Eucalyptus camaldulensis Dehnh Leaf Essential Oil from Palestine Exhibits Antimicrobial and Antioxidant Activity but No Effect on Porcine Pancreatic Lipase and α-Amylase
Abstract
:1. Introduction
2. Results and Discussion
2.1. Phytochemistry
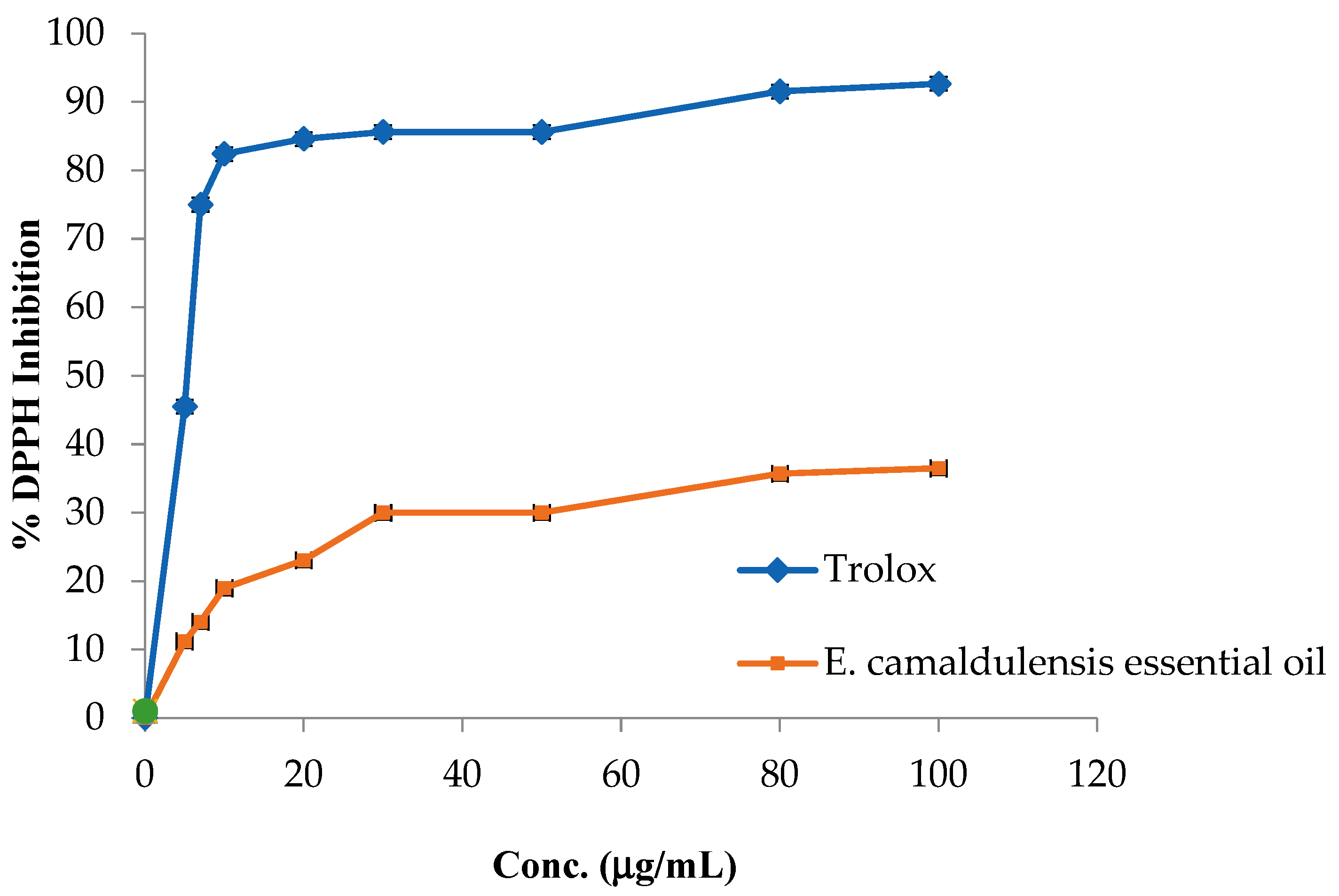
2.2. Evaluation of the Antioxidant, Antilipase, and Anti-α-Amylase Effects
2.3. Antimicrobial Effect
3. Materials and Methods
3.1. Plant Material
3.2. Chemicals and Instruments
3.3. Extraction of Essential Oil
3.4. Gas Chromatography-Mass Spectrometry
3.5. Free Radical Scavenging Activity
- B = absorbance of blank
- Z = absorbance of the tested samples
3.6. Porcine Pancreatic Lipase Inhibition Assay
3.7. α-Amylase Inhibitory Method
- B: is the absorbance of blank;
- S: is the absorbance of a tested sample [45].
3.8. Antimicrobial Activity
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Angane, M.; Swift, S.; Huang, K.; Butts, C.A.; Quek, S.Y. Essential oils and their major components: An updated review on antimicrobial activities, mechanism of action and their potential application in the food industry. Foods 2022, 11, 464. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.K.; Chaudhari, A.K.; Das, S.; Tiwari, S.; Maurya, A.; Singh, V.K.; Dubey, N.K. Chitosan encompassed Aniba rosaeodora essential oil as innovative green candidate for antifungal and antiaflatoxigenic activity in millets with emphasis on cellular and its mode of action. Front. Microbiol. 2022, 13, 970670. [Google Scholar] [CrossRef]
- Hou, T.; Sana, S.S.; Li, H.; Xing, Y.; Nanda, A.; Netala, V.R.; Zhang, Z. Essential oils and its antibacterial, antifungal and anti-oxidant activity applications: A review. Food Biosci. 2022, 47, 101716. [Google Scholar] [CrossRef]
- Maurya, A.; Prasad, J.; Das, S.; Dwivedy, A.K. Essential oils and their application in food safety. Front. Sustain. Food Syst. 2021, 5, 653420. [Google Scholar] [CrossRef]
- Sabo, V.A.; Knezevic, P. Antimicrobial activity of Eucalyptus camaldulensis Dehn. plant extracts and essential oils: A review. Ind. Crops Prod. 2019, 132, 413–429. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.; Abebe, W.; Sousa, S.; Duarte, V.; Machado, M.; Matos, F. Analgesic and anti-inflammatory effects of essential oils of Eucalyptus. J. Ethnopharmacol. 2003, 89, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sarma, V.; Sing, A.; Kamla, S. Antimicrobial properties of different Eucalyptus oils. Fitoterapia 1988, 59, 141–144. [Google Scholar]
- Bruneton, J. Pharmacognosy, Phytochemistry, Medicinal Plants, 2nd ed.; Intercept Ltd.: London, UK, 1999. [Google Scholar]
- Huang, H.-C.; Ho, Y.-C.; Lim, J.-M.; Chang, T.-Y.; Ho, C.-L.; Chang, T.-M. Investigation of the anti-melanogenic and antioxidant characteristics of Eucalyptus camaldulensis flower essential oil and determination of its chemical composition. Int. J. Mol. Sci. 2015, 16, 10470–10490. [Google Scholar] [CrossRef]
- Sebei, K.; Sakouhi, F.; Herchi, W.; Khouja, M.L.; Boukhchina, S. Chemical composition and antibacterial activities of seven Eucalyptus species essential oils leaves. Biol. Res. 2015, 48, 7. [Google Scholar] [CrossRef]
- Muhammad, A.; Qasim, A.; Farooq, A.; Hussain, A.I. Composition of leaf essential oil of Eucalyptus camaldulensis. Asian J. Chem. 2010, 22, 1779–1786. [Google Scholar]
- Farah, A.; Fechtal, M.; Chaouch, A.; Zrira, S. The essential oils of Eucalyptus camaldulensis and its natural hybrid (clone 583) from Morocco. Flavour Fragr. J. 2002, 17, 395–397. [Google Scholar] [CrossRef]
- Moudachirou, M.; Gbenou, J.; Chalchat, J.; Chabard, J.; Lartigue, C. Chemical composition of essential oils of Eucalyptus from Bénin: Eucalyptus citriodora and E. camaldulensis. Influence of location, harvest time, storage of plants and time of steam distillation. J. Essent. Oil Res. 1999, 11, 109–118. [Google Scholar] [CrossRef]
- Dellacassa, E.; Menéndez, P.; Moyna, P.; Soler, E. Chemical composition of Eucalyptus essential oils grown in Uruguay. Flavour Fragr. J. 1990, 5, 91–95. [Google Scholar] [CrossRef]
- Elgat, W.A.A.; Kordy, A.M.; Böhm, M.; Černý, R.; Abdel-Megeed, A.; Salem, M.Z. Eucalyptus camaldulensis, Citrus aurantium, and Citrus sinensis Essential Oils as Antifungal Activity against Aspergillus flavus, Aspergillus niger, Aspergillus terreus, and Fusarium culmorum. Processes 2020, 8, 1003. [Google Scholar] [CrossRef]
- Barra, A.; Coroneo, V.; Dessi, S.; Cabras, P.; Angioni, A. Chemical variability, antifungal and antioxidant activity of Eucalyptus camaldulensis essential oil from Sardinia. Nat. Prod. Commun. 2010, 5, 1934578X1000500232. [Google Scholar] [CrossRef]
- Cheng, J.Y.S.; Ngok, K.L.; Huang, Y. Multinational corporations, global civil society and Chinese labour: Workers’ solidarity in China in the era of globalization. Econ. Ind. Democr. 2012, 33, 379–401. [Google Scholar] [CrossRef]
- Ez-Zriouli, R.; ElYacoubi, H.; Imtara, H.; Mesfioui, A.; ElHessni, A.; Al Kamaly, O.; Zuhair Alshawwa, S.; Nasr, F.A.; Benziane Ouaritini, Z.; Rochdi, A. Chemical Composition, Antioxidant and Antibacterial Activities and Acute Toxicity of Cedrus atlantica, Chenopodium ambrosioides and Eucalyptus camaldulensis Essential Oils. Molecules 2023, 28, 2974. [Google Scholar] [CrossRef] [PubMed]
- Oyedeji, A.O.; Ekundayo, O.; Olawore, O.N.; Adeniyi, B.A.; Koenig, W.A. Antimicrobial activity of the essential oils of five Eucalyptus species growing in Nigeria. Fitoterapia 1999, 70, 526–528. [Google Scholar] [CrossRef]
- Shieh, J. Yields and chemical components of essential oils in Eucalyptus camaldulensis leaves. Taiwan J. For. Sci 1996, 11, 149–157. [Google Scholar]
- Williams, C. Medicinal Plants in Australia Volume 2: Gums, Resins, Tannin and Essential Oils; Rosenberg Publishing: Kenthurst, Australia, 2011; Volume 2. [Google Scholar]
- Tsiri, D.; Kretsi, O.; Chinou, I.B.; Spyropoulos, C.G. Composition of fruit volatiles and annual changes in the volatiles of leaves of Eucalyptus camaldulensis Dehn. growing in Greece. Flavour Fragr. J. 2003, 18, 244–247. [Google Scholar] [CrossRef]
- Hidayat, M.A.; Sari, P.; Kuswandi, B. Simple scanometric assay based on DPPH immobilized on pharmaceutical blister for determination of antioxidant capacity in the herbal extracts. Marmara Pharm. J. 2018, 22, 450–459. [Google Scholar] [CrossRef]
- Sliti, S.; Ayadi, S.; Kachouri, F.; Khouja, M.A.; Abderrabba, M.; Bouzouita, N. Leaf essential oils chemical composition, antibacterial and antioxidant activities of Eucalyptus camaldulensis and E. rudis from korbous (Tunisia). J. Mater. Environ. Sci. 2015, 6, 743–748. [Google Scholar]
- Siramon, P.; Ohtani, Y. Antioxidative and antiradical activities of Eucalyptus camaldulensis leaf oils from Thailand. J. Wood Sci. 2007, 53, 498–504. [Google Scholar] [CrossRef]
- Sahin Basak, S.; Candan, F. Chemical composition and in vitro antioxidant and antidiabetic activities of Eucalyptus camaldulensis Dehnh. essential oil. J. Iran. Chem. Soc. 2010, 7, 216–226. [Google Scholar] [CrossRef]
- Chaves, T.P.; Pinheiro, R.E.E.; Melo, E.S.; Soares, M.J.d.S.; Souza, J.S.N.; de Andrade, T.B.; de Lemos, T.L.G.; Coutinho, H.D. Essential oil of Eucalyptus camaldulensis Dehn potentiates β-lactam activity against Staphylococcus aureus and Escherichia coli resistant strains. Ind. Crops Prod. 2018, 112, 70–74. [Google Scholar] [CrossRef]
- Abubakar, E.-M.M. Antibacterial potential of crude leaf extracts of Eucalyptus camaldulensis against some pathogenic bacteria. Afr. J. Plant Sci. 2010, 4, 202–209. [Google Scholar]
- El-Baz, F.K.; Mahmoud, K.; El-Senousy, W.M.; Darwesh, O.; ElGohary, A. Antiviral–antimicrobial and schistosomicidal activities of Eucalyptus camaldulensis essential oils. Int. J. Pharm. Sci. Rev. Res. 2015, 31, 262–268. [Google Scholar]
- May, T.; Ito, A.; Okabe, S. Induction of multidrug resistance mechanism in Escherichia coli biofilms by interplay between tetracycline and ampicillin resistance genes. Antimicrob. Agents Chemother. 2009, 53, 4628–4639. [Google Scholar] [CrossRef]
- Jaradat, N.; Al-Masri, M.; Abu Shanab, B.; Hussein, F.; Qneibi, M.; Nasser Eldin, A.; Yassin, T.; Khawaja, M. Phytochemical and antibacterial assessment of Rhagadiolus stellatus Plant in Jerusalem Area-Palestine. Palest. Med. Pharm. J. 2017, 2, 4. [Google Scholar] [CrossRef]
- Jaradat, N.A. Review of the taxonomy, ethnobotany, phytochemistry, phytotherapy and phytotoxicity of germander plant (Teucrium polium L.). Asian J. Pharm. Clin. Res. 2015, 8, 13–19. [Google Scholar]
- Takó, M.; Kerekes, E.B.; Zambrano, C.; Kotogán, A.; Papp, T.; Krisch, J.; Vágvölgyi, C. Plant phenolics and phenolic-enriched extracts as antimicrobial agents against food-contaminating microorganisms. Antioxidants 2020, 9, 165. [Google Scholar] [CrossRef] [PubMed]
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Fazly Bazzaz, B.S. Review on plant antimicrobials: A mechanistic viewpoint. Antimicrob. Resist. Infect. Control. 2019, 8, 118. [Google Scholar] [CrossRef]
- Marchese, A.; Arciola, C.R.; Barbieri, R.; Silva, A.S.; Nabavi, S.F.; Tsetegho Sokeng, A.J.; Izadi, M.; Jafari, N.J.; Suntar, I.; Daglia, M. Update on monoterpenes as antimicrobial agents: A particular focus on p-cymene. Materials 2017, 10, 947. [Google Scholar] [CrossRef] [PubMed]
- Mulyaningsih, S.; Sporer, F.; Reichling, J.; Wink, M. Antibacterial activity of essential oils from Eucalyptus and of selected components against multidrug-resistant bacterial pathogens. Pharm. Biol. 2011, 49, 893–899. [Google Scholar] [CrossRef]
- Sikkema, J.; de Bont, J.A.; Poolman, B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol. Rev. 1995, 59, 201–222. [Google Scholar] [CrossRef]
- Wink, M. Evolutionary advantage and molecular modes of action of multi-component mixtures used in phytomedicine. Curr. Drug Metab. 2008, 9, 996–1009. [Google Scholar] [CrossRef]
- Farhanghi, A.; Aliakbarlu, J.; Tajik, H.; Mortazavi, N.; Manafi, L.; Jalilzadeh-Amin, G. Antibacterial interactions of pulegone and 1, 8-cineole with monolaurin ornisin against Staphylococcus aureus. Food Sci. Nutr. 2022, 10, 2659–2666. [Google Scholar] [CrossRef]
- Li, L.; Li, Z.-W.; Yin, Z.-Q.; Wei, Q.; Jia, R.-Y.; Zhou, L.-J.; Xu, J.; Song, X.; Zhou, Y.; Du, Y.-H. Antibacterial activity of leaf essential oil and its constituents from Cinnamomum longepaniculatum. Int. J. Clin. Exp. Med. 2014, 7, 1721. [Google Scholar]
- Hoch, C.C.; Petry, J.; Griesbaum, L.; Weiser, T.; Werner, K.; Ploch, M.; Verschoor, A.; Multhoff, G.; Dezfouli, A.B.; Wollenberg, B. 1, 8-cineole (eucalyptol): A versatile phytochemical with therapeutic applications across multiple diseases. Biomed. Pharmacother. 2023, 167, 115467. [Google Scholar] [CrossRef]
- Kind, T.; Wohlgemuth, G.; Lee, D.Y.; Lu, Y.; Palazoglu, M.; Shahbaz, S.; Fiehn, O. FiehnLib: Mass spectral and retention index libraries for metabolomics based on quadrupole and time-of-flight gas chromatography/mass spectrometry. Anal. Chem. 2009, 81, 10038–10048. [Google Scholar] [CrossRef]
- Alqub, M.; Jaradat, N. In vitro studies of Bituminaria bituminosa L. extracts from Palestine for their antioxidant, qualitative, and quantitative properties. Palest. Med. Pharm. J. 2023, 8, 8. [Google Scholar] [CrossRef]
- Bustanji, Y.; Issa, A.; Mohammad, M.; Hudaib, M.; Tawah, K.; Alkhatib, H.; Almasri, I.; Al-Khalidi, B. Inhibition of hormone sensitive lipase and pancreatic lipase by Rosmarinus officinalis extract and selected phenolic constituents. J. Med. Plants Res. 2010, 4, 2235–2242. [Google Scholar]
- Nishiyama, J.; Kuninori, T.; Matsumoto, H. The use of 1, 1-diphenyl-2-picrylhydrazyl for detecting free radicals in wheat flour dough. J. Sci. Food Agric. 1978, 29, 267–273. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]


| Compounds | Retention Time (min) | Kovats’ Retention Index * | Area | Relative Area (%) |
|---|---|---|---|---|
| α-Thujene | 9.471 | 923 | 1,599,865 | 0.57 |
| α-Pinene | 9.791 | 931 | 2,615,981 | 0.94 |
| Thuja-2,4(10)-diene | 10.246 | 941 | 480,772 | 0.17 |
| Sabinene | 11.541 | 970 | 139,685 | 0.05 |
| β-Pinene | 11.741 | 974 | 86,508 | 0.03 |
| Myrcene | 12.337 | 987 | 235,294 | 0.09 |
| α-Phellandrene | 13.022 | 1002 | 733,936 | 0.27 |
| α-Terpinene | 13.497 | 1014 | 628,687 | 0.23 |
| p-Cymene | 13.867 | 1022 | 106,797,504 | 38.64 |
| Sylvestrene | 14.067 | 1027 | 6,566,526 | 2.38 |
| 1,8-Cineole | 14.152 | 1029 | 17,823,158 | 6.45 |
| γ-Terpinene | 15.298 | 1056 | 936,553 | 0.34 |
| Terpinolene | 16.433 | 1083 | 152,116 | 0.06 |
| p-Cymenene | 16.613 | 1087 | 929,263 | 0.34 |
| cis-Thujone | 17.264 | 1102 | 835,748 | 0.30 |
| cis-p-Menth-2-en-1-ol | 17.719 | 1114 | 549,290 | 0.20 |
| α-Campholenal | 18.079 | 1123 | 113,198 | 0.04 |
| Allo-ocimene | 18.394 | 1131 | 533,340 | 0.19 |
| 4-Ketoisophorone | 18.574 | 1136 | 318,614 | 0.12 |
| Sabina ketone | 18.984 | 1147 | 1,808,566 | 0.65 |
| Borneol | 19.77 | 1167 | 526,830 | 0.19 |
| 4-Ethyl-3,4-dimethyl-2-cyclohexene | 19.92 | 1171 | 402,158 | 0.15 |
| Terpinen-4-ol | 20.25 | 1179 | 8,409,609 | 3.04 |
| Verbenone | 20.8 | 1193 | 417,699 | 0.15 |
| γ-Terpineol | 21 | 1198 | 1,443,225 | 0.52 |
| α-Terpineol | 21.23 | 1204 | 248,590 | 0.09 |
| Citronellol | 21.885 | 1223 | 114,947 | 0.04 |
| Ascaridole | 22.361 | 1236 | 224,751 | 0.08 |
| Cuminaldehyde | 22.486 | 1239 | 14,441,493 | 5.22 |
| Ethyl oct-(2E)-enoate | 22.781 | 1248 | 381,282 | 0.14 |
| Piperitone | 22.896 | 1251 | 646,349 | 0.23 |
| n-Decanol | 23.291 | 1262 | 237,868 | 0.09 |
| Phellandral | 23.756 | 1279 | 11,838,921 | 4.28 |
| α-Terpinen-7-al | 24.056 | 1283 | 427,241 | 0.15 |
| Carvacrol | 24.862 | 1306 | 641,374 | 0.23 |
| Myrtenyl acetate | 25.347 | 1321 | 313,433 | 0.11 |
| 3-oxo-ρ-Menth-1-en-7-al | 25.807 | 1334 | 392,091 | 0.14 |
| Bicyclo [3,3,1]non-6-en-3-ol, 7-methyl | 26.377 | 1351 | 142,510 | 0.05 |
| α-Ylangene | 27.132 | 1374 | 64,722 | 0.02 |
| n-Tetradecane | 27.908 | 1397 | 144,223 | 0.05 |
| α-Santalene | 28.493 | 1416 | 119,993 | 0.04 |
| α-Humulene | 29.633 | 1452 | 201,051 | 0.07 |
| Allo-aromadendrene | 29.849 | 1459 | 2,210,432 | 0.80 |
| n-Pentadecane | 31.039 | 1497 | 159,306 | 0.06 |
| 14-Hydroxy-9-epi-(E)-caryophyllene | 35.751 | 1660 | 1,375,888 | 0.50 |
| Isobicyclogermacrenal | 37.732 | 1733 | 567,702 | 0.21 |
| Cyclocolorenone | 38.322 | 1755 | 477,660 | 0.17 |
| Methyl octanoate | 17.564 | 1110 | 129,609 | 0.05 |
| β-Santalene | 30.084 | 1467 | 489,471 | 0.18 |
| α-Copaene | 30.734 | 1488 | 1,912,648 | 0.69 |
| Aromadendrene | 33.47 | 1598 | 81,966,904 | 29.65 |
| epi-α-Cadinol | 34.455 | 1614 | 1,450,303 | 0.57 |
| Total identified | 276,404,887 | 100.00 | ||
| Phytochemical classification | ||||
| Monocyclic monoterpene | 44.27 | |||
| Oxygenated monoterpenoid | 21.62 | |||
| Sesquiterpenes hydrocarbon | 31.46 | |||
| Oxygenated sesquiterpenoid | 1.44 | |||
| Others | 1.21 | |||
| Total | 100.00 | |||
| Bacteria | Fungus | ||||||
|---|---|---|---|---|---|---|---|
| Gram-Positive | Gram-Negative | Yeast | |||||
| ATCC Number/Strain | Clinical Strain | ATCC 25923 | ATCC 25922 | ATCC 13883 | ATCC 8427 | ATCC 9027 | ATCC 90028 |
| Microbe | MRSA | S. aureus | E. coli | K. pneumoniae | P. vulgaris | P. aeruginosa | C. albicans |
| E. camaldulensis essential oil | 0.2 ± 0.01 | 0.2 ± 0.01 | 12.5 ± 0.09 | 12.5 ± 0.09 | 6.25 ± 0.1 | 50 ± 1.19 | 0.2 ± 0.01 |
| Ciprofloxacin | 12.5 ± 0.09 | 0.78 ± 0.01 | 1.56 ± 0.1 | 0.13 ± 0.01 | 15 ± 1.1 | 3.12 ± 0.35 | R |
| Ampicillin | 25 ± 0.1 | 25 ± 0.125 | 3.12 ± 0.35 | 1.25 ± 0.1 | 18 ± 1.04 | R | R |
| Fluconazole | R | R | R | R | R | R | 1.56 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaradat, N.; Al-Maharik, N.; Hawash, M.; Qadi, M.; Issa, L.; Anaya, R.; Daraghmeh, A.; Hijleh, L.; Daraghmeh, T.; Alyat, A.; et al. Eucalyptus camaldulensis Dehnh Leaf Essential Oil from Palestine Exhibits Antimicrobial and Antioxidant Activity but No Effect on Porcine Pancreatic Lipase and α-Amylase. Plants 2023, 12, 3805. https://doi.org/10.3390/plants12223805
Jaradat N, Al-Maharik N, Hawash M, Qadi M, Issa L, Anaya R, Daraghmeh A, Hijleh L, Daraghmeh T, Alyat A, et al. Eucalyptus camaldulensis Dehnh Leaf Essential Oil from Palestine Exhibits Antimicrobial and Antioxidant Activity but No Effect on Porcine Pancreatic Lipase and α-Amylase. Plants. 2023; 12(22):3805. https://doi.org/10.3390/plants12223805
Chicago/Turabian StyleJaradat, Nidal, Nawaf Al-Maharik, Mohammed Hawash, Mohammad Qadi, Linda Issa, Rashad Anaya, Ayham Daraghmeh, Lobna Hijleh, Tasneem Daraghmeh, Amal Alyat, and et al. 2023. "Eucalyptus camaldulensis Dehnh Leaf Essential Oil from Palestine Exhibits Antimicrobial and Antioxidant Activity but No Effect on Porcine Pancreatic Lipase and α-Amylase" Plants 12, no. 22: 3805. https://doi.org/10.3390/plants12223805
APA StyleJaradat, N., Al-Maharik, N., Hawash, M., Qadi, M., Issa, L., Anaya, R., Daraghmeh, A., Hijleh, L., Daraghmeh, T., Alyat, A., & Aboturabi, R. (2023). Eucalyptus camaldulensis Dehnh Leaf Essential Oil from Palestine Exhibits Antimicrobial and Antioxidant Activity but No Effect on Porcine Pancreatic Lipase and α-Amylase. Plants, 12(22), 3805. https://doi.org/10.3390/plants12223805








