1. Introduction
In the Mediterranean basin, the high concentration of NaCl in irrigation water poses a major problem [
1]. Globally, approximately 7% of soil is affected by salinity [
2]. Salinity can be classified as primary salinity, which occurs due to environmental conditions such as reduced rainfall, wind, or natural processes like erosion, or secondary salinity, resulting from human activities such as excessive water and fertilizer usage [
3]. Salinity induced by climate change refers to the excess accumulation of Na
+ and/or Cl
− in the rhizosphere [
4]. Ions can accumulate in water when the plant’s concentration is lower than that of the initial irrigation water [
5]. Salt stress poses severe challenges for vegetable crops, particularly tomatoes, as it inhibits growth and biomass production by negatively impacting cell division and elongation [
6]. Furthermore, it adversely affects the yield and tomato fruit size [
7]. The reduced yield is a consequence of a decrease in the number of fruits, which, according to Cuartero and Fernandez-Muñoz (1998) [
8], is associated with a reduction in flower production with increasing salinity. According to Gama et al. (2007) [
9], the increased NaCl concentration in the roots leads to imbalances in different plant parts, especially for nutrients such as K, Ca, and Mg ions, for which assimilation problems are caused [
10].
Tomato (
Solanum lycopersicum L.) is the second most important crop worldwide in terms of production and consumption after potato, with cultivation covering an area of 5.03 Mha and a production of 180 Mt (FAO) [
11]. In the Mediterranean basin, tomato is the predominant vegetable crop, both in outdoor and greenhouse cultivation [
12]. A tomato is a plant characterized by a moderate resistance to salt stress [
13,
14], with variations in tolerance depending on the genotype [
15]. Consequently, tomatoes can be successfully grown with an electrical conductivity (EC) of up to 2.5 dS/m without compromising yield [
16]. According to Campos et al. (2006) [
17], for each unit increase in salinity above 2.5 dS/m, there is an approximate 10% reduction in tomato yield.
Landraces, unlike modern cultivated varieties [
18], offer the advantage of being able to adapt to adverse environments (e.g., salinity, drought, heat) without compromising yields [
19,
20]. Extensive research has been dedicated to identifying tolerance traits in tomato landraces that confer resilience to abiotic stresses such as drought and salinity [
21,
22]. Salt stress tolerant landraces or tomato genotypes exhibit the ability to alleviate the adverse effects of stress through mechanisms such as enhanced root development to overcome saline zones [
23] or activation of biochemical and physiological processes that can enable ion and water homeostasis restoration [
24].
Considering the aforementioned background, the present study aims to assess the impact of salinity on the growth, yield, and macro- and micronutrient concentration in the roots, leaves, and fruit of several Mediterranean tomato landraces. Their divergent response to the moderate salinity stress caused by a concentration of 30 mM NaCl in the rhizosphere is of great interest, as these traditional cultivars hold potential as valuable genetic resources for integration into breeding programs or as a promising tomato rootstock material in the near future. Additionally, they offer promising avenues for future research aimed at identifying genetic traits and mechanisms that contribute to improved fruit quality and enhanced resilience to salinity stress.
2. Results
2.1. Plant Growth Responses
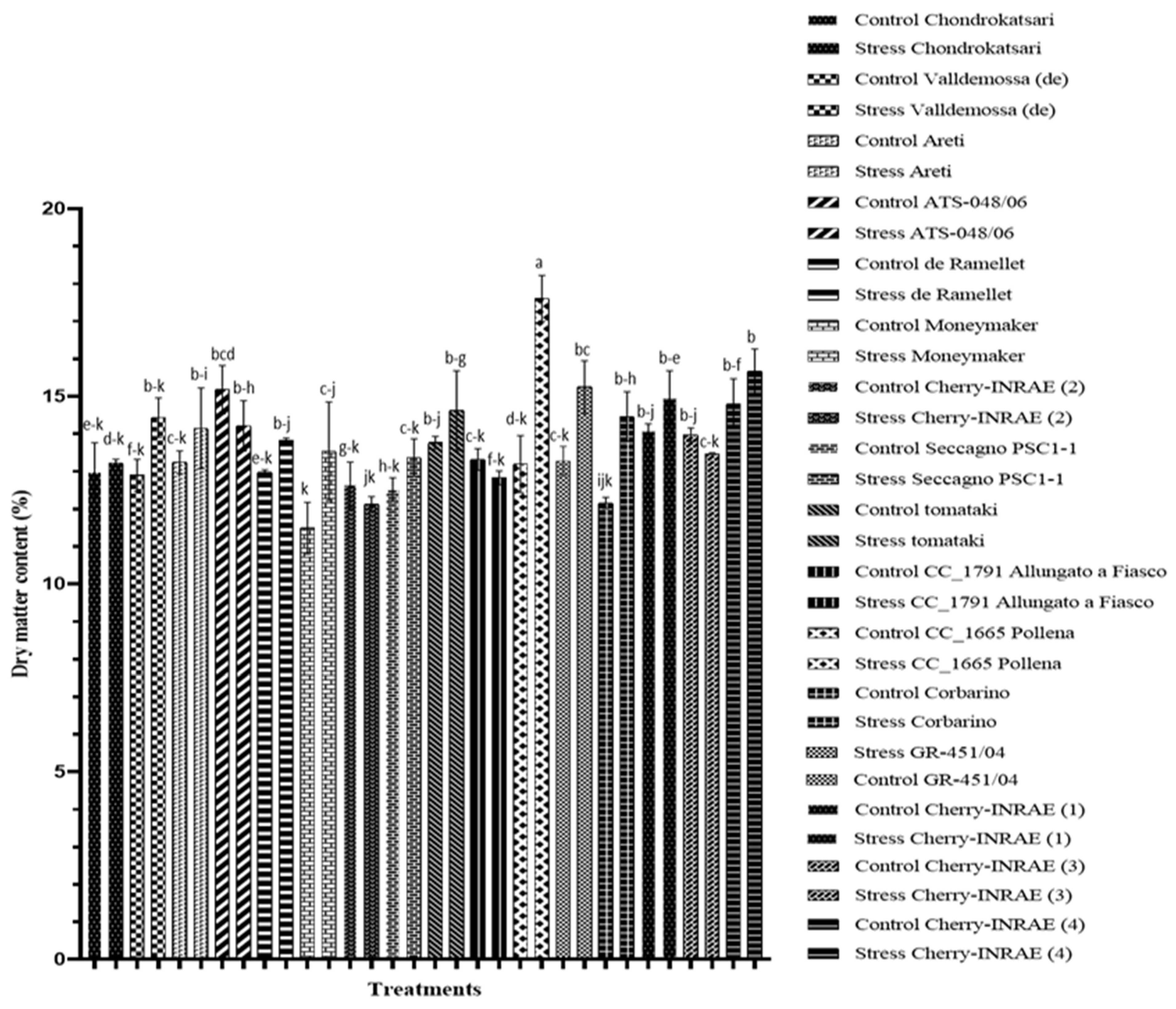
The growth of tomato plants, as indicated by the dry matter content of the leaves (
Figure 1), is significantly affected by salinity. Furthermore, this study reveals statistically significant differences among different cultivated genotypes and their interactions with salinity. According to
Figure 1, the leaf dry matter content of the two cherry-type landraces, ‘CC_1665 Pollena’ and ‘Corbarino’, significantly increased by 25 and 16%, respectively, while at the same time the dry matter content of the leaves of the reference variety ‘Moneymaker’ increased by 15% when subjected to salinity stress.
2.2. Yield
Significant differences were observed among the different genotypes under salinity stress in terms of fruit production, as indicated by the mean fruit weight and the number of fruits per plant (
Table 1). The impact of salinity on the number of fruits varied across different tomato genotypes. Notably, the mid-type landrace ‘Cherry-INRAE (2)’ and the cherry-type landraces ‘Corbarino’, ‘Cherry-INRAE (1)’, and ‘Cherry-INRAE (3)’ exhibit a reduction in fruit number when grown in a nutrient solution supplemented with 30 mM NaCl. Specifically, the above-mentioned genotypes produced approximately 19, 18, 13, and 21 fruits less than the control, respectively, when subjected to salinity stress. When considering mean fruit weight, the two beef-steak landraces, ‘Chondrokatsari’ and ‘Areti’, were the ones that, under stress conditions, had significantly reduced their mean fruit weight by 23 and 14%, respectively, compared to normal growth conditions.
As illustrated in
Figure 2, 30 mM of NaCl in the root environment of tomatoes differentially impacted the genotypes under study, with some showing a decrease in marketable yield while others remained unaffected. Among the genotypes evaluated, approximately half of them exhibit a decrease in marketable yield under stress conditions. Specifically, all four beef-steak landraces ‘Chondrokatsari’, ‘Valldemossa (de)’, ‘Areti’, and ‘ATS-048/06’ displayed significant reductions in marketable yield, with decreases of 28, 30, 25, and 21%, respectively, under the stress applied. In the mid-type ‘Moneymaker’ and ‘Cherry-INRAE (2)’, marketable yield was reduced by 22 and 41%, respectively, under stress conditions. Among the cherry-type landraces, ‘CC_1791 Allungato a Fiasco’ and ‘Corbarino’ exhibit significant reductions of 20 and 63%, respectively, in marketable yield when grown under salinity stress.
2.3. Concentration of Macro- and Micronutrients in Roots
The effect of salinity stress, the different genotypes, and their interaction on the concentrations of macronutrients (K, Na, Ca, and Mg) in plant roots was found to be significant (
Table 2). Specifically, under salinity stress, the cherry-type landrace ‘GR 451/04’ exhibits a 50% reduction in K concentration in the roots compared to plants grown under normal conditions. The cherry-type landrace ‘CC_1665 Pollena’ and the mid-type reference variety ‘Moneymaker’ also show significant reductions of 43%, and the large-fruited ‘de Ramellet’ had a reduction of 37% in K concentration compared to those plants grown under normal conditions.
Regarding the K/Na ratio in the roots, it is observed that of the large-fruited landraces, only the ‘de Ramellet’ does not show a difference under salinity conditions, while among the cherry-type ‘CC_1665 Pollena’, ‘Cherry-INRAE (1)’, ‘Cherry-INRAE (3)’, and ‘Cherry-INRAE (4)’ are the ones where the K/Na ratio did not show a significant difference between the two treatments (0.5 and 30 mM NaCl).
Regarding Ca concentration, the mid-type landrace ‘de Ramellet’ shows a significant decrease of 50% in the roots under high NaCl concentration in the nutrient solution. However, the cherry-type varieties exhibit a lesser decrease in Ca concentration under salt stress. In particular, ‘CC_1665 Pollena’ shows an increase of 13%, and ‘CC_1791 Allungato a Fiasco’ shows an increase of 22% in Ca concentration in the roots compared to the control.
The beefsteak landrace ‘Chondrokatsari’ displayed a significant decrease of 55% in Mg concentration in the roots under salinity stress conditions. In contrast, no significant decrease in root Mg concentration of the cherry-type landraces ‘CC_1791 Allungato a Fiasco’, ‘CC_1665 Pollena’ and ‘Corbarino’ was found.
Regarding the salinity factor, the addition of NaCl affected the Na concentration in the roots, which increased under stress conditions. The cherry-type landraces ‘CC_1791 Allungato a Fiasco’ and ‘Corbarino’ exhibit the highest percentage of sodium in the roots. On the other hand, the large-type landrace ‘de Ramellet’ and the cherry-type ‘Cherry-INRAE (4)’ had the lowest Na concentration in their roots. No significant difference in the Na root concentration was observed between the two treatments (0.5 and 30 mM NaCl) for the three other cherry-type landraces ‘Cherry-INRAE (1)’, ‘Cherry-INRAE (3)’, and ‘CC_1665 Pollena’.
Among the four trace elements studied (Fe, Cu, Mn, and Zn), three of them (Fe, Cu, and Mn) show statistically significant differences in response to stress in the different landraces, while zinc (Zn) did not exhibit significant changes. Regarding the concentration of Fe in plant roots, the variety ‘Moneymaker’ used as a reference shows the greatest reduction of about 67% under stress conditions. On the other hand, the cherry-type landrace ‘Cherry-INRAE (1)’ displayed a slight increase in Fe concentration with a tendency towards increasing levels under salt stress.
For Cu concentration, the landrace ‘de Ramellet’ exhibits the greatest reduction of 37% under salinity conditions. In contrast, the cherry-type landrace ‘Cherry-INRAE (1)’ did not show any significant change in Cu concentration in the roots when exposed to salinity stress.
The concentration of Mn in the roots of plants of the beef-steak landrace ‘Chondrokatsari’ shows the greatest decrease compared to the other varieties, with a reduction of about 41%. On the other hand, the cherry-type landraces ‘Cherry-INRAE (4)’, ‘Cherry-INRAE (1)’, and the mid-type ‘Seccagno PSC1-1’ did not display any significant changes in Mn concentration in their roots when subjected to salinity stress. As shown in
Table 3, salinity stress significantly reduced the concentrations of Zn in the roots of tomato plants by about 9%. Furthermore, the different cultivars had statistically significant differences in the concentration of Zn in their roots. Specifically, the Zn concentration of the cherry-type landrace ‘tomataki’ was close to the highest concentration of the cherry-type landrace ‘Cherry-INRAE (1)’ (122,42 μg/g DW).
According to
Table 4, salt stress significantly increased the concentration of potassium (K) by 18% while the concentration of magnesium (Mg) remained unaffected.
2.4. Concentration of Macro- and Micronutrients in Leaves
The different cultivars show significant differences for leaf K and Mg contents. Among the tested genotypes, the mid-type landrace ‘Seccagno PSC1-1’ and the cherry-type landraces ‘GR-451/04’, ‘Corbarino’, and ‘Cherry-INRAE (4)’ displayed the highest K concentration, while the two beefsteaks ‘ATS-048/06’, ‘Valldemossa (de)’, and a cherry-type landrace ‘tomataki’ had the lowest K concentrations, which were approximately half of the concentration observed in the genotypes with the highest K levels. The genotypes in which the leaf K/Na ratio did not change under salt stress conditions were the cherry-type landraces ‘CC_1665 Pollena’, ‘Cherry-Inrae (3)’, and ‘Cherry-Inrae (4)’.
Among the studied genotypes, the beef-steak landrace ‘Valldemossa (de)’ and the two cherry-type landraces ‘Cherry-INRAE (4)’ and ‘Corbarino’ exhibit the highest Mg concentration, reaching approximately 10 mg/g dry weight (DW).
In terms of leaf Na content, all cultivars grown under salt stress displayed increased concentrations in their leaves. The beef-steak ‘Chondrokatsari’ was the one with the highest increase under stress conditions, approximately 88%, while the smallest increase of 61% was observed in the beef-steak landrace ‘ATS-048/06’. No significant difference in the Na leaf concentration was observed between the two treatments (0.5 and 30 mM NaCl) for the landraces ‘Cherry-INRAE (3)’, ‘Cherry-INRAE (4)’, ‘CC_1665 Pollena’, and ‘GR_451/04’. As for Ca, the reference cultivar ‘Moneymaker’ demonstrates the largest decrease of about 30% in leaf concentration under salt stress.
A significant interaction among the treatments (salinity × genotype) was evident for all micronutrients in the leaves (
Table 5). For Fe, the cherry-type landrace ‘GR-451/04’ increased its leaf Fe concentration by 49% under stress conditions, while the beefsteak landrace ‘Chondrokatsari’ shows a decrease of 35% in Fe concentration.
In terms of Cu, the beef-steak landrace ‘Areti’ increased its leaf concentration under salinity stress conditions, while the cherry-type variety ‘Cherry-INRAE (1)’ exhibits a 66% decrease. Regarding Mn concentration in the leaves, a decrease was observed in the beef-steak and cherry-type varieties under salinity stress conditions. The decrease ranged from 60% in ‘de Ramellet’, 32% in ‘Chondrokatsari’, 26% in ‘Cherry-INRAE (2)’, and 39% in ‘Moneymaker’, to 60% and 46% in the cherry-type ‘tomataki’. Finally, for Zn, some beef-steak and cherry-type varieties show a decrease in leaf concentration under saline conditions, while others exhibit significantly higher concentrations. Specifically, the large-type ‘de Ramellet’, ‘Valldemossa (de)’, ‘ATS-048/06’, and ‘Areti’ and a cherry-type variety ‘GR-451/04’ were the ones that show a decrease of the micronutrient in their leaves when grown under salinity conditions, while the mid-type ‘Seccagno PSC1-1’ and the cherry-type Cherry-INRAE (4)’ were the ones that show a significantly higher concentration of Zn under stress conditions. The cherry-type landraces ‘CC_1791 Allungato a Fiasco’ and CC_1665 Pollena’ did not show a significant difference in any of the measured micronutrients between the salinity treatments.
2.5. Concentration of Macro- and Micronutrients in Fruit
As shown in
Table 6, a significant interaction among the treatments was found for all macronutrients in the tomato fruit. Specifically, the beef-steak landraces ‘de Ramellet’ and ‘Cherry-INRAE (2)’ and the two cherry-type landraces ‘CC_1665 Pollena’ and ‘tomataki’ exhibit a reduction in K concentration under stress conditions by 15%, 19%, 19%, and 16%, respectively. Τhe K/Na ratio in fruit did not have any statistically significant difference for the cultivation of the different genotypes under saline stress. A significant decrease in the Ca concentration in the fruits was observed in the beef-steak landrace ‘Areti’ and in the cherry-type landrace ‘GR-451/04’ cultivated under increased NaCl in the nutrient solution. A corresponding decrease was also observed in the Mg concentration in the fruit of large-type landraces ‘Valldemossa (de)’ and ‘de Ramellet’ as well as the cherry-type ‘CC_1665 Pollena’, ‘Corbarino’, and ‘Cherry-INRAE (1)’. On the other hand, Na concentration increased in almost all varieties under salinity stress conditions. However, variations among the varieties were identified. The landrace ‘CC_1665 Pollena’ shows the same Na fruit concentration in both treatments, indicating a limited response to salinity. The landraces ‘Cherry-INRAE (1)’, ‘Cherry-INRAE (4)’, and ‘Corbarino’ exhibit the lowest increase of this element in the fruit compared to all other varieties in the study.
The interaction between salinity and the different cultivars shows statistically significant differences for all measured micronutrients (
Table 7). Among the landraces, the large-type variety ‘de Ramellet’ was the one with the largest decrease in all fruit trace element concentrations under stress conditions. Specifically, the fruit of this variety shows a reduction of approximately 40% in Fe, 50% in Cu, 30% in Mn, and 14% in Zn. The cherry-type landrace ‘GR-451/04’ also displayed significant reductions in Fe, Mn, and Zn fruit concentrations, with decreases of 43%, 25%, and 18%, respectively. Interestingly, this variety shows a significant increase of 40% in Cu content in the fruit of stressed plants. Finally, the beef-steak landrace ‘Areti’ shows reductions of 37% in Fe concentration and 15% in Mn concentration in the stressed fruit.
2.6. Sodium Content (%)
As shown in
Figure 3, the distribution of sodium was different in the plant parts determined in the present study for the different genotypes. In a general context, the highest Na content was found in the leaves, then in the roots, and the lowest Na content was found in the fruit. However, this distribution was genotype-dependent. It is observed that the large-sized landraces ‘Chondrokatsari’ and ‘de Ramellet’ have a similar distribution of Na in the parts of the plant. However, there are also genotypes such as the large-sized landrace ‘Areti’ and the cherry-type ‘GR-451/04’ in which the Na content appears to be almost equally distributed in leaves, roots, and fruits. Conversely, in the cherry-type landraces, only a small percentage was found in the fruit, while the rest was distributed in the leaves and the roots.
3. Discussion
High salinity has a detrimental impact on tomato plant biomass [
25]. Romero-Aranda et al. (2001) [
26] explained this phenomenon as a decrease in water potential in leaves due to the presence of high salinity in their rhizosphere, which adversely affects various plant processes. Additionally, under salt stress, the excessive accumulation of Na and the concomitant nutrient imbalances in plants further impede their growth [
27]. In the present study, the exposure of tomatoes to a NaCl concentration of 30 mM in the nutrient solution retained in the root zone (root solution) increased the dry matter content of the leaves. This finding aligns with the research conducted by Douglas McCall and Aušra Brazaityte (1997) [
28], where increasing EC resulted in increased shoot dry matter content. According to Adams et al. (1990) [
29], a negative correlation exists between NaCl concentration and leaf area in tomato plants, as increased NaCl levels led to a reduction in leaf size [
30,
31]. Nonetheless, in the present study, notable variations among the landraces were found, indicating the divergent tolerance of different tomato landraces to salinity stress [
32,
33]. Among the landraces examined, ‘CC_1665 Pollena’, ‘Corbarino’, and ‘GR-451/04’ exhibit a statistically significant increase in dry matter content under saline conditions (
Figure 1). However, it is worth noting that only ‘Corbarino’ displayed a significant decrease in marketable yield (
Figure 2).
To evaluate the effect of salinity on the different tomato landraces under study, fruit yield was assessed at a moderate concentration of NaCl (30 mM) in the root zone. According to Saranga et al. (1991) [
34], a yield reduction of approximately 10% occurs for each 1 dSm
−1 increase above the threshold of 2.5 dSm
−1, which signifies a decline in production. Furthermore, Rodríguez-Ortega et al. (2019) [
35] reported a reduction of 35% and 58% in marketable yield in soilless tomato crops exposed to 40 mM and 80 mM NaCl, respectively, compared to the control. However, the response of tomato genotypes to salt stress exhibits notable variation. Caro et al. (1991) [
36] demonstrated that cherry-type cultivars display higher tolerance to saline stress compared to those with normal-sized fruits. In our study, eight of the tested genotypes did not show a statistically significant decrease in marketable yield. More specifically, the large-sized ‘de Ramellet’ and the mid-type ‘Seccagno PSC1-1’ with reduction percentages of 17% and 26%, respectively, were the two genotypes that did not show a statistically significant difference in their yield under salinity stress conditions. In addition, a non-significant reduction in marketable yield was observed in six cherry-type landraces. These landraces were ‘CC-1665 Pollena’, ‘GR-451/04’, ‘tomataki’, ‘Cherry-INRAE (1)’, ‘Cherry-INRAE (3)’, and ‘Cherry-INRAE (4)’ with 35%, 18%, 14%, 22%, 24%, and 5% reduction, respectively (
Figure 2).
In the study conducted by Liu et al. (2014) [
37], it was found that the addition of 50 mM of nutrient solution resulted in a 21% reduction in the number of fruit per plant in the tomato variety (TA19) compared to the control. Similarly, Magán et al. (2008) [
7] reported that the reduced yield in tomato production was attributed to a decrease in the number of fruits per plant with increasing salinity. Consistent with these findings, the present study reveals a significant decrease in fruit number per plant under moderate salinity conditions of 30 mM in the root zone, amounting to approximately 14%. The cherry-type landraces ‘Corbarino’, ‘Cherry-INRAE (1)’, ‘Cherry-INRAE (3)’, and ‘Cherry-INRAE (4)’ were the landraces in which a statistically significant reduction of 18, 14, 19, and 21%, respectively, was observed. In contrast, the other genotypes did not change their fruit number under salinity stress conditions (
Table 1). This decline in fruit number can be attributed to the disruption of the plant’s physiological functions caused by osmotic stress and the consequent imbalance of nutrients resulting from the increased concentration of salt [
38]. Therefore, our results indicate that the decline in production was primarily driven by a decrease in the average fresh fruit weight and the number of fruits per plant [
39], and this effect was genotype-dependent. Therefore, landraces that, under saline stress conditions, did not reduce their yield can be characterized as tolerant to this salinity level (30 mM NaCl), as according to Maas and Hoffman (1977) [
16], crops are considered resistant to the level of salinity that does not affect their yield.
Τhe addition of NaCl to the nutrient solution leads to an increase in the Na concentration within the plant tissues [
40]. Sodium accumulation varies across different plant parts [
24], reflecting its distinct distribution within plant organs. According to Babu et al. (2012) [
41], the Na content of tomato leaves increases more significantly than in fruits when cultivated under high NaCl concentrations. Similarly, in the present study, the Na content in the leaves of the stressed plants was approximately five times higher compared to the control plant, while the increase in fruits was twice as high and in roots was three times higher than in the control plant (
Figure 3). Alfocea et al. (1993) [
42] reported that different genotypes of
L.
esculentum exhibit diverse responses to salinity, either by replacing K with Na or through K selectivity. In addition, the K/Na ratio determined in the present study is considered a selection factor for resistant cultivated species to salinity [
43]. The ability of plants to exclude salt is one of the mechanisms of salt tolerance [
44]. ‘Cherry-INRAE (1)’, ‘Cherry-INRAE (3)’, ‘Cherry-INRAE (4)’, and ‘de Ramellet’ landraces can be considered tolerant to moderate salinity as these genotypes did not show a significant difference in Na concentration or K/Na in the roots of stressed plants compared to the control (
Table 6). Additionally, these cultivars did not exhibit a significant difference in yield under the stress applied.
Potassium is a crucial macronutrient for tomato plants and plays a significant role in achieving high fruit quality [
45,
46]. However, under increased concentrations of NaCl, a nutrient imbalance occurs due to the substitution of K+ by n plants by Na+ in plant tissues. In the present study, a reduction in K concentration under salinity stress conditions was observed, with roots showing a decrease of 25% and leaves exhibiting a decrease of 20%. Specifically, the genotypes that show a significant reduction of K in their roots were the large-sized landraces ‘Areti’ at a 29% and 38% reduction, respectively, the mid-type variety ‘Moneymaker’ at 43%, and the cherry-type landraces ‘CC_1665 Pollena’ and ‘GR-451/04’ with a reduction at 43% and 53%. Regarding leaf K concentration, there was no statistically significant interaction for the genotypes under salinity conditions. This finding is consistent with the study conducted by Yan Li et al. (2009) [
47], who reported a greater decrease in K ions in roots compared to leaves. Furthermore, a 5% decrease in Κ concentration was observed in the fruit of the present study, in agreement with the study of Babu et al. (2012) [
41], where the concentration of potassium ions in the fruit decreased with increasing NaCl concentration. The large-type landrace ‘de Ramellet’ and the mid-type ‘Cherry-INRAE (2)’ under salinity stress conditions show a 16% and 19% reduction of K in their fruits, respectively. At the same time, the cherry-type landraces ‘CC_1665Pollena’ and ‘tomataki’ show a decrease of 19% and 16%, respectively, in K under stress conditions. According to Adams and Ho (1995) [
48], the competition of these two macronutrients leads to reduced uptake of potassium by plants under salinity conditions, and this reduction is more strongly related to decreased water uptake. Among the landraces studied, the large-fruited landrace ‘de Ramellet’ and the cherry-type ‘CC_1665 Pollena’ exhibit the most significant reductions in K concentration in both roots and fruit when grown under salinity conditions. Similarly, the K/Na ratio decreased in the leaves, fruits, and roots of tomatoes with the addition of NaCl to the nutrient solution. This observation aligns with the finding of Taffouo et al. (2010) [
49], who observed a decrease in the K/Na ratio in the leaves and roots of tomato cultivars grown under salinity stress. In a broader context, the small-sized tomatoes in this study were those with the highest K/Na ratios, indicating greater salinity tolerance. This observation is consistent with several studies [
50,
51,
52,
53] that highlight the ability of plants with high K/Na in their tissues to exhibit salinity tolerance.
Under high NaCl conditions, tomato plants exhibit reduced Ca uptake [
54], which is not solely due to competition with Na but also attributed to a decrease in transpiration rate caused by salinity stress [
8]. In the present study, salinity negatively affected the Ca concentrations in the roots, leaves, and fruit of tomato plants. Similar findings have been reported in other studies, where plants subjected to salinity stress exhibited a decrease in Ca concentration in leaves [
55] and fruit [
56]. This reduction in Ca concentration is associated with increased hydraulic resistance caused by high NaCl levels, resulting in reduced water and Ca transport. The roots of the large-type landraces ‘de Ramellet’, ‘Chondrokatsari’, and ‘Valldemossa (de)’ show a significant decrease of 50% in Ca concentration. In contrast, the other landraces did not display a significant reduction in the concentration of this ion. The retention of Ca in roots can be attributed to either a decrease in Na uptake and an increase in K uptake [
44,
57] or the maintenance of K concentration [
58], which contributes to the proper growth and the maintenance of adequate K levels in plants.
The concentration of Mg in the leaves of the cultivated tomato landraces was not significantly affected by salinity. This finding aligns with previous studies that reported no significant impact of NaCl on Mg content in tomato leaves [
59]. On the contrary, a significant decrease in Mg concentration was observed in the roots of plants subjected to salinity, which is consistent with the findings of Li et al. [
60] and Yunus and Zari [
59]. In particular, the large-type landraces ‘de Ramellet’, ‘Chondrokatsari’, and ‘Valldemossa (de)’ exhibit the highest decrease in Mg content in their roots. Furthermore, under high NaCl, a decrease in Mg concentration in tomato fruits was observed, although to a lesser extent (approximately 5%) compared to the leaves. Among the landraces, ’de Ramellet’ and ‘Valldemossa (de)’ also show a decrease in fruit Mg content under saline conditions.
The concentration of micronutrients (Fe, Cu, Mn, and Zn) in tomato tissues has been found to decrease with increasing NaCl [
61]. Consistent with previous studies on tomato cultivars [
62], the present study also observed a decrease in fruit micronutrient concentrations under salinity conditions. Nouck et al. [
63] similarly found that NaCl addition in the nutrient solution reduced the concentrations of Fe, Cu, Mn, and Zn in the roots of different tomato cultivars, confirming the findings of the present study. Variations in salinity tolerance and micronutrient accumulation were also observed among different tomato varieties, as reported by Nouck et al. [
63]. In terms of leaf responses to salinity stress, an increase in Fe concentration was observed, in line with the findings of García Fuentes et al. [
64], while the Mn concentration of tomato leaves decreased, as also reported by Alam et al. [
65]. The Cu concentration in leaves did not show significant differences, consistent with the study of El-Fouly et al. [
66]. Likewise, no differences were observed in leaf Zn concentration, consistent with the findings of García Fuentes et al. [
64] and Kowalska and Smolen [
67]. In the present study, variations in the micronutrient concentrations (Fe, Cu, Mn, and Zn) among different landraces under salt stress were observed. Notably, the variety ‘CC_1791 Allungato a Fiasco’ did not show any changes in micronutrient concentrations in response to salinity. Furthermore, for the landraces ‘Cherry-INRAE (4)’ and ‘Seccagno PSC1-1’, the increase in NaCl either had no effect or increased the concentration of Fe and Zn, but only in their leaves and not in other plant parts analyzed.
4. Materials and Methods
4.1. Experimental Design and Plant Material
The experiment was carried out at the greenhouse facilities of the Laboratory of Vegetable Production of the Agricultural University of Athens (AUA), located at coordinates 37°59′2″ N and 23°42′19″ E. Fifteen different Mediterranean tomato landraces and one tomato variety, ‘Moneymaker’, as a reference, were cultivated in an open soilless culture system by applying two different concentrations (0.5 and 30 mM) of NaCl in the nutrient solution. The name and the seed source of the cultivated tomatoes, including the fifteen landraces and the variety ‘Moneymaker’, are provided in
Table 8. The experiment was laid out as a randomized complete block design (RCBD) by applying two different concentrations of NaCl (0.5 and 30 mM) in the nutrient solution retained in the root zone (root solution).
4.2. Growth Conditions and Cultivation Practices
On 30 January 2021, the tomato seeds underwent a disinfection process using a 15% v/w trisodium phosphate (Na3PO4) solution. Following disinfection, the tomato seeds were placed in a temperature-controlled (25 °C) incubation chamber for germination. After three days, the germinated seeds were transplanted into sowing trays using turf as a substrate. On March 10, when the seedlings had developed four or five true leaves, they were transplanted into perlite bags (33 L), and the cultivation was performed in an open hydroponic system. Each treatment was replicated three times (3 perlite bags/treatment). Each perlite bag accommodated 3 plants of the same variety and was fed with nutrient solution (NS) from a ‘supply-tank’ via a pump and a drip irrigation system. The bottom of each bag was slit to allow the free drainage of NS supplied in excess of the plants’ demand. The mean temperature during the whole cultivation period was 21 °C (day) and 17 °C (night), respectively.
4.3. Nutrient Solution Formula
To calculate the nutrient solution (NS) applied to tomato plants, the NUTRISENSE online tool (accessible at
https://nutrisense.online) was used. The concentration of nutrients in the supplied nutrient solution varied depending on the treatment. Half of the plants were grown using a nutrient solution (NS) containing 30 mM NaCl (salinity treatment), while the other half were grown with a nutrient solution containing 0.5 mM NaCl (control treatment). The macro- and micronutrient concentrations in the nutrient solution for each treatment and developmental stage can be found in
Table 9. The pH in the nutrient solution was adjusted to 5.6 daily by adding appropriate amounts of 1 N HNO
3 stock solution.
4.4. Application of Salt Stress in Tomato Experiments Conducted in an Open Hydroponic System
To apply salt stress at this level, we followed the procedure described below:
A starter nutrient solution was prepared with a final concentration of 30 mmol L−1 (including the NaCl of the irrigation water) and an EC of 6.4–6.5 dS m−1. The starter NS was used to moisten the substrate in substrate-grown crops up to saturation, and then action was taken by silting the bottom of the substrate to enable free drainage, thereby reducing the moisture status to container capacity in the perlite.
The NaCl concentration was calculated in the NS used to fertigate the plants after transplanting (C
t) using Equation (1):
The target drainage fraction (a) in (1) was substituted by a suitable value (normally ranging from 0.1 to 0.35). Furthermore, C
d in (1) was replaced by 30 mmol L
−1 since this is the target NaCl concentration in the root zone. The uptake concentration of Na (C
u) in (1) was calculated using the relationship suggested by Carmassi et al. (2005) [
68] for tomatoes:
Equation (2) enables the calculation of the actual Na UC (Cu) in tomato crops as a relationship of the actual Na+ concentration in the root environment (Cr). Substituting Cr by 30 mmol L−1 (the target Na+ concentration in the root zone) in (2) renders a Cu of 5.4 mmol L−1. Replacing Cu with 5.4 mmol L−1 in (1) and with 0.3 (a standard drainage fraction) renders a Ct of 12.8 mmol L−1.
A standard NS for open soilless cultivation of tomato with an initial EC of 2.6–2.8 dS m−1 was prepared after the addition of the fertilizers and NaCl at a concentration of 12.8 mmol L−1. The addition of NaCl at this concentration is anticipated to increase the EC by 1.47 dS m−1, thereby rendering a final EC of about 4.2 dS m−1. This NS was used to supply the tomato plants in the salt stress treatment after their transplanting.
Sodium concentration was measured weekly in the drainage solution to control the NaCl level. If the measured Na concentration was substantially different than 30 mmol L
−1, C
ra was replaced by the measured value in (3) to estimate the actual C
u. Then use (1) again, as described in step 2, to calculate a new value for C
t. At the first measurement of the Na concentration in the drainage solution, C
rp by 30 mmol L
−1 was replaced, while in all subsequent measurements, the Na concentration was measured in the previous week as C
rp to adjust C
t. Subsequently, we repeated step 3 using the new C
t in place of 12.8 mmol L
−1.
4.5. Sampling and Measurements
At the end of the experiment, all tomato plants per treatment replication were sampled. From every plant, the fresh weight of the 3rd, 4th, and 5th leaf from the top was weighed. Its root was cleaned from perlite and rinsed with water. In addition, during the harvest, fruit samples were taken and their fresh weight was determined. The roots, leaves, and fruit were placed for drying at 65 °C to a constant weight.
4.6. Growth Parameter: Dry Matter Content
The measurements of fresh weight and dry weight after drying at 65 °C were used to determine the dry matter content. Leaf dry matter content (%) was calculated using the relationship:
4.7. Yield
The harvest period started on 11 May 2021, and ended on 28 June 2021, and the fruits were harvested twice per week when they reached their commercial ripe stage. The total fruit number per plant, the total fruit fresh weight per plant (g plant−1), and the mean fresh fruit weight (g) were recorded. Marketable yield was classified as fruit without cracking, blossom-end rot (BER), blotchy ripening, or deformations.
4.8. Nutrient Concentrations
After drying, all samples were crushed in a mill MF 10 Basic Micro Fine Grinder (IKA Werke, Staufen, Germany). This powder was placed in porcelain capsules and placed for dry ashing in a muffle furnace at 550 for 8 h. The ash was dissolved in 0.5 N HCl. This extract was used for the determination of K and Na (flame photometer method, Sherwood Model 410, Cambridge, UK), and the concentration of Ca, Mg, Zn, Fe, Cu, and Mn was also determined using an atomic absorption spectrophotometer (AA-7000, Shimadzu Co., Tokyo, Japan).
4.9. Statistical Analysis
In the current study, a two-way ANOVA analysis was applied to identify the main effects of the NaCl stress on growth parameters, marketable yield, and nutrient composition of plant tissue of the different tomato genotypes. The data were statistically evaluated by applying ANOVA using the STATISTICA software package, version 12.0 for Windows. When either salinity stress at the different landraces had a significant impact on a measured parameter, the means within each factor were separated using the Duncan’s Multiple Range test (p ≤ 5%).