Abstract
Brassica carinata has received considerable attention as a renewable biofuel crop for semi-arid zones due to its high oil content and polyunsaturated fatty acids contents. It is important to develop new drought-resistant cultivars of B. carinata production to expand its areas into more arid regions. The accumulation of leaf cuticular wax on plant surfaces is one mechanism that reduces non-stomatal water loss, thus increasing drought resistance in plants. To explore phenotypic variations in cuticular wax in B. carinata, leaf waxes were extracted and quantified from a diversity panel consisting of 315 accessions. The results indicate that the accessions have a wide range of total leaf wax content (289–1356 µg dm−2), wax classes, and their components. The C29 and C31 homologues of alkanes, C29 ketone homologue, C29 secondary alcohol, and C30 aldehyde were the most abundant leaf waxes extracted from B. carinata accessions. The high heritability values of these waxes point to the positive selection for high wax content during early generations of future B. carinata breeding programs. Positive correlation coefficients, combined with the effects of these waxes on leaf wax content accumulation, suggest that modifying specific wax content could increase the total wax content and enhance cuticle composition. The identified leaf wax content and compositions in B. carinata will lead to the future discovery of wax biosynthetic pathways, the dissection of its genetic regulatory networks, the identification of candidate genes controlling production of these waxes, and thus, develop and release new B. carinata drought-tolerant cultivars.
Keywords:
Brassica carinata; cuticle; drought; cuticular wax; wax classes; wax components; stress tolerance 1. Introduction
Vegetable oils from several traditional crops, including soybean (Glycine max (L.) Merr), corn (Zea mays L.), canola (Brassica napus subsp. napus), and sunflower (Helianthus annuus L.), as well new industrial crops such as carinata (Brassica carinata A. Braun), camelina (Camelina sativa L.), pennycress (Thlaspi arvense L.), crambe (Crambe abyssinica R.E.Fr.), jojoba (Simmondsia chinensis (Link) C. K.) and jatropha (Jatropha curcas L.), are used to meet the increasing demand for biofuels, such as a biodiesel source. Drenth et al. [1] concluded that oils extracted from industrial crops have very similar engine performance to traditional oils crops. An ideal biofuel crop should have low agricultural inputs, a high oil content, a high polyunsaturated fatty acid content, be compatible with existing farm equipment and infrastructure, be tolerant to low-input growth conditions, have low irrigation requirements, have definable growth seasons, and maintain uniform seed maturation rates [2]. There is a pressing need to develop high-yielding, non-food oil crops that can be cultivated in underutilized farming areas, thus avoiding the food vs. fuel debate. Based on these criteria, plants such as carinata, camelina, and pennycress promise non-food crops for low-input and marginal agricultural systems in the U.S.
Brassica carinata A. Braun is a species native to Ethiopian highlands and has recently become the subject of increasing interest due to its high oil and erucic fatty acid content (~40%). Brassica carinata oil can be refined into biofuels that meet the specifications of petroleum-based fuels [3,4,5]. This crop has better agronomic performance (seed yield, resistance to various biotic stresses and diseases, tolerant to abiotic stresses, and resistance to pod shattering) compared to other brassicas, especially camelina and canola, in semi-arid areas of the Northern Great Plains, Canadian Prairies, and Southern Europe [6,7,8,9,10,11,12]. Brassica carinata could prove more adaptable to marginal environmental conditions that require crops with low irrigation requirements to grow [13]. These growing conditions make B. carinata an ideal crop for marginal lands in the wheat-producing regions of the Northern Great Plains (NGP) of the U.S. Historically, the semi-arid regions of the NGP were dominated by monoculture cereal cropping systems that include a 14-month fallow period and extensive use of mechanical tillage [14].
Abiotic stresses are estimated to reduce crop yields to less than half of the gain under ideal growing conditions [15]. Notably, vascular plants have evolved mechanisms to survive abiotic stress. However, the underlying molecular mechanisms are not well understood in most agricultural crops. Plants adapted to avoid tissue dehydration possess more efficient root systems underground that increase soil water extraction, and/or exhibit highly efficient above-ground systems to reduce water evaporation, such as control over stomatal conductance, modification of solar radiation absorption, and/or modification of cuticle water permeability [16]. Non-stomatal water loss is primarily controlled by the cuticle, an extracellular lipophilic polymer that covers and protects the aerial organs of plants. The cuticle also provides protection from environmental stresses, such as pathogens, insects [17,18], supra-optimal air temperature, and high solar radiation [18,19,20,21,22,23]. The cuticle consists of two lipid classes; the non-polymerized cuticular wax components and cutin polyester layers. Cuticular waxes are mostly saturated very-long-chain (C20–C34) fatty acids that occur as epicuticular and intra-cuticular lipids. Cutin monomers consist of C16 and C18 fatty acid derivatives (e.g., hydroxy fatty acids and dicarboxylic acids) linked primarily by ester bonds to form a polyester matrix. Two major pathways involved in the synthesis of cuticular wax includes the primary alcohol-forming pathway, which produces very long-chain alcohols and wax esters, and the alkane-forming pathway, which produces aldehydes, alkanes, secondary alcohols, and ketones [18]. Additional components of waxes include cyclic triterpenes and other aliphatic components, some of which can account for a significant proportion of total wax composition [24]. Among the 40 leaf wax components identified in Camelina species and the Camelina sativa diversity panel [25,26], primary alcohols (mainly C24, C26, and C28) and alkanes (mainly C31) are the predominant leaf wax classes, followed by wax esters, fatty acids, and aldehydes. These wax components show wide phenotypic variations among Camelina accessions. Previous studies have shown that there is a positive correlation between drought resistance and wax accumulation deposited on the leaf surface in plants, including Arabidopsis [20], tobacco [27], peanut [28], soybean [29], wheat [30,31], and cotton [32]. Wax composition often varies significantly among plant species and organs [33,34,35], developmental stages [33,36], and in response to environmental conditions [21,37]. As leaf wax components vary among species, organs, and in response to environmental stresses, it is expected that the genes involved in wax accumulation also vary and belong to different pathways. For example, under drought stress conditions, a transgenic camelina line overexpressing a transcription factor MYB96 accumulated leaf wax by 52% more than the null control line [38].
The nature and inheritance of B. carinata’s response to abiotic stresses, such as drought, is unknown. The goal of this current research is to explore the variation in leaf wax composition in B. carinata. The objectives of the current study are to characterize the phenotypic variations in B. carinata leaf wax content and compositions and estimate the heritability and genetic components of the accumulations of these waxes.
2. Results
The first step in expanding our understanding of leaf wax-accumulating mechanisms and identifying candidate genes controlling wax biosynthetic pathways in B. carinata is to identify and quantify leaf wax content, waxes classes and components in large diverse populations. The analysis of 315 B. carinata accessions, collected from its area of origin and different growing areas, revealed wide variations in wax content, classes, and components (Supplementary Table S1).
The B. carinata diversity panel showed a wide range of total wax contents, where the highest accession, collected from the Bonga region in Ethiopia, accumulated 1356 µg dm−2 of total wax on the leaf surface, while one of the lowest accessions, collected from Sweden, accumulated 289 µg dm−2 (Table 1, Figure 1). The extracted leaf waxes from B. carinata consist of seven main classes.

Table 1.
Means, ranges (min-max), and standard deviations (SDs) of BLUP values for total wax, wax classes, and components extracted from 315 Brassica carinata accessions.
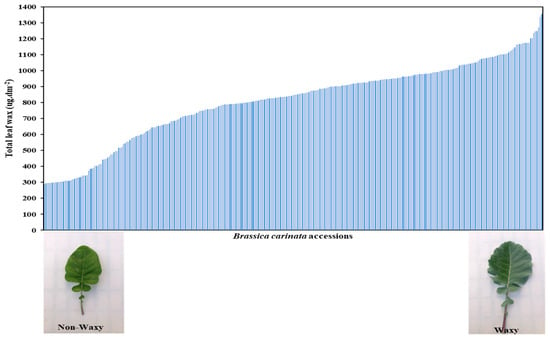
Figure 1.
Total leaf wax BLUP values of 315 Brassica carinata accessions.
Among these classes, alkanes and ketones accounted for 77% of the accumulated leaf wax; primary alcohols and secondary alcohols accounted for 15%, and aldehydes, methyl alcohols, free fatty acids, and wax esters accounted for around 8% (Table 1). B. carinata’s alkane content ranged from 145 µg dm−2 to 637 µg dm−2. The C29 and C31 alkanes accounted for 35% and 12% of the total leaf waxes in B. carinata, respectively (Table 1). The C29 ketone accounted for 27% of the total wax accumulated on B. carinata leaves, making it the second most abundant wax after C29 alkane (Table 1). B. carinata accessions showed a wide phenotypic variation, with a 79 µg dm−2 to 453 µg dm−2 range for C29 Ket (Table 1). Primary alcohols contributed 8% to the total waxes (Table 1), with B. carinata accessions ranging from 48 µg dm−2 to 86 µg dm−2 in primary alcohols. Among primary alcohols in B. carinata, C26 Alc and C28 Alc were the predominant molecular species, together accounting for 7% of total waxes (Table 1). Secondary alcohols accounted for 7% of the total leaf wax in B. carinata, with the lowest amount at 16 µg dm−2 and the highest amount at 87 µg dm−2 (Table 1). The prominent secondary alcohol is C29 Alc-2, accounting for 5% of the total leaf waxes (ranging from 15 µg dm−2 to 75 µg dm−2). The C30 Ald (an aldehyde wax component) accounted for 7% of the total wax, with a range from 20 µg dm−2 to 52 µg dm−2. Other wax classes each accounted for less than 5% of the total wax content but still showed significant phenotypic variation within each class; where methyl alcohols ranged from 13 µg dm−2 to 41 µg dm−2, free fatty acids ranged from 8 µg dm−2 to 17 µg dm−2, and C43 WE (a wax ester component) ranged from 7.28 µg dm−2 to 7.94 µg dm−2 (Table 1). Even though these wax components have a small contribution to the total wax content, they could play a role in stress tolerance.
Cuticular wax classes are produced via two main pathways: acyl reduction and decarbonylation [19,34,39,40]. In the decarbonylation pathway (alkane-forming pathway), aldehydes are produced from VLCFA precursors, followed by aldehyde decarbonylation to produce alkanes that may be converted into ketones via secondary alcohols. Highly significant positive correlation coefficients were observed between C30 Ald and C29 Alk (r = 0.658), C29 Alk and C29 Alc-2 (r = 0.915), and C29 Alc-2 and C29 Ket (r = 0.918) (Figure 2, Supplementary Table S2). These findings suggest that the decarbonylation pathway is the main pathway for wax biosynthesis in B. carinata. In the acyl reduction pathway (alcohol-forming pathway), primary alcohols are produced by reducing very long-chain fatty acid (VLCFA) precursors to produce aldehydes, primary alcohols, and wax esters via the esterification of fatty acids and primary alcohols. In B. oleracea, it was shown that acyl-CoA reductase converts fatty-CoA into aldehydes, which are then reduced by aldehyde reductase to produce primary alcohols [41].
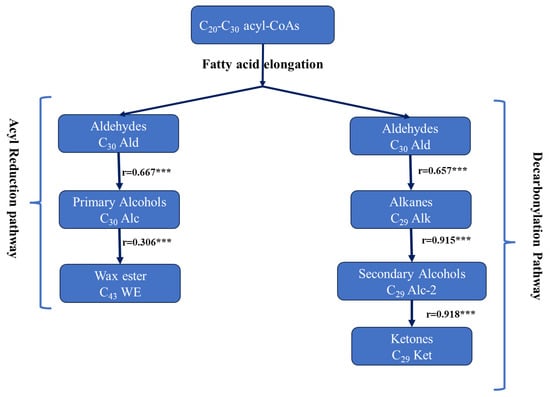
Figure 2.
Correlation coefficients for selected wax components in cuticular wax biosynthesis. *** is the significance levels at p < 0.001.
Significant positive correlation coefficients were observed between C30 Ald and C29 Alc (r = 0.812), C29 Alc and C43 WE (r = 0.439), and C30 Ald and C43 WE (r = 0.474) (Figure 2, Supplementary Table S2). These observations suggest that primary fatty alcohols, wax esters, and free fatty acids have a lesser effect on the variations in B. carinata leaf waxes. Tassone et al. [42] obtained similar results in B. napus and suggested that both pathways are coupled in their effects to produce and accumulate leaf waxes.
Highly significant correlation coefficients were detected between total wax and free fatty acids (r = 0.602), primary alcohols (r = 0.619), secondary alcohols (r = 0.947), methyl alcohols (r = 0.838), alkanes (r = 0.983), wax esters (r = 0.0.465), aldehydes (r = 0.653), and ketones (r = 0.970) (Table S2). These correlations could indicate that these leaf wax compounds are dependently regulated. To gain a deeper understanding of the correlations among wax classes in B. carinata, path analysis was conducted to partition the correlation coefficients for total wax accumulation and its wax classes into direct and indirect effects contributing to total wax accumulation. Alkanes had the highest positive direct effect on total wax accumulation, followed by C29 Ket and secondary alcohols with 0.509, 0.294, and 0.108, respectively (Figure 3, Supplementary Table S3). Among the detected alkanes, C29 Alk and C31 Alk had indirect positive effects on total wax accumulation, displaying 0.415 and 0.163, respectively (with direct positive effects of 0.816 and 0.320 on alkanes accumulation). C29 Alc-2 and C29 Alc-2 had the highest positive indirect effects, with 0.092 and 0.016, respectively (with direct effects of 0.853 and 0.148 on secondary alcohols accumulation). The detected C30 Ald had a positive direct effect of 0.039 on total wax accumulation. Even though total free fatty acid was highly correlated with total wax (r = 0.602), its direct effect on accumulation was the lowest among wax classes (0.09). The path analysis results indicated that the alkane-forming pathway exhibits a higher influence on leaf wax accumulation in B. carinata, with C29 Alk, C29 Ket, C31 Alk, C29 Alc-2, and C30 Ald being the most prominent waxes (from the higher effect to the lower effect).
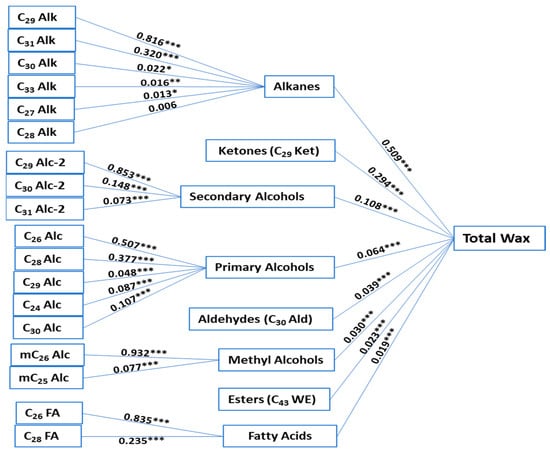
Figure 3.
Direct effects of the total wax content related to wax classes and components. *, **, *** are the significance levels at p < 0.05, p < 0.01, and p < 0.001, respectively.
High heritability estimates indicate the feasibility of selecting a trait of interest during the early generations of breeding programs [34]. The total wax content in the current study showed a high heritability estimate (H2 = 0.68, based on broad-sense heritability), indicating a high genetic component controlling leaf wax content in B. carinata. For wax classes and components, heritability values ranged from 0.02 (C43 Wax ester) to 0.68 (C29 Alkane) (Figure 4), demonstrating the variable environmental effects on the inheritance of these waxes. In general, alkanes, secondary alcohols, and C29 ketone showed high heritability values, each greater than H2 = 0.65, while secondary and methyl alcohols showed moderate heritability values (H2 is more than 30 and less than 0.60).
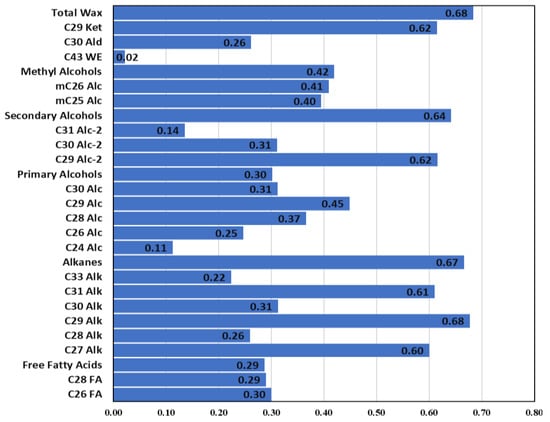
Figure 4.
Broad-sense heritability values (H2) of total wax content, wax classes, and components.
3. Discussion
Plant adaptation to drought stress mechanisms can be grouped into four main categories: escape, avoidance, tolerance, and recovery [43]. The avoidance mechanism generally increases a plant’s ability to delay tissue dehydration when soil moisture depletes. Accumulating cuticular wax on plant surfaces is one of the strategies to reduce nonstomatal water loss under abiotic stresses [43,44]. A large diversity panel of B. carinata accessions collected from different geographical areas was used to explore the phenotypic diversity in leaf waxes, aiming to identify candidate genes controlling wax biosynthesis pathways. To date, there have been few reports describing leaf wax structure on the B. carinata leaf surface [45,46,47]. This is the first report on identifying wax classes and components in B. carinata. The B. carinata diversity panel showed a wide range of total wax contents, in similar fashion to B. napus [42], with seven main wax classes extracted and classified. In B. carinata, alkanes constituted the most abundant wax class. Alkanes positively increased under drought stresses on the leaves of alfalfa [48], Arabidopsis [20,49], sesame [50] soybean [29], and Populus euphratica [51]. The C29 Alk accounted for up to 45% of the waxes accumulated on B. napus leaves [42]. Li et al. [52] found that the C29 Alk and C31 Alk contents increased in watermelon leaves in response to drought stress. The abundant C29 Alk accounted for 35% of the total leaf waxes in B. carinata, indicating the possible role of C29 Alk in abiotic stress tolerance. The second most abundant wax class was ketones, mainly represented as C29 Ket wax. An analysis of 504 B. napus accessions indicated that the C29 Ket ranged from 200 µg dm−2 to 574 µg dm−2 [42]. Ketones were found to be a major wax class in species such as B. oleracea and Allium porrum, with 31% and 52% of the total wax [53]. Kosma et al. [20] found a significant increase in Arabidopsis C29 Ket under NaCl stress. Both primary and secondary alcohols were identified in B. carinata leaf waxes. Primary alcohols accounted for 8% of the waxes accumulated on Arabidopsis leaves [34] and are the main wax class in camelina [25,26]. Primary alcohols showed an increase in soybean [29] and maritime pine [54] leaves under drought stress conditions.
Both cuticular wax biosynthesis pathways, acyl reduction (alcohol-forming pathway) and decarbonylation (alkane-forming pathway) [19,34,39,40], were identified in B. carinata. The abundance of alkane-forming pathway waxes (alkanes, ketones, and secondary alcohols) and their strong effects on total wax accumulation suggest that the decarbonylation pathway is the main pathway in B. carinata. Nonetheless, the acryl reduction pathway classes (primary fatty alcohols, wax esters, and free fatty acids) contributed to the variations in B. carinata leaf waxes. Tassone [42] obtained similar results in B. napus and suggested that both pathways are coupled in their effects on the production and accumulation of leaf waxes. The highly significant correlation coefficients between total wax and wax classes and components could indicate that these leaf wax compounds are dependently regulated, and selections for one wax class could affect another wax class. The C29 Alk, C31 Alk, C29 Alc-2, and C29 Ket are found to be the most significant wax components affecting the total leaf wax in B. carinata. These components had high heritability scores. These findings suggest that these components are heritable and strong potential targets for modifying B. carinata wax content and/or composition through selection during the early generations of Brassica carinata breeding programs for drought stress tolerance.
4. Materials and Methods
4.1. Plant Materials
A Brassica carinata diversity panel was assembled from 315 accessions collected from different parts of the world and stored at the USDA-ARS National Plant Germplasm System, Ames, IA, USA, the Leibniz Institute of Plant Genetics and Crop Plant Research, Gatersleben, Germany, and the Centre for Genetic Resources the Netherlands, Wageningen, The Netherlands (Supplementary Table S1). The accessions were planted in the greenhouse at the US Arid-Land Agricultural Research Center in Maricopa, Arizona at two different times: 22 March 2019, and 2 December, 2019. Accessions were arranged in a completely randomized design (CRD) with three replications each. The seeds of each accession were planted in two-gallon pots. Plants were regularly irrigated and fertilized. Leaf samples were collected from each pot at 35 days after planting. Each sample consisted of three middle leaves.
4.2. Wax Extraction and Analyses
Leaf waxes were extracted and analyzed following the protocols described in Tomasi et al. [25,26]. Waxes were extracted from each greenhouse and replicated separately. Immediately after harvesting leaf samples, the samples were submerged in 10 mL hexane (Sigma-Aldrich, St. Louis, MO, USA) and internal standards in a 20 mL glass scintillation vial. The wax extract vials were loaded onto the Agilent 7890A gas chromatograph, equipped with a 5975C mass spectrometer (Agilent, Santa Clara, CA, USA). Molecular identities of compounds were determined by the characteristic quadrupole electron impact mass spectra method. Leaf surface areas were calculated using ImageJ v1.53g software (https://imagej.net/ij/index.html, accessed 15 December 2020), and leaf waxes were calculated as µg dm−2.
4.3. Statistical Analyses
The Best Linear Unbiased Predictors (BLUPs) for each B. carinata accession for each wax component were estimated using SAS mixed linear models. The effects were partitioned into genetic effects, greenhouse effects (referred to as the environment), genotype x environment interactions (GxEs), and replication effects. The observed trait Y was analyzed as the response from the ith genotype in the kth replicate over the jth environment, using the model Yijkl = μ + gi + ej + geij + r(e)jk + errorijkl, where μ is the grand mean, gi is the effect of the ith genotype, ej is the effect of the jth environment, geij is the interaction effect between the ith genotype and jth environment, and r(e)jk is the kth replicate effect nested in the jth environment.
Correlation Coefficients (rs) were calculated using PROC CORR of SAS software to assess the relationship among wax classes and compositions. Path analysis using Proc CALIS of SAS 9.4 software was used to partition the correlation coefficients for wax classes and compositions into direct and indirect effects contributing to total wax accumulation. The broad-sense heritability (H2) was calculated as: H2 = σ2G/(σ2G + σ2error/re + σ2G×E/e), where σ2G is the genetic variance, σ2G×E is the genotype by environment interaction variance, σ2error is the residual error variance, e represents the number of environments, and r is the number of replicates [55,56,57].
5. Conclusions
The accumulation of leaf cuticular wax in plants can be one of the strategies to reduce non-stomatal water loss and thus, enhance resistance to abiotic stresses. A wide range of phenotypic variation in leaf total wax content and composition were characterized in the B. carinata diversity panel collected from different growing regions. The discovery of wide variations in leaf waxes is the first step in exploring wax biosynthetic pathways in B. carinata, moving toward identifying candidate genes that control wax accumulation, identifying its genetic networks, and developing molecular markers for molecular breeding and genomic selection programs to increase drought stress resistance in B. carinata. Among the detected waxes, C29 Alk (alkanes), C29 Alc-2 (secondary alcohol), and C29 Ket (ketone), products of the alkane forming pathway, were found to be the dominant waxes in all studied B. carinata accessions. These waxes had a high heritable nature. The possibility of identifying these waxes suggests their potential as good biomarkers in breeding for drought resistance in B. carinata, through modification of cuticle composition. The high heritability values of these waxes suggest the possibility of identifying candidate genes that control wax accumulation and selecting for these wax traits during the early generations of genetic improvement programs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants12213716/s1; Table S1: List of the 315 Brassica carina accessions; Table S2: Correlation coefficients (r) of total wax, wax classes and components in Brassica carinata accessions, r values are above the horizontal axis, corresponding p-values are below the horizontal axis; Table S3: Direct (green) and indirect (black) effects of the total wax content related wax classes and components. Effect values are above the horizontal axis; corresponding p-values are below the horizontal axis.
Author Contributions
H.A.-H. conceived and designed the study; P.T. performed wax extraction, identification and analysis; H.A.-H. and P.T. coordinated the experiment; H.A.-H. analyzed data and wrote the manuscript; P.T. provided suggestions and comments for the manuscriptใ All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the United States Department of Agriculture-Agricultural Research Service (USDA-ARS), project number: 2020-21410-008-00D and National Institute of Food and Agriculture (NIFA), Grant number: 2016-67009-25639.
Data Availability Statement
All data generated or analyzed during this study are available upon request to the corresponding author (Hussein Abdel-Haleem) at hussein.abdel-haleem@usda.gov.
Acknowledgments
We acknowledgment the funded by the United States Department of Agriculture-Agricultural Research Service (USDA-ARS), project number: 2020-21410-008-00D and National Institute of Food and Agriculture (NIFA), Grant number: 2016-67009-25639.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Mention of trade names or commercial products in this publication is solely for providing specific information and does not imply recommendation or endorsement by the United States Department of Agriculture. The USDA is an equal opportunity provider and employer.
References
- Drenth, A.C.; Olsen, D.B.; Cabot, P.E.; Johnson, J.J. Compression ignition engine performance and emission evaluation of industrial oilseed biofuel feedstocks camelina, carinata, and pennycress across three fuel pathways. Fuel 2014, 136, 143–155. [Google Scholar] [CrossRef]
- Moser, B.R. Biodiesel production, properties, and feedstocks. Vitr. Cell. Dev. Biol. Plant 2009, 45, 229–266. [Google Scholar] [CrossRef]
- Pinzi, S.; Garcia, I.L.; Lopez-Gimenez, F.J.; de Castro, M.D.L.; Dorado, G.; Dorado, M.P. The Ideal Vegetable Oil-based Biodiesel Composition: A Review of Social, Economical and Technical Implications. Energy Fuels 2009, 23, 2325–2341. [Google Scholar] [CrossRef]
- Cardone, M.; Prati, M.V.; Rocco, V.; Seggiani, M.; Senatore, A.; Vitolo, S. Brassica carinata as an Alternative Oil Crop for the Production of Biodiesel in Italy: Engine Performance and Regulated and Unregulated Exhaust Emissions. Environ. Sci. Technol. 2002, 36, 4656–4662. [Google Scholar] [CrossRef] [PubMed]
- Dorado, M.P.; Ballesteros, E.; López, F.J.; Mittelbach, M. Optimization of Alkali-Catalyzed Transesterification of Brassica Carinata Oil for Biodiesel Production. Energy Fuels 2004, 18, 77–83. [Google Scholar] [CrossRef]
- Moeller, D.; Sieverding, H.L.; Stone, J.J. Comparative Farm-Gate Life Cycle Assessment of Oilseed Feedstocks in the Northern Great Plains. Biophys. Econ. Resour. Qual. 2017, 2, 13. [Google Scholar] [CrossRef]
- Gesch, R.W.; Isbell, T.A.; Oblath, E.A.; Allen, B.L.; Archer, D.W.; Brown, J.; Hatfield, J.L.; Jabro, J.D.; Kiniry, J.R.; Long, D.S.; et al. Comparison of several Brassica species in the north central U.S. for potential jet fuel feedstock. Ind. Crops Prod. 2015, 75, 2–7. [Google Scholar] [CrossRef]
- Del Gatto, A.; Melilli, M.G.; Raccuia, S.A.; Pieri, S.; Mangoni, L.; Pacifico, D.; Signor, M.; Duca, D.; Foppa Pedretti, E.; Mengarelli, C. A comparative study of oilseed crops (Brassica napus L. subsp. oleifera and Brassica carinata A. Braun) in the biodiesel production chain and their adaptability to different Italian areas. Ind. Crops Prod. 2015, 75, 98–107. [Google Scholar] [CrossRef]
- Marillia, E.-F.; Francis, T.; Falk, K.C.; Smith, M.; Taylor, D.C. Palliser’s promise: Brassica carinata, An emerging western Canadian crop for delivery of new bio-industrial oil feedstocks. Biocatal. Agric. Biotechnol. 2014, 3, 65–74. [Google Scholar] [CrossRef]
- Hossain, Z.; Johnson, E.N.; Wang, L.; Blackshaw, R.E.; Cutforth, H.; Gan, Y. Plant establishment, yield and yield components of Brassicaceae oilseeds as potential biofuel feedstock. Ind. Crops Prod. 2019, 141, 111800. [Google Scholar] [CrossRef]
- Seepaul, R. Carinata, the Jet Fuel Cover Crop: 2016 Production Recommendations for the Southeastern United States; SS-AGR-384; Agronomy Department, UF/IFAS Extension; University of Florida: Gainesville, FL, USA, 2015; pp. 1–8. [Google Scholar]
- Gasol, C.M.; Gabarrell, X.; Anton, A.; Rigola, M.; Carrasco, J.; Ciria, P.; Solano, M.L.; Rieradevall, J. Life cycle assessment of a Brassica carinata bioenergy cropping system in southern Europe. Biomass Bioenergy 2007, 31, 543–555. [Google Scholar] [CrossRef]
- Gugel, R.K.; Falk, K.C. Agronomic and seed quality evaluation of Camelina sativa in western Canada. Can. J. Plant Sci. 2006, 86, 1047–1058. [Google Scholar] [CrossRef]
- Padbury, G.; Waltman, S.; Caprio, J.; Coen, G.; McGinn, S.; Mortensen, D.; Nielsen, G.; Sinclair, R. Agroecosystems and Land Resources of the Northern Great Plains. Agron. J. 2002, 94, 251–261. [Google Scholar] [CrossRef]
- Boyer, J.S. Plant productivity and environment. Science 1982, 218, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.M.; Turner, N.C.; Osmond, C.B. Mechanisms of drought resistance. In The Physiology and Biochemistry of Drought Resistance in Plants; Paleg, L.G., Aspinall, D., Eds.; Academic Press: New York, NY, USA, 1981; pp. 15–37. [Google Scholar]
- Jenks, M.A.; Joly, R.J.; Peters, P.J.; Rich, P.J.; Axtell, J.D.; Ashworth, E.N. Chemically Induced Cuticle Mutation Affecting Epidermal Conductance to Water Vapor and Disease Susceptibility in Sorghum bicolor (L.) Moench. Plant Physiol. 1994, 105, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Yeats, T.H.; Rose, J.K.C. The Formation and Function of Plant Cuticles. Plant Physiol. 2013, 163, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, T.; Wynne Griffiths, D. The effects of stress on plant cuticular waxes. New Phytol. 2006, 171, 469–499. [Google Scholar] [CrossRef] [PubMed]
- Kosma, D.K.; Bourdenx, B.; Bernard, A.; Parsons, E.P.; Lü, S.; Joubès, J.; Jenks, M.A. The Impact of Water Deficiency on Leaf Cuticle Lipids of Arabidopsis. Plant Physiol. 2009, 151, 1918–1929. [Google Scholar] [CrossRef]
- Shaheenuzzamn, M.; Shi, S.D.; Sohail, K.; Wu, H.Q.; Liu, T.X.; An, P.P.; Wang, Z.H.; Hasanuzzaman, M. Regulation of cuticular wax biosynthesis in plants under abiotic stress. Plant Biotechnol. Rep. 2021, 15, 1–12. [Google Scholar] [CrossRef]
- Shaheenuzzamn, M.; Liu, T.X.; Shi, S.D.; Wu, H.Q.; Wang, Z.H. Research Advances on Cuticular Waxes Biosynthesis in Crops: A Review. Int. J. Agric. Biol. 2019, 21, 911–921. [Google Scholar] [CrossRef]
- Ormeno, E.; Ruffault, J.; Gutigny, C.; Madrigal, J.; Guijarro, M.; Hernando, C.; Ballini, C. Increasing cuticular wax concentrations in a drier climate promote litter flammability. For. Ecol. Manag. 2020, 473, 118242. [Google Scholar] [CrossRef]
- Buschhaus, C.; Jetter, R. Composition and Physiological Function of the Wax Layers Coating Arabidopsis Leaves: β-Amyrin Negatively Affects the Intracuticular Water Barrier. Plant Physiol. 2012, 160, 1120–1129. [Google Scholar] [CrossRef] [PubMed]
- Tomasi, P.; Dyer, J.M.; Jenks, M.A.; Abdel-Haleem, H. Characterization of leaf cuticular wax classes and constituents in a spring Camelina sativa diversity panel. Ind. Crops Prod. 2018, 112, 247–251. [Google Scholar] [CrossRef]
- Tomasi, P.; Wang, H.; Lohrey, G.; Park, S.; Dyer, J.; Jenks, M.; Abdel-Haleem, H.J.I.C. Characterization of leaf cuticular waxes and cutin monomers of Camelina sativa and closely-related Camelina species. Ind. Crops Prod. 2017, 98, 130–138. [Google Scholar] [CrossRef]
- Cameron, K.D.; Teece, M.A.; Smart, L.B. Increased accumulation of cuticular wax and expression of lipid transfer protein in response to periodic drying events in leaves of tree tobacco. Plant Physiol. 2006, 140, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Samdur, M.Y.; Manivel, P.; Jain, V.K.; Chikani, B.M.; Gor, H.K.; Desai, S.; Misra, J.B. Genotypic differences and water-deficit induced enhancement in epicuticular wax load in peanut. Crop Sci. 2003, 43, 1294–1299. [Google Scholar] [CrossRef]
- Kim, K.S.; Park, S.H.; Kim, D.K.; Jenks, M.A. Influence of water deficit on leaf cuticular waxes of soybean (Glycine max [L.] Merr.). Int. J. Plant Sci. 2007, 168, 307–316. [Google Scholar] [CrossRef]
- Mohammed, S.; Huggins, T.; Mason, E.; Beecher, F.; Chick, C.; Sengodon, P.; Paudel, A.; Ibrahim, A.; Tilley, M.; Hays, D. Mapping the genetic loci regulating leaf epicuticular wax, canopy temperature, and drought susceptibility index in Triticum aestivum. Crop Sci. 2021, 61, 2294–2305. [Google Scholar] [CrossRef]
- Mohammed, S.; Huggins, T.D.; Beecher, F.; Chick, C.; Sengodon, P.; Mondal, S.; Paudel, A.; Ibrahim, A.M.H.; Tilley, M.; Hays, D.B. The Role of Leaf Epicuticular Wax in the Adaptation of Wheat (Triticum aestivum L.) to High Temperatures and Moisture Deficit Conditions. Crop Sci. 2018, 58, 679–689. [Google Scholar] [CrossRef]
- Bondada, B.R.; Oosterhuis, D.M.; Murphy, J.B.; Kim, K.S. Effect of water stress on the epicuticular wax composition and ultrastructure of cotton (Gossypium hirsutum L.) leaf, bract, and boll. Environ. Exp. Bot. 1996, 36, 61–69. [Google Scholar] [CrossRef]
- Lee, S.B.; Suh, M.C. Advances in the understanding of cuticular waxes in Arabidopsis thaliana and crop species. Plant Cell Rep. 2015, 34, 557–572. [Google Scholar] [CrossRef] [PubMed]
- Bernard, A.; Joubès, J. Arabidopsis cuticular waxes: Advances in synthesis, export and regulation. Prog. Lipid Res. 2013, 52, 110–129. [Google Scholar] [CrossRef] [PubMed]
- Baales, J.; Zeisler-Diehl, V.V.; Schreiber, L. Analysis of Extracellular Cell Wall Lipids: Wax, Cutin, and Suberin in Leaves, Roots, Fruits, and Seeds. In Plant Lipids: Methods and Protocols; Bartels, D., Dörmann, P., Eds.; Springer US: New York, NY, USA, 2021; pp. 275–293. [Google Scholar]
- Samuels, L.; Kunst, L.; Jetter, R. Sealing Plant Surfaces: Cuticular Wax Formation by Epidermal Cells. Annu. Rev. Plant Biol. 2008, 59, 683–707. [Google Scholar] [CrossRef] [PubMed]
- Baker, E.A. The influence of environment on leaf wax development in Brassica oleracea var. gemmifera. New Phytol. 1974, 73, 955–966. [Google Scholar] [CrossRef]
- Lee, S.B.; Kim, H.; Kim, R.J.; Suh, M.C. Overexpression of Arabidopsis MYB96 confers drought resistance in Camelina sativa via cuticular wax accumulation. Plant Cell Rep. 2014, 33, 1535–1546. [Google Scholar] [CrossRef] [PubMed]
- Millar, A.A.; Clemens, S.; Zachgo, S.; Giblin, E.M.; Taylor, D.C.; Kunst, L. CUT1, an Arabidopsis Gene Required for Cuticular Wax Biosynthesis and Pollen Fertility, Encodes a Very-Long-Chain Fatty Acid Condensing Enzyme. Plant Cell 1999, 11, 825–838. [Google Scholar] [CrossRef] [PubMed]
- Xue, D.; Zhang, X.; Lu, X.; Chen, G.; Chen, Z.-H. Molecular and Evolutionary Mechanisms of Cuticular Wax for Plant Drought Tolerance. Front. Plant Sci. 2017, 8, 621. [Google Scholar] [CrossRef] [PubMed]
- Kolattukudy, P.E. Enzymatic synthesis of fatty alcohols in Brassica oleracea. Arch. Biochem. Biophys. 1971, 142, 701–709. [Google Scholar] [CrossRef]
- Tassone, E.E.; Lipka, A.E.; Tomasi, P.; Lohrey, G.T.; Qian, W.; Dyer, J.M.; Gore, M.A.; Jenks, M.A. Chemical variation for leaf cuticular waxes and their levels revealed in a diverse panel of Brassica napus L. Ind. Crops Prod. 2016, 79, 77–83. [Google Scholar] [CrossRef]
- Fang, Y.; Xiong, L. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell. Mol. Life Sci. 2015, 72, 673–689. [Google Scholar] [CrossRef]
- Riederer, M.; Schreiber, L. Protecting against water loss: Analysis of the barrier properties of plant cuticles. J. Exp. Bot. 2001, 52, 2023–2032. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Campo, C.; Tortosa, M.E.; Tewari, I.; Tewari, J.P. Epicuticular Wax Columns in Cultivated Brassica Species and in their Close Wild Relatives. Ann. Bot. 1999, 83, 515–519. [Google Scholar] [CrossRef]
- Gunasinghe, N.; You, M.P.; Clode, P.L.; Barbetti, M.J. Mechanisms of resistance in Brassica carinata, B. napus and B. juncea to Pseudocercosporella capsellae. Plant Pathol. 2016, 65, 888–900. [Google Scholar] [CrossRef]
- Bodnaryk, R.P. Leaf epicuticular wax, an antixenotic factor in Brassicaceae that affects the rate and pattern of feeding of flea beetles, Phyllotreta cruciferae (Goeze). Can. J. Plant Sci. 1992, 72, 1295–1303. [Google Scholar] [CrossRef]
- Ni, Y.; Guo, Y.J.; Han, L.; Tang, H.; Conyers, M. Leaf cuticular waxes and physiological parameters in alfalfa leaves as influenced by drought. Photosynthetica 2012, 50, 458–466. [Google Scholar] [CrossRef]
- Bourdenx, B.; Bernard, A.; Domergue, F.; Pascal, S.; Léger, A.; Roby, D.; Pervent, M.; Vile, D.; Haslam, R.P.; Napier, J.A.; et al. Overexpression of Arabidopsis ECERIFERUM1 Promotes Wax Very-Long-Chain Alkane Biosynthesis and Influences Plant Response to Biotic and Abiotic Stresses. Plant Physiol. 2011, 156, 29–45. [Google Scholar] [CrossRef]
- Kim, K.S.; Park, S.H.; Jenks, M.A. Changes in leaf cuticular waxes of sesame (Sesamum indicum L.) plants exposed to water deficit. J. Plant Physiol. 2007, 164, 1134–1143. [Google Scholar] [CrossRef]
- Xu, X.; Xiao, L.; Feng, J.; Chen, N.; Chen, Y.; Song, B.; Xue, K.; Shi, S.; Zhou, Y.; Jenks, M.A. Cuticle lipids on heteromorphic leaves of Populus euphratica Oliv. growing in riparian habitats differing in available soil moisture. Physiol. Plant. 2016, 158, 318–330. [Google Scholar] [CrossRef]
- Li, H.; Guo, Y.; Cui, Q.; Zhang, Z.; Yan, X.; Ahammed, G.J.; Yang, X.; Yang, J.; Wei, C.; Zhang, X. Alkanes (C29 and C31)-Mediated Intracuticular Wax Accumulation Contributes to Melatonin- and ABA-Induced Drought Tolerance in Watermelon. J. Plant Growth Regul. 2020, 39, 1441–1450. [Google Scholar] [CrossRef]
- Post-Beittenmiller, D. Biochemistry and Molecular Biology of Wax Production in Plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996, 47, 405–430. [Google Scholar] [CrossRef]
- Le Provost, G.; Domergue, F.; Lalanne, C.; Ramos Campos, P.; Grosbois, A.; Bert, D.; Meredieu, C.; Danjon, F.; Plomion, C.; Gion, J.-M. Soil water stress affects both cuticular wax content and cuticle-related gene expression in young saplings of maritime pine (Pinus pinaster Ait). BMC Plant Biol. 2013, 13, 95. [Google Scholar] [CrossRef]
- Nyquist, W.E.; Baker, R. Estimation of heritability and prediction of selection response in plant populations. Crit. Rev. Plant Sci. 1991, 10, 235–322. [Google Scholar] [CrossRef]
- Holland, J.B.; Nyquist, W.E.; Cervantes-Martínez, C.T. Estimating and interpreting heritability for plant breeding: An update. Plant Breed. Rev. 2003, 22, 9–112. [Google Scholar]
- Piepho, H.P.; Mohring, J. Computing heritability and selection response from unbalanced plant breeding trials. Genetics 2007, 177, 1881–1888. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).