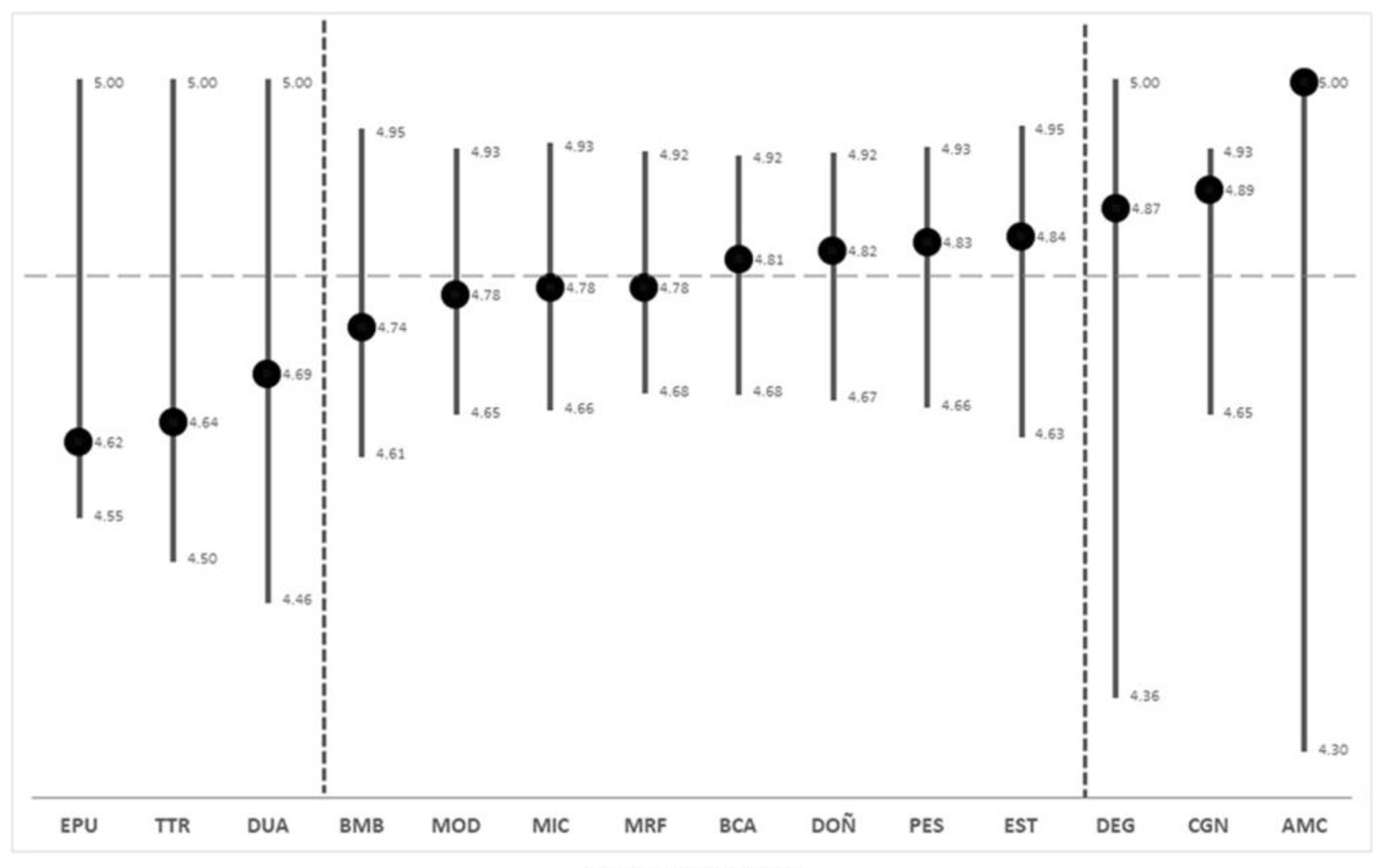
2.1. Syntaxonomic Distinctness (STD)
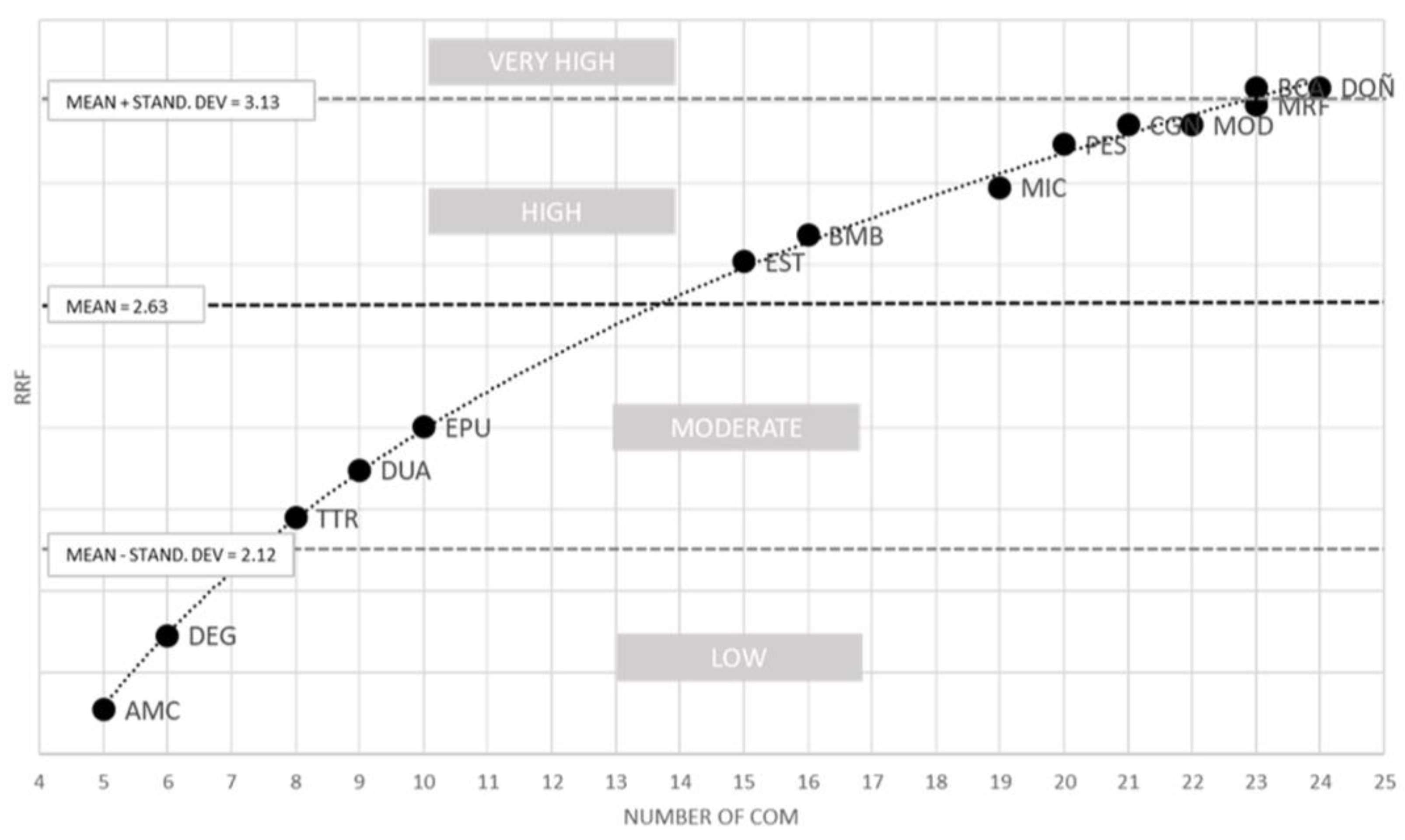
2.2. Rarefaction Index (RRF)
2.3. Priority Conservation Areas (PCA)
2.4. Correlation of the Legal Protection Index and DRA
- (1)
- those residuals whose absolute value is >1 (red bars) corresponded to PAs with a significant divergence between LPI and DRA (DEG, BCA, BMB and EPU);
- (2)
- a central range with absolute values between 0–0.50 (green bars), corresponding to those PAs where LPI and DRA showed a significant convexity (DOÑ, AMC, MRF and MIC);
- (3)
- finally, those with absolute values between 0.51–1 (orange bars), which required a specific interpretation through the individual analysis of the ARD components (CGN, DUA, PES, MOD, EST and TTR).
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Method
4.3. Syntaxonomic Distinctness (STD)
4.4. Rarefaction (RRF)
4.5. Priority Conservation Areas (PCA)
4.6. Calculation of the DRA Index
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Syntaxonomic Scheme of the Protected Areas [25]. Abbreviations of Plant Communities
| Cakiletea maritimae Tüxen & Preising ex Br.-Bl. & Tüxen 1952 Cakmar |
| Cakiletalia integrifoliae Tüxen ex Oberdorfer 1949 corr. Rivas-Martínez, Costa & Loidi 1992 Caklia |
| Cakilion maritimae Pignatti 1953 Cakion |
| Hypochoerido radicatae-Glaucietum flavi Rivas Goday & Rivas-Martínez 1958 HypGla |
| Salsolo kali-Cakiletum maritimae Costa & Mansanet 1981 SalCak |
| Cisto-Lavanduletea stoechadis Br.-Bl. in Br.-Bl., Molinier & Wagner 1940 CisLav |
| Stauracantho genistoidis-Halimietalia calycini Rivas-Martínez, Lousã, T.E. Díaz, Fernández-González & J.C. Costa 1990 StaHal |
| Coremation albi Rothmaler 1943 Coralb |
| Cisto salviifolii-Ulicetum australis A.V. Pérez, Nieto & Cabezudo 1993 CisSal |
| Fumano juniperinae-Cistetum crispi Sánchez & Galán 1996 FumCis |
| Halimio halimifolii-Stauracanthetum genistoidis Rivas-Martínez 1979 HalSta |
| Thymo albicantis-Stauracanthetum genistoidis Galán, I. Sánchez & Vicente 1997 ThyAlb |
| Crithmo maritimi-Limonietea Br.-Bl. in Br.-Bl., Roussine & Nègre 1952 Cretea |
| Crithmo maritimi-Limonietalia Molinier 1934 Cralia |
| Crithmo maritimi-Daucion halophili Rivas-Martínez, Lousã, T. E. Díaz, Fernández-González & J. C. Costa 1990 Cdnion |
| Limonietum emarginati Asensi 1984 Limemar |
| Crithmo maritimi-Limonion pseudominuti Molinier 1934Clnion |
| Crithmo maritimi-Limonietum malacitani Diez Garretas 1977 corr. Diez Garretas 1981 CriLim |
| Limonio cossoniani-Lycietum intricate Esteve 1976 corr. Alcaraz, P. Sánchez, De la Torre, Ríos & J. Alvarez 1991 LimLyc |
| Cytisetea-Scopario striati Rivas-Martínez 1974 CytSco |
| Cytisetalia scopario-striati Rivas-Martínez 1974 Cyalia |
| Retamion monospermae Rivas-Martínez & Cantó 2002 Retmon |
| Pycnocomono rutaefolii-Retametum monospermae Pérez Chiscano 1983 PycRet |
| Euphorbio paraliae-Ammophiletea australis Géhu & Rivas-Martínez 2011 EupAmm |
| Ammophiletalia australis Br.-Bl. 1933 Amalia |
| Ammophilion australis Br.-Bl. 1921 Amlion |
| Loto cretici-Ammophiletum australis Rivas-Martínez 1965 corr. Rivas-Martínez, T.E. Díaz, Fernández-González, Izco, Loidi, Lousã & Penas 2002 LotAmm |
| Honckenyo peploidis-Elytrigion boreoatlanticae Tüxen in Br.-Bl. & Tüxen 1952 HonEly |
| Cypero mucronati-Elytrigietum junceae Br.-Bl. 1933 CypEly |
| Euphorbio paraliae-Elytrigietum boreoatlanticae Tüxen in Br.-Bl. & Tüxen 1952 EupEly |
| Sporobolion arenarii (Géhu & Géhu-Franck ex Géhu & Biondi 1994) Rivas-Martínez & Cantó 2002 Spoare |
| Eryngio maritimi-Sporoboletum arenarii (Arenes ex Géhu & Biondi 1994) Rivas-Martínez & Cantó 2002 ErySpo |
| Sporoboletum arenarii Rothmaler 1943 Spoari |
| Crucianelletalia maritimae Sissing 1974 Crulia |
| Crucianellion maritimae Rivas Goday & Rivas-Martínez 1959 Cruion |
| Loto cretici-Crucianelletum maritimae Alcaraz, T.E. Díaz, Rivas-Martínez & P. Sánchez 1989 LotCru |
| Helichrysion picardii (Rivas-Martínez, Costa & Izco in Rivas-Martínez, Lousã, T.E. Díaz, Fernández-González & J.C. Costa 1990) Rivas-Martínez, Fernández-González & Loidi 1999 Hesion |
| Artemisio crithmifoliae-Armerietum pungentis Rivas Goday & Rivas-Martínez 1959 ArtArm |
| Halodulo wrightii-Thalassietea testudinum Den Hartog ex Rivas-Martínez, Fernández Gonzalez & Loidi 1999 HalTha |
| Thalassio-Syringodietalia filiformis Borhidi, Muñiz & Del Risco in Borhidi 1996 ThaSyr |
| Syringodio-Thalassion testudinum Borhidi 1996 SyrTha |
| Cymodoceetum nodosae Feldmann 1937 Cymnod |
| Juncetea maritimi Br.-Bl. in Br.-Bl., Roussine & Nègre 1952 Juetea |
| Juncetalia maritimi Br.-Bl. in Br.-Bl., Roussine & Nègre 1952 Jualia |
| Juncion maritimi Br.-Bl. in Br.-Bl., Roussine & Nègre 1952 Jucion |
| Elymo elongati-Juncetum maritimi Alcaraz, Garre, Peinado & Martínez Parras 1986 ElyJun |
| Polygono equisetiformis-Juncetum maritimi J. C. Costa in J. C. Costa, Lousã & Espírito Santo 1997 PolJun |
| Magnocarici elatae-Phragmitetea australis Klika in Klika & V. Novák 1941 MagPhr |
| Bolboschoenetalia compacti Dahl & Hadac 1941 corr. Rivas-Martínez, Costa, Castroviejo & E. Valdés 1980 Boalia |
| Bolboschoenion compacti Dahl & Hadac 1941 corr. Rivas-Martínez, Costa, Castroviejo & E. Valdés 1980 Bolion |
| Bolboschoeno maritimi-Schoenoplectetum litoralis Br.-Bl. in Br.-Bl., Roussine & Nègre 1952 corr. Rivas-Martínez, Costa, Castroviejo & E. Valdés 1980 BolJun |
| Nerio-Tamaricetea Br.-Bl. & O. Bolòs 1958 NerTam |
| Tamaricetalia Br.-Bl. & O. Bolós 1958 Tamlia |
| Tamaricion africanae Br.-Bl. & O. Bolós 1958 Tamafr |
| Polygono equisetiformis-Tamaricetum africanae Rivas-Martínez & Costa in Rivas- Martínez, Costa, Castroviejo & E. Valdés 1980 PolTam |
| Tamaricion boveano-canariensis Izco, Fernández-González & A. Molina 1984 Tambov |
| Inulo crithmoidis-Tamaricetum boveanae Izco, Fernández-González & A. Molina 1984 InuTam |
| Parietarietea judaicae Rivas-Martínez in Rivas Goday 1964 Paetea |
| Parietarietalia judaicae (Rivas-Martínez 1960) Rivas Goday 1964 Paalia |
| Lavaterion maritimae Rivas-Martínez & Cantó 2002 Lavmar |
| Rosmarinetum tomentosi F. Casas & M. López in F. Casas 1972 Rostom |
| Pegano harmalae-Salsoletea vermiculatae Br.-Bl. & O. Bolós 1958. PegSal |
| Salsolo vermiculatae-Peganetalia harmalae Br.-Bl. & O. Bolòs 1954 SalPeg |
| Salsolo oppositifoliae-Suaedion mollis Rigual 1972 SalSua |
| Frankenio laevis-Salsoletum vermiculatae J. C. Costa in J. C. Costa, Lousã & Espírito Santo 1997 FraSal |
| Withanio frutescentis-Lycietum intricate Alcaraz, P. Sánchez, De la Torre, Ríos & J. Alvárez 1991 WhiLyc |
| Posidonietea Den Hartog 1976 Poetea |
| Posidonietalia Den Hartog 1976 Poalia |
| Posidonion Br.-Bl., Roussine & Nègre 1952 Ponion |
| Posidonietum oceanicae Funk 1927 Posoce |
| Quercetea ilicis Br.-Bl. ex A. & O. Bolòs 1950 Quetea |
| Pistacio lentisci-Rhamnetalia alaterni Rivas-Martínez 1975 PisRha |
| Asparago albi-Rhamnion oleoidis Rivas Goday ex Rivas-Martínez 1975 |
| AspRha |
| Cneoro tricocci-Buxetum balearicae Rivas Goday & Rivas-Martínez 1969 CneBux |
| Juniperion turbinatae Rivas-Martínez 1975 corr. Rivas-Martínez 1987 Juntur |
| Osyrio quadripartitae-Juniperetum turbinatae Rivas-Martínez ex Rivas-Martínez, Lousã, T.E. Díaz, Fernández-González & J.C. Costa 1990 OsyJun |
| Chamaeropo humilis-Juniperetum navicularis Sánchez García, Sánchez Gullón, Linares Perea & Galán de Mera 2014 ChaJun |
| Rhamno angustifoliae-Juniperetum turbinatae Rivas-Martínez ex Freitag 1971 RanJun |
| Rhamno oleoidis-Juniperetum macrocarpae Rivas-Martínez 1965 RolJun |
| Periplocion angustifoliae Rivas-Martínez 1975 Perang |
| Ziziphetum loti Rivas Goday & Bellot 1944 Zizlot |
| Rubio longifoliae-Coremation albi Rivas-Martínez in Rivas-Martínez, Costa, Castroviejo & E. Valdés 1980 Rution |
| Rubio longifoliae-Corematetum albi Rivas-Martínez in Rivas-Martínez, Costa, Castroviejo & E. Valdés 1980 RubCor |
| Querco rotundifoliae-Oleion sylvestris Barbéro, Quézel & Rivas-Martínez in Rivas-Martínez, Costa & Izco 1986 QueOle |
| Aro neglecti-Quercetum suberis Rivas-Martínez & Díez-Garretas 2011 AroQue |
| Rosmarinetea officinalis Rivas-Martínez, T.E. Díaz, F. Prieto, Loidi & Penas 2002 Rosoff |
| Anthyllidetalia terniflorae Rivas Goday, Rigual, Esteve, Borja & Rivas-Martínez in Rivas Goday & Borja 1961 Antter |
| Thymo moroderi-Sideritidion leucanthae O. Bolòs 1957 corr. Alcaraz, T.E. Díaz, Rivas-Martínez & P. Sánchez 1989 ThySid |
| Teucrio belionis-Helianthemetum scopulorum Peinado, Martínez Parras, Alcaraz, Garre & de la Cruz 1985 TeuHel |
| Saginetea maririmae Westhoff, Van Leeuwen & Adriani 1962 Sagmar |
| Frankenietalia pulverulentae Rivas-Martínez ex Castroviejo & Porta 1976 Fralia |
| Frankenion pulverulentae Rivas-Martínez ex Castroviejo & Porta 1976 Fraion |
| Parapholido incurvae-Frankenietum pulverulentae Rivas-Martínez ex Castroviejo & Porta 1976. ParFra |
| Hordeion marini Ladero, F. Navarro, C. Valle, Marcos, Ruiz & M. T. Santos 1984 Hormar |
| Hainardio cylindricae-Lophochloetum hispidae Rivas-Martínez, Costa, Castroviejo & E. Valdés 1980 HaiLop |
| Sarlicornietea fruticosae Br.-Bl. & Tüxen ex A. & O. Bolós 1950 Saetea |
| Limonietalia Br.-Bl. & O. Bolós 1958 Lialia |
| Limoniastrion monopetali Pignatti 1953 Limmon |
| Polygono equisetiformis-Limoniastretum monopetali Rivas-Martínez & Costa in Rivas- Martínez, Costa, Castroviejo & E. Valdés 1980 PolLim |
| Limonion confusi (Br.-Bl. 1933) Rivas-Martínez & Costa 1984 Limcon |
| Limonietum ferulacei Rothmaler 1943 Limfer |
| Lygeo sparti-Limonion furfuracei Rigual 1972 LygLim |
| Limonietum angustebracteato-delicatuli Rivas-Martínez & Alcaraz in Alcaraz 1984 Limang |
| Arthrocnemo macrostachyi-Suaedetalia braun-blanquetii Rufo, Fuente & Sánchez Mata 2016 ArtSua |
| Arthrocnemion glauci (Rivas-Martínez in Rivas-Martínez & al. 1980) Rivas-Martínez & Costa 1984 Artgla |
| Arthrocnemo macrostachyi-Sarcocornietum hispanicae Fuente, Rufo, Teijeiro & Sánchez-Mata 2013 ArtSar |
| Inulo crithmoidis-Arthrocnemetum macrostachyi Fontes ex Géhu & Géhu Franck 1977 InuArt |
| Salicornietalia fruticosae Br.-Bl. 1933 Sarlia |
| Sarlicornion fruticosae Br.-Bl. 1933 Sarion |
| Limonio cossoniani-Sarcocornietum lagascae M.A. Alonso & De la Torre 2002 corr. Rufo et al. 2016 LimSar |
| Sarcocornio pruinosae-Halimionetalia portulacoidis Rufo, Fuente & Sánchez Mata 2016 SarHal |
| Halimionion portulacoidis Géhu 1976 Halpor |
| Cistancho phelypaeae-Sarcocornietum pruinosae Géhu ex Géhu & Géhu-Franck 1977 corr. Rufo et al. 2016 CisSar |
| Sarcocornio perennis-Puccinellietum ibericae J. C. Costa in J. C. Costa, Lousã & Espírito Santo 1997 corr. Rivas-Martínez, Fernández-González, Loidi, Lousã, Penas & Izco 2002 SarPuc |
| Sarcocornion alpini (Rivas-Martínez et al. 1990) Brullo et al. 2002 Sarcon |
| Halimiono portulacoidis-Sarcocornietum alpini Rivas-Martínez & Costa 1984 HalSar |
| Sarcocornietum alpini Br.-Bl. 1933 corr. Rivas-Martínez, Lousã, T. E. Díaz, Fernández González & J. C. Costa 1990 Saralp |
| Suaedion verae (Rivas-Martínez, Lousã, T. E. Díaz, Fernández González & J. C. Costa 1990) Rivas-Martínez, Fernández González & Loidi 1999 Suaver |
| Cistancho phelypaeae-Suaedetum verae Géhu & Géhu-Franck 1977 CisSua |
| Frankenio corymbosae-Suaedetum verae Alonso & De la Torre 2002 FraSua |
| Spartinetea maritimae Tüxen in Beeftink 1962 Spetea |
| Spartinetalia maritimae Conard 1935 Spalia |
| Spartinion maritimae Beeftink & Géhu 1973 Spaion |
| Spartinetum densiflorae Rivas-Martínez, Costa, Castroviejo & E. Valdés 1980 Spaden |
| Spartinetum maritimae Béguinot ex Corillion 1953 Spamar |
| Thero-Salicornietea Tüxen in Tüxen & Oberdorfer ex Géhu & Géhu-Frank 1984 Thetea |
| Thero-Salicornietalia Tüxen in Tüxen & Oberdorfer ex Géhu & Géhu-Frank 1984 Tsalia |
| Salicornion patulae Géhu & Géhu-Frank 1984 Salpat |
| Suaedo spicatae-Salicornietum patulae Brullo & Furnari ex Géhu & Géhu-Franck 1984 corr. Alcaraz, Ríos, De la Torre, Delgado & Inocencio 1998 Suaspi |
| Suaedo splendentis-Salicornietum patulae Rivas-Martínez, Costa, Castroviejo & E. Valdés 1980 corr. Rivas-Martínez 1991 Suasal |
| Thero-Suaedetalia Br.-Bl. & O. Bolòs 1958 Sualia |
| Thero-Suaedion Br.-Bl in Br.-Bl, Roussine & Nègre 1952 Theion |
| Suaedo splendentis-Salsoletum sodae Br.-Bl. 1933 Salsod |
| Tuberarietea guttatae (Br.-Bl. in Br.-Bl., Roussine & Nègre 1952) Rivas Goday & Rivas-Martínez 1963 em Rivas-Martínez 1978 Tubgut |
| Cutandietalia maritimae Rivas-Martínez, Díez Garretas & Asensi 2002 Cutmar |
| Alkanno-Maresion nanae Rivas Goday ex Rivas Goday & Rivas-Martínez 1963 corr. Diez-Garretas, Asensi & Rivas-Martínez 2001 AlkMar |
| Wahlenbergio nutabundae-Loeflingietum pentandrae Alcaraz, Díez Garretas & Asensi in Ferre, Díez-Garretas & Asensi 1985 WalLoe |
| Linarion pedunculatae Díez-Garretas, Asensi & Esteve in Díez-Garretas 1984 Linped |
| Ononido variegatae-Linarietum pedunculatae Diez-Garretas, Asensi & Esteve ex Izco, P. & J. Guitián 1988 OnoLin |
| Triplachno nitentis-Silenetum ramosissimae Peinado, Martínez Parras, Alcaraz, Garre & de La Cruz 1985 TriSil |
| Malcolmietalia Rivas Goday 1958 Maalia |
| Anthyllido hamosae-Malcolmion lacerae Rivas Goday 1958 em. Rivas-Martínez 1978 AntMal |
| Linario donyanae-Loeflingietum baeticae Rivas-Martínez, Costa, Castroviejo & E. Valdés 1980 LinLoe |
| Zosteretea marinae Pignatti 1954 ZonMar |
| Zosteretalia Béguinot 1941 Zoalia |
| Zosterion Christiansen 1934 Zorion |
| Zosteretum noltii Harmsen 1936 Zosnol |
References
- Géhu, J.-M. Pour une approche nouvelle des paysages végétaux: La symphytosociologle. Bull. Soc. Bot. Fr. Lett. Bot. 1979, 26, 213–223. [Google Scholar] [CrossRef] [Green Version]
- Géhu, J.-M.; Rivas-Martínez, S. Notions fondamentales de phytosociologie. In Berlchte der Internationalen Symposien der Internationalen Vereinigung für Vegetationskunde; Hrsg von R. Tüxen «Syntaxonomie»; (Rinteln 31 mars-3 avril 1980); Rinteln, Germany, 1981; pp. 5–33. [Google Scholar]
- Biondi, E. L’analisi fitosociológica nello studio integrato del paesaggio. In Avances en Fitosociología/Advances in Phytosociology; Loidi, J., Ed.; Servicio Editorial, Universidad del País Vasco: Bilbao, Spain, 1996; pp. 13–22. [Google Scholar]
- Loidi, J. (Ed.) Avances en Fitosociología/Advances in Phytosociology; Servicio Editorial, Universidad del País Vasco: Bilbao, Spain, 1996; p. 191. [Google Scholar]
- Capelo, J. Conceitos e métodos da Fitossociologia. Formulaçao Contemporânea e Métodos Numéricos de Análise da Vegetaçao; Stação Florestal Nacional, Sociedades Portuguesa de Ciências Florestais: Oeiras, Portugal, 2003; p. 102. [Google Scholar]
- Frattegiani, M.; Gigante, D.; Maneli, F.; Venanzoni, R. Applicaziones del medtodo fitosociologico per la definizione dei criteri gestionali di hábitat forestali dell’all. I alla Direttiva 92/43/CEE. Braun-Blanquetia Centen. De La Phytosociologie 2010, 46, 255–259. [Google Scholar]
- Hruska, K. Ruolo della Fitosociologia nella ricerche interdisciplinaire. Braun-Blanquetia Centen. De La Phytosociologie 2010, 46, 287–290. [Google Scholar]
- Lalanne, A.; Bioret, F.; Boullet, V. Phytosociologie paysagère et applications a la bioévaluation. Docs. Phytosociol. 2016, 8, 208–212. [Google Scholar]
- Braun-Blanquet, J. Essai sur les notions d’Élements et de Territoires Phytogeographiques Earch. Sci. Phys. Nat. 1919, 1, 497–512. [Google Scholar]
- Rivas-Martínez, S. Mapa de series, geoseries y geopermaseries de vegetación de España. Parte I. Itinera Geobot. 2007, 17, 5–436. [Google Scholar]
- Escudero, A.; Gavilán, R.; Rubio, A. Una breve revisión de técnicas de análisis multivariantes aplicables en Fitosociología. Bot. Complut. 1995, 19, 9–38. [Google Scholar]
- Cristea, V.; Gafta, D.; Pedrotti, F. Fitosociología; Temi, Ed.; Koeltz Botanical Books: Trento, Italy, 2015; p. 408. [Google Scholar]
- Blandin, P. Bioindicateurs et diagnostic des systèmes écologiques. Bull. Écol. 1986, 17, 214–307. [Google Scholar]
- Loidi, J. Phytosociology Applied to Nature Conservation and Land Management. In Applied Vegetation Ecology; Song, Y., Dierschke, H., Wang, X., Eds.; Proc. 35th. Symp. IAVS; East China Normal University Press: Shanghai, China, 1994; pp. 17–30. [Google Scholar]
- Asensi, A.; Díez-Garretas, B. Modelos de cartografía de la vegetación actual y de los hábitats naturales y seminaturales en el Parque Natural del Estrecho (Cádiz, España). Estado de conservación. Fitosociología 2007, 44 (Suppl. S1), 17–22. [Google Scholar]
- Asensi, A.; Díez-Garretas, B. Empleo de los hábitats naturales y seminaturales en la evaluación en las sierras del Levante almeriense (Almería, España). Braun-Blanquetia 2010, 46, 97–100. [Google Scholar]
- Bioret, F.; Lazare, J.J.; Géhu, J.M. Evaluation patrimoniale et vulnérabilité des associations végétales du littoral atlantique français. J. Bot. Soc. Bot. 2011, 56, 39–67. [Google Scholar]
- Loidi, J. La fitosociología como proveedora de herramientas de gestión. Lazaroa 2008, 29, 7–17. [Google Scholar]
- Asensi, A.; Díez-Garretas, B. Jean-Marie Géhu y la evaluación biológica del territorio. Su desarrollo en España. Docs. Phytosociol. 2016, 8, 213–217. [Google Scholar]
- Pérez Hernández, C.X. Distintividad taxonómica: Evaluación de la diversidad en la estructura taxonómica en los ensambles. In La Biodiversidad en un Mundo Cambiante: Fundamentos Teóricos y Metodológicos Para su Estudio; Moreno, C.E., Ed.; Universidad Autónoma del Estado de Hidalgo/Libermex: Ciudad de México, México, 2019; pp. 285–306. [Google Scholar]
- Ricklefs, R.E.; Miller, G.L. Ecology, 4th ed.; W. H. Freeman: New York, NY, USA, 2000; p. 822. [Google Scholar]
- Jiménez-Valverde, A.; y Hortal, J. Las curvas de acumulación de especies y la necesidad de evaluar la calidad de los inventarios biológicos. Rev. Iber. Aracnol. 2003, 8, 151–161. [Google Scholar]
- Moreno, C.E. Métodos Para Medir La Biodiversidad; Manuales y tesis SEA, Ed.; Sociedad Entomológica Aragonesa: Zaragoza, Spain, 2000; Volume 1. [Google Scholar]
- Tierra, Á.E.S.; Ortiz, J.P.; Cortes-Molino, A.; Rodríguez, D.R.; Martínez-Vega, J. Modelo de evaluación de la efectividad, continuidad y conectividad del sistema de áreas protegidas costeras mediante el análisis de su flora y vegetación. Bot. Complut. 2020, 44, 73–95. [Google Scholar] [CrossRef]
- Pereña, J. Valor Patrimonial y Estado de Conservación de Hábitats Litorales en Espacios Naturales Protegidos del sur de España. Modelos de Gestión. Doctor’s Thesis, Riuma, Universidad de Málaga, Málaga, Spain, 10 July 2018. [Google Scholar]
- Dudley, N. (Ed.) Guidelines for Applying Protected Area Management Categories; IUCN: Gland, Suiza, 2008; p. x+96. [Google Scholar]
- Castaño-Villa, G.J. Áreas protegidas, criterios para su selección y problemáticas en su conservación. Boletín Científico-Cent. De Mus. -Mus. De Hist. Nat. 2005, 10, 79–101. [Google Scholar]
- Clarke, K.R.; Warwick, R.M. A taxonomic distinctness index and its statistical properties. J. Appl. Ecol. 1998, 35, 523–531. [Google Scholar] [CrossRef]
- López-Mejía, M.; Claudia, E.M.; Zuria, I.; Sánchez-Rojas, E.; Rojas-Martínez, A. Comparación de dos métodos para analizar la proporción de riqueza de especies entre comunidades: Un ejemplo con murciélagos de selvas y hábitats modificados. Rev. Mex. Biodivers. 2017, 88, 183–191. [Google Scholar] [CrossRef]
- Sanders, H.L. Marine benthic diversity: A comparative study. Am. Nat. 1968, 102, 243–282. [Google Scholar] [CrossRef]
- Hulbert, S.H. The nonconcept of species diversity: A critique and alternative parameters. Ecology 1971, 52, 577–585. [Google Scholar] [CrossRef] [Green Version]
- Clarke, K.R.; Warwick, R.M. The taxonomic distinctness measure of biodiversity: Weighting of step lengths between hierarchical levels. Mar. Ecol. Prog. Ser. 1999, 184, 21–29. [Google Scholar] [CrossRef]
- Clarke, K.R.; Warwick, R.M. A further biodiversity index applicable to species lists: Variation in taxonomic distinctness. Mar. Ecol. Prog. Ser. 2001, 216, 265–278. [Google Scholar] [CrossRef]
- Tucker, C.M.; Cadotte, M.W.; Carvalho, S.B.; Davies, J.; Ferrier, S.; Fritz, S.A.; Grenyer, R.; Helmus, M.R.; Jin, L.S.; Mooers, A.O.; et al. A guide to phylogenetic metrics for conservation, community ecology and macroecology. Biol. Rev. Biol. Proc. Camb. Philos. Soc. 2017, 92, 698–715. [Google Scholar] [CrossRef] [PubMed]
- Given, D.R. Changing Aspects of Endemism and Endangerment in Pteridophyta. J. Biogeogr. 1993, 20, 293–302. [Google Scholar] [CrossRef]
- Warwick, R.M.; Clarke, K.R. New ’biodiversity’ measures reveal a decrease in taxonomic distinctness with increasing stress. Mar. Ecol. Prog. Ser. 1995, 129, 301–305. [Google Scholar] [CrossRef] [Green Version]
- Colwell, R.K.; Mao, C.X.; Chang, J. Interpolating, extrapolating, and comparing incidence-based species accumulation curves. Ecology 2004, 8, 2717–2727. [Google Scholar] [CrossRef] [Green Version]
- Vane-Wright, R.I.; Humphries, C.J.; Williams, P.H. What to protect? Systematics and the agony of choice. Biol. Conserv. 1991, 55, 235–254. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Hammer, Ø. PAST Manual Version 4.11. Available online: https://www.nhm.uio.no/english/research/resources/past/ (accessed on 7 January 2022).






| PC | Eigenvalue | % Variance | PC1 | |
|---|---|---|---|---|
| 1 | 0.287115 | 91.78 | STD | −0.0061 |
| 2 | 0.018848 | 6.02 | RRF | 0.9869 |
| 3 | 0.006894 | 2.20 | CPA | 0.1610 |
| LPI | |
|---|---|
| DRA | 0.82 |
| STD | 0.50 |
| RRF | 0.72 |
| PCA | 0.74 |
| DESIGNATED STATUS | AMC | BCA | BMB | CGN | DEG | DOÑ | DUA | EPU | EST | MFR | MIC | MOD | PES | TTR | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Designated status at regional or national level | National Park | + | |||||||||||||
| Natural Park | + | + | + | + | + | ||||||||||
| Natural Area | + | + | + | + | + | + | + | ||||||||
| Natural Monument | + | + | |||||||||||||
| Natural Reserve | + | ||||||||||||||
| Designated status by EU | Special Conservation Area | + | + | + | + | + | + | + | + | + | + | + | + | ||
| Special Protection Area for Birds | + | + | + | + | + | + | + | + | + | + | |||||
| Designated status at international level | Biosphere Reserve | + | + | + | + | ||||||||||
| RAMSAR site | + | + | + | + | + | ||||||||||
| Specially Protected Areas of Mediterranean Importance | + | + | |||||||||||||
| Geopark | + | ||||||||||||||
| World Heritage Site | + | ||||||||||||||
| Legal Protection Index | 3.62 | 5.27 | 3.85 | 6.83 | 0.38 | 10 | 0.2 | 1.22 | 4.64 | 2.8 | 2.74 | 4.39 | 5.29 | 0.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereña-Ortiz, J.F.; Salvo-Tierra, Á.E.; Sánchez-Mata, D. Application of Phytosociological Information in the Evaluation of the Management of Protected Areas. Plants 2023, 12, 406. https://doi.org/10.3390/plants12020406
Pereña-Ortiz JF, Salvo-Tierra ÁE, Sánchez-Mata D. Application of Phytosociological Information in the Evaluation of the Management of Protected Areas. Plants. 2023; 12(2):406. https://doi.org/10.3390/plants12020406
Chicago/Turabian StylePereña-Ortiz, Jaime F., Ángel Enrique Salvo-Tierra, and Daniel Sánchez-Mata. 2023. "Application of Phytosociological Information in the Evaluation of the Management of Protected Areas" Plants 12, no. 2: 406. https://doi.org/10.3390/plants12020406
APA StylePereña-Ortiz, J. F., Salvo-Tierra, Á. E., & Sánchez-Mata, D. (2023). Application of Phytosociological Information in the Evaluation of the Management of Protected Areas. Plants, 12(2), 406. https://doi.org/10.3390/plants12020406








