Cosexuality Reduces Pollen Production and Fitness in Cannabis sativa L.
Abstract
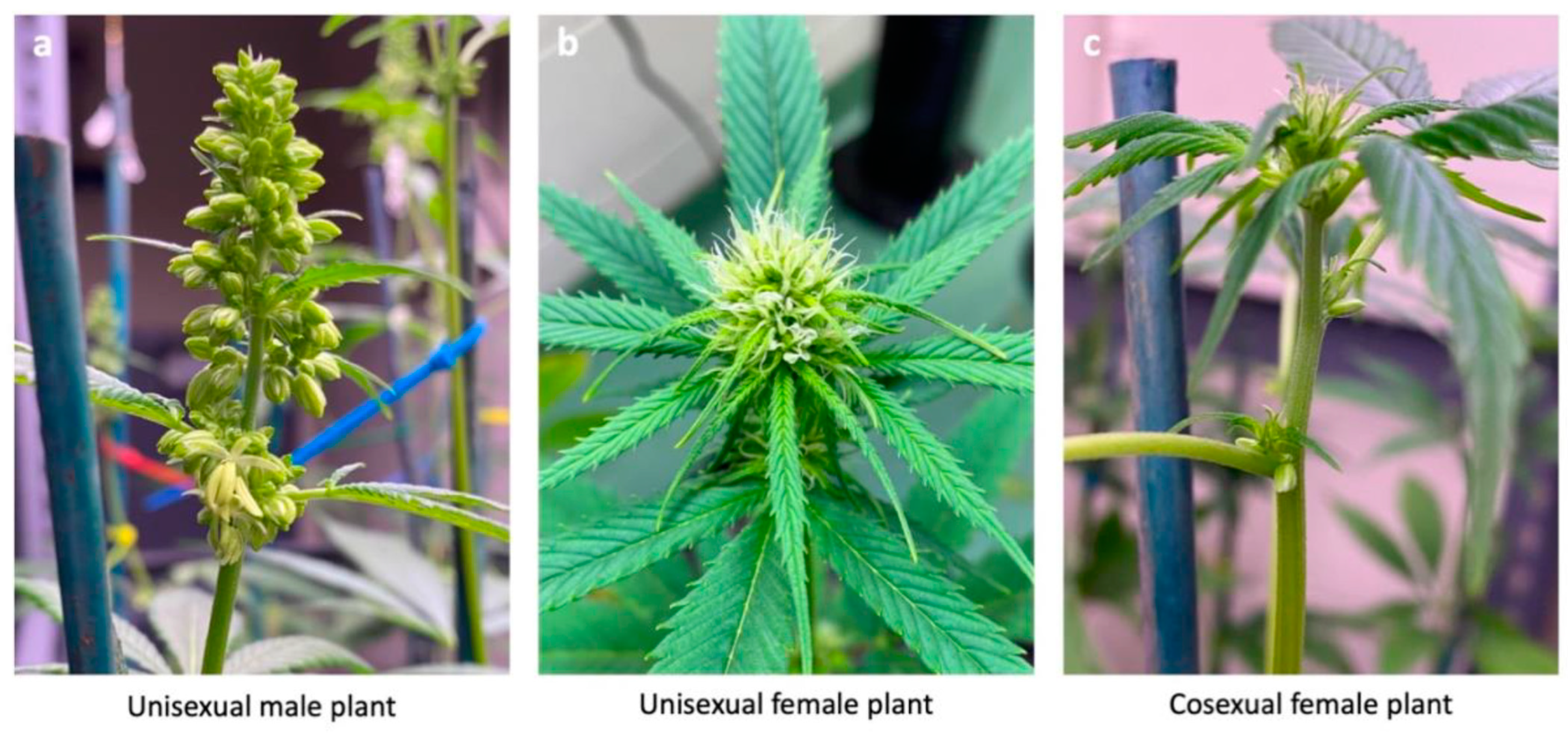
1. Introduction
2. Results
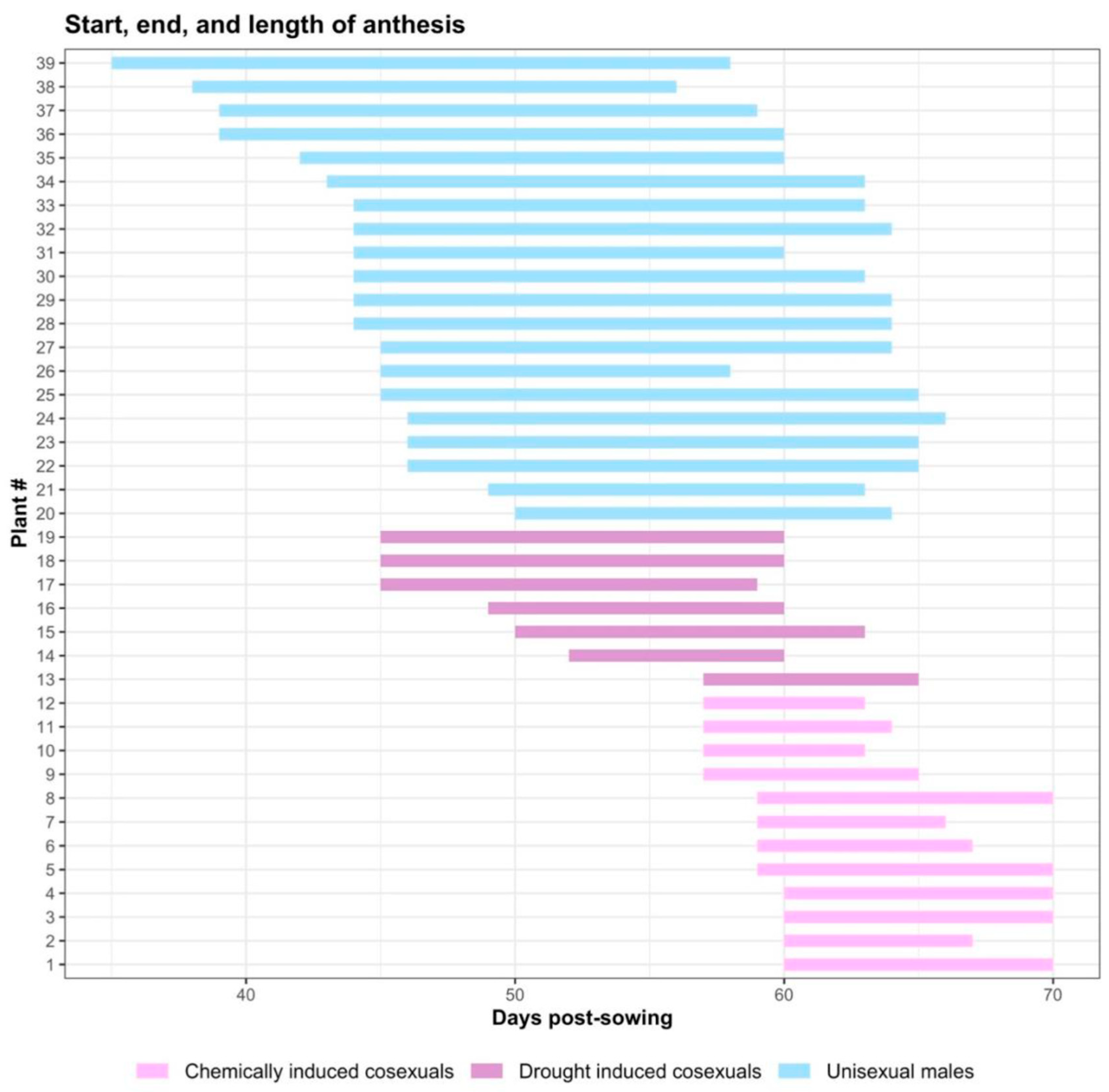
2.1. Phenology
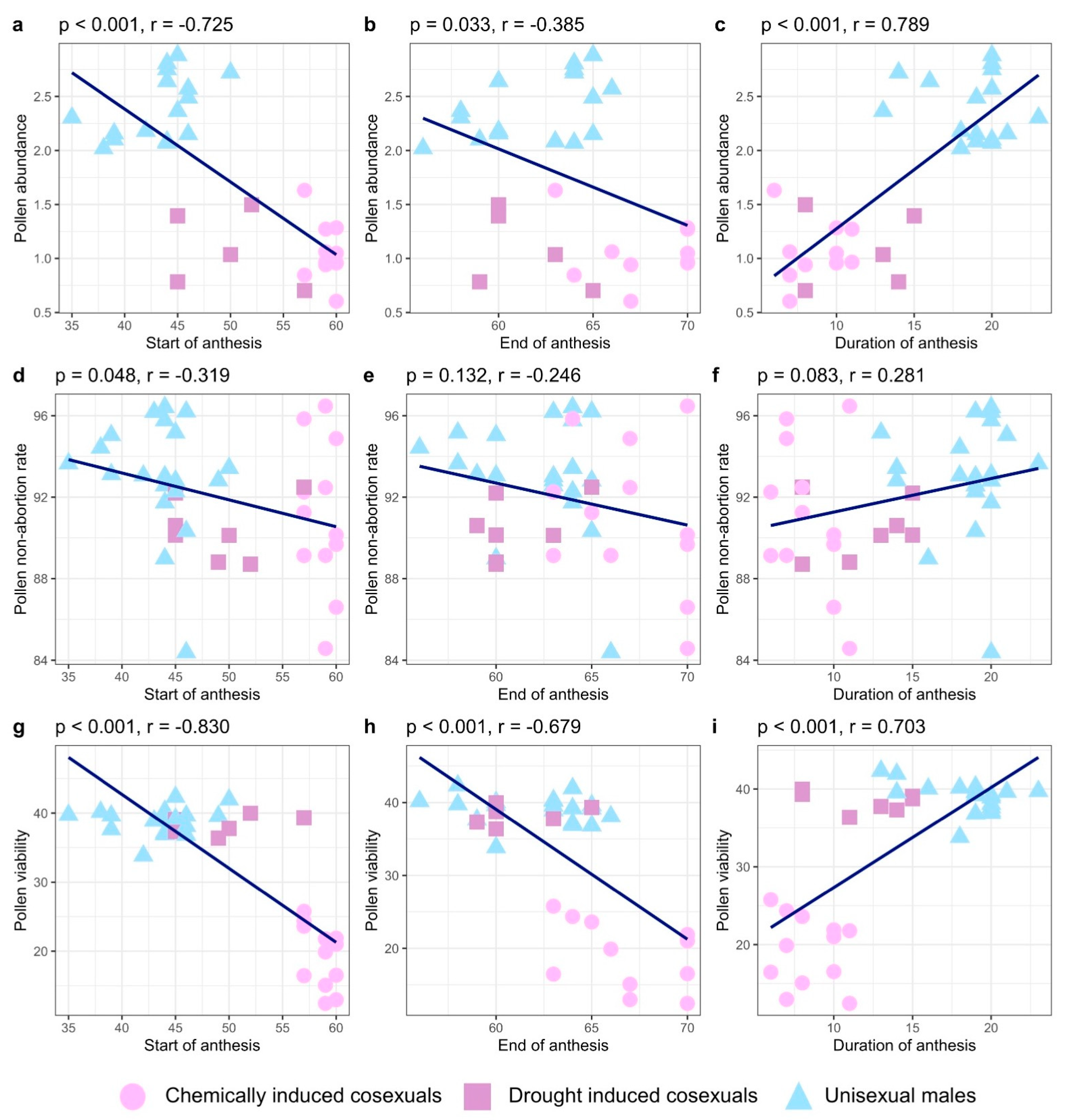
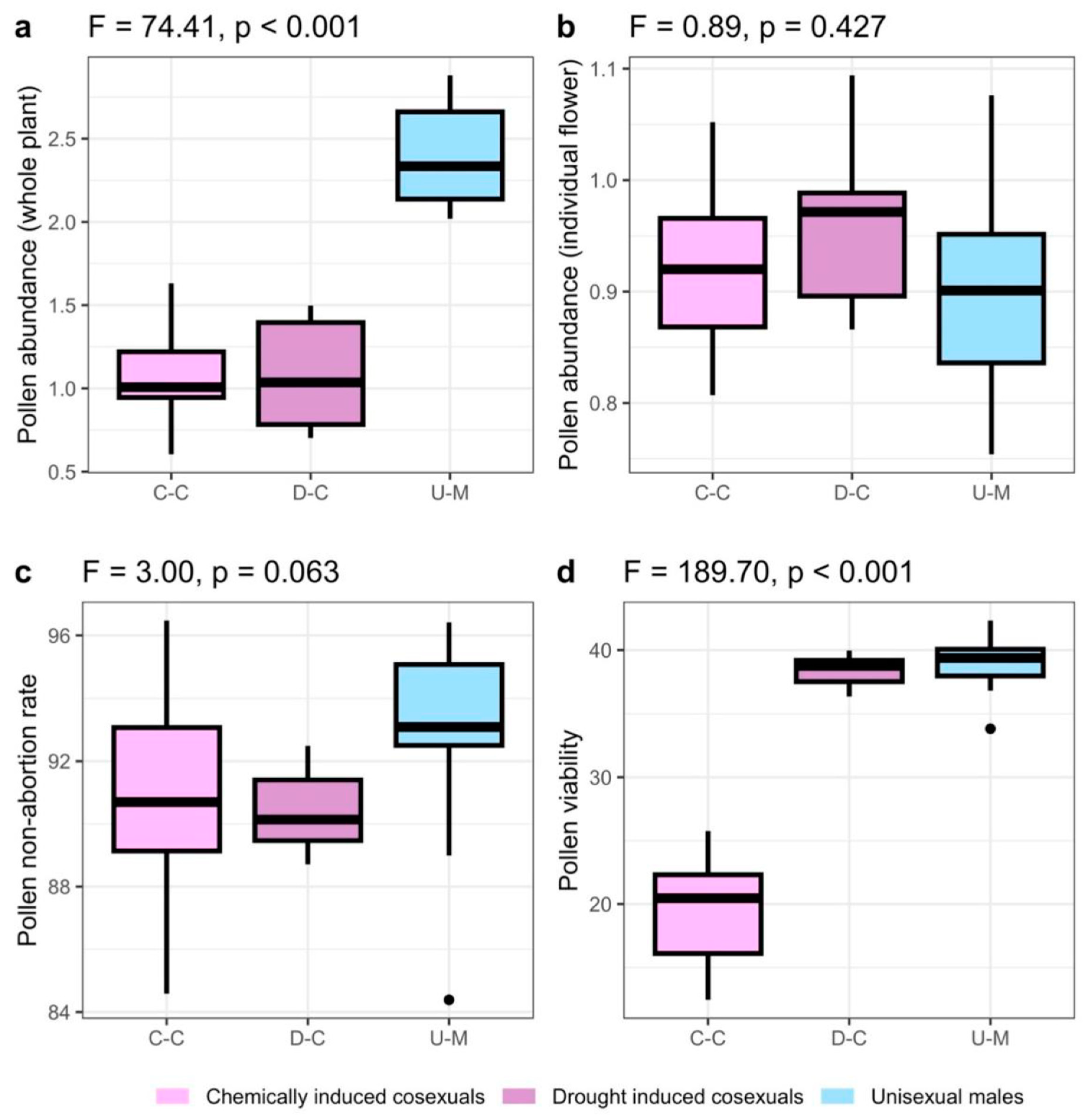
2.2. Pollen Abundance
2.3. Pollen Non-Abortion Rates
2.4. Pollen Viability
3. Discussion
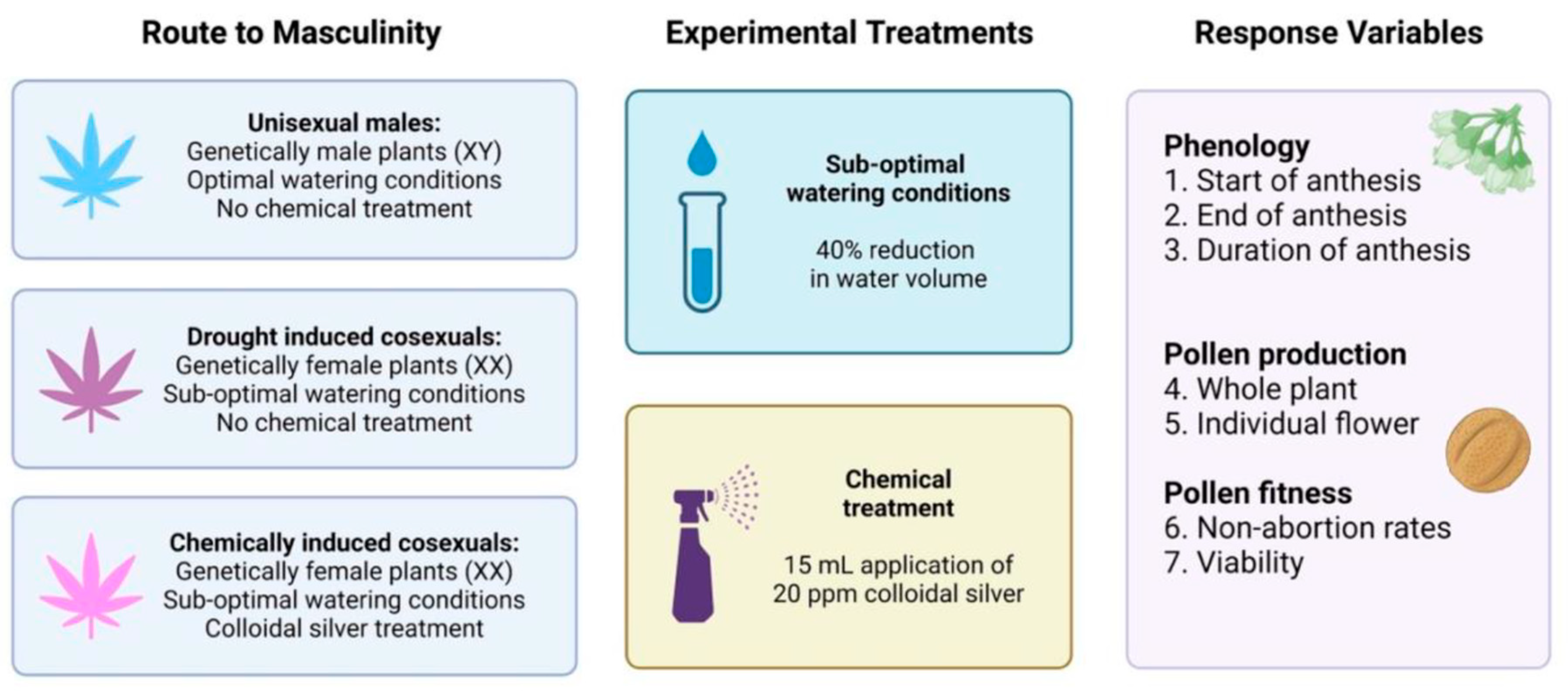
4. Materials and Methods
4.1. Plant Genotype and Cultivation
4.2. Phenology
4.3. Pollen Abundance
4.4. Pollen Non-Abortion Rates
4.5. Pollen Viability
4.6. Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Käfer, J.; Méndez, M.; Mousset, S. Labile sex expression in angiosperm species with sex chromosomes. Philos. Trans. R. Soc. B 2022, 377, 20210216. [Google Scholar] [CrossRef] [PubMed]
- Campbell, L.G.; Peach, K.; Wizenberg, S.B. Dioecious hemp (Cannabis sativa L.) plants do not express significant sexually dimorphic morphology in the seedling stage. Sci. Rep. 2021, 11, 16825. [Google Scholar] [CrossRef] [PubMed]
- Faux, A.M.; Draye, X.; Lambert, R.; d’Andrimont, R.; Raulier, P.; Bertin, P. The relationship of stem and seed yields to flowering phenology and sex expression in monoecious hemp (Cannabis sativa L.). Eur. J. Agron. 2013, 47, 11–22. [Google Scholar] [CrossRef]
- Korpelainen, H. Labile sex expression in plants. Biol. Rev. 1998, 73, 157–180. [Google Scholar] [CrossRef]
- Freeman, D.C.; Harper, K.T.; Charnov, E.L. Sex change in plants: Old and new observations and new hypotheses. Oecologia 1980, 47, 222–232. [Google Scholar] [CrossRef]
- Flajšman, M.; Slapnik, M.; Murovec, J. Production of feminized seeds of high CBD Cannabis sativa L. by manipulation of sex expression and its application to breeding. Front. Plant Sci. 2021, 12, 718092. [Google Scholar] [CrossRef]
- Ram, H.M.; Sett, R. Modification of growth and sex expression in Cannabis sativa by aminoethoxyvinylglycine and ethephon. Z. Fuer Pflanzenphysiol. 1982, 105, 165–172. [Google Scholar]
- Mohan Ram, H.Y.; Sett, R. Induction of fertile male flowers in genetically female Cannabis sativa plants by silver nitrate and silver thiosulphate anionic complex. Theor. Appl. Genet. 1982, 62, 369–375. [Google Scholar] [CrossRef]
- Ram, H.M.; Jaiswal, V.S. Sex reversal in the male plants of Cannabis sativa L. by ethyl hydrogen-L-propylphosphonate. Z. Für Pflanzenphysiol. 1972, 68, 181–183. [Google Scholar]
- Sarath, G.; Mohan Ram, H.Y. Comparative effect of silver ion and gibberellic acid on the induction of male flowers on female Cannabis plants. Experientia 1979, 35, 333–334. [Google Scholar] [CrossRef]
- Cronk, Q. The distribution of sexual function in the flowering plant: From monoecy to dioecy. Philos. Trans. R. Soc. B 2022, 377, 20210486. [Google Scholar] [CrossRef]
- Käfer, J.; Marais, G.A.; Pannell, J.R. On the rarity of dioecy in flowering plants. Mol. Ecol. 2017, 26, 1225–1241. [Google Scholar] [CrossRef]
- Zhang, D.Y. Resource allocation and the evolution of self-fertilization in plants. Am. Nat. 2000, 155, 187–199. [Google Scholar] [CrossRef]
- Barrett, S.C. The evolution of plant sexual diversity. Nat. Rev. Genet. 2022, 3, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Charlesworth, D. Theories of the evolution of dioecy. In Gender and Sexual Dimorphism in Flowering Plants; Springer: Berlin/Heidelberg, Germany, 1999; pp. 33–60. [Google Scholar]
- Bawa, K.S. Evolution of dioecy in flowering plants. Annu. Rev. Ecol. Syst. 1980, 11, 15–39. [Google Scholar] [CrossRef]
- Roach, D.A.; Smith, E.F. Life-history trade-offs and senescence in plants. Funct. Ecol. 2020, 34, 17–25. [Google Scholar] [CrossRef]
- Cao, G.X.; Worley, A.C. Life history trade-offs and evidence for hierarchical resource allocation in two monocarpic perennials. Plant Biol. 2013, 15, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Burd, M. Flower number and floral components in ten angiosperm species: An examination of assumptions about trade-offs in reproductive evolution. Biol. J. Linn. Soc. 1999, 68, 579–592. [Google Scholar] [CrossRef][Green Version]
- Delph, L.F.; Meagher, T.R. Sexual dimorphism masks life history trade-offs in the dioecious plant Silene latifolia. Ecology 1995, 76, 775–785. [Google Scholar] [CrossRef]
- Friedman, J.; Barrett, S.C. The evolution of ovule number and flower size in wind-pollinated plants. Am. Nat. 2011, 177, 246–257. [Google Scholar] [CrossRef]
- Doust, J.L. Plant reproductive strategies and resource allocation. Trends Ecol. Evol. 1989, 4, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Ross, M.D.; Gregorius, H.R. Outcrossing and sex function in hermaphrodites: A resource-allocation model. Am. Nat. 1983, 121, 204–222. [Google Scholar] [CrossRef] [PubMed]
- Bonini, S.A.; Premoli, M.; Tambaro, S.; Kumar, A.; Maccarinelli, G.; Memo, M.; Mastinu, A. Cannabis sativa: A comprehensive ethnopharmacological review of a medicinal plant with a long history. J. Ethnopharmacol. 2018, 227, 300–315. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.C.; Merlin, M.D. Cannabis domestication, breeding history, present-day genetic diversity, and future prospects. Crit. Rev. Plant Sci. 2016, 35, 293–327. [Google Scholar] [CrossRef]
- Bewley-Taylor, D.; Blickman, T.; Jelsma, M. The Rise and Decline of Cannabis Prohibition. In The History of Cannabis in the UN Drug Control System and Options for Reform; Global Drug Policy Observatory: Amsterdam, The Netherlands; Transnational Institute: Swansea, UK, 2014. [Google Scholar]
- Russo, E.B. History of cannabis and its preparations in saga, science, and sobriquet. Chem. Biodivers. 2007, 4, 1614–1648. [Google Scholar] [CrossRef]
- Zuardi, A.W. History of cannabis as a medicine: A review. Braz. J. Psychiatry 2006, 28, 153–157. [Google Scholar] [CrossRef]
- Dang, M.; Arachchige, N.M.; Campbell, L.G. Optimizing Photoperiod Switch to Maximize Floral Biomass and Cannabinoid Yield in Cannabis sativa L.: A Meta-Analytic Quantile Regression Approach. Front. Plant Sci. 2022, 12, 797425. [Google Scholar] [CrossRef]
- Saloner, A.; Bernstein, N. Nitrogen supply affects cannabinoid and terpenoid profile in medical cannabis (Cannabis sativa L.). Ind. Crops Prod. 2021, 167, 113516. [Google Scholar] [CrossRef]
- Campbell, L.G.; Naraine, S.G.; Dusfresne, J. Phenotypic plasticity influences the success of clonal propagation in industrial pharmaceutical Cannabis sativa. PLoS ONE 2019, 14, e0213434. [Google Scholar] [CrossRef]
- Spitzer-Rimon, B.; Duchin, S.; Bernstein, N.; Kamenetsky, R. Architecture and florogenesis in female Cannabis sativa plants. Front. Plant Sci. 2019, 10, 350. [Google Scholar] [CrossRef]
- Techen, N.; Chandra, S.; Lata, H.; ElSohly, M.A.; Khan, I.A. Genetic identification of female Cannabis sativa plants at early developmental stage. Planta Medica 2010, 76, 1938–1939. [Google Scholar] [CrossRef]
- McCallum, B.; Chang, S.M. Pollen competition in style: Effects of pollen size on siring success in the hermaphroditic common morning glory, Ipomoea purpurea. Am. J. Bot. 2016, 103, 460–470. [Google Scholar] [CrossRef]
- Lankinen, Å.; Karlsson Green, K. Using theories of sexual selection and sexual conflict to improve our understanding of plant ecology and evolution. AoB Plants 2015, 7, plv008. [Google Scholar] [CrossRef] [PubMed]
- Lyons, E.E.; Waser, N.M.; Price, M.V.; Antonovics, J.; Motten, A.F. Sources of variation in plant reproductive success and implications for concepts of sexual selection. Am. Nat. 1989, 134, 409–433. [Google Scholar] [CrossRef]
- Malti; Shivanna, K.R. The role of the pistil in screening compatible pollen. Theor. Appl. Genet. 1985, 70, 684–686. [Google Scholar] [CrossRef]
- Heslop-Harrison, J. Incompatibility and the pollen-stigma interaction. Annu. Rev. Plant Physiol. 1975, 26, 403–425. [Google Scholar] [CrossRef]
- Sorojsrisom, E.S.; Haller, B.C.; Ambrose, B.A.; Eaton, D.A. Selection on the gametophyte: Modeling alternation of generations in plants. Appl. Plant Sci. 2022, 10, e11472. [Google Scholar] [CrossRef] [PubMed]
- Otto, S.P.; Scott, M.F.; Immler, S. Evolution of haploid selection in predominantly diploid organisms. Proc. Natl. Acad. Sci. USA 2015, 112, 15952–15957. [Google Scholar] [CrossRef]
- Walbot, V.; Evans, M.M. Unique features of the plant life cycle and their consequences. Nat. Rev. Genet. 2003, 4, 369–379. [Google Scholar] [CrossRef]
- Valero, M.; Richerd, S.; Perrot, V.; Destombe, C. Evolution of alternation of haploid and diploid phases in life cycles. Trends Ecol. Evol. 1992, 7, 25–29. [Google Scholar] [CrossRef]
- Hormaza, J.I.; Herrero, M. Gametophytic competition and selection. In Genetic Control of Self-Incompatibility and Reproductive Development in Flowering Plants; Springer: Dordrecht, The Netherlands, 1994; pp. 372–400. [Google Scholar]
- Ottaviano, E. Selection pressure on pollen and its relevance to plant breeding. In Proceedings of the International Congress of Plant Physiology, New Delhi, India, 15–20 February 1990; Volume 2, pp. 1315–1321. [Google Scholar]
- Wizenberg, S.B.; Weis, A.E.; Campbell, L.G. Comparing methods for controlled capture and quantification of pollen in Cannabis sativa. Appl. Plant Sci. 2020, 8, e11389. [Google Scholar] [CrossRef] [PubMed]
- Wizenberg, S.B.; Dang, M.; Campbell, L.G. Methods for characterizing pollen fitness in Cannabis sativa L. PLoS ONE 2022, 17, e0270799. [Google Scholar] [CrossRef] [PubMed]
- Salentijn, E.M.; Petit, J.; Trindade, L.M. The complex interactions between flowering behavior and fiber quality in hemp. Front. Plant Sci. 2019, 10, 614. [Google Scholar] [CrossRef]
- Haney, A.; Kutscheid, B.B. Quantitative variation in the chemical constituents of marihuana from stands of naturalized Cannabis sativa L. in east-central Illinois. Econ. Bot. 1973, 27, 193–203. [Google Scholar] [CrossRef]
- Charlesworth, D.; Charlesworth, B. Allocation of resources to male and female functions in hermaphrodites. Biol. J. Linn. Soc. 1981, 15, 57–74. [Google Scholar] [CrossRef]
- Heath, D.J. Brooding and the evolution of hermaphroditism. J. Theor. Biol. 1979, 81, 151–155. [Google Scholar] [CrossRef]
- Mahesha, H.S.; Vinay, J.U.; Ravikumar, M.R.; Visweswarashastry, S.; Keerthi, M.C.; Halli, H.M.; Elansary, H.O. Colloidal Silver Hydrogen Peroxide: New Generation Molecule for Management of Phytopathogens. Horticulturae 2021, 7, 573. [Google Scholar] [CrossRef]
- Adal, A.M.; Doshi, K.; Holbrook, L.; Mahmoud, S.S. Comparative RNA-Seq analysis reveals genes associated with masculinization in female Cannabis sativa. Planta 2021, 253, 17. [Google Scholar] [CrossRef]
- Lubell, J.D.; Brand, M.H. Foliar sprays of silver thiosulfate produce male flowers on female hemp plants. HortTechnology 2018, 28, 743–747. [Google Scholar] [CrossRef]
- Owen, L.C.; Suchoff, D.H.; Chen, H. A Novel Method for Stimulating Cannabis sativa L. Male Flowers from Female Plants. Plants 2023, 12, 3371. [Google Scholar] [CrossRef]
- Vanhove, W.; Van Damme, P.; Meert, N. Factors determining yield and quality of illicit indoor cannabis (Cannabis spp.) production. Forensic Sci. Int. 2011, 212, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Amaducci, S.; Zatta, A.; Pelatti, F.; Venturi, G. Influence of agronomic factors on yield and quality of hemp (Cannabis sativa L.) fibre and implication for an innovative production system. Field Crops Res. 2008, 107, 161–169. [Google Scholar] [CrossRef]
- Law, T.F.; Lebel-Hardenack, S.; Grant, S.R. Silver enhances stamen development in female white campion (Silene latifolia [Caryophyllaceae]). Am. J. Bot. 2002, 89, 1014–1020. [Google Scholar] [CrossRef] [PubMed]
- Alexander, M.P. Differential staining of aborted and nonaborted pollen. Stain Technol. 1969, 44, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Peterson, R.; Slovin, J.P.; Chen, C. A simplified method for differential staining of aborted and non-aborted pollen grains. Int. J. Plant Biol. 2010, 1, e13. [Google Scholar] [CrossRef]
- Gaudet, D.; Yadav, N.S.; Sorokin, A.; Bilichak, A.; Kovalchuk, I. Development and optimization of a germination assay and long-term storage for Cannabis sativa pollen. Plants 2020, 9, 665. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wizenberg, S.B.; Muir-Guarnaccia, J.; Campbell, L.G. Cosexuality Reduces Pollen Production and Fitness in Cannabis sativa L. Plants 2023, 12, 3731. https://doi.org/10.3390/plants12213731
Wizenberg SB, Muir-Guarnaccia J, Campbell LG. Cosexuality Reduces Pollen Production and Fitness in Cannabis sativa L. Plants. 2023; 12(21):3731. https://doi.org/10.3390/plants12213731
Chicago/Turabian StyleWizenberg, Sydney B., Jillian Muir-Guarnaccia, and Lesley G. Campbell. 2023. "Cosexuality Reduces Pollen Production and Fitness in Cannabis sativa L." Plants 12, no. 21: 3731. https://doi.org/10.3390/plants12213731
APA StyleWizenberg, S. B., Muir-Guarnaccia, J., & Campbell, L. G. (2023). Cosexuality Reduces Pollen Production and Fitness in Cannabis sativa L. Plants, 12(21), 3731. https://doi.org/10.3390/plants12213731




