Effects of Climate Change and Drought Tolerance on Maize Growth
Abstract
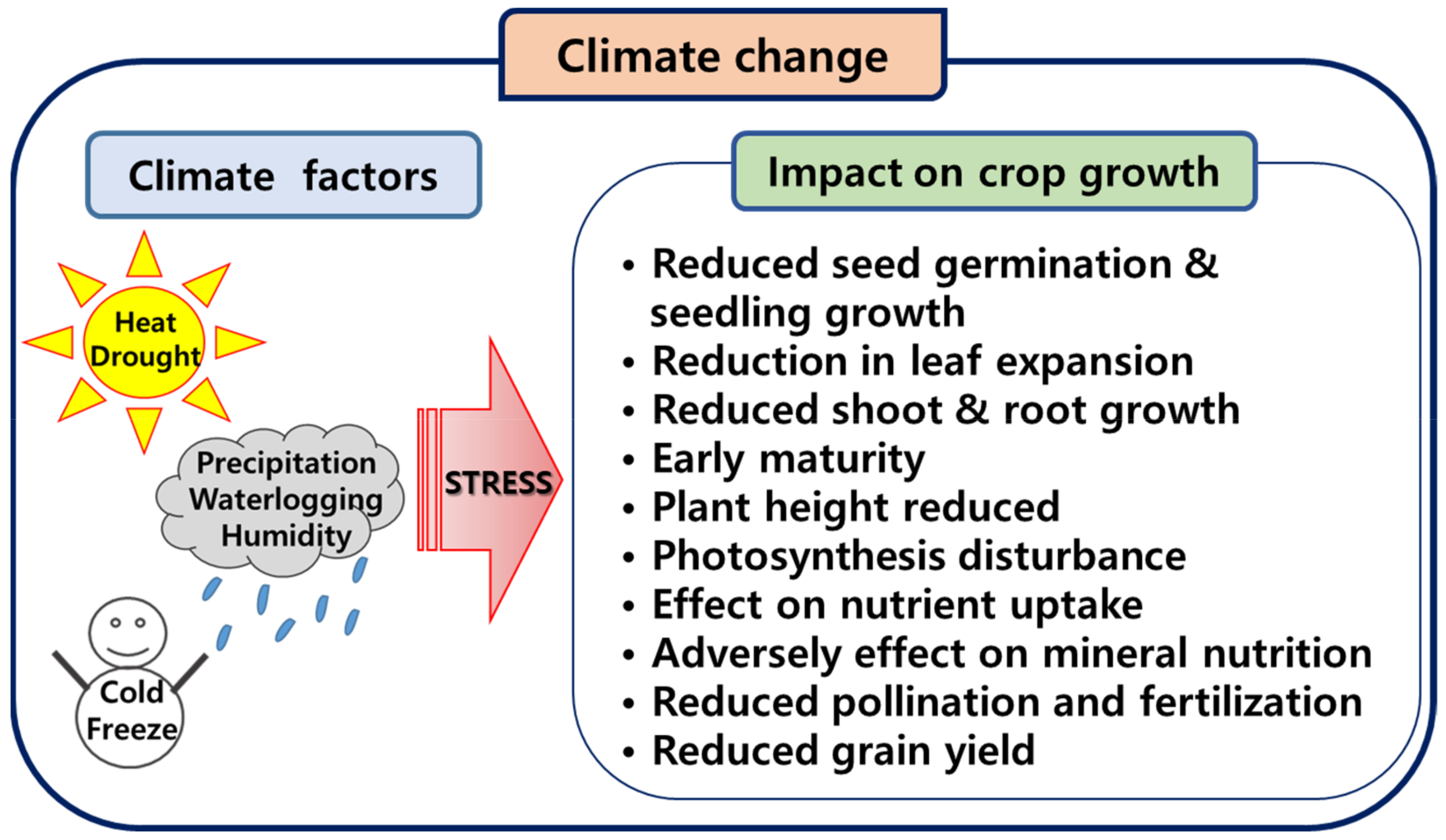
:1. Introduction
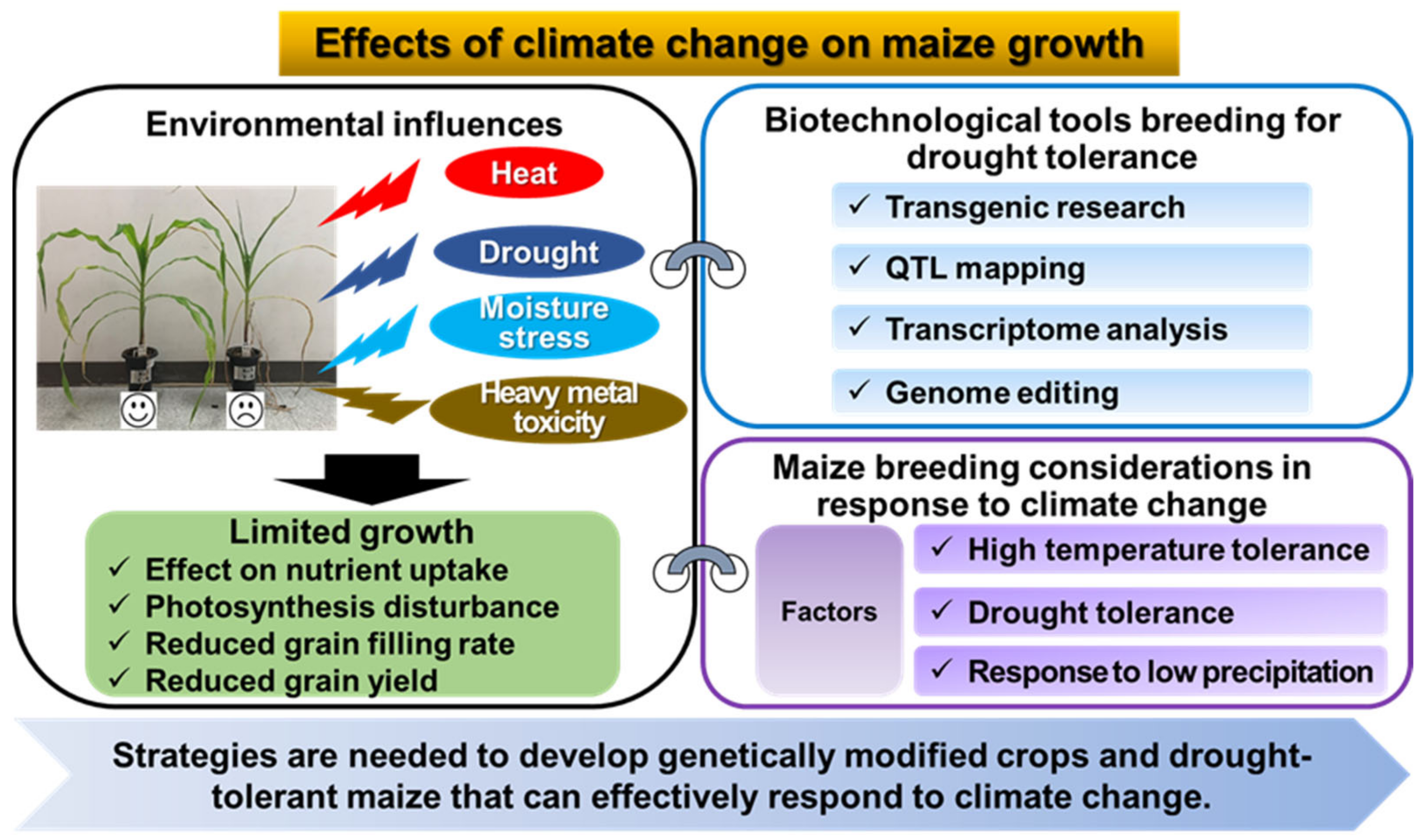
2. Effects of Climate Change on Maize Growth
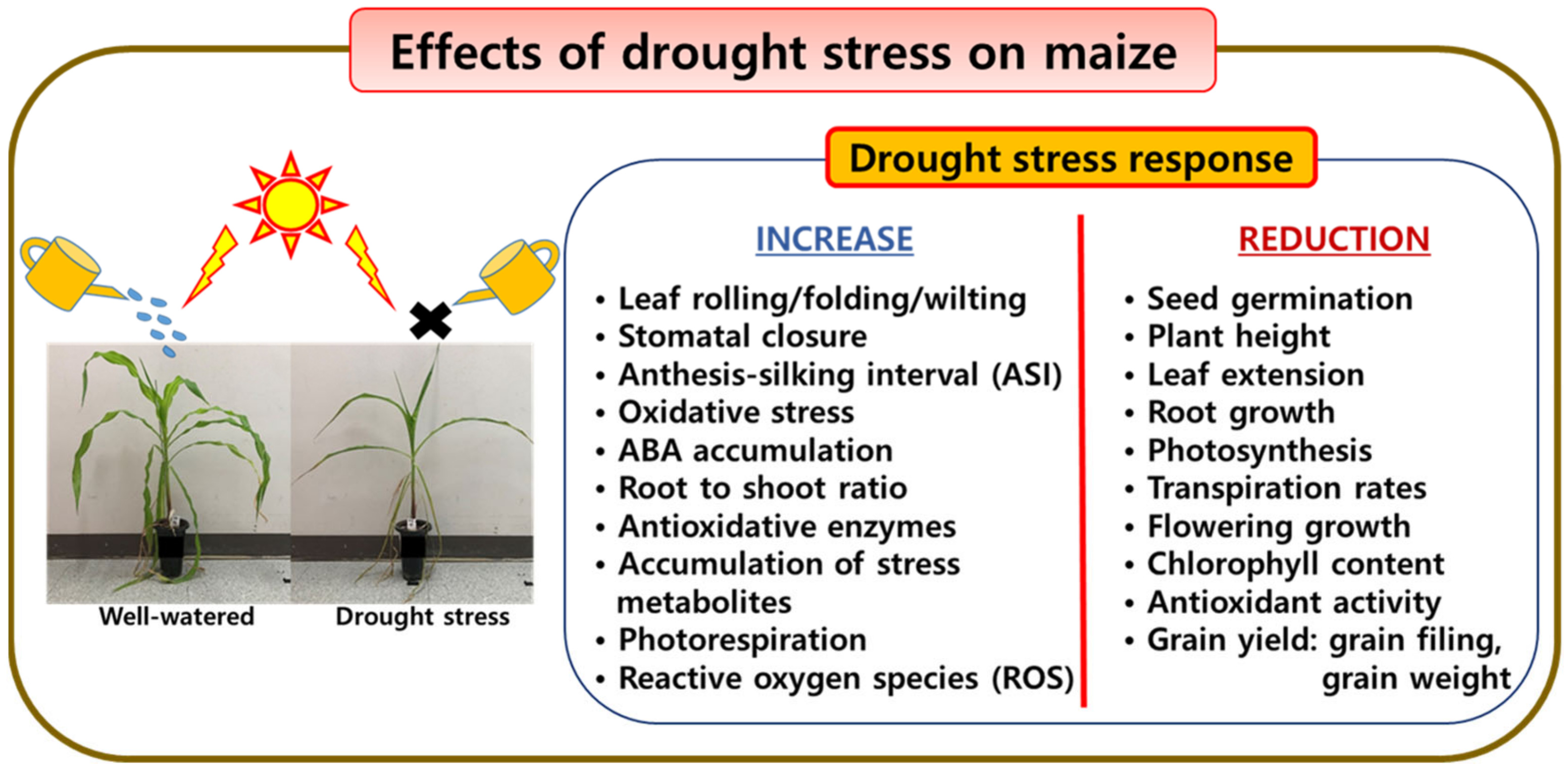
2.1. Drought Stress
2.2. Heat Stress
2.3. Moisture Stress and Precipitation Variability
3. Effects of Drought and Heat Stress on Maize Yields
4. Application of Biotechnological Tools Breeding for Drought Tolerance in Maize
4.1. Transgenic Research to Develop Drought-Tolerant Maize
4.2. QTL Mapping for Drought Tolerance in Maize
4.3. Impact of Transcriptome Analysis for Drought Tolerance in Maize
4.4. Genome Editing: Genetic Improvement of Drought Tolerance in Maize
5. Future Prospects of Maize Breeding Research in Response to Climate Change
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lee, H.; Calvin, K.; Dasgupta, D.; Krinner, G.; Mukherji, A.; Thorne, P.; Trisos, C.; Romero, J.; Aldunce, P.; Barrett, K. AR6 Synthesis Report: Climate Change 2023. Summary for Policymakers. 2023. Available online: https://www.ipcc.ch/report/ar6/syr/downloads/report/IPCC_AR6_SYR_SPM.pdf (accessed on 2 June 2023).
- Aliniaeifard, S.; Rezayian, M.; Mousavi, S.H. Drought stress: Involvement of Plant Hormones in Perception, Signaling, and response. In Plant Hormones and Climate Change; Springer: Cham, Switzerland, 2023; pp. 227–250. [Google Scholar]
- Khalili, M.; Naghavi, M.R.; Aboughadareh, A.P.; Rad, H.N. Effects of drought stress on yield and yield components in maize cultivars (Zea mays L.). Int. J. Agron. Plant Prod. 2013, 4, 809–812. [Google Scholar]
- Song, K.; Kim, K.; Kim, H.; Moon, J.; Kim, J.; Baek, S.; Kwon, Y.; Lee, B. Evaluation of drought tolerance in maize seedling using leaf rolling. Korean J. Crop Sci. Jakmul Hakhoe Chi 2015, 60, 8–16. [Google Scholar] [CrossRef]
- Lu, Y.; Hao, Z.; Xie, C.; Crossa, J.; Araus, J.-L.; Gao, S.; Vivek, B.S.; Magorokosho, C.; Mugo, S.; Makumbi, D. Large-scale screening for maize drought resistance using multiple selection criteria evaluated under water-stressed and well-watered environments. Field Crop. Res. 2011, 124, 37–45. [Google Scholar] [CrossRef]
- Saglam, A.; Kadioglu, A.; Demiralay, M.; Terzi, R. Leaf rolling reduces photosynthetic loss in maize under severe drought. Acta Bot. Croat. 2014, 73, 315–332. [Google Scholar] [CrossRef]
- Effendi, R.; Priyanto, S.B.; Aqil, M.; Azrai, M. Drought adaptation level of maize genotypes based on leaf rolling, temperature, relative moisture content, and grain yield parameters. IOP Conf. Ser. Earth Environ. Sci. 2019, 270, 012016. [Google Scholar] [CrossRef]
- Aslam, M.; Zamir, M.S.I.; Afzal, I.; Yaseen, M.; Mubeen, M.; Shoaib, A. Drought stress, its effect on maize production and development of drought tolerance through potassium application. Agron. Res. Mold. 2013, 46, 99–144. [Google Scholar]
- Sah, R.P.; Chakraborty, M.; Prasad, K.; Pandit, M.; Tudu, V.K.; Chakravarty, M.K.; Narayan, S.C.; Rana, M.; Moharana, D. Impact of water deficit stress in maize: Phenology and yield components. Sci. Rep. 2020, 10, 2944. [Google Scholar] [CrossRef] [PubMed]
- Herrero, M.P.; Johnson, R.R. Drought stress and its effects on maize reproductive systems. Crop Sci. 1981, 21, 105–110. [Google Scholar] [CrossRef]
- Roy, S.K.; Cho, S.W.; Kwon, S.J.; Kamal, A.H.M.; Kim, S.W.; Oh, M.W.; Lee, M.S.; Chung, K.Y.; Xin, Z.; Woo, S.H. Morpho-physiological and proteome level responses to cadmium stress in sorghum. PLoS ONE 2016, 11, e0150431. [Google Scholar] [CrossRef]
- Siebers, N.; Godlinski, F.; Leinweber, P. Bone char as phosphorus fertilizer involved in cadmium immobilization in lettuce, wheat, and potato cropping. J. Plant Nutr. Soil Sci. 2014, 177, 75–83. [Google Scholar] [CrossRef]
- Retamal-Salgado, J.; Hirzel, J.; Walter, I.; Matus, I. Bioabsorption and bioaccumulation of cadmium in the straw and grain of maize (Zea mays L.) in growing soils contaminated with cadmium in different environment. Int. J. Environ. Res. Public Health 2017, 14, 1399. [Google Scholar] [CrossRef] [PubMed]
- Marquez, J.E.; Pourret, O.; Faucon, M.P.; Weber, S.; Hoàng, T.B.H.; Martinez, R.E. Effect of Cadmium, Copper and lead on the growth of rice in the coal mining region of Quang Ninh, Cam-Pha (Vietnam). Sustainability 2018, 10, 1758. [Google Scholar] [CrossRef]
- Naveed, M.; Mustafa, A.; Majeed, S.; Naseem, Z.; Saeed, Q. Through Enterobacter sp. MN17 Inoculation Together. Plants 2020, 9, 530. [Google Scholar] [CrossRef] [PubMed]
- Javed, N.; Ashraf, M.; Akram, N.A.; Al-Qurainy, F. Alleviation of adverse effects of drought stress on growth and some potential physiological attributes in maize (Zea mays L.) by seed electromagnetic treatment. Photochem. Photobiol. 2011, 87, 1354–1362. [Google Scholar] [CrossRef] [PubMed]
- Van Slycken, S.; Witters, N.; Meers, E.; Peene, A.; Michels, E.; Adriaensen, K.; Ruttens, A.; Vangronsveld, J.; Du Laing, G.; Wierinck, I.; et al. Safe use of metal-contaminated agricultural land by cultivation of energy maize (Zea mays). Environ. Pollut. 2013, 178, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Dong, M.; Mao, P.; Zhuang, P.; Paz-Ferreiro, J.; Li, Y.; Li, Y.; Hu, X.; Netherway, P.; Li, Z. Evaluation of phytoremediation potential of five Cd (hyper)accumulators in two Cd contaminated soils. Sci. Total Environ. 2020, 721, 137581. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Habib, M.; Kakavand, S.N.; Zahid, Z.; Zahra, N.; Sharif, R.; Hasanuzzaman, M. Phytoremediation of cadmium: Physiological, biochemical, and molecular mechanisms. Biology 2020, 9, 177. [Google Scholar] [CrossRef] [PubMed]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S. Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef]
- Daryanto, S.; Wang, L.; Jacinthe, P.-A. Global synthesis of drought effects on maize and wheat production. PLoS ONE 2016, 11, e0156362. [Google Scholar] [CrossRef]
- Lauer, J. Concerns about Drought as Corn Pollination Begins. Field Crop. 2006, 28, 493–542. [Google Scholar]
- Nielsen, R.L. Drought and heat stress effects on corn pollination. J. Agron. 2002, 196, 19–25. [Google Scholar]
- Nielsen, R.L. A Fast & Accurate Pregnancy Test for Corn. 2002. Available online: http://www.kingcorn.org/news/timeless/EarShake.html (accessed on 1 July 2022).
- Sánchez, B.; Rasmussen, A.; Porter, J.R. Temperatures and the growth and development of maize and rice: A review. Glob. Chang. Biol. 2014, 20, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Calleja-Cabrera, J.; Boter, M.; Oñate-Sánchez, L.; Pernas, M. Root growth adaptation to climate change in crops. Front. Plant Sci. 2020, 11, 544. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.A.; Kiniry, J.R.; Dyke, P.T. CERES-Maize: A Simulation Model of Maize Growth and Development; Texas A&M University Press: College Station, TX, USA, 1986. [Google Scholar]
- Lobell, D.B.; Burke, M.B. On the use of statistical models to predict crop yield responses to climate change. Agric. For. Meteorol. 2010, 150, 1443–1452. [Google Scholar] [CrossRef]
- Stone, P. The effects of heat stress on cereal yield and quality. In Crop Responses and Adaptations to Temperature Stress; CRC Press: Boca Raton, FL, USA, 2023; pp. 243–291. [Google Scholar]
- Schoper, J.B.; Lambert, R.J.; Vasilas, B.L. Pollen viability, pollen shedding, and combining ability for tassel heat tolerance in maize. Crop Sci. 1987, 27, 27–31. [Google Scholar] [CrossRef]
- Sinsawat, V.; Leipner, J.; Stamp, P.; Fracheboud, Y. Effect of heat stress on the photosynthetic apparatus in maize (Zea mays L.) grown at control or high temperature. Environ. Exp. Bot. 2004, 52, 123–129. [Google Scholar] [CrossRef]
- Hussain, T.; Khan, I.A.; Malik, M.A.; Ali, Z. Breeding potential for high temperature tolerance in corn (Zea mays L.). Pakistan J. Bot. 2006, 38, 1185–1195. [Google Scholar]
- McNellie, J.P.; Chen, J.; Li, X.; Yu, J. Genetic mapping of foliar and tassel heat stress tolerance in maize. Crop Sci. 2018, 58, 2484–2493. [Google Scholar] [CrossRef]
- Shao, R.; Yu, K.; Li, H.; Jia, S.; Yang, Q.; Zhao, X.; Zhao, Y.; Liu, T. The effect of elevating temperature on the growth and development of reproductive organs and yield of summer maize. J. Integr. Agric. 2021, 20, 1783–1795. [Google Scholar] [CrossRef]
- Liu, P.; Yin, B.; Gu, L.; Zhang, S.; Ren, J.; Wang, Y.; Duan, W.; Zhen, W. Heat stress affects tassel development and reduces the kernel number of summer maize. Front. Plant Sci. 2023, 14, 1186921. [Google Scholar] [CrossRef]
- Claassen, M.M.; Shaw, R.H. Water deficit effects on corn. II. Grain components. Agron. J. 1970, 62, 652–655. [Google Scholar] [CrossRef]
- Westgate, M.E.; Grant, D.L.T. Water deficits and reproduction in maize: Response of the reproductive tissue to water deficits at anthesis and mid-grain fill. Plant Physiol. 1989, 91, 862–867. [Google Scholar] [CrossRef] [PubMed]
- NeSmith, D.S.; Ritchie, J.T. Effects of soil water-deficits during tassel emergence on development and yield component of maize (Zea mays). Field Crop. Res. 1992, 28, 251–256. [Google Scholar] [CrossRef]
- Udomprasert, N.; Kijjanon, J.; Chusri-Iam, K.; Machuay, A. Effects of Water Deficit at Tasseling on Photosynthesis, Development, and Yield of Corn. Kasetsart J. (Nat. Sci.) 2005, 39, 546–551. [Google Scholar]
- Heinigre, R.W. Irrigation and Drought Management. 2000. Available online: https://www.scirp.org/%28S%28351jmbntvnsjt1aadkozje%29%29/reference/referencespapers.aspx?referenceid=1315258 (accessed on 2 June 2023).
- Nam, H.-H.; Seo, M.-C.; Cho, H.-S.; Lee, Y.-H.; Seo, Y.-J. Growth and yield responses of corn (Zea mays L.) as affected by growth period and irrigation intensity. Korean J. Soil Sci. Fertil. 2017, 50, 674–683. [Google Scholar] [CrossRef]
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Jaldhani, V.; Rao, D.S.; Beulah, P.; Nagaraju, P.; Suneetha, K.; Veronica, N.; Kondamudi, R.; Sundaram, R.M.; Madhav, M.S.; Neeraja, C.N. Drought and heat stress combination in a changing climate. In Climate Change and Crop Stress; Elsevier: Amsterdam, The Netherlands, 2022; pp. 33–70. [Google Scholar]
- Ray, D.K.; Gerber, J.S.; MacDonald, G.K.; West, P.C. Climate variation explains a third of global crop yield variability. Nat. Commun. 2015, 6, 5989. [Google Scholar] [CrossRef] [PubMed]
- Shaw, R.H. Climate requirement. Corn Corn Improv. 1988, 18, 609–638. [Google Scholar]
- Legg, S. IPCC, 2021: Climate change 2021-the physical science basis. Interaction 2021, 49, 44–45. [Google Scholar]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Naveenkumar, K.L.; Sen, D.; Khanna, V.K. Effect of maize production in a changing climate: Its impacts, adaptation, and mitigation strategies through breeding. Open Access J. Oncol. Med. 2018, 2, 2018. [Google Scholar]
- Duveiller, E.; Singh, R.P.; Nicol, J.M. The challenges of maintaining wheat productivity: Pests, diseases, and potential epidemics. Euphytica 2007, 157, 417–430. [Google Scholar] [CrossRef]
- Schussler, J.R.; Westgate, M.E. Maize kernel set at low water potential: I. Sensitivity to reduced assimilates during early kernel growth. Crop Sci. 1991, 31, 1189–1195. [Google Scholar] [CrossRef]
- Abrecht, D.G.; Carberry, P.S. The influence of water deficit prior to tassel initiation on maize growth, development and yield. Field Crop. Res. 1993, 31, 55–69. [Google Scholar] [CrossRef]
- Bolaños, J.; Edmeades, G.O. Eight cycles of selection for drought tolerance in lowland tropical maize. II. Responses in reproductive behavior. Field Crop. Res. 1993, 31, 253–268. [Google Scholar] [CrossRef]
- Ribaut, J.-M.; Hoisington, D.A.; Deutsch, J.A.; Jiang, C.; Gonzalez-de-Leon, D. Identification of quantitative trait loci under drought conditions in tropical maize. 1. Flowering parameters and the anthesis-silking interval. Theor. Appl. Genet. 1996, 92, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Serna, L. Maize stomatal responses against the climate change. Front. Plant Sci. 2022, 13, 952146. [Google Scholar] [CrossRef] [PubMed]
- Maitah, M.; Malec, K.; Maitah, K. Influence of precipitation and temperature on maize production in the Czech Republic from 2002 to 2019. Sci. Rep. 2021, 11, 10467. [Google Scholar] [CrossRef]
- Khan, A.; Ali, S.; Shah, S.A.; Khan, A.; Ullah, R. Impact of climate change on maize productivity in Khyber Pakhtunkhwa, Pakistan. Sarhad J. Agric. 2019, 35, 594–601. [Google Scholar] [CrossRef]
- Webber, H.; Ewert, F.; Olesen, J.E.; Müller, C.; Fronzek, S.; Ruane, A.C.; Bourgault, M.; Martre, P.; Ababaei, B.; Bindi, M. Diverging importance of drought stress for maize and winter wheat in Europe. Nat. Commun. 2018, 9, 4249. [Google Scholar] [CrossRef]
- Dellal, İ.; McCarl, B.A.; Butt, T. The economic assessment of climate change on Turkish agriculture. J. Environ. Prot. Ecol. 2011, 12, 376–385. [Google Scholar]
- Yu, C.; Miao, R.; Khanna, M. Maladaptation of US corn and soybeans to a changing climate. Sci. Rep. 2021, 11, 12351. [Google Scholar] [CrossRef] [PubMed]
- Notununu, I.; Moleleki, L.; Roopnarain, A.; Adeleke, R. Effects of plant growth-promoting rhizobacteria on the molecular responses of maize under drought and heat stresses: A review. Pedosphere 2022, 32, 90–106. [Google Scholar] [CrossRef]
- Pei, Y.-Y.; Lei, L.; Fan, X.-W.; Li, Y.-Z. Effects of high air temperature, drought, and both combinations on maize: A case study. Plant Sci. 2023, 327, 111543. [Google Scholar] [CrossRef] [PubMed]
- Kooyers, N.J. The evolution of drought escape and avoidance in natural herbaceous populations. Plant Sci. 2015, 234, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Shavrukov, Y.; Kurishbayev, A.; Jatayev, S.; Shvidchenko, V.; Zotova, L.; Koekemoer, F.; De Groot, S.; Soole, K.; Langridge, P. Early flowering as a drought escape mechanism in plants: How can it aid wheat production? Front. Plant Sci. 2017, 8, 1950. [Google Scholar] [CrossRef] [PubMed]
- Cattivelli, L.; Rizza, F.; Badeck, F.-W.; Mazzucotelli, E.; Mastrangelo, A.M.; Francia, E.; Marè, C.; Tondelli, A.; Stanca, A.M. Drought tolerance improvement in crop plants: An integrated view from breeding to genomics. Field Crop. Res. 2008, 105, 1–14. [Google Scholar] [CrossRef]
- Tardieu, F.; Simonneau, T.; Muller, B. The physiological basis of drought tolerance in crop plants: A scenario-dependent probabilistic approach. Annu. Rev. Plant Biol. 2018, 69, 733–759. [Google Scholar] [CrossRef]
- Saini, H.S.; Westgate, M.E. Reproductive development in grain crops during drought. Adv. Agron. 1999, 68, 59–96. [Google Scholar]
- Boyer, J.S.; Westgate, M.E. Grain yields with limited water. J. Exp. Bot. 2004, 55, 2385–2394. [Google Scholar] [CrossRef]
- Lobell, D.B.; Roberts, M.J.; Schlenker, W.; Braun, N.; Little, B.B.; Rejesus, R.M.; Hammer, G.L. Greater sensitivity to drought accompanies maize yield increase in the US Midwest. Science 2014, 344, 516–519. [Google Scholar] [CrossRef]
- Babu, R.C.; Nguyen, B.D.; Chamarerk, V.; Shanmugasundaram, P.; Chezhian, P.; Jeyaprakash, P.; Ganesh, S.K.; Palchamy, A.; Sadasivam, S.; Sarkarung, S. Genetic analysis of drought resistance in rice by molecular markers: Association between secondary traits and field performance. Crop Sci. 2003, 43, 1457–1469. [Google Scholar] [CrossRef]
- Tester, M.; Langridge, P. Breeding technologies to increase crop production in a changing world. Science 2010, 327, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Qin, F. Genetic dissection of maize drought tolerance for trait improvement. Mol. Breed. 2021, 41, 8. [Google Scholar] [CrossRef] [PubMed]
- Voytas, D.F.; Gao, C. Precision genome engineering and agriculture: Opportunities and regulatory challenges. PLoS Biol. 2014, 12, e1001877. [Google Scholar] [CrossRef] [PubMed]
- Gao, C. Genome editing in crops: From bench to field. Natl. Sci. Rev. 2015, 2, 13–15. [Google Scholar] [CrossRef]
- Yang, S.; Vanderbeld, B.; Wan, J.; Huang, Y. Narrowing down the targets: Towards successful genetic engineering of drought-tolerant crops. Mol. Plant 2010, 3, 469–490. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.E.; Repetti, P.P.; Adams, T.R.; Creelman, R.A.; Wu, J.; Warner, D.C.; Anstrom, D.C.; Bensen, R.J.; Castiglioni, P.P.; Donnarummo, M.G. Plant nuclear factor Y (NF-Y) B subunits confer drought tolerance and lead to improved corn yields on water-limited acres. Proc. Natl. Acad. Sci. USA 2007, 104, 16450–16455. [Google Scholar] [CrossRef]
- Zeng, T.; Zhang, D.; Li, Y.; Li, C.; Liu, X.; Shi, Y.; Song, Y.; Li, Y.; Wang, T. Identification of genomic insertion and flanking sequences of the transgenic drought-tolerant maize line “SbSNAC1-382” using the single-molecule real-time (SMRT) sequencing method. PLoS ONE 2020, 15, e0226455. [Google Scholar] [CrossRef]
- Wang, B.; Li, Z.; Ran, Q.; Li, P.; Peng, Z.; Zhang, J. ZmNF-YB16 overexpression improves drought resistance and yield by enhancing photosynthesis and the antioxidant capacity of maize plants. Front. Plant Sci. 2018, 9, 709. [Google Scholar] [CrossRef]
- Muppala, S.; Gudlavalleti, P.K.; Malireddy, K.R.; Puligundla, S.K.; Dasari, P. Development of stable transgenic maize plants tolerant for drought by manipulating ABA signaling through Agrobacterium-mediated transformation. J. Genet. Eng. Biotechnol. 2021, 19, 96. [Google Scholar] [CrossRef]
- Quan, R.; Shang, M.; Zhang, H.; Zhao, Y.; Zhang, J. Engineering of enhanced glycine betaine synthesis improves drought tolerance in maize. Plant Biotechnol. J. 2004, 2, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qin, L.; Han, L.; Xiang, Y.; Zhao, D. Overexpression of maize SDD1 (ZmSDD1) improves drought resistance in Zea mays L. by reducing stomatal density. Plant Cell Tissue Organ Cult. 2015, 122, 147–159. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.; Liu, S.; Ferjani, A.; Li, J.; Yan, J.; Yang, X.; Qin, F. Genetic variation in ZmVPP1 contributes to drought tolerance in maize seedlings. Nat. Genet. 2016, 48, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Virlouvet, L.; Jacquemot, M.-P.; Gerentes, D.; Corti, H.; Bouton, S.; Gilard, F.; Valot, B.; Trouverie, J.; Tcherkez, G.; Falque, M. The ZmASR1 protein influences branched-chain amino acid biosynthesis and maintains kernel yield in maize under water-limited conditions. Plant Physiol. 2011, 157, 917–936. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, M.; Guo, Z.; Zhu, X.; Xia, Z. Identification of a 119-bp promoter of the maize sulfite oxidase gene (ZmSO) that confers high-level gene expression and ABA or drought inducibility in transgenic plants. Int. J. Mol. Sci. 2019, 20, 3326. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, Y.; Yin, Y.; Liu, Q.; Li, N.; Li, X.; He, W.; Hao, D.; Liu, X.; Guo, C. Expression of AtGA2ox1 enhances drought tolerance in maize. Plant Growth Regul. 2019, 89, 203–215. [Google Scholar] [CrossRef]
- Sheoran, S.; Gupta, M.; Kumari, S.; Kumar, S.; Rakshit, S. Meta-QTL analysis and candidate genes identification for various abiotic stresses in maize (Zea mays L.) and their implications in breeding programs. Mol. Breed. 2022, 42, 26. [Google Scholar] [CrossRef]
- Welcker, C.; Boussuge, B.; Bencivenni, C.; Ribaut, J.M.; Tardieu, F. Are source and sink strengths genetically linked in maize plants subjected to water deficit? A QTL study of the responses of leaf growth and of anthesis-silking interval to water deficit. J. Exp. Bot. 2007, 58, 339–349. [Google Scholar] [CrossRef]
- Ruta, N.; Liedgens, M.; Fracheboud, Y.; Stamp, P.; Hund, A. QTLs for the elongation of axile and lateral roots of maize in response to low water potential. Theor. Appl. Genet. 2010, 120, 621–631. [Google Scholar] [CrossRef]
- Liu, Y.; Subhash, C.; Yan, J.; Song, C.; Zhao, J.; Li, J. Maize leaf temperature responses to drought: Thermal imaging and quantitative trait loci (QTL) mapping. Environ. Exp. Bot. 2011, 71, 158–165. [Google Scholar] [CrossRef]
- Landi, P.; Giuliani, S.; Salvi, S.; Ferri, M.; Tuberosa, R.; Sanguineti, M.C. Characterization of root-yield-1.06, a major constitutive QTL for root and agronomic traits in maize across water regimes. J. Exp. Bot. 2010, 61, 3553–3562. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.F.; Li, X.H.; Xie, C.X.; Li, M.S.; Zhang, D.G.; Bai, L.; Zhang, S.H. Two consensus quantitative trait loci clusters controlling anthesis–silking interval, ear setting and grain yield might be related with drought tolerance in maize. Ann. Appl. Biol. 2008, 153, 73–83. [Google Scholar] [CrossRef]
- Agrama, H.A.S.; Moussa, M.E. Mapping QTLs in breeding for drought tolerance in maize (Zea mays L.). Euphytica 1996, 91, 89–97. [Google Scholar] [CrossRef]
- Almeida, G.D.; Makumbi, D.; Magorokosho, C.; Nair, S.; Borém, A.; Ribaut, J.-M.; Bänziger, M.; Prasanna, B.M.; Crossa, J.; Babu, R. QTL mapping in three tropical maize populations reveals a set of constitutive and adaptive genomic regions for drought tolerance. Theor. Appl. Genet. 2013, 126, 583–600. [Google Scholar] [CrossRef] [PubMed]
- Tuberosa, R.; Sanguineti, M.C.; Landi, P.; Salvi, S.; Casarini, E.; Conti, S. RFLP mapping of quantitative trait loci controlling abscisic acid concentration in leaves of drought-stressed maize (Zea mays L.). Theor. Appl. Genet. 1998, 97, 744–755. [Google Scholar] [CrossRef]
- Sanguineti, M.C.; Tuberosa, R.; Landi, P.; Salvi, S.; Maccaferri, M.; Casarini, E.; Conti, S. QTL analysis of drought-related traits and grain yield in relation to genetic variation for leaf abscisic acid concentration in field-grown maize. J. Exp. Bot. 1999, 50, 1289–1297. [Google Scholar] [CrossRef]
- Abdelghany, M.; Liu, X.; Hao, L.; Gao, C.; Kou, S.; Su, E.; Zhou, Y.; Wang, R.; Zhang, D.; Li, Y.; et al. QTL analysis for yield-related traits under different water regimes in maize. Maydica 2019, 64, 10. [Google Scholar]
- Leng, P.; Khan, S.U.; Zhang, D.; Zhou, G.; Zhang, X.; Zheng, Y.; Wang, T.; Zhao, J. Linkage mapping reveals QTL for flowering time-related traits under multiple abiotic stress conditions in maize. Int. J. Mol. Sci. 2022, 23, 8410. [Google Scholar] [CrossRef]
- Hu, X.; Wang, G.; Du, X.; Zhang, H.; Xu, Z.; Wang, J.; Chen, G.; Wang, B.; Li, X.; Chen, X. QTL analysis across multiple environments reveals promising chromosome regions associated with yield-related traits in maize under drought conditions. Crop J. 2021, 9, 759–766. [Google Scholar] [CrossRef]
- Semagn, K.; Beyene, Y.; Warburton, M.L.; Tarekegne, A.; Mugo, S.; Meisel, B.; Sehabiague, P.; Prasanna, B.M. Meta-analyses of QTL for grain yield and anthesis silking interval in 18 maize populations evaluated under water-stressed and well-watered environments. BMC Genom. 2013, 14, 313. [Google Scholar] [CrossRef]
- Zhao, X.Q.; Zhong, Y. Genetic dissection of the photosynthetic parameters of maize (Zea mays L.) in drought-stressed and well-watered environments. Russ. J. Plant Physiol. 2021, 68, 1125–1134. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, J.; Ma, Z.; Xia, L.; Wang, X.; Zhang, L.; Ding, Y.; Qi, J.; Mu, X.; Zhao, F. Bulk analysis by resequencing and RNA-seq identifies candidate genes for maintaining leaf water content under water deficit in maize. Physiol. Plant. 2021, 173, 1935–1945. [Google Scholar] [CrossRef] [PubMed]
- Araus, J.L.; Slafer, G.A.; Royo, C.; Serret, M.D. Breeding for yield potential and stress adaptation in cereals. CRC Crit. Rev. Plant Sci. 2008, 27, 377–412. [Google Scholar] [CrossRef]
- Collins, N.C.; Tardieu, F.; Tuberosa, R. Quantitative trait loci and crop performance under abiotic stress: Where do we stand? Plant Physiol. 2008, 147, 469–486. [Google Scholar] [CrossRef] [PubMed]
- Eathington, S.R.; Crosbie, T.M.; Edwards, M.D.; Reiter, R.S.; Bull, J.K. Molecular markers in a commercial breeding program. Crop Sci. 2007, 47, S-154–S-163. [Google Scholar] [CrossRef]
- Tsonev, S.; Todorovska, E.; Avramova, V.; Kolev, S.; Abu-Mhadi, N.; Christov, N.K. Genomics assisted improvement of drought tolerance in maize: QTL approaches. Biotechnol. Biotechnol. Equip. 2009, 23, 1410–1413. [Google Scholar] [CrossRef]
- Xu, Y.; Skinner, D.J.; Wu, H.; Palacios-Rojas, N.; Araus, J.L.; Yan, J.; Gao, S.; Warburton, M.L.; Crouch, J.H. Advances in maize genomics and their value for enhancing genetic gains from breeding. Int. J. Plant Genom. 2009, 2009, 957602. [Google Scholar] [CrossRef]
- Stuber, C.W.; Edwards, M.D.; Wendel, J.F. Molecular Marker-Facilitated Investigations of Quantitative Trait Loci in Maize. II. Factors Influencing Yield and its Component Traits. Crop Sci. 1987, 27, 639–648. [Google Scholar] [CrossRef]
- Lebreton, C.; Lazić-Jančić, V.; Steed, A.; Pekić, S.; Quarrie, S.A. Identification of QTL for drought responses in maize and their use in testing causal relationships between traits. J. Exp. Bot. 1995, 46, 853–865. [Google Scholar] [CrossRef]
- Messmer, R.; Fracheboud, Y.; Bänziger, M.; Vargas, M.; Stamp, P.; Ribaut, J.M. Drought stress and tropical maize: QTL-by-environment interactions and stability of QTLs across environments for yield components and secondary traits. Theor. Appl. Genet. 2009, 119, 913–930. [Google Scholar] [CrossRef] [PubMed]
- Szalma, S.J.; Hostert, B.M.; LeDeaux, J.R.; Stuber, C.W.; Holland, J.B. QTL mapping with near-isogenic lines in maize. Theor. Appl. Genet. 2007, 114, 1211–1228. [Google Scholar] [CrossRef] [PubMed]
- Tuberosa, R.; Salvi, S.; Sanguineti, M.C.; Landi, P.; Maccaferri, M.; Conti, S. Mapping QTLS regulating morpho-physiological traits and yield: Case studies, shortcomings and perspectives in drought-stressed maize. Ann. Bot. 2002, 89, 941–963. [Google Scholar] [CrossRef] [PubMed]
- Campos, H.; Cooper, M.; Habben, J.E.; Edmeades, G.O.; Schussler, J.R. Improving drought tolerance in maize: A view from industry. Field Crop. Res. 2004, 90, 19–34. [Google Scholar] [CrossRef]
- Thatcher, S.R.; Danilevskaya, O.N.; Meng, X.; Beatty, M.; Zastrow-Hayes, G.; Harris, C.; Van Allen, B.; Habben, J.; Li, B. Genome-wide analysis of alternative splicing during development and drought stress in maize. Plant Physiol. 2016, 170, 586–599. [Google Scholar] [CrossRef] [PubMed]
- Danilevskaya, O.N.; Yu, G.; Meng, X.; Xu, J.; Stephenson, E.; Estrada, S.; Chilakamarri, S.; Zastrow-Hayes, G.; Thatcher, S. Developmental and transcriptional responses of maize to drought stress under field conditions. Plant Direct 2019, 3, e00129. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Qiao, P.; Matschi, S.; Vasquez, M.; Ramstein, G.P.; Bourgault, R.; Mohammadi, M.; Scanlon, M.J.; Molina, I.; Smith, L.G. Integrating GWAS and TWAS to elucidate the genetic architecture of maize leaf cuticular conductance. Plant Physiol. 2022, 189, 2144–2158. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zhang, F.; Hu, H.; Bakshi, A.; Robinson, M.R.; Powell, J.E.; Montgomery, G.W.; Goddard, M.E.; Wray, N.R.; Visscher, P.M. Integration of summary data from GWAS and eQTL studies predicts complex trait gene targets. Nat. Genet. 2016, 48, 481–487. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, H.; Zhu, H. CRISPR technology is revolutionizing the improvement of tomato and other fruit crops. Hortic. Res. 2019, 6, 77. [Google Scholar] [CrossRef]
- Shi, J.; Gao, H.; Wang, H.; Lafitte, H.R.; Archibald, R.L.; Yang, M.; Hakimi, S.M.; Mo, H.; Habben, J.E. ARGOS 8 variants generated by CRISPR-Cas9 improve maize grain yield under field drought stress conditions. Plant Biotechnol. J. 2017, 15, 207–216. [Google Scholar] [CrossRef]
- Pan, Z.; Liu, M.; Zhao, H.; Tan, Z.; Liang, K.; Sun, Q.; Gong, D.; He, H.; Zhou, W.; Qiu, F. ZmSRL5 is involved in drought tolerance by maintaining cuticular wax structure in maize. J. Integr. Plant Biol. 2020, 62, 1895–1909. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gao, Y.; Wu, W.; Chen, L.; Wang, Y. Two calcium-dependent protein kinases enhance maize drought tolerance by activating anion channel ZmSLAC1 in guard cells. Plant Biotechnol. J. 2022, 20, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, C.; Wang, H.; Wang, S.; Yang, S.; Liu, X.; Yan, J.; Li, B.; Beatty, M.; Zastrow-Hayes, G. Mapping regulatory variants controlling gene expression in drought response and tolerance in maize. Genome Biol. 2020, 21, 163. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Habben, J.E.; Archibald, R.L.; Drummond, B.J.; Chamberlin, M.A.; Williams, R.W.; Lafitte, H.R.; Weers, B.P. Overexpression of ARGOS genes modifies plant sensitivity to ethylene, leading to improved drought tolerance in both Arabidopsis and maize. Plant Physiol. 2015, 169, 266–282. [Google Scholar] [CrossRef] [PubMed]
- Sammons, B.; Whitsel, J.; Stork, L.G.; Reeves, W.; Horak, M. Characterization of drought-tolerant maize MON 87460 for use in environmental risk assessment. Crop Sci. 2014, 54, 719–729. [Google Scholar] [CrossRef]
- Tesfaye, K.; Kruseman, G.; Cairns, J.E.; Zaman-Allah, M.; Wegary, D.; Zaidi, P.H.; Boote, K.J.; Rahut, D.; Erenstein, O. Potential benefits of drought and heat tolerance for adapting maize to climate change in tropical environments. Clim. Risk Manag. 2018, 19, 106–119. [Google Scholar] [CrossRef]
- Jiang, L.-G.; Li, B.; Liu, S.-X.; Wang, H.-W.; Li, C.-P.; Song, S.-H.; Beatty, M.; Zastrow-Hayes, G.; Yang, X.-H.; Qin, F. Characterization of Proteome Variation During Modern Maize Breeding. Mol. Cell. Proteom. 2019, 18, 263–276. [Google Scholar] [CrossRef] [PubMed]
- EL Sabagh, A.; Hossain, A.; Aamir Iqbal, M.; Barutçular, C.; Islam, M.S.; Çiğ, F.; Erman, M.; Sytar, O.; Brestic, M.; Wasaya, A.; et al. Maize Adaptability to Heat Stress under Changing Climate. In Plant Stress Physiology; Intechopen: London, UK, 2021. [Google Scholar] [CrossRef]
- Chukwudi, U.P.; Kutu, F.R.; Mavengahama, S. Influence of heat stress, variations in soil type, and soil amendment on the growth of three drought–tolerant maize varieties. Agronomy 2021, 11, 1485. [Google Scholar] [CrossRef]
- Niu, S.; Du, X.; Wei, D.; Liu, S.; Tang, Q.; Bian, D.; Zhang, Y.; Cui, Y.; Gao, Z. Heat stress after pollination reduces kernel number in maize by insufficient assimilates. Front. Genet. 2021, 12, 728166. [Google Scholar] [CrossRef]
- Li, Y.; Han, X.; Ren, H.; Zhao, B.; Zhang, J.; Ren, B.; Gao, H.; Liu, P. Exogenous SA or 6-BA maintains photosynthetic activity in maize leaves under high temperature stress. Crop J. 2023, 11, 605–617. [Google Scholar] [CrossRef]
- Ahmad, M.; Imtiaz, M.; Nawaz, S.; Mubeen, F.; Sarwar, Y.; Hayat, M.; Asif, M.; Naqvi, R.; Imran, A. Thermotolerant PGPR consortium B3P modulates physio-biochemical and molecular machinery for enhanced heat tolerance in maize during early vegetative growth. Ann. Microbiol. 2023, 73, 34. [Google Scholar] [CrossRef]
- Chekole, F.C.; Mohammed Ahmed, A. Future climate implication on maize (Zea mays) productivity with adaptive options at Harbu district, Ethiopia. J. Agric. Food Res. 2023, 11, 100480. [Google Scholar] [CrossRef]
- Ghani, A.; Yousaf, M.I.; Hussain, K.; Hussain, S.; Razaq, A.; Akhtar, N.; Ibrar, I.; Kamal, N.; Ali, B.; Khan, A.M. Relationship between high-temperature stress and key physio-chemical, reactive oxygen species and antioxidants in spring maize hybrids under semi-arid conditions. Biol. Clin. Sci. Res. J. 2023, 2023, 199. [Google Scholar] [CrossRef]
- Muruo, R.M.; Nchore, S.B.; Oduor, R.O.; Ngugi, M.P. Overexpressing the IPT gene improves drought tolerance and nutritional value of tropical maize (Zea mays L.). bioRxiv 2023. [Google Scholar] [CrossRef]
- Mansour, E.; El-Sobky, E.S.E.A.; Abdul-Hamid, M.I.E.; Abdallah, E.; Zedan, A.M.I.; Serag, A.M.; Silvar, C.; El-Hendawy, S.; Desoky, E.S.M. Enhancing Drought Tolerance and Water Productivity of Diverse Maize Hybrids (Zea mays) Using Exogenously Applied Biostimulants under Varying Irrigation Levels. Agronomy 2023, 13, 1320. [Google Scholar] [CrossRef]
- Messina, C.D.; Gho, C.; Hammer, G.L.; Cooper, M. Two decades of harnessing standing genetic variation for physiological traits to improve drought tolerance in maize (Zea mays L.). J. Exp. Bot. 2023, 74, 4847–4861. [Google Scholar] [CrossRef] [PubMed]
- Nihranz, C.T.; Guzchenko, I.A.; Casteel, C.L. Silencing ZmPP2C-A10 with a foxtail mosaic virus (FoMV) derived vector benefits maize growth and development following water limitation. Plant Biol. 2023, 25, 956–964. [Google Scholar] [CrossRef]
- Abaza, A.S.D.; Elshamly, A.M.S.; Alwahibi, M.S.; Elshikh, M.S.; Ditta, A. Impact of different sowing dates and irrigation levels on NPK absorption, yield and water use efficiency of maize. Sci. Rep. 2023, 13, 12956. [Google Scholar] [CrossRef]
- Xu, J.; Mu, Q.; Ding, Y.; Sun, S.; Zou, Y.; Yu, L.; Zhang, P.; Yang, N.; Guo, W.; Cai, H. Considering spatio-temporal dynamics of soil water with evapotranspiration partitioning helps to clarify water utilization characteristics of summer maize under deficit irrigation. J. Hydrol. 2023, 617, 129102. [Google Scholar] [CrossRef]
- Te, X.; Din, A.M.U.; Cui, K.; Raza, M.A.; Faraz Ali, M.; Xiao, J.; Yang, W. Inter-specific root interactions and water use efficiency of maize/soybean relay strip intercropping. Field Crop. Res. 2023, 291, 108793. [Google Scholar] [CrossRef]
- Setti, A.; Castelli, G.; Villani, L.; Ferrise, R.; Bresci, E. Modelling the impacts of water harvesting and climate change on rainfed maize yields in Senegal. J. Agric. Eng. 2023, 54. [Google Scholar] [CrossRef]
- Jiao, F.; Hong, S.; Zhang, Q.; Li, M.; Shi, R.; Ma, Y.; Li, Q. Subsoiling before winter wheat cultivation increases photosynthetic characteristics and leaf water-use efficiency of summer maize in a double-cropping system. Arch. Agron. Soil Sci. 2023, 69, 847–860. [Google Scholar] [CrossRef]
- Hütsch, B.W.; Schubert, S. Grain yield, harvest index, water-use efficiency and nitrogen partitioning to grain can be improved by application of the plant growth regulator paclobutrazol to maize plants with reduced N supply. J. Agron. Crop Sci. 2023, 209, 261–272. [Google Scholar] [CrossRef]
| Target Gene | Technique | Comments | Reference |
|---|---|---|---|
| ZmNF-YB16 | Agrobacterium-mediated method | ZmNF-YB16 overexpression: maintains higher photosynthesis, improves drought stress resistance, and increases grain yield under drought stress conditions | [77] |
| nced, rpk | Agrobacterium-mediated method | Development of drought-resistant maize by introducing two genes involved in the ABA pathway and manipulating ABA signaling | [78] |
| betA | Agrobacterium-mediated method | Enhanced Glycine Betaine Accumulation: Improved osmotic/drought stress tolerance in transgenic maize | [79] |
| ZmSDD1 | Agrobacterium-mediated method | Overexpression of ZmSDD1: reduced stomatal density and transpiration rate, improved drought tolerance | [80] |
| Population | Trait | QTL | Reference |
|---|---|---|---|
| Ac7643 x Ac7729/TZSRW RIL | Leaf elongation rate in correspondence with ASI | 5 | [86] |
| Ac7643 x Ac7729/TZSRW RIL | Seedling root traits in PEG solution | 13 | [87] |
| Ac7643S5 x Ac7729/TZSRWS5 F2 families | Flowering parameters, ASI | 7 | [53] |
| Zong3 x 87-1 RIL | LTD, RSDW, ESFW | 9 | [88] |
| Lo964 x Lo1016 NIL F3:4 families | Root trait and yield | 1 (root-yield-1.06) | [89] |
| X178 x B73 F2:3 families | Yield and ASI | 2 | [90] |
| SD34 x SD35 F3 families | Yield, plant height, days to silking, ear number | 5 | [91] |
| CML444 x MALAWI RIL, CML440 x CML504 F2:3 families, CML444 x CML441 F2:3 families | GY and ASI | GY: 83, ASI: 62 | [92] |
| Os420 (high L-ABA) x IABO78 (low L-ABA) F3:4 families | L-ABA | 16 | [93] |
| Os420 (high L-ABA) x IABO78 (low L-ABA) F4 families | L-ABA and yield | 17 | [94] |
| H082183 (drought-tolerant) x Lv28 (drought sensitive) | Yield | 2 (qEL4s, qKW4s) | [95] |
| Huangzaosi x Mo17 RIL F7 families | Flowering time: DTA | 2 (qDTA3-3, qDTA10) | [96] |
| Han21 (drought-tolerant) x Ye478 (drought sensitive) BC3F6 families | GY, ESP, ASI | 2 (qWS-GY3-1, qWS-ESP3-1) | [97] |
| Technique | Comments | Reference |
|---|---|---|
| CRISPR/Cas9 system to edit ARGOS8 Delivery: particle bombardment | Flowering: Increases grain yield by 2–3% Grain ripening: Does not increase grain yield | [117,121] |
| Transgenic maize with homologous ZmNF-YB2 | Increased grain yield by 50% | [75] |
| Transgenic maize preserves RNA stability and translation of cold shock protein B | Maintain cell function under water stress conditions | [122] |
| Gene knockout using CRISPR/Cas9 | Generating abh2-CRISPR knockout drought-tolerant maize: i1, d2, and d35 | [120] |
| Gene knockout using CRISPR/Cas9 Delivery: Agrobacterium | Generating ZmSRL5-CRISPR knockout maize: KO1, KO2 ZmSRL5 is involved in drought tolerance by maintaining waxy structures in maize | [118] |
| CRISPR/Cas9 Delivery: Agrobacterium | Generating zmslac1-2 and zmcpk37 mutant-maize ZmSLAC1: stomatal closure in maize ZmCPK35 and ZmCPK37: improve drought tolerance and reduce yield loss under drought stress | [119] |
| Factors | Description | References |
| High-temperature tolerance |
| [125,126,127,128,129,130,131] |
| Drought tolerance |
| [132,133,134,135] |
| Response to low precipitation |
| [136,137,138,139,140,141] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.-H.; Lee, B.-M. Effects of Climate Change and Drought Tolerance on Maize Growth. Plants 2023, 12, 3548. https://doi.org/10.3390/plants12203548
Kim K-H, Lee B-M. Effects of Climate Change and Drought Tolerance on Maize Growth. Plants. 2023; 12(20):3548. https://doi.org/10.3390/plants12203548
Chicago/Turabian StyleKim, Kyung-Hee, and Byung-Moo Lee. 2023. "Effects of Climate Change and Drought Tolerance on Maize Growth" Plants 12, no. 20: 3548. https://doi.org/10.3390/plants12203548
APA StyleKim, K.-H., & Lee, B.-M. (2023). Effects of Climate Change and Drought Tolerance on Maize Growth. Plants, 12(20), 3548. https://doi.org/10.3390/plants12203548






