Comprehensive Genome-Wide Identification of the RNA-Binding Glycine-Rich Gene Family and Expression Profiling under Abiotic Stress in Brassica oleracea
Abstract
:1. Introduction
2. Results
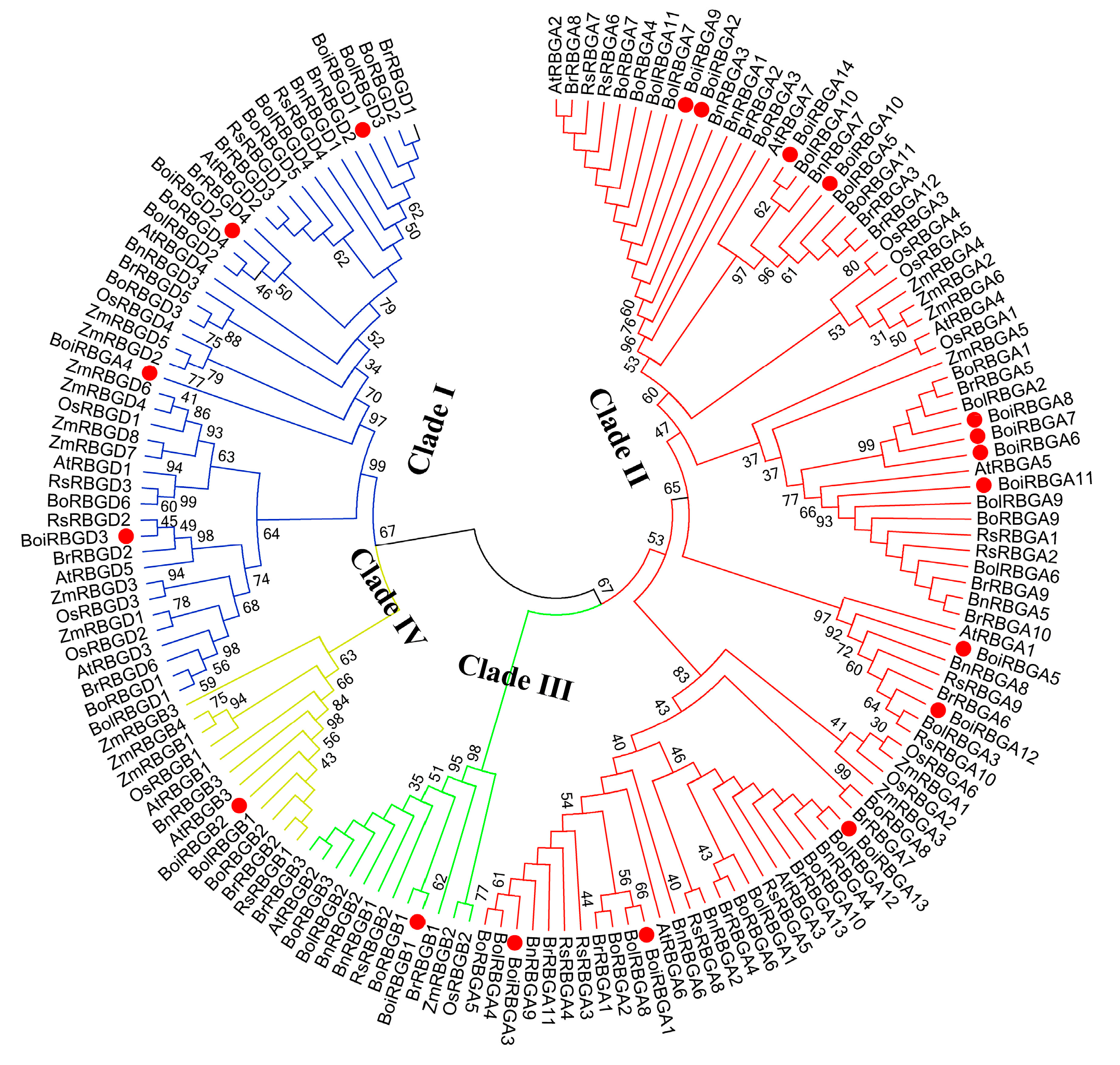
2.1. Genome-Wide Identification and Characterization of the RBG Gene Family Members
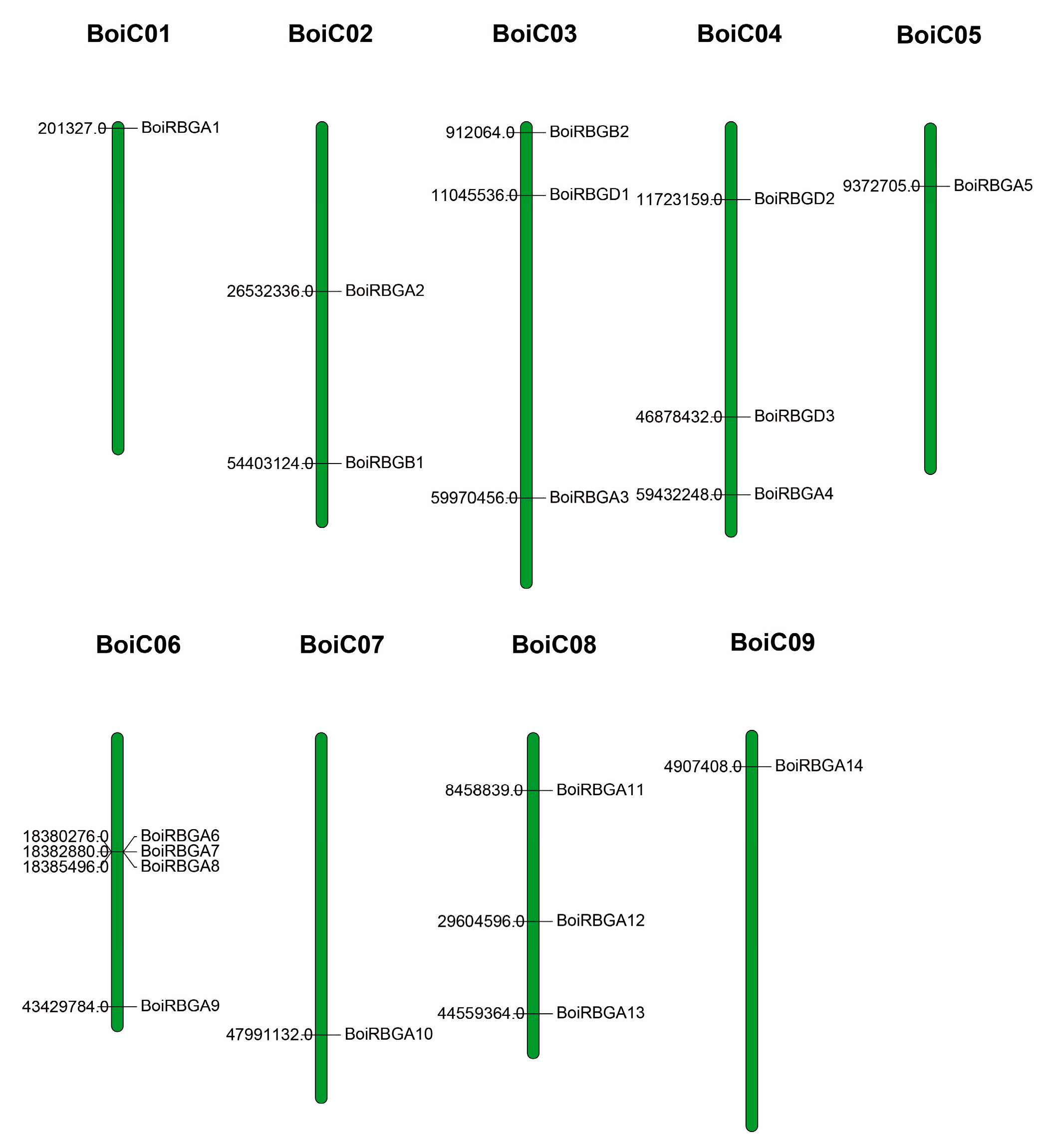
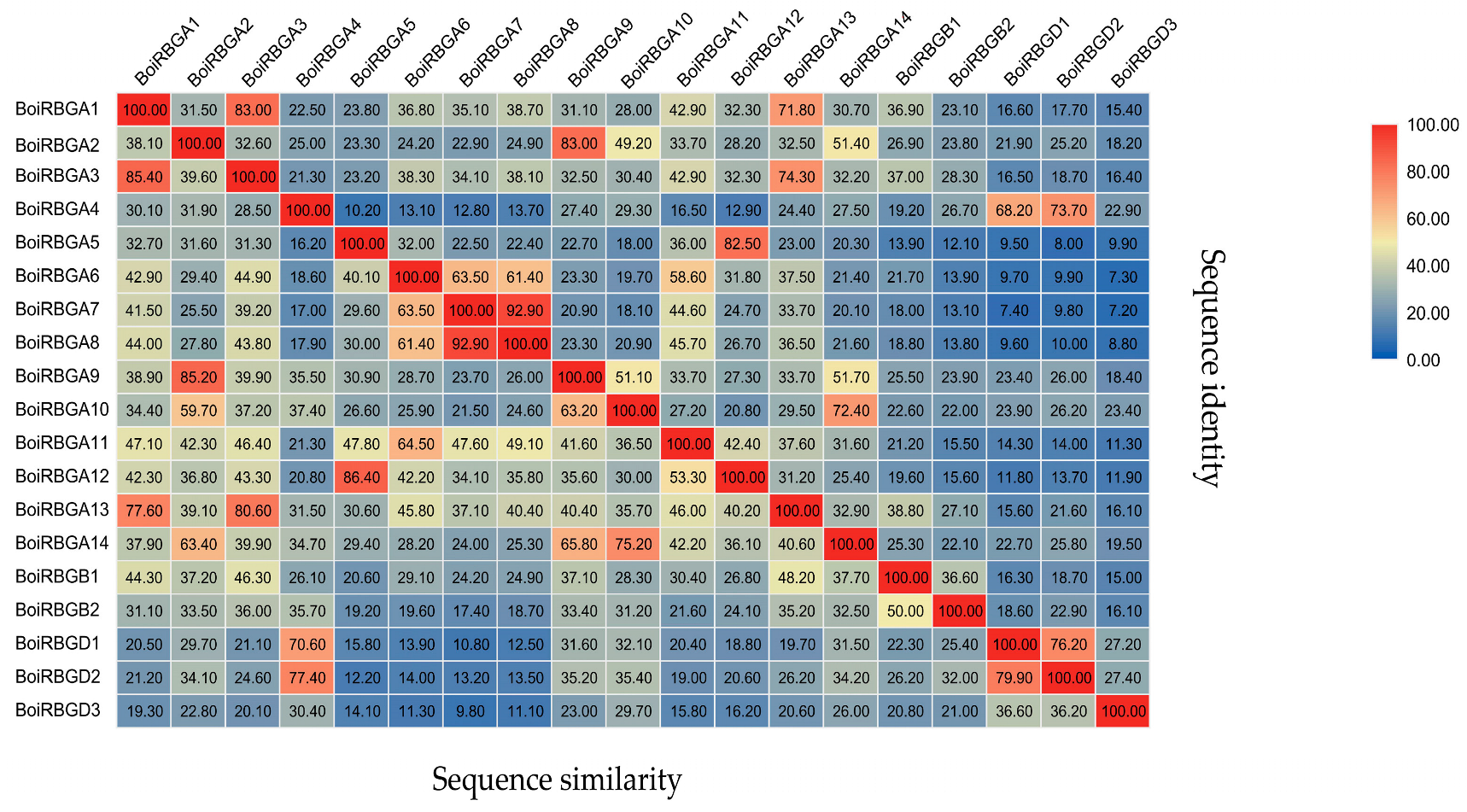
2.2. Chromosome Location and Sequence Similarity Analysis of BoiRBG Genes
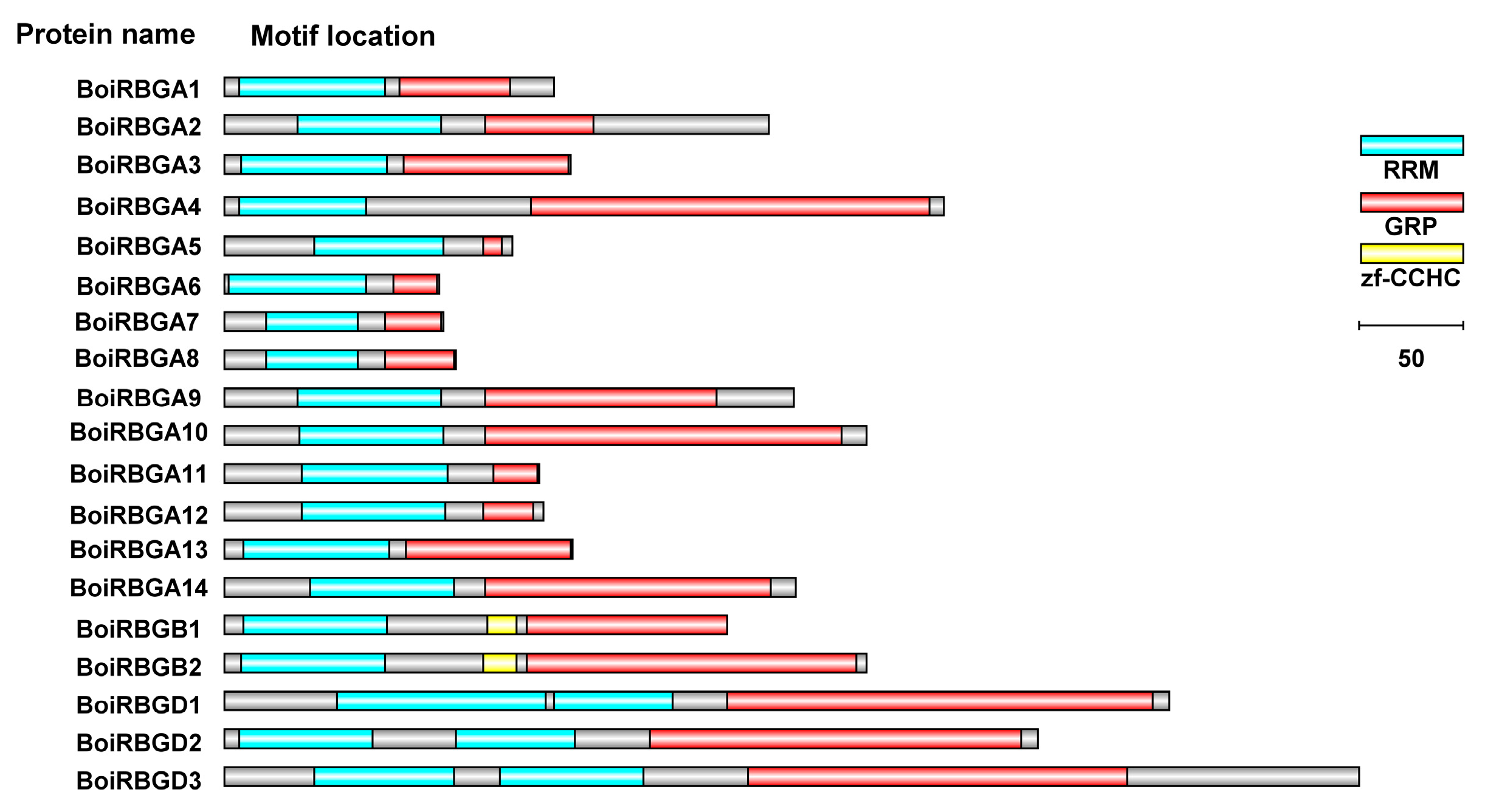
2.3. Conserved Domain Compositions and Structural Analysis of BoiRBG Genes
2.4. Synteny Tandem Duplications of RBG Genes
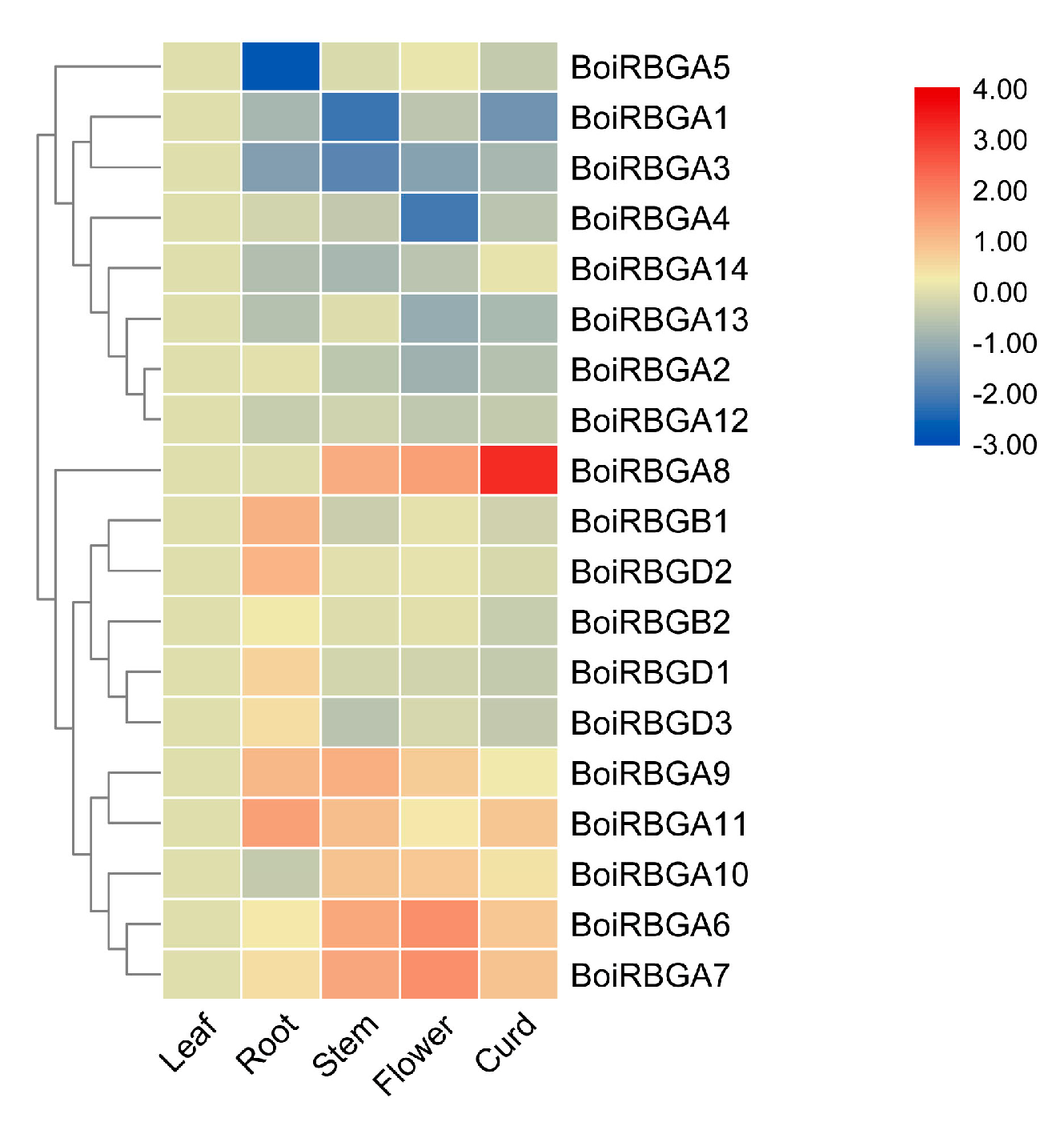
2.5. Expression Profiles of BoiRBG Genes in Various B. oleracea HDEM Organs
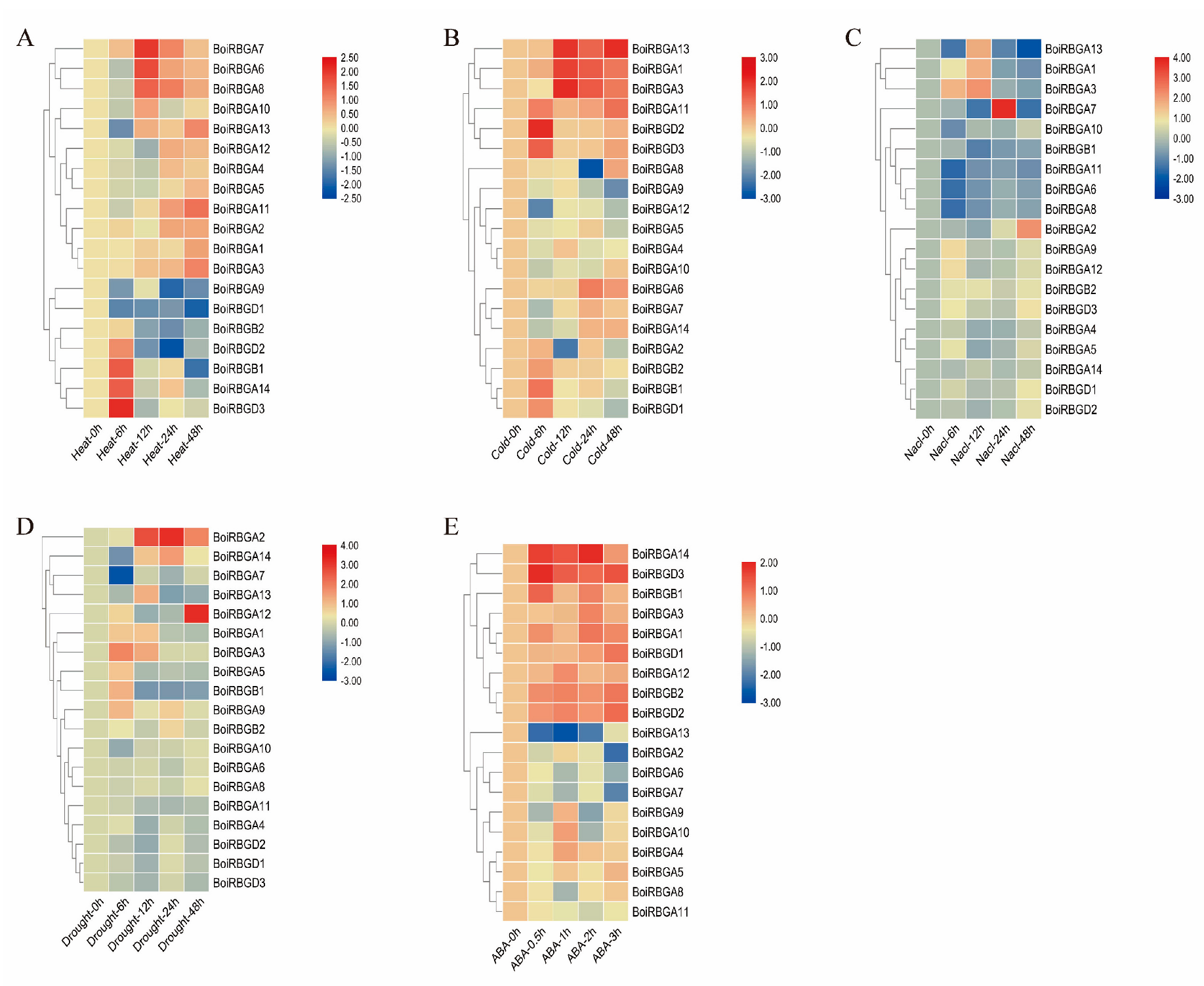
2.6. Analysis of BoiRBG Expression in Response to Different Abiotic Stresses in Broccoli
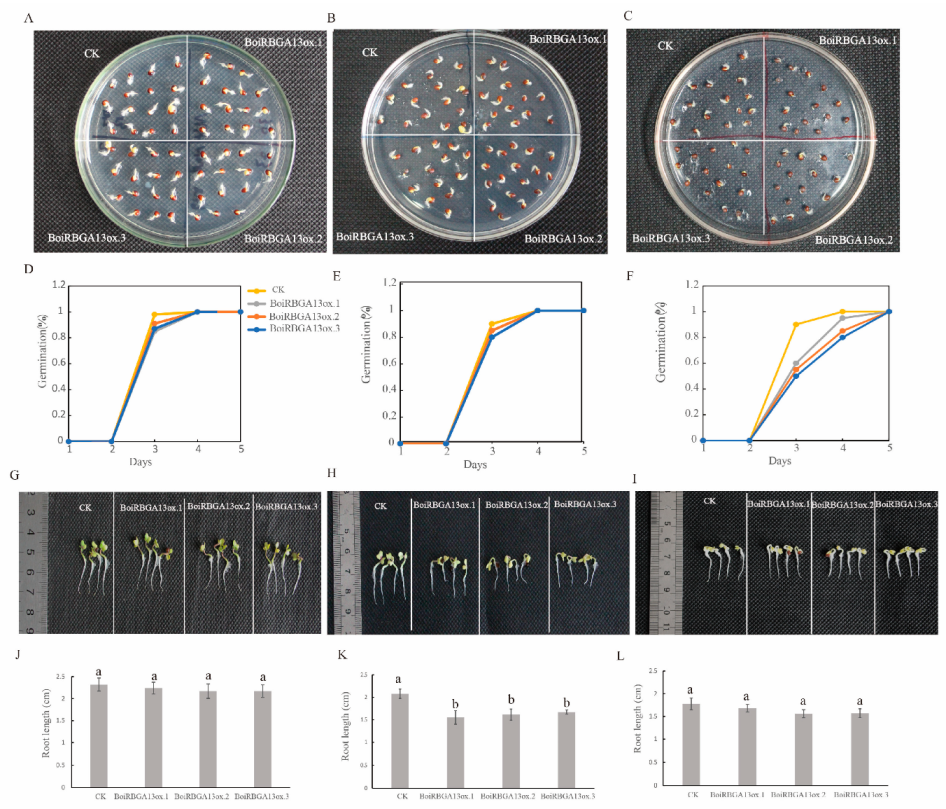
2.7. BoiRBGA13 Was Involved in the Regulation of Seed Germination and Seedling Growth under NaCl and Cold Stress in Broccoli
3. Discussion
4. Materials and Methods
4.1. Sequence Acquisition and Genome-Wide Identification of RBG Genes in B. oleracea
4.2. Sequence Alignment and Phylogenetic Analyses of RBG Genes
4.3. Detection of Collinear Tandem Duplications and Synteny
4.4. Conserved Motif, Gene Structure, and Subcellular Localization Analyses of RBG Family Members
4.5. Plant Materials and Stress Treatments
4.6. BoiRBGA13 Ectopic Expressed in Broccoli
4.7. NaCl and Cold Stress in Transgenic Broccoli
4.8. RNA Isolation and Expression Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Condit, C.M.; Meagher, R.B. A gene encoding a novel glycine-rich structural protein of petunia. Nature 1986, 323, 178–181. [Google Scholar] [CrossRef]
- Sachettomartins, G.; Franco, L.O.; De Oliveira, D.E. Plant glycine-rich proteins: A family or just proteins with a common motif? Biochim. Biophys. Acta Gene Struct. 2000, 1492, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mangeon, A.; Junqueira, R.M.; Sachetto-Martins, G. Functional diversity of the plant glycine-rich proteins superfamily. Plant Signal. Behav. 2010, 5, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Ambrosone, A.; Costa, A.; Leone, A.; Grillo, S. Beyond transcription: RNA-binding proteins as emerging regulators of plant response to environmental constraints. Plant Sci. 2012, 182, 12–18. [Google Scholar] [CrossRef]
- Jung, H.J.; Park, S.J.; Kang, H. Regulation of RNA metabolism in plant development and stress responses. J. Plant Biol. 2013, 56, 123–129. [Google Scholar] [CrossRef]
- Magdalena, C.; Rurek, M. Plant Glycine-Rich Proteins in Stress Response: An Emerging, Still Prospective Story. Front. Plant Sci. 2018, 9, 302. [Google Scholar] [CrossRef]
- Fusaro, A.; Mangeon, A.; Junqueira, R.M.; Rocha, C.A.B.; Coutinho, T.C.; Margis, R.; Sachetto-Martins, G. Classification, expression pattern and comparative analysis of sugarcane expressed sequences tags (ESTs) encoding glycine-rich proteins (GRPs). Genet. Mol. Biol. 2001, 24, 263–273. [Google Scholar] [CrossRef]
- Mousavi, A.; Hotta, Y. Glycine-rich proteins—A class of novel proteins. Appl. Biochem. Biotechnol. 2005, 120, 169–174. [Google Scholar] [CrossRef]
- Steinert, P.M.; Mack, J.W.; Korge, B.P.; Gan, S.Q.; Haynes, S.R.; Steven, A.C. Glycine loops in proteins: Their occurence in certain intermediate filament chains, loricrins and single-stranded RNA binding proteins. Int. J. Biol. Macromol. 1991, 13, 130–139. [Google Scholar] [CrossRef]
- Ciuzan, O.; Hancock, J.; Pamfil, D.; Wilson, I.; Ladomery, M. The evolutionarily conserved multifunctional glycine-rich RNA binding proteins play key roles in development and stress adaptation. Physiol. Plant. 2014, 153, 1–11. [Google Scholar] [CrossRef]
- Wang, S.; Wang, R.; Liang, D.; Ma, F.; Shu, H. Molecular characterization and expression analysis of a glycine-rich RNA-binding protein gene from Malus hupehensis Rehd. Mol. Biol. Rep. 2012, 39, 4145–4153. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.H.; Kwak, K.J.; Kim, M.K.; Park, S.J.; Yang, K.Y.; Kang, H. Expression of Arabidopsis glycine-rich RNA-binding protein AtGRP2 or AtGRP7 improves grain yield of rice (Oryza sativa) under drought stress conditions. Plant Sci. 2014, 214, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kim, W.Y.; Kwak, K.J.; Oh, S.H.; Han, Y.S.; Kang, H. Glycine-rich RNA-binding proteins are functionally conserved in Arabidopsis thaliana and Oryza sativa during cold adaptation process. J. Exp. Bot. 2010, 61, 2317–2325. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, P.; Jin, A.K.; Jeong, M.J.; Chang, H.K.; Lee, S.I. Defining the RNA-binding glycine-rich (RBG) gene superfamily: New insights into nomenclature, phylogeny, and evolutionary trends obtained by genome-wide comparative analysis of Arabidopsis, Chinese cabbage, rice and maize genomes. Mol. Genet. Genom. 2015, 290, 2279–2295. [Google Scholar] [CrossRef]
- Lewinski, M.; Hallmann, A.; Staiger, D. Genome-wide identification and phylogenetic analysis of plant RNA binding proteins comprising both RNA recognition motifs and contiguous glycine residues. Mol. Genet. Genom. 2016, 291, 763–773. [Google Scholar] [CrossRef]
- Sahi, C.; Agarwal, M.; Singh, A.; Grover, A. Molecular characterization of a novel isoform of rice (Oryza sativa L.) glycine rich-RNA binding protein and evidence for its involvement in high temperature stress response. Plant Sci. 2007, 173, 144–155. [Google Scholar] [CrossRef]
- Zhang, J. Genome-wide identification, evolution, and expression analysis of RNA-binding glycine-rich protein family in maize. J. Integr. Plant Biol. 2014, 56, 1020–1031. [Google Scholar] [CrossRef]
- Lu, X.; Cheng, Y.; Gao, M.; Li, M.; Xu, X. Molecular Characterization, Expression Pattern and Function Analysis of Glycine-Rich Protein Genes under Stresses in Chinese Cabbage (Brassica rapa L. ssp. pekinensis). Front. Genet. 2020, 11, 774. [Google Scholar] [CrossRef]
- Lu, Y.; Sun, J.; Yang, Z.; Zhao, C.; Zhu, M.; Ma, D.; Dong, T.; Zhou, Z.; Liu, M.; Yang, D.; et al. Genome-wide identification and expression analysis of glycine-rich RNA-binding protein family in sweet potato wild relative Ipomoea trifida. Gene 2019, 686, 177–186. [Google Scholar] [CrossRef]
- Yang, W.; Yu, M.; Zou, C.; Lu, C.; Yu, D.; Cheng, H.; Jang, P.; Feng, X.; Zhang, Y.; Wang, Q. Genome-wide comparative analysis of RNA-binding Glycine-rich protein family genes between Gossypium arboreum and Gossypium raimondii. PLoS ONE 2019, 14, e0218938. [Google Scholar] [CrossRef]
- Tang, Y.; Huang, C.; Li, Y.; Wang, Y.; Zhang, C. Genome-wide identification, phylogenetic analysis, and expression profiling of glycine-rich RNA-binding protein (GRPs) genes in seeded and seedless grapes (Vitis vinifera). Physiol. Mol. Biol. Plants 2021, 27, 2231–2243. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Park, S.J.; Kwak, K.J.; Kim, Y.O.; Kim, J.Y.; Song, J.; Jang, B.; Jung, C.H.; Kang, H. Cold shock domain proteins and glycine-rich RNA-binding proteins from Arabidopsis thaliana can promote the cold adaptation process in Escherichia coli. Nucleic Acids Res. 2007, 35, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Park, S.J.; Jang, B.; Jung, C.H.; Ahn, S.J.; Goh, C.H.; Cho, K.; Han, O.; Kang, H. Functional characterization of a glycine-rich RNA-binding protein 2 in Arabidopsis thaliana under abiotic stress conditions. Plant J. 2007, 50, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.K.; Jung, H.J.; Lee, H.J.; Kim, K.A.; Kang, H. Glycine-rich RNA-binding protein 7 affects abiotic stress responses by regulating stomata opening and closing in Arabidopsis thaliana. Plant J. 2008, 55, 455–466. [Google Scholar] [CrossRef]
- Steffen, A.; Elgner, M.; Staiger, D. Regulation of Flowering Time by the RNA-Binding Proteins AtGRP7 and AtGRP8. Plant Cell Physiol. 2019, 60, 2040–2050. [Google Scholar] [CrossRef]
- Xiao, J.; Li, C.; Xu, S.; Xing, L.; Xu, Y.; Chong, K. JACALIN-LECTIN LIKE1 Regulates the Nuclear Accumulation of GLYCINE-RICH RNA-BINDING PROTEIN7, Influencing the RNA Processing of FLOWERING LOCUS C Antisense Transcripts and Flowering Time in Arabidopsis. Plant Physiol. 2015, 169, 2102–2117. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Y.; Yang, X.; Tong, C.; Edwards, D.; Parkin, I.A.P.; Zhao, M.; Ma, J.; Yu, J.; Huang, S. The Brassica oleracea genome reveals the asymmetrical evolution of polyploid genomes. Nat. Commun. 2014, 5, 3930. [Google Scholar] [CrossRef]
- Parkin, I.A.P.; Koh, C.; Tang, H.; Robinson, S.J.; Kagale, S.; Clarke, W.E.; Town, C.D.; Nixon, J.; Krishnakumar, V.; Bidwell, S.L. Transcriptome and methylome profiling reveals relics of genome dominance in the mesopolyploid Brassica oleracea. Genome Biol. 2014, 15, R77. [Google Scholar] [CrossRef]
- Xiaohui, Z.; Zhen, Y.; Shiyong, M.; Yang, Q.; Xinhua, Y.; Xiaohua, C.; Feng, C.; Zhangyan, W.; Yuyan, S.; Yi, J. A de novo Genome of a Chinese Radish Cultivar. Hortic. Plant J. 2015, 1, 155–164. [Google Scholar] [CrossRef]
- Yang, J.; Liu, D.; Wang, X.; Ji, C.; Zhang, M. The genome sequence of allopolyploid Brassica juncea and analysis of differential homoeolog gene expression influencing selection. Nat. Genet. 2016, 48, 1225. [Google Scholar] [CrossRef]
- Belser, C.; Istace, B.; Denis, E.; Dubarry, M.; Baurens, F.C.; Falentin, C.; Genete, M.; Berrabah, W.; Chevre, A.M.; Delourme, R. Chromosome-scale assemblies of plant genomes using nanopore long reads and optical maps. Nat. Plants 2018, 4, 879. [Google Scholar] [CrossRef]
- Graumann, P.L.; Marahiel, M.A. A superfamily of proteins that contain the cold-shock domain. Trends Biochem. Sci. 1998, 23, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Karlson, D.; Imai, R. Conservation of the Cold Shock Domain Protein Family in Plants. Plant Physiol. 2003, 131, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, L.; Guo, H.; Liao, B.; Zhang, X. Genome evolutionary dynamics followed by diversifying selection explains the complexity of the Sesamum indicum genome. BMC Genom. 2017, 18, 257. [Google Scholar] [CrossRef]
- El-Gebali, S.; Mistry, J.; Bateman, A.; Eddy, S.R.; Luciani, A.; Potter, S.C.; Qureshi, M.; Richardson, L.J.; Salazar, G.A.; Smart, A. The Pfam protein families database in 2019. Nucleic Acids Res. 2018, 47, D427–D432. [Google Scholar] [CrossRef]
- Chaikam, V.; Karlson, D. Functional characterization of two cold shock domain proteins from Oryza sativa. Plant Cell Environ. 2008, 31, 995–1006. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Yu, R.; Li, W.; Geng, L.; Jing, X.; Zhu, C.; Liu, H. Identification and characterisation of a glycine-rich RNA-binding protein as an endogenous suppressor of RNA silencing from Nicotiana glutinosa. Planta 2019, 249, 1811–1822. [Google Scholar] [CrossRef]
- Kar, B.; Nayak, S.; Joshi, R.K. Classification and comparative analysis of Curcuma longa L. expressed sequences tags (ESTs) encoding glycine-rich proteins (GRPs). Bioinformation 2012, 8, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Paterson, A.H.; Chapman, B.A.; Kissinger, J.C.; Bowers, J.E.; Feltus, F.A.; Estill, J.C. Many gene and domain families have convergent fates following independent whole-genome duplication events in Arabidopsis, Oryza, Saccharomyces and Tetraodon. Trends Genet. 2006, 22, 597–602. [Google Scholar] [CrossRef]
- Chauve, C.; Doyon, J.P.; El-Mabrouk, N. Gene Family Evolution by Duplication, Speciation, and Loss. J. Comput. Biol. 2008, 15, 1043–1062. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Tang, H.; Tan, X.; Ficklin, S.P.; Feltus, F.A.; Paterson, A.H. Modes of gene duplication contribute differently to genetic novelty and redundancy, but show parallels across divergent angiosperms. PLoS ONE 2011, 6, e28150. [Google Scholar] [CrossRef]
- Wang, Y. Locally Duplicated Ohnologs Evolve Faster Than Nonlocally Duplicated Ohnologs in Arabidopsis and Rice. Genome Biol. Evol. 2013, 5, 362–369. [Google Scholar] [CrossRef]
- Tang, H.; Bowers, J.E.; Wang, X.; Ming, R.; Alam, M.; Paterson, A.H. Synteny and Collinearity in Plant Genomes. Science 2008, 320, 486–488. [Google Scholar] [CrossRef]
- Chen, T.W.; Wardill, T.J.; Sun, Y.; Pulver, S.R.; Renninger, S.L.; Baohan, A.; Schreiter, E.R.; Kerr, R.A.; Orger, M.B.; Jayaraman, V.; et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 2013, 499, 295–300. [Google Scholar] [CrossRef]
- Long, M.; Betrán, E.; Thornton, K.; Wang, W. The origin of new genes: Glimpses from the young and old. Nat. Rev. Genet. 2003, 4, 865–875. [Google Scholar] [CrossRef]
- Kwak, K.J.; Kim, Y.O.; Kang, H. Characterization of transgenic Arabidopsis plants overexpressing GR-RBP4 under high salinity, dehydration, or cold stress. J. Exp. Bot. 2005, 56, 3007–3016. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zeng, Q.; Lu, X.; Yu, D.; Li, W. Characterization and Expression Analysis of Four Glycine-Rich RNA-Binding Proteins Involved in Osmotic Response in Tobacco (Nicotiana tabacum cv. Xanthi). Agric. Sci. China 2010, 9, 1577–1587. [Google Scholar] [CrossRef]
- Chory, J.; Peto, N.C.A. Phenotypic and Genetic Analysis of det2, a New Mutant That Affects Light-Regulated Seedling Development in Arabidopsis. Plant Cell 1991, 3, 445–459. [Google Scholar] [CrossRef]
- An, F.; Zhang, X.; Zhu, Z.; Ji, Y.; He, W.; Jiang, Z.; Li, M.; Guo, H. Coordinated regulation of apical hook development by gibberellins and ethylene in etiolated Arabidopsis seedlings. Cell Res. 2012, 22, 915–927. [Google Scholar] [CrossRef] [PubMed]
- Smet, D.; Žádníková, P.; Vandenbussche, F.; Benková, E.; Straeten, D.V.D. Dynamic infrared imaging analysis of apical hook development in Arabidopsis: The case of brassinosteroids. New Phytol. 2014, 202, 1398–1411. [Google Scholar] [CrossRef]
- Finn, R.D.; Clements, J.; Eddy, S.R. HMMER web server: Interactive sequence similarity searching. Nucleic Acids Res. 2011, 39 (Suppl. S2), W29–W37. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. 20 years of the SMART protein domain annotation resource. Nucleic Acids Res. 2018, 46, D493–D496. [Google Scholar] [CrossRef] [PubMed]
- Hunter, S.; Apweiler, R.; Attwood, T.K.; Bairoch, A.; Bateman, A.; Binns, D.; Bork, P.; Das, U.; Daugherty, L.; Duquenne, L. InterPro: The integrative protein signature database. Nucleic Acids Res. 2009, 37 (Suppl. S1), D211–D215. [Google Scholar] [CrossRef] [PubMed]
- Corpet, F. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 1988, 16, 10881–10890. [Google Scholar] [CrossRef]
- Gasteiger, E.; Gattiker, A.; Hoogland, C.; Ivanyi, I.; Appel, R.D.; Bairoch, A. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003, 31, 3784–3788. [Google Scholar] [CrossRef]
- Voorrips, R.E. MapChart: Software for the Graphical Presentation of Linkage Maps and QTLs. J. Hered. 2002, 93, 77–78. [Google Scholar] [CrossRef] [PubMed]
- Rice, P.; Longden, I.; Bleasby, A. EMBOSS: The European molecular biology open software suite. Trends Genet. 2000, 16, 276–277. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple Sequence Alignment with High Accuracy and High Throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Sudhir, K.; Glen, S.; Koichiro, T. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Cheng, F.; Wu, J.; Fang, L.; Wang, X. Syntenic gene analysis between Brassica rapa and other Brassicaceae species. Front. Plant Sci. 2012, 3, 198. [Google Scholar] [CrossRef]
- Krzywinski, M.; Schein, J.; Birol, I.; Connors, J.; Gascoyne, R.; Horsman, D.; Jones, S.J.; Marra, M.A. Circos: An information aesthetic for comparative genomics. Genome Res. 2009, 19, 1639–1645. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.Z.; Xie, Y.B.; Ma, J.Y.; Luo, X.T.; Nie, P.; Zuo, Z.X.; Lahrmann, U.; Zhao, Q.; Zheng, Y.Y.; Zhao, Y.; et al. IBS: An illustrator for the presentation and visualization of biological sequences. Bioinformatics 2015, 31, 3359–3361. [Google Scholar] [CrossRef]
- Hu, B.; Jin, J.P.; Guo, A.Y.; Zhang, H.; Luo, J.C.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef]
- Horton, P.; Park, K.J.; Obayashi, T.; Fujita, N.; Harada, H.; Adams-Collier, C.J.; Nakai, K. WoLF PSORT: Protein localization predictor. Nucleic Acids Res. 2007, 35, W585–W587. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zong, M.; Liu, D.; Wu, Y.; Tian, S.; Han, S.; Guo, N.; Duan, M.; Miao, L.; Liu, F. Efficient Generation of Targeted Point Mutations in the Brassica oleracea var. botrytis Genome via a Modified CRISPR/Cas9 System. Hortic. Plant J. 2022, 8, 527–530. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, M.; Zong, M.; Guo, N.; Han, S.; Wang, G.; Miao, L.; Liu, F. Comprehensive Genome-Wide Identification of the RNA-Binding Glycine-Rich Gene Family and Expression Profiling under Abiotic Stress in Brassica oleracea. Plants 2023, 12, 3706. https://doi.org/10.3390/plants12213706
Duan M, Zong M, Guo N, Han S, Wang G, Miao L, Liu F. Comprehensive Genome-Wide Identification of the RNA-Binding Glycine-Rich Gene Family and Expression Profiling under Abiotic Stress in Brassica oleracea. Plants. 2023; 12(21):3706. https://doi.org/10.3390/plants12213706
Chicago/Turabian StyleDuan, Mengmeng, Mei Zong, Ning Guo, Shuo Han, Guixiang Wang, Liming Miao, and Fan Liu. 2023. "Comprehensive Genome-Wide Identification of the RNA-Binding Glycine-Rich Gene Family and Expression Profiling under Abiotic Stress in Brassica oleracea" Plants 12, no. 21: 3706. https://doi.org/10.3390/plants12213706
APA StyleDuan, M., Zong, M., Guo, N., Han, S., Wang, G., Miao, L., & Liu, F. (2023). Comprehensive Genome-Wide Identification of the RNA-Binding Glycine-Rich Gene Family and Expression Profiling under Abiotic Stress in Brassica oleracea. Plants, 12(21), 3706. https://doi.org/10.3390/plants12213706






