Foliar Application of Melatonin Positively Affects the Physio-Biochemical Characteristics of Cotton (Gossypium hirsutum L.) under the Combined Effects of Low Temperature and Salinity Stress
Abstract
:1. Introduction
2. Results
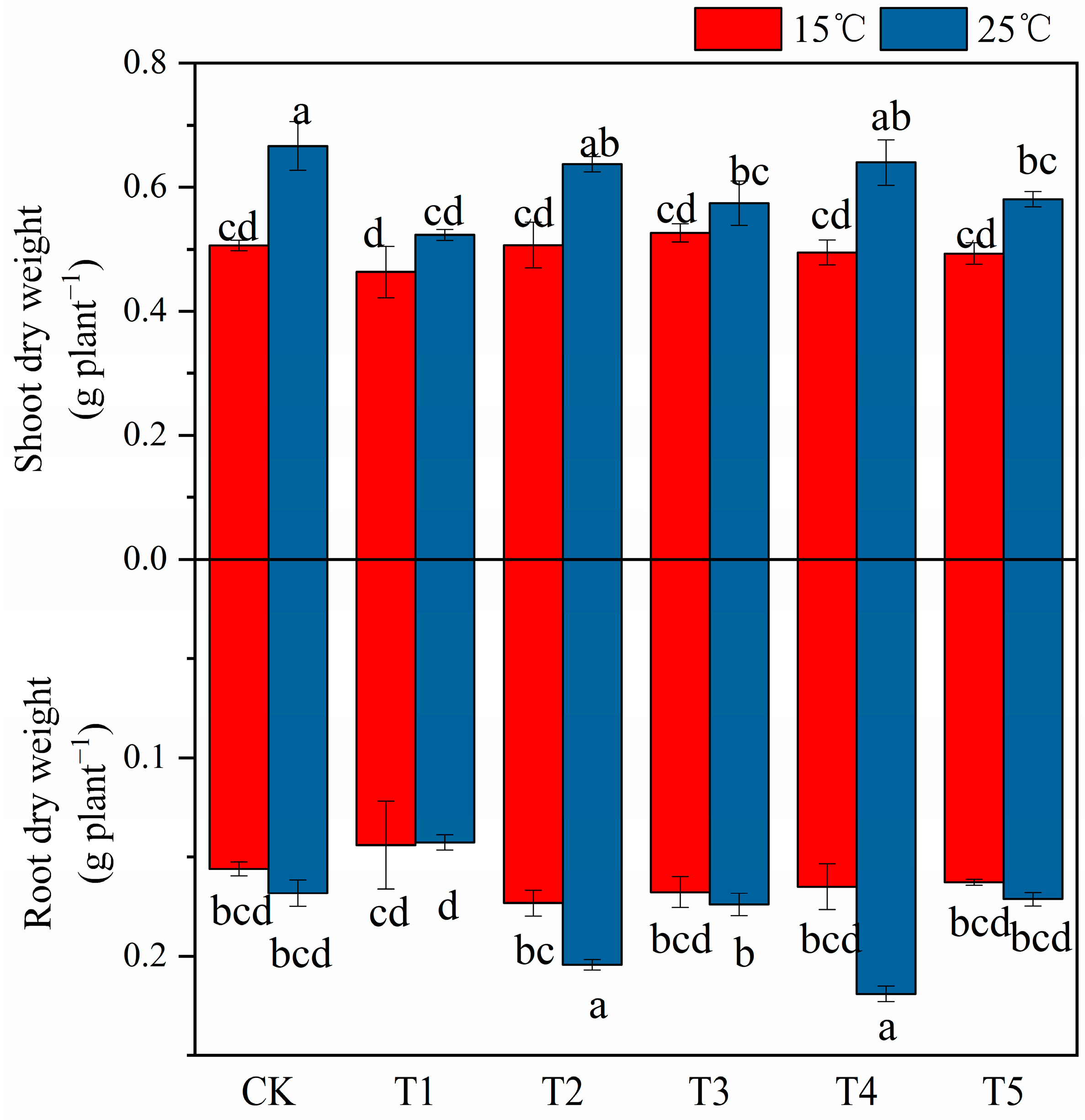
2.1. The Effect of MT on Cotton Seedling Biomass under Low Temperature and Salt Stress
2.2. Effects of MT on Gas Exchange Parameters of Cotton Seedling Leaves under Low Temperature and Salt Stress
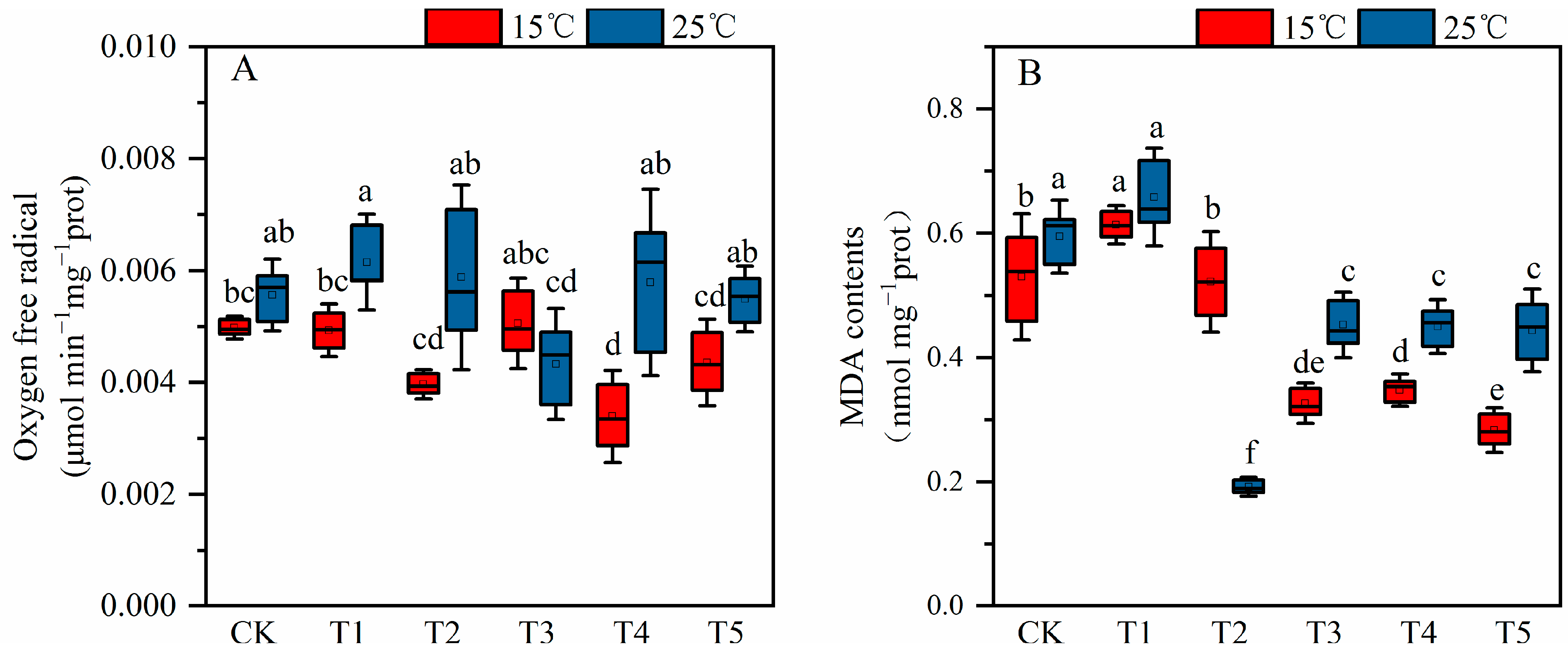
2.3. The Effect of MT on Cotton Seedling Lipid Peroxidationunder Low Temperature and Salt Stress
2.4. Effect of MT on Antioxidant Enzyme Activities under Low Temperature and Salt Stress
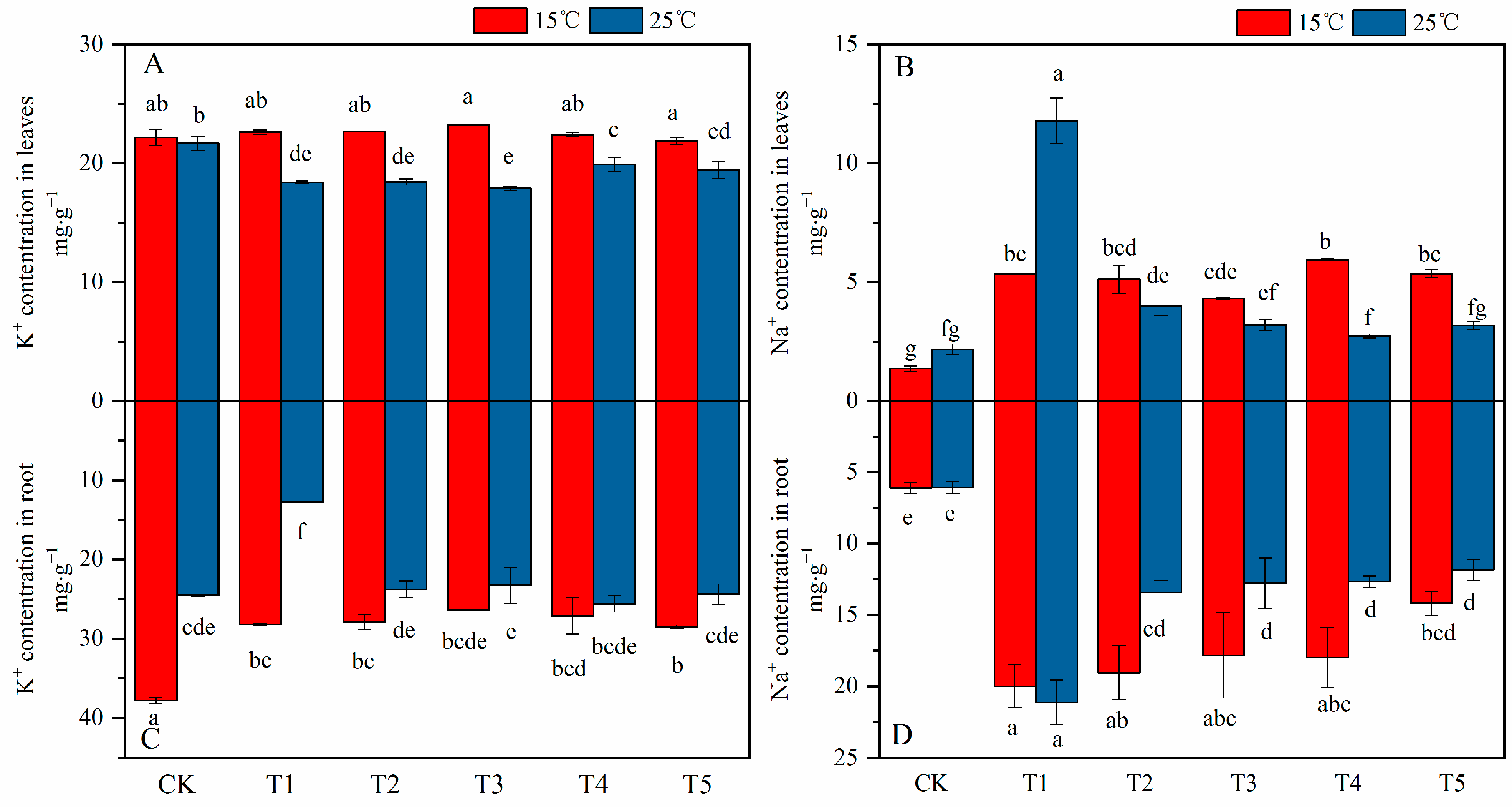
2.5. Effect of MT on Ion Homeostasis and Absorption under Different Temperatures and Salt Stress
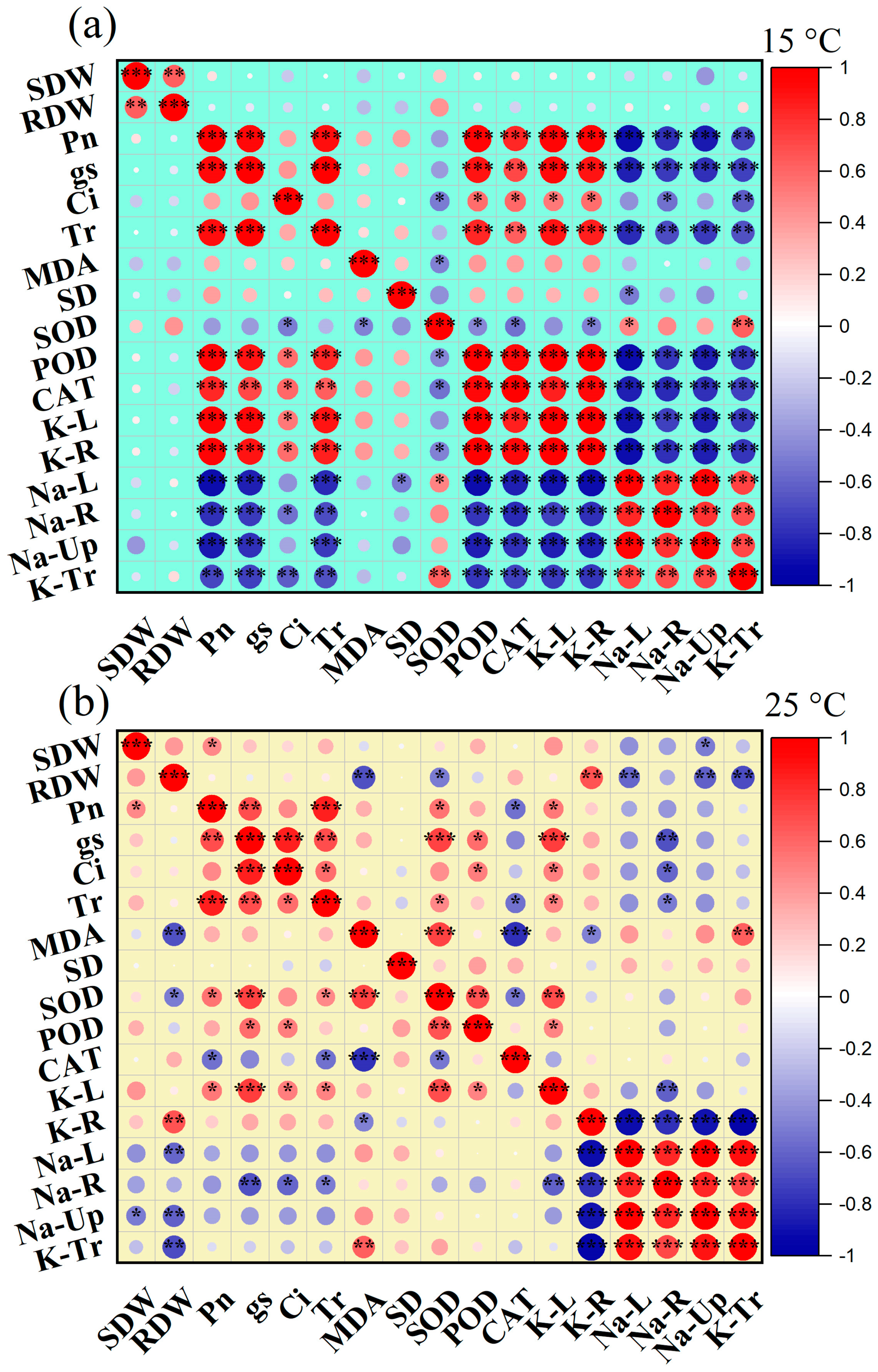
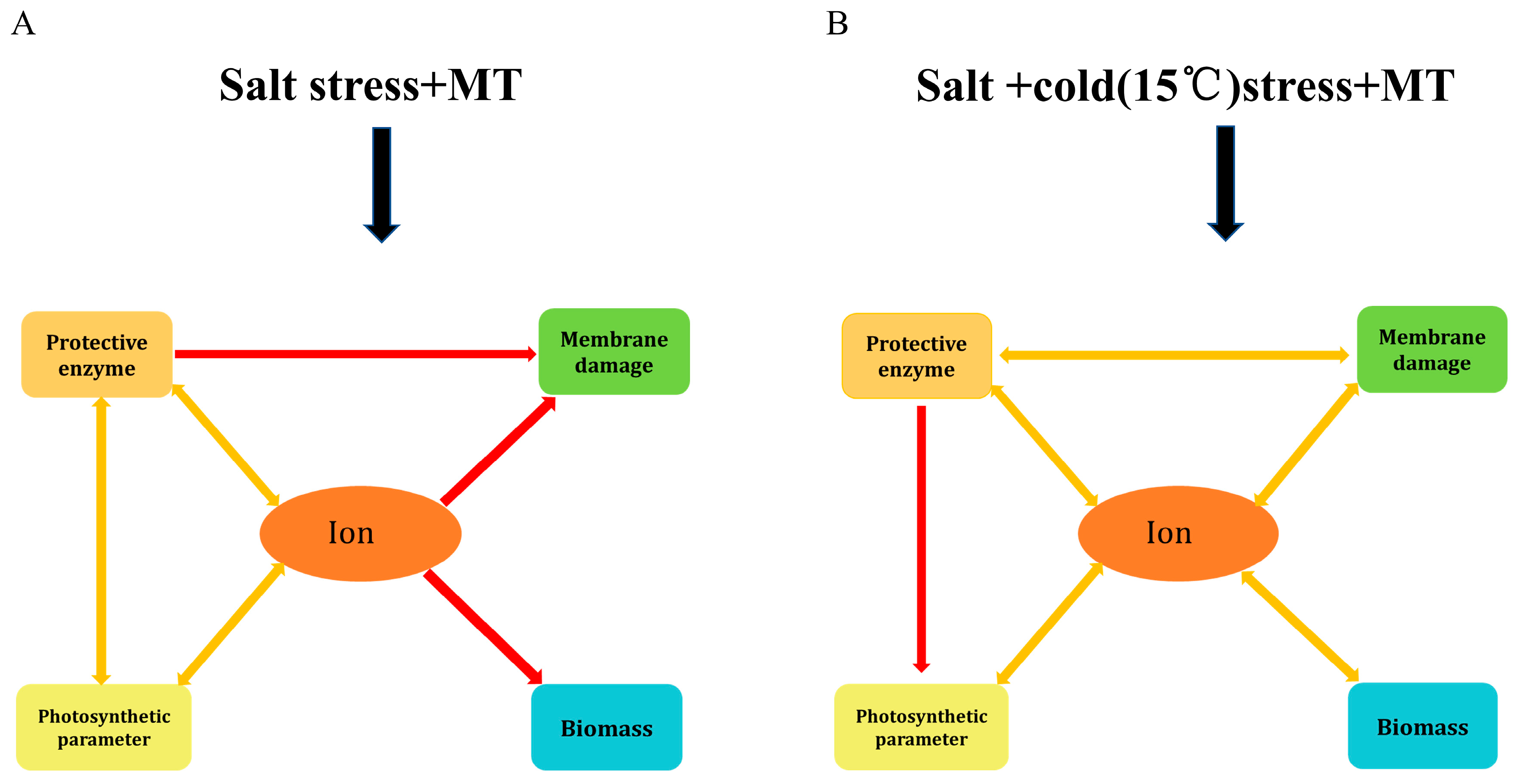
3. Discussion
4. Materials and Methods
4.1. Plant Material and Experimental Design
4.2. Measurements
4.2.1. Determination of Cotton Seeding Biomass and Gas Exchange Parameters
4.2.2. Determination of MDA, Superoxide Anion and Antioxidant Enzyme Activities
4.2.3. Determination of Seeding Biomass and Ion Content
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Mishra, S.; Kumar, S.; Saha, B.; Awasthi, J.; Dey, M.; Panda, S.K.; Sahoo, L. Crosstalk between Salt, Drought, and Cold Stress in Plants: Toward Genetic Engineering for Stress Tolerance. In Abiotic Stress Response in Plants; Springer: New York, NY, USA, 2016; pp. 57–88. [Google Scholar]
- Naing, A.H.; Kim, C.K. Abiotic stress-induced anthocyanins in plants: Their role in tolerance to abiotic stresses. Physiol. Plant. 2021, 172, 1711–1723. [Google Scholar] [CrossRef] [PubMed]
- Demiral, T.; Türkan, I. Exogenous glycinebetaine affects growth and proline accumulation and retards senescence in two rice cultivars under NaCl stress. Environ. Exp. Bot. 2006, 56, 72–79. [Google Scholar] [CrossRef]
- Saeed, N.A.; Ahmad, M.; Mukhtar, Z. GM Cotton for Stress Environments. In Cotton Precision Breeding; Springer: Berlin/Heidelberg, Germany, 2021; pp. 257–280. [Google Scholar]
- Çiçek, N.; Çakırlar, H. Effects of salt stress on some physiological and photosynthetic parameters at three different temperatures in six soya bean (Glycine max L. Merr.) cultivars. J. Agron. Crop Sci. 2008, 194, 34–46. [Google Scholar] [CrossRef]
- Prajapati, P.; Gupta, P.; Kharwar, R.N.; Seth, C.S. Nitric oxide mediated regulation of ascorbate-glutathione pathway alleviates mitotic aberrations and DNA damage in Allium cepa L. under salinity stress. Int. J. Phytoremediation 2023, 25, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Jinc, S.; Liu, S.; Xu, L.; Kong, J. Effects of exogenous nitric oxide on growth of cotton seedlings under NaCl stress. J. Soil Sci. Plant Nutr. 2014, 14, 1–13. [Google Scholar] [CrossRef]
- Hamani, A.K.M.; Chen, J.; Soothar, M.K.; Wang, G.; Shen, X.; Gao, Y.; Qiu, R. Application of Exogenous Protectants Mitigates Salt-Induced Na(+) Toxicity and Sustains Cotton (Gossypium hirsutum L.) Seedling Growth: Comparison of Glycine Betaine and Salicylic Acid. Plants 2021, 10, 380. [Google Scholar] [CrossRef]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef]
- Mariyam, S.; Bhardwaj, R.; Khan, N.A.; Sahi, S.V.; Seth, C.S. Review on nitric oxide at the forefront of rapid systemic signaling in mitigation of salinity stress in plants: Crosstalk with calcium and hydrogen peroxide. Plant Sci. 2023, 336, 111835. [Google Scholar] [CrossRef]
- Yan, P.; Li, G.; Sun, H.; Zhang, Z.; Yang, R.; Sun, J. Can arbuscular mycorrhizal fungi and biochar enhance plant resistance to low-temperature stress? Agron. J. 2021, 113, 1457–1466. [Google Scholar] [CrossRef]
- Airaki, M.; Leterrier, M.; Mateos, R.M.; Valderrama, R.; Chaki, M.; Barroso, J.B.; Del Rio, L.A.; Palma, J.M.; Corpas, F.J. Metabolism of reactive oxygen species and reactive nitrogen species in pepper (Capsicum annuum L.) plants under low temperature stress. Plant Cell Environ. 2012, 35, 281–295. [Google Scholar] [CrossRef]
- Sun, B.; Liu, G.-L.; Phan, T.T.; Yang, L.-T.; Li, Y.-R.; Xing, Y.-X. Effects of cold stress on root growth and physiological metabolisms in seedlings of different sugarcane varieties. Sugar Tech. 2017, 19, 165–175. [Google Scholar] [CrossRef]
- Srinivasan, V.; Pandi-Perumal, S.R.; Trahkt, I.; Spence, D.W.; Poeggeler, B.; Hardeland, R.; Cardinali, D.P. Melatonin and Melatonergic Drugs on Sleep: Possible Mechanisms of Action. Int. J. Neurosci. 2009, 119, 821–846. [Google Scholar] [CrossRef] [PubMed]
- Calvo, J.R.; Gonzalez-Yanes, C.; Maldonado, M. The role of melatonin in the cells of the innate immunity: A review. J. Pineal Res. 2013, 55, 103–120. [Google Scholar] [CrossRef]
- Zhan, Y.; Wu, T.; Zhao, X.; Wang, Z.; Chen, Y. Comparative physiological and full-length transcriptome analyses reveal the molecular mechanism of melatonin-mediated salt tolerance in okra (Abelmoschus esculentus L.). BMC Plant Biol. 2021, 21, 1–16. [Google Scholar]
- Hattori, A.; Migitaka, H.; Iigo, M.; Itoh, M.; Yamamoto, K.; Ohtani-Kaneko, R.; Hara, M.; Suzuki, T.; Reiter, R.J. Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates. Biochem. Mol. Biol. Int. 1995, 35, 627–634. [Google Scholar] [PubMed]
- Byeon, Y.; Back, K. An increase in melatonin in transgenic rice causes pleiotropic phenotypes, including enhanced seedling growth, delayed flowering, and low grain yield. J. Pineal Res. 2014, 56, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Melatonin in plants–diversity of levels and multiplicity of functions. Front. Plant Sci. 2016, 7, 198. [Google Scholar] [CrossRef]
- Wei, J.; Li, D.X.; Zhang, J.R.; Shan, C.; Rengel, Z.; Song, Z.B.; Chen, Q. Phytomelatonin receptor PMTR 1-mediated signaling regulates stomatal closure in Arabidopsis thaliana. J. Pineal Res. 2018, 65, e12500. [Google Scholar] [CrossRef]
- Back, K. Melatonin metabolism, signaling and possible roles in plants. Plant J. 2021, 105, 376–391. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-F.; Lu, K.-K.; Li, T.-T.; Zhang, Y.; Guo, J.-X.; Song, R.-F.; Liu, W.-C. Maize Phytomelatonin Receptor1 functions in plant tolerance to osmotic and drought stress. J. Exp. Bot. 2021, 73, 5961–5973. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Zheng, G.; Li, W.; Wang, Y.; Hu, B.; Wang, H.; Wu, H.; Qian, Y.; Zhu, X.G.; Tan, D.X. Melatonin delays leaf senescence and enhances salt stress tolerance in rice. J. Pineal Res. 2015, 59, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Wang, J.; Xu, D.; Tao, S.; Chong, S.; Yan, D.; Li, Z.; Yuan, H.; Zheng, B. Melatonin regulates the functional components of photosynthesis, antioxidant system, gene expression, and metabolic pathways to induce drought resistance in grafted Carya cathayensis plants. Sci. Total Environ. 2020, 713, 136675. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Jiang, C.; Ye, T.; Tan, D.-X.; Reiter, R.J.; Zhang, H.; Liu, R.; Chan, Z. Comparative physiological, metabolomic, and transcriptomic analyses reveal mechanisms of improved abiotic stress resistance in bermudagrass [Cynodon dactylon (L). Pers.] by exogenous melatonin. J. Exp. Bot. 2015, 66, 681–694. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: A new plant hormone and/or a plant master regulator? Trends Plant Sci. 2019, 24, 38–48. [Google Scholar] [CrossRef]
- Moustafa-Farag, M.; Almoneafy, A.; Mahmoud, A.; Elkelish, A.; Arnao, M.B.; Li, L.; Ai, S. Melatonin and its protective role against biotic stress impacts on plants. Biomolecules 2020, 10, 54. [Google Scholar] [CrossRef]
- Turk, H.; Genisel, M. Melatonin-related mitochondrial respiration responses are associated with growth promotion and cold tolerance in plants. Cryobiology 2020, 92, 76–85. [Google Scholar] [CrossRef]
- Zhan, H.; Nie, X.; Zhang, T.; Li, S.; Wang, X.; Du, X.; Tong, W.; Song, W. Melatonin: A small molecule but important for salt stress tolerance in plants. Int. J. Mol. Sci. 2019, 20, 709. [Google Scholar] [CrossRef]
- Fan, J.; Xie, Y.; Zhang, Z.; Chen, L. Melatonin: A multifunctional factor in plants. Int. J. Mol. Sci. 2018, 19, 1528. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, X.; Dong, Y.; Zhang, F.; He, Q.; Chen, J.; Zhu, S.; Zhao, T. Seed priming with melatonin improves salt tolerance in cotton through regulating photosynthesis, scavenging reactive oxygen species and coordinating with phytohormone signal pathways. Ind. Crops Prod. 2021, 169, 113671. [Google Scholar] [CrossRef]
- Bajwa, V.S.; Shukla, M.R.; Sherif, S.M.; Murch, S.J.; Saxena, P.K. Role of melatonin in alleviating cold stress in A rabidopsis thaliana. J. Pineal Res. 2014, 56, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.M.; Zhang, Y. Melatonin: A well-documented antioxidant with conditional pro-oxidant actions. J. Pineal Res. 2014, 57, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Kamiab, F. Exogenous melatonin mitigates the salinity damages and improves the growth of pistachio under salinity stress. J. Plant Nutr. 2020, 43, 1468–1484. [Google Scholar] [CrossRef]
- Li, J.; Yang, Y.; Sun, K.; Chen, Y.; Chen, X.; Li, X. Exogenous melatonin enhances cold, salt and drought stress tolerance by improving antioxidant defense in tea plant (Camellia sinensis (L.) O. Kuntze). Molecules 2019, 24, 1826. [Google Scholar] [CrossRef]
- Mukherjee, S.; David, A.; Yadav, S.; Baluška, F.; Bhatla, S.C. Salt stress-induced seedling growth inhibition coincides with differential distribution of serotonin and melatonin in sunflower seedling roots and cotyledons. Physiol. Plant. 2014, 152, 714–728. [Google Scholar] [CrossRef]
- Ding, F.; Liu, B.; Zhang, S. Exogenous melatonin ameliorates cold-induced damage in tomato plants. Sci. Hortic. 2017, 219, 264–271. [Google Scholar] [CrossRef]
- Shen, J.; Chen, D.; Zhang, X.; Song, L.; Dong, J.; Xu, Q.; Hu, M.; Cheng, Y.; Shen, F.; Wang, W. Mitigation of salt stress response in upland cotton (Gossypium hirsutum) by exogenous melatonin. J. Plant Res. 2021, 134, 857–871. [Google Scholar] [CrossRef]
- Li, J.; Arkorful, E.; Cheng, S.; Zhou, Q.; Li, H.; Chen, X.; Sun, K.; Li, X. Alleviation of cold damage by exogenous application of melatonin in vegetatively propagated tea plant (Camellia sinensis (L.) O. Kuntze). Sci. Hortic. 2018, 238, 356–362. [Google Scholar] [CrossRef]
- Fan, J.; Hu, Z.; Xie, Y.; Chan, Z.; Chen, K.; Amombo, E.; Chen, L.; Fu, J. Alleviation of cold damage to photosystem II and metabolisms by melatonin in Bermudagrass. Front. Plant Sci. 2015, 6, 925. [Google Scholar] [CrossRef]
- Hamani, A.K.M.; Wang, G.; Soothar, M.K.; Shen, X.; Gao, Y.; Qiu, R.; Mehmood, F. Responses of leaf gas exchange attributes, photosynthetic pigments and antioxidant enzymes in NaCl-stressed cotton (Gossypium hirsutum L.) seedlings to exogenous glycine betaine and salicylic acid. BMC Plant Biol. 2020, 20, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Wang, Q.; Sun, R.; Zhang, B. Deep sequencing reveals important roles of microRNAs in response to drought and salinity stress in cotton. J. Exp. Bot. 2015, 66, 789–804. [Google Scholar] [CrossRef] [PubMed]
- Valliyodan, B.; Nguyen, H.T. Understanding regulatory networks and engineering for enhanced drought tolerance in plants. Curr. Opin. Plant Biol. 2006, 9, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Fan, J.; Xie, Y.; Amombo, E.; Liu, A.; Gitau, M.M.; Khaldun, A.; Chen, L.; Fu, J. Comparative photosynthetic and metabolic analyses reveal mechanism of improved cold stress tolerance in bermudagrass by exogenous melatonin. Plant Physiol. Biochem. 2016, 100, 94–104. [Google Scholar] [CrossRef]
- Mittler, R. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 2006, 11, 15–19. [Google Scholar] [CrossRef]
- Mittler, R.; Blumwald, E. Genetic engineering for modern agriculture: Challenges and perspectives. Annu. Rev. Plant Biol. 2010, 61, 443–462. [Google Scholar] [CrossRef]
- Suzuki, N.; Rivero, R.M.; Shulaev, V.; Blumwald, E.; Mittler, R. Abiotic and biotic stress combinations. N. Phytol. 2014, 203, 32–43. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Balfagón, D.; Arbona, V.; Gómez-Cadenas, A.; Inupakutika, M.A.; Mittler, R. ABA is required for the accumulation of APX1 and MBF1c during a combination of water deficit and heat stress. J. Exp. Bot. 2016, 67, 5381–5390. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Rivero, R.M.; Martínez, V.; Gómez-Cadenas, A.; Arbona, V. Tolerance of citrus plants to the combination of high temperatures and drought is associated to the increase in transpiration modulated by a reduction in abscisic acid levels. BMC Plant Biol. 2016, 16, 1–16. [Google Scholar] [CrossRef]
- Khalil, R.; Yusuf, M.; Bassuony, F.; Haroun, S.; Gamal, A. Alpha-tocopherol reinforce selenium efficiency to ameliorates salt stress in maize plants through carbon metabolism, enhanced photosynthetic pigments and ion uptake. South Afr. J. Bot. 2022, 144, 1–9. [Google Scholar] [CrossRef]
- Sarropoulou, V.N.; Therios, I.N.; Dimassi-Theriou, K.N. Melatonin promotes adventitious root regeneration in in vitro shoot tip explants of the commercial sweet cherry rootstocks CAB-6P (Prunus Cerasus L.), Gisela 6 (P. cerasus × P. canescens), and MxM 60 (P. avium × P. mahaleb). J. Pineal Res. 2012, 52, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Chatrath, A.; Mandal, P.; Anuradha, M. Effect of secondary salinization on photosynthesis in fodder oat (Avena sativa L.) genotypes. J. Agron. Crop Sci. 2000, 184, 13–16. [Google Scholar] [CrossRef]
- Irshad, A.; Rehman, R.N.U.; Kareem, H.A.; Yang, P.; Hu, T. Addressing the challenge of cold stress resilience with the synergistic effect of Rhizobium inoculation and exogenous melatonin application in Medicago truncatula. Ecotoxicol. Environ. Saf. 2021, 226, 112816. [Google Scholar] [CrossRef]
- Parveen, A.; Ahmar, S.; Kamran, M.; Malik, Z.; Ali, A.; Riaz, M.; Abbasi, G.H.; Khan, M.; Sohail, A.B.; Rizwan, M. Abscisic acid signaling reduced transpiration flow, regulated Na+ ion homeostasis and antioxidant enzyme activities to induce salinity tolerance in wheat (Triticum aestivum L.) seedlings. Environ. Technol. Innov. 2021, 24, 101808. [Google Scholar] [CrossRef]
- Hernandez, J.A.; Jiménez, A.; Mullineaux, P.; Sevilia, F. Tolerance of pea (Pisum sativum L.) to long-term salt stress is associated with induction of antioxidant defences. Plant Cell Environ. 2000, 23, 853–862. [Google Scholar] [CrossRef]
- Anjum, S.A.; Ashraf, U.; Tanveer, M.; Khan, I.; Hussain, S.; Shahzad, B.; Zohaib, A.; Abbas, F.; Saleem, M.F.; Ali, I. Drought induced changes in growth, osmolyte accumulation and antioxidant metabolism of three maize hybrids. Front. Plant Sci. 2017, 8, 69. [Google Scholar] [CrossRef]
- Wang, L.; Liu, J.; Wang, W.; Sun, Y. Exogenous melatonin improves growth and photosynthetic capacity of cucumber under salinity-induced stress. Photosynthetica 2016, 54, 19–27. [Google Scholar] [CrossRef]
- Turk, H.; Erdal, S.; Genisel, M.; Atici, O.; Demir, Y.; Yanmis, D. The regulatory effect of melatonin on physiological, biochemical and molecular parameters in cold-stressed wheat seedlings. Plant Growth Regul. 2014, 74, 139–152. [Google Scholar] [CrossRef]
- Marta, B.; Szafrańska, K.; Posmyk, M.M. Exogenous melatonin improves antioxidant defense in cucumber seeds (Cucumis sativus L.) germinated under chilling stress. Front. Plant Sci. 2016, 7, 575. [Google Scholar] [CrossRef]
- Martinez, V.; Nieves-Cordones, M.; Lopez-Delacalle, M.; Rodenas, R.; Mestre, T.C.; Garcia-Sanchez, F.; Rubio, F.; Nortes, P.A.; Mittler, R.; Rivero, R.M. Tolerance to stress combination in tomato plants: New insights in the protective role of melatonin. Molecules 2018, 23, 535. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, H.; Song, C.; Zhu, J.-K.; Shabala, S. Mechanisms of plant responses and adaptation to soil salinity. Innovation 2020, 1, 100017. [Google Scholar] [CrossRef]
- Shahzad, R.; Khan, A.L.; Bilal, S.; Waqas, M.; Kang, S.-M.; Lee, I.-J. Inoculation of abscisic acid-producing endophytic bacteria enhances salinity stress tolerance in Oryza sativa. Environ. Exp. Bot. 2017, 136, 68–77. [Google Scholar] [CrossRef]
- Rasouli, F.; Kiani-Pouya, A.; Tahir, A.; Shabala, L.; Chen, Z.; Shabala, S. A comparative analysis of stomatal traits and photosynthetic responses in closely related halophytic and glycophytic species under saline conditions. Environ. Exp. Bot. 2021, 181, 104300. [Google Scholar] [CrossRef]
- Perin, E.C.; da Silva Messias, R.; Borowski, J.M.; Crizel, R.L.; Schott, I.B.; Carvalho, I.R.; Rombaldi, C.V.; Galli, V. ABA-dependent salt and drought stress improve strawberry fruit quality. Food Chem. 2019, 271, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Lv, Y.; Shi, Y.; Li, T.; Chen, Y.; Zhao, D.; Zhao, Z. The role of phyto-melatonin and related metabolites in response to stress. Molecules 2018, 23, 1887. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Is phytomelatonin a new plant hormone? Agronomy 2020, 10, 95. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Tian, M.; Gu, Q.; Zhu, M. The involvement of hydrogen peroxide and antioxidant enzymes in the process of shoot organogenesis of strawberry callus. Plant Sci. 2003, 165, 701–707. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Lacan, D.; Baccou, J.-C. High levels of antioxidant enzymes correlate with delayed senescence in nonnetted muskmelon fruits. Planta 1998, 204, 377–382. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, B. Effects of calcium on antioxidant activities and water relations associated with heat tolerance in two cool-season grasses. J. Exp. Bot. 2001, 52, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Shabala, S.; Mackay, A. Ion transport in halophytes. Adv. Bot. Res. 2011, 57, 151–199. [Google Scholar]
- Malik, R.N.; Husain, S.Z.; Nazir, I. Heavy metal contamination and accumulation in soil and wild plant species from industrial area of Islamabad, Pakistan. Pak. J. Bot. 2010, 42, 291–301. [Google Scholar]


| Antioxidant Enzyme Activities in Leaf Tissues | ||||||
|---|---|---|---|---|---|---|
| Treatment | SOD/U × g−1 × FW | POD/U × g−1 × FW | CAT/U × g−1 × FW | |||
| 15 °C | 25 °C | 15 °C | 25 °C | 15 °C | 25 °C | |
| CK | 67.32 cd | 108.32 b | 13.32 a | 7.38 b | 0.46 a | 0.01 b |
| T1 | 69.27 cd | 78.52 c | 4.38 d e | 5.09 cd | 0.11 b | 0.02 b |
| T2 | 147.18 a | 45.32 f | 5.17 cd | 5.95 c | 0.03 b | 0.07 b |
| T3 | 137.57 a | 51.96 ef | 4.46 de | 3.55 ef | 0.04 b | 0.02 b |
| T4 | 140.41 a | 58.09 d ef | 4.45 de | 3.79 ef | 0.04 b | 0.02 b |
| T5 | 101.91 b | 63.78 de | 3.14 f | 3.95 ef | 0.03 b | 0.04 b |
| Temp | 11.79 ** | 1.43 | 29.48 ** | |||
| MT | 2.60 | 1.46 ** | 20.29 ** | |||
| Temp× MT | 1.35 | 9.90 ** | 33.43 ** | |||
| Independent Variable | Low Temperature (15 °C) + Salt Stress (150 mM) | 25 °C + Salt Stress (150 mM) | |||||
|---|---|---|---|---|---|---|---|
| Stepwise Regression Equation | R2 | p | Stepwise Regression Equation | R2 | p | ||
| X1 | shoot dry weight | y = 1.443x2 + 0.266 | 0.40 | 0.01 | y = 0.20x12 + 0.192x14 − 0.101x16 + 0.147 | 0.87 | 0.00 |
| X2 | root dry weight | y = 0.278x1 + 0.023 | 0.40 | 0.01 | y = −0.070x17 + 0.244 | 0.47 | 0.00 |
| X3 | Pn | y = −0.014x5 + 3.090x6 + 0.398x10 + 2.046 | 0.97 | 0.00 | y = 3.558x6 + 2.126 | 0.72 | 0.00 |
| X4 | gs | y = −0.002x12 + 0.001x10 + 0.061x6 + 0.034 | 1.00 | 0.00 | y = 0.002x13 + 0.001x9 + 0.000x5−0.108 | 0.92 | 0.00 |
| X5 | Ci | y = −135.623x17 − 269.164 | 0.39 | 0.06 | y = 891.435x4 + 122.900 | 0.72 | 0.00 |
| X6 | Tr | y = 0.025x12 − 0.016x10 + 15.698x4−0.468 | 0.99 | 0.00 | y = 0.203x3−0.033 | 0.72 | 0.00 |
| X7 | MDA | y = −0.002x9 + 0.635 | 0.23 | 0.04 | y = −0.070x10 + 0.007x9 + 0.209x17 + 0.120 | 0.92 | 0.00 |
| X8 | Superoxide anion | y = 0.000x14 − 0.005 | 0.26 | 0.03 | / | / | / |
| X9 | SOD | y = 205.005x17 − 49.711 | 0.40 | 0.01 | y = −1.211x15 + 7.685x10 + 101.543x7 − 1.916 | 0.96 | 0.00 |
| X10 | POD | y = 0.125x15 + 130.185x4 + 2.404x11 − 0.422 | 0.98 | 0.00 | y = −4.530x7 + 32.189x11 + 0.083x9 + 0.531 | 0.85 | 0.00 |
| X11 | CAT | y = −0.052x15 − 2.979x6 + 0.329x10 − 0.498 | 0.96 | 0.00 | y = 0.002x14 − 0.114x7 + 0.073 | 0.73 | 0.00 |
| X12 | K+-L | y = −0.057x16 + 0.042x15 − 35.334x4 + 0.604x13 + 22.223x17 − 11.876 | 0.96 | 0.00 | y = 37.733x4 + 16.815 | 0.57 | 0.00 |
| X13 | K+-R | y = 0.42x11 + 48.308x4 + 1.020x12 − 33.413x17 + 31.137 | 1.00 | 0.00 | y = 0.237x16 + 1.078x12−23.081x17 + 20.755 | 0.99 | 0.00 |
| X14 | Na+-L | y = 4.983x2 + 9.996x1 + 0.492x16 − 5.787 | 0.99 | 0.00 | y = −0.896x7 + 4.021x1 + 0.534x16 − 1.744 | 1.00 | 0.00 |
| X15 | Na+-R | y = 0.758x13−4.227x11 + 4.298x12 + 2.025x14 − 110.436 | 0.87 | 0.00 | y = 17.741x17 − 0.150x9 + 6.932 | 0.91 | 0.00 |
| X16 | Na+-uptake | y = −0.993x2 − 20.381x1 + 2.027x14 + 11.795 | 1.00 | 0.00 | y = 1.713x7 − 7.595x1 + 1.869x14 + 3.308 | 1.00 | 0.00 |
| X17 | K+ translocation | y = 0.011x11 + 1.427x4 + 0.031x12 − 0.030x13 + 0.912 | 1.00 | 0.00 | y = 0.119x7 + 0.012x16 + 0.040x12 − 0.039x13 + 0.856 | 0.99 | 0.00 |
| Temperature | Treatment Label | NaCl Dose (mM) | MT Dose (μM) |
|---|---|---|---|
| 15 °C | CK | 0 | 0 |
| T1 | 150 | 0 | |
| T2 | 150 | 50 | |
| T3 | 150 | 100 | |
| T4 T5 | 150 150 | 150 200 | |
| 25 °C | CK | 0 | 0 |
| T1 | 150 | 0 | |
| T2 | 150 | 50 | |
| T3 | 150 | 100 | |
| T4 T5 | 150 150 | 150 200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Y.; Xin, L.; Mounkaila Hamani, A.K.; Sun, W.; Wang, H.; Amin, A.S.; Wang, X.; Qin, A.; Gao, Y. Foliar Application of Melatonin Positively Affects the Physio-Biochemical Characteristics of Cotton (Gossypium hirsutum L.) under the Combined Effects of Low Temperature and Salinity Stress. Plants 2023, 12, 3730. https://doi.org/10.3390/plants12213730
Fu Y, Xin L, Mounkaila Hamani AK, Sun W, Wang H, Amin AS, Wang X, Qin A, Gao Y. Foliar Application of Melatonin Positively Affects the Physio-Biochemical Characteristics of Cotton (Gossypium hirsutum L.) under the Combined Effects of Low Temperature and Salinity Stress. Plants. 2023; 12(21):3730. https://doi.org/10.3390/plants12213730
Chicago/Turabian StyleFu, Yuanyuan, Lang Xin, Abdoul Kader Mounkaila Hamani, Weihao Sun, Hongbo Wang, Abubakar Sunusi Amin, Xingpeng Wang, Anzhen Qin, and Yang Gao. 2023. "Foliar Application of Melatonin Positively Affects the Physio-Biochemical Characteristics of Cotton (Gossypium hirsutum L.) under the Combined Effects of Low Temperature and Salinity Stress" Plants 12, no. 21: 3730. https://doi.org/10.3390/plants12213730
APA StyleFu, Y., Xin, L., Mounkaila Hamani, A. K., Sun, W., Wang, H., Amin, A. S., Wang, X., Qin, A., & Gao, Y. (2023). Foliar Application of Melatonin Positively Affects the Physio-Biochemical Characteristics of Cotton (Gossypium hirsutum L.) under the Combined Effects of Low Temperature and Salinity Stress. Plants, 12(21), 3730. https://doi.org/10.3390/plants12213730








