Biochemical and Molecular Characterization of Musa sp. Cultured in Temporary Immersion Bioreactor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
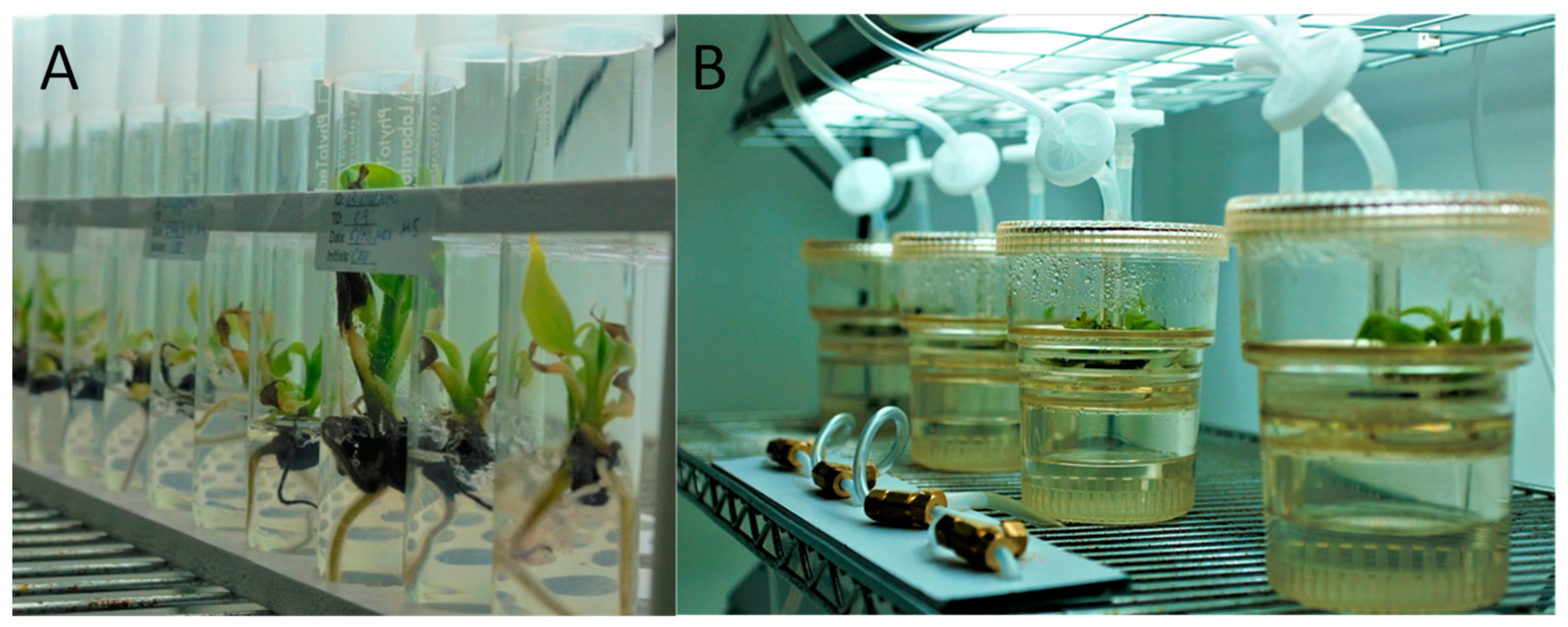
2.2. Growth Comparison of TIB and Conventional Micropropagation
2.3. RNA Isolation and cDNA Preparation
2.4. Oligonucleotide Design
2.5. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.6. Photosynthetic Activity Measurement and Acclimatization
2.7. ICP-OES Qualitative Analysis
2.8. Scanning Electron Microscopy
2.9. Statistical Analysis
3. Results
3.1. Comparison of Growth Parameters in TIB vs. Conventional Micropropagation
3.2. Gene Expression
3.3. Photosynthetic Activity
3.4. Digestion and ICP-OES Qualitative Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huang, H.; Jing, G.; Wang, H.; Duan, X.; Qu, H.; Jiang, Y. The combined effects of phenylurea and gibberellins on quality maintenance and shelf life extension of banana fruit during storage. Sci. Hortic. 2014, 167, 36–42. [Google Scholar] [CrossRef]
- Nelson, S.; Ploetz, R.C.; Kepler, A.K. Musa Species (bananas and plantains) Species Profiles for Pacific Island Agroforestry. Permanent Agriculture Resource. 2006. Available online: https://agroforestry.org/images/pdfs/Musa-banana-plantain.pdf (accessed on 28 November 2021).
- Kumar, N.; Reddy, M. In vitro Plant Propagation: A Review. J. For. Sci. 2011, 27, 61–72. [Google Scholar]
- Rodríguez Cruz, L.; Niles, M. Hurricane Maria’s Impacts on Puerto Rican Farmers: Experience, Challenges, and Perceptions; University of Vermont: Burlington, VT, USA, 2018. [Google Scholar]
- Smith, M.; Meade, B. Global Food Security.USDA. Available online: https://www.ers.usda.gov/amber-waves/2019/june/who-are-the-world-s-food-insecure-identifying-the-risk-factors-of-food-insecurity-around-the-world/ (accessed on 3 June 2019).
- Bellido, M.S. Food Security in Puerto Rico: Vulnerable Food Supply. American Society for Nutrition. Available online: https://nutrition.org/food-security-puerto-rico-vulnerable-food-supply/ (accessed on 10 September 2014).
- Roels, S.; Escalona, M.; Cejas, I.; Noceda, C.; Rodriguez, R.; Canal, M.; Debergh, P. Optimization of plantain (Musa AAB) micropropagation by temporary immersion system. Plant Cell Tissue Organ Cult. 2005, 82, 57–66. [Google Scholar] [CrossRef]
- Nhut, D.T.; Trinh Don, N.; Hong Vu, N.; Quoc Thien, N.; Thi Thu Thuy, D.; Duy, N.; Teixeira da Silva, J.A. Advanced Technology in Micropropagation of Some Important Plants; Global Science Book: UK, 2006. [Google Scholar]
- Escalona, M.; Lorenzo, J.C.; Gonza’lez, B.; Daquinta, M.; Gonza´lez, J.; Desjardins, Y.; Borroto, C.G. Pineapple (Ananas comosus L. Merr) micropropagation in temporary immersion systems. Plant Cell Rep. 1999, 18, 743–748. [Google Scholar] [CrossRef]
- Florez, S.L.; Curtis, M.S.; Shaw, S.E.; Hamaker, N.K.; Larsen, J.S.; Curtis, W.R. A temporary immersion plant propagation bioreactor with decoupled gas and liquid flows for enhanced control of gas phase. Biotechnol. Prog. 2016, 32, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Albarrán, J.; Bertrand, B.; Lartaud, M.; Etienne, H. Cycle characteristics in a temporary immersion bioreactor affect regeneration, morphology, water, and mineral status of coffee (Coffea arabica) somatic embryos. Plant Cell Tissue Organ Cult. 2005, 81, 27–36. [Google Scholar] [CrossRef]
- Georgiev, V.; Schumann, A.; Pavlov, A.; Bley, T. Temporary immersion systems in plant biotechnology. Eng. Life Sci. 2014, 14, 607–621. [Google Scholar] [CrossRef]
- Paek, K.; Chakrabarty, D.; Hahn, E. Application of bioreactor systems for large-scale production of horticultural and medicinal plants. Plant Cell Tissue Organ Cult. 2005, 81, 287–300. [Google Scholar] [CrossRef]
- Alvard, D.; Cote, F.; Teisson, C. Comparison of methods of liquid medium culture for banana micropropagation. Plant Cell Tissue Organ Cult. 1993, 32, 55–60. [Google Scholar] [CrossRef]
- Etienne, H.; Berthouly, M. Temporary immersion in plant micropropagation. Plant Cell Tissue Organ Cult. 2002, 69, 215–231. [Google Scholar] [CrossRef]
- Tumer, N.; Clark, W.; Tabor, G.; Hironaka, C.; Fraley, R.; Shah, D. The genes encoding the small subunit of ribulose-1,5-bisphosphate carboxylase are expressed differentially in petunia leaves. Nucleic Acids Res. 1986, 14, 3325–3342. [Google Scholar] [CrossRef]
- Scafaro, A.P.; Bautsoens, N.; Boer, B.; Rie, J.; Gallé, A. A conserved sequence from heat-adapted species improves Rubisco activase thermostability in wheat1. Plant Physiol. 2019, 181, 43–54. [Google Scholar] [CrossRef]
- Marcus, Y.; Altman-Gueta, H.; Snir, A.; Wolff, Y.; Gurevitz, M. Does Rubisco limit the rate of photosynthesis? In Photosynthesis. Energy from the Sun: 14th International Congress on Photosynthesis; Springer: Berlin/Heidelberg, Germany, 2008; pp. 863–866. [Google Scholar]
- Suzuki, Y.; Makino, A. Availability of Rubisco small subunit up-regulates the transcript levels of large subunit for stoichiometric assembly of its holoenzyme in rice. Plant Physiol. 2012, 160, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Kanai, R.; Edward, G. The biochemistry of C4 photosynthesis. In C4 Plant Biology; Monson, R., Ed.; Academic Press: Cambridge, MA, USA, 1999. [Google Scholar]
- Cousins, A.; Baroli, I.; Badger, M.; Ivakov, A.; Lea, P.; Leegood, R.; Caemmerer, S. The Role of phosphoenolpyruvate carboxylase during C4 photosynthetic isotope exchange and stomatal conductance. Plant Physiol. 2007, 145, 1006–1017. [Google Scholar] [CrossRef]
- Aragón, C.; Escalona, M.; Capote, I.; Pina, D.; Cejas, I.; Rodriguez, R.; Canal, M.; Sandoval, J.; Roels, S.; Debergh, P.; et al. Photosynthesis and carbon metabolism in plantain (Musa AAB) growing in temporary immersion bioreactor (TIB) and ex vitro acclimatization. Vitr. Cell. Dev. Biol. Plant 2005, 41, 550–554. [Google Scholar] [CrossRef]
- Aragón, C.; Sanchez, C.; Gonzalez-Olmedo, J.; Escalona, M.; Carvalho, L.; Amancio, S. Comparison of plantain plantlets propagated in temporary immersion bioreactors and gelled medium during in vitro growth and acclimatization. Biol. Plant. 2014, 1, 29–38. [Google Scholar] [CrossRef]
- Ravet, K.; Pilon, M. Copper and iron homeostasis in plants: The challenges of oxidative stress. Antioxid. Redox Signal. 2013, 19, 5084. [Google Scholar] [CrossRef]
- Yruela, I. Transition metals in plant photosynthesis. Metallomics 2013, 5, 1090–1109. [Google Scholar] [CrossRef]
- Merchant, S.; Wise, R.; Hoober, J. Trace metal utilization in chloroplasts. In The Structure and Function of Plastids; Springer: Berlin/Heidelberg, Germany, 2006; pp. 199–218. [Google Scholar]
- Raven, J.; Evans, M.; Korb, R. The role of trace metals in photosynthetic electron transport in O2-evolving organisms. Photosynth. Res. 1999, 60, 111–149. [Google Scholar] [CrossRef]
- Valderrama-Cháirez, M.L.; Cruz-Hernández, A.; Paredes-López, O. Isolation of functional RNA from cactus fruit. Plant Mol. Biol. Rep. 2002, 20, 279–286. [Google Scholar] [CrossRef]
- Rodríguez-García, C.M.; Peraza-Echeverría, L.; Islas-Flores, I.R.; Canto-Canché, B.B.; Grijalva-Arango, R. Isolation of retro-transcribed RNA from in vitro Mycosphaerella fijiensis-infected banana leaves. Genet. Mol. Res. GMR 2010, 9, 1460–1468. [Google Scholar] [CrossRef]
- Uma, S.; Karthic, R.; Kalpana, S.; Backiyarani, S.; Saraswathi, M.S. A novel temporary immersion bioreactor system for large-scale multiplication of banana (Rasthali AAB-Silk). Sci. Rep. 2021, 11, 20371. [Google Scholar] [CrossRef] [PubMed]
- Izzo, L.G.; Aronne, G. Root tropisms: New insights leading the growth direction of the hidden half. Plants 2021, 10, 220. [Google Scholar] [CrossRef]
- Muthert, L.W.F.; Izzo, L.G.; van Zanten, M.; Aronne, G. Root tropisms: Investigations on earth and in space to unravel plant growth direction. Front. Plant Sci. 2019, 10, 1807. [Google Scholar] [CrossRef]
- Yang, L.; Zambrano, Y.; Hu, C.-J.; Carmona, E.R.; Bernal, A.; Pérez, A.; Zayas, C.M.; Li, Y.-R.; Guerra, A.; Santana, I.; et al. Sugarcane metabolites produced in CO2-rich temporary immersion bioreactors (TIBs) induce tomato (Solanum lycopersicum) resistance against bacterial wilt (Ralstonia solanacearum). Vitr. Cell. Dev. Biology. Plant J. Tissue Cult. Assoc. 2010, 46, 558–568. [Google Scholar] [CrossRef]
- Aragón, C.; Escalona, M.; Rodriguez, R.; Cañal, M. Effect of sucrose, light, and carbon dioxide on plantain micropropagation in temporary immersion bioreactors. Vitr. Cell. Dev. Biol. Plant 2009, 46, 89–94. [Google Scholar] [CrossRef]
- Michelet, L.; Zaffagnini, M.; Morisse, S.; Sparla, F.; Pérez-Pérez, M.; Francia, F.; Lemaire, S. Redox regulation of the Calvin–Benson cycle: Something old, something new. Front. Plant Sci. 2013, 4, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Matthijs, D.; Gielis, J.; Debergh, P. Automation and Environmental Control in Plant Tissue Culture; Kluwer Academic Publishers: Alphen aan den Rijn, The Netherlands, 1995. [Google Scholar]
- Maes, K.; Debergh, P. Volatiles emitted from in vitro grown tomato shoots during abiotic and biotic stress. Plant Cell Tissue Organ Cult. 2003, 75, 73–78. [Google Scholar] [CrossRef]
- Buddendorf-Joosten, J.; Woltering, E. Components of the gaseous environment and their effect on plant growth and development in-vitro. Plant Growth Regul. 1994, 15, 1–16. [Google Scholar] [CrossRef]
- González-Guerrero, M.; Escudero, V.; Saéz, Á.; Tejada-Jiménez, M. Transition metal transport in plants and associated endosymbionts: Arbuscular mycorrhizal fungi and rhizobia. Front. Plants Sci. 2016, 7, 1088. [Google Scholar] [CrossRef]
- Gonzalez, E. Mass Propagation of Tropical Crops in Temporary Immersion Systems; Springer: Dordrecht, The Netherlands, 2005. [Google Scholar]
- Hepler, P.K. Calcium: A central regulator of plant growth and development. Plant Cell 2005, 17, 2142–2155. [Google Scholar] [CrossRef] [PubMed]






| Genes | Amplicon Size | Sequences | NCBI Accession Number |
|---|---|---|---|
| Actin | 121 bp | 5″AAGTACAGTGTCTGGATTGG 3″ 3″GTTTCGCTGCTATTTCATGA 5″ | EF672732.1 |
| rbcS1 | 73 bp | 5″ TGTTTTTGTTTCCCCAAGAA 3″ 3″ ATGGACGTTTCGCTTTATTTA 5″ | AF008214 |
| PEPC | 223 bp | 5″ GGTAGTGGAAATGTCTCGCTTGG 3″ 3″ GCAATCCATGAACCTGAGAAGCC 5″ | Z99987.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sambolín Pérez, C.A.; Aybar Batista, R.; Morales Marrero, S.; Andino Santiago, D.; Reyes Colón, A.; Negrón Berríos, J.A.; Núñez Marrero, Á.; Arun, A. Biochemical and Molecular Characterization of Musa sp. Cultured in Temporary Immersion Bioreactor. Plants 2023, 12, 3770. https://doi.org/10.3390/plants12213770
Sambolín Pérez CA, Aybar Batista R, Morales Marrero S, Andino Santiago D, Reyes Colón A, Negrón Berríos JA, Núñez Marrero Á, Arun A. Biochemical and Molecular Characterization of Musa sp. Cultured in Temporary Immersion Bioreactor. Plants. 2023; 12(21):3770. https://doi.org/10.3390/plants12213770
Chicago/Turabian StyleSambolín Pérez, Christopher A., Rosalinda Aybar Batista, Sullymar Morales Marrero, Dinorah Andino Santiago, Axel Reyes Colón, Juan A. Negrón Berríos, Ángel Núñez Marrero, and Alok Arun. 2023. "Biochemical and Molecular Characterization of Musa sp. Cultured in Temporary Immersion Bioreactor" Plants 12, no. 21: 3770. https://doi.org/10.3390/plants12213770
APA StyleSambolín Pérez, C. A., Aybar Batista, R., Morales Marrero, S., Andino Santiago, D., Reyes Colón, A., Negrón Berríos, J. A., Núñez Marrero, Á., & Arun, A. (2023). Biochemical and Molecular Characterization of Musa sp. Cultured in Temporary Immersion Bioreactor. Plants, 12(21), 3770. https://doi.org/10.3390/plants12213770





