Abstract
Different drying techniques may alter the chemical composition of plant extracts and consequently affect their bioactivity potential. The current study was designed to reveal the effect of four different drying methods on the phytochemical composition and antioxidant activity of hydrodistilled essential oil (HD-EO) and methanolic (APM) extract obtained from the aerial part of Anthemis palestina from Jordan. Aerial parts of A. palestina in their fresh (FR) form and after drying in shade (ShD), sun (SD), oven at 40 °C (O40D) and 60 °C (O60D), in addition to microwave (MWD), were used to extract their essential oils by hydrodistillation and to prepare the different methanolic extracts (APM). GC/MS analysis of the different HD-EOs revealed qualitative and quantitative differences among the different samples. While FR, O40D, O60D, and MWD EO samples contained mainly sesquiterpene hydrocarbons (35.43%, 29.04%, 53.69%, and 59.38%, respectively), ShD sample was rich in oxygenated monoterpenes (33.57%), and SD-EO contained mainly oxygenated sesquiterpenes (40.36%). Principal component analysis (PCA) and Cluster analysis (CA) grouped the different drying methods based on their impact on the concentration of chemical constituents. SD-EO demonstrated high DPPH and ABTS antioxidant activity (1.31 ± 0.03) × 10−2; (1.66 ± 0.06) × 10−2 μg/mL, respectively). Furthermore, A. paleistina methanolic extracts (APM) obtained after subjecting the plant to different drying methods showed interesting patterns in terms of their TPC, TFC, antioxidant activity, and phytochemical profiling. Of all extracts, SD-APM extract had the highest TPC (105.37 ± 0.19 mg GA/g DE), highest TFC (305.16 ± 3.93 mg Q/g DE) and demonstrated the highest DPPH and ABTS scavenging activities ((4.42 ± 0.02) × 10−2; (3.87 ± 0.02) × 10−2 mg/mL, respectively); all were supported by correlation studies. LC-MS/MS analysis of the different extracts revealed the richness of the SD-APM extract in phenolic acids and flavonoids.
1. Introduction
Essential oils (EOs) are complex natural combinations of volatile molecules that are usually extracted from the plant material through hydrodistillation and are well recognized for their wide spectrum of bioactivities. EOs and their constituents have been classified as antioxidants [1,2,3,4,5], food preservatives [6,7,8], and insect repellents [9,10,11] in the past. Exogenous parameters such as soil characteristics, harvest time, plant growth stage, geographical location, extraction technique, and/or drying method were all found to have a significant impact on the extractive yield, chemistry, and related bioactivities [12].
Due to the short lifetime of most medicinal herbs, fresh plant material is seldom used for a long time during the year. Herbal remedies are usually collected and then preserved by drying for prolonged use during the whole year. While drying can preserve plant material by preventing enzymatic spoilage, it can alter the yield and chemical composition of EOs and extracts, including constituents like polyphenols, pigments, and vitamins [13]. Different drying methods may have pronounced effects on the chemical composition of EOs/extracts [14,15,16], and thus their bioactivity might change. According to the literature, the most common method employed for plant drying is drying in oven [14,17,18,19,20]. Accordingly, preservation of medicinal plants requires the employment of the optimal drying method to preserve and enhance the content and bioactivity.
Anthemis is one of the largest genera of the Asteraceae (Compositeae) family that comprises approximately 210 species [21]. Plants belonging to this genus are known for their wild geographical distribution including Europe, Southwestern Asia, Northern and Northeastern Africa, Southern Arabia, and tropical East Africa [22,23]. Here in Jordan, the Anthemis genus is represented by 16 species only [24]. Anthemis species are reputed in folk medicine in many cultures for their anti-inflammatory, antioxidant, antibacterial, and antispasmodic effects [25,26]. Different classes of secondary metabolites were isolated from several Anthemis plants including sesquiterpene lactones [27], polyacetylenes [28,29,30,31], and flavonoids [32]. Anthemis species assayed previously for their essential oil composition [33,34] contained β-pinene, α-pinene, spathulenol, germacrene D, caryophyllene oxide, and limonene [33,34,35,36,37,38,39] as main constituents.
The current study was designed to investigate the effect of different drying methods (natural ones (drying in shade (ShD); Sun drying (SD)) and artificial (oven drying at two different temperatures (40 °C: O40D); 60 °C (O60D); microwave drying (MWD))) on the volatile (essential oil) and nonvolatile chemistry of Anthemis palestina, from Jordan. The impact of these different drying methods was assessed in terms of their effect on the chemical constituents, total phenol content (TPC), total flavonoid content (TFC), and antioxidant activity, determined by the DPPH and ABTS methods, in addition to their effect on the methanolic extract composition.
2. Results and Discussion
2.1. Composition of HDEO Obtained from the Plant Material Subjected to Different Drying Methods
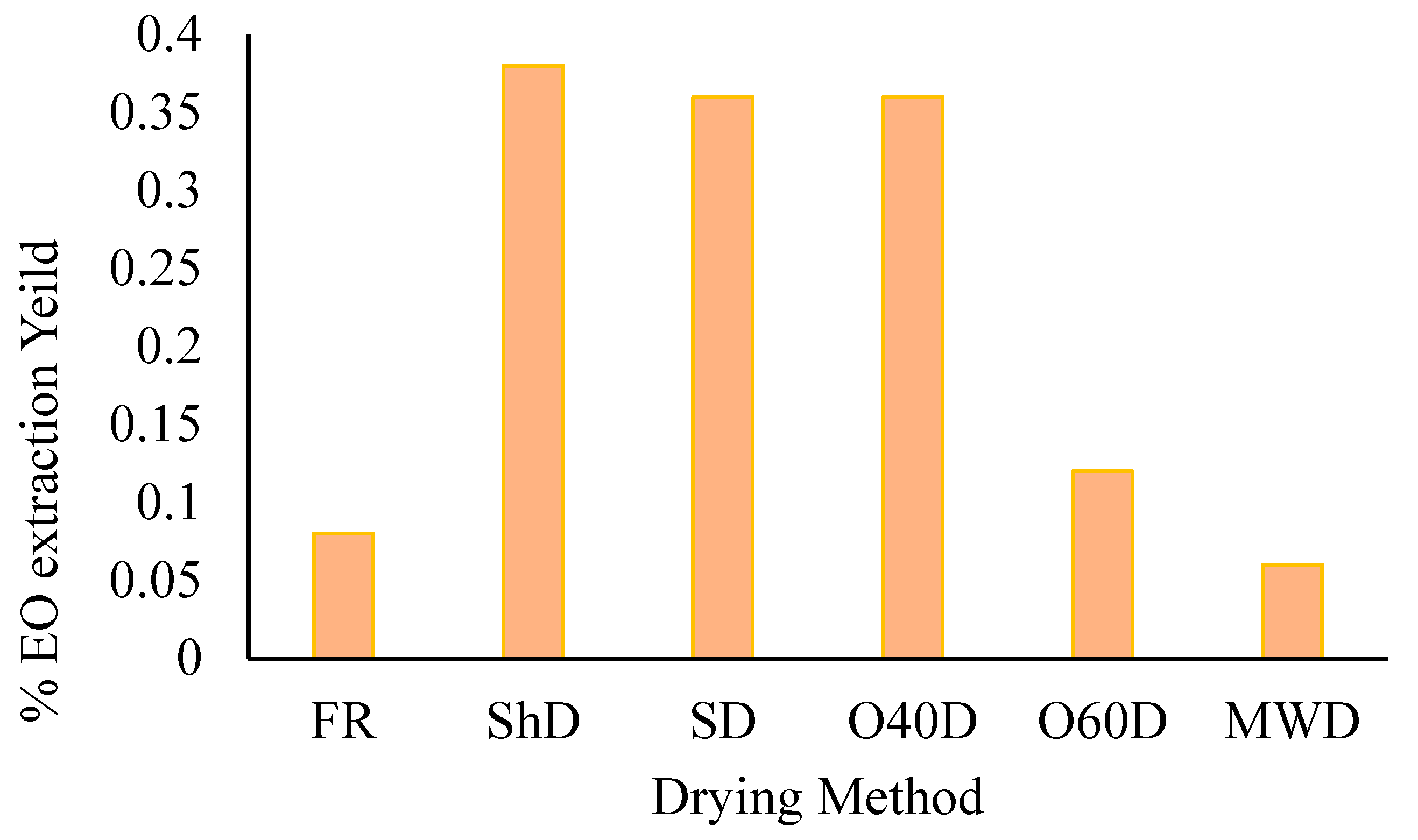
Hydrodistillation of the aerial parts of A. palestina afforded essential oils at varying yields (Figure 1), with the highest yield obtained from the plant material subjected to ShD (0.38%, by weight) and the lowest resulting from microwave (100 W)-dried plant material (0.06% by weight).
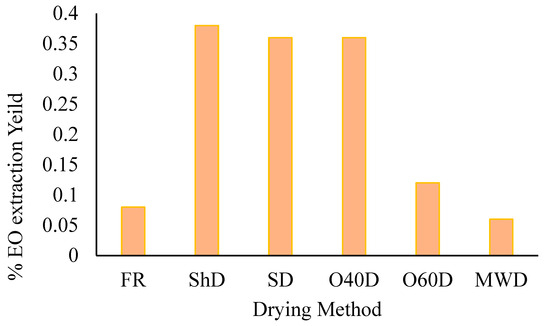
Figure 1.
A. palestina EO yields variation in essential oil extraction yield (w/w) with the employed drying method (FR: Fresh; ShD: Shade-dried; SD: Sun-dried; O40D: Oven-dried at 40 °C; O60D: Oven-dried at 60 °C; MWD: Microwaved-dried).
2.2. GC/MS Analysis of HD-EOs Obtained from A. palestina Aerial Parts Subjected to Different Drying Methods
Each HD-EO obtained from the plant material after being subjected to the selected drying method was analyzed by GC/MS technique to reveal its chemical constituents; the results are listed in Table 1 (Figure S1). The analysis resulted in the identification of 165 constituents with notable qualitative and quantitative variations. In total, 102, 72, 78, 84, 81, and 75 components were identified in the HD-EOs, representing 95.21%, 97.74%, 96.83%, 97.36%, 66.69%, and 99.09% of the total content in FR, ShD, SD, O40D, O60D, and MWD samples, respectively. The identified compounds were grouped into six main groups based on their chemical structures. These were aliphatic compounds (AC), carboxylic acids and esters (CE), monoterpene hydrocarbons (MH), oxygenate monoterpenes (OM), sesquiterpene hydrocarbons (SH), and oxygenate sesquiterpenes (OS).

Table 1.
Chemical compositions of HD-EO obtained from A. palestina aerial parts, subjected to different drying methods, in comparison to the fresh (FR) sample.
The HD-EO obtained from FR plant sample was dominated by SH and OS, which amounted to 35.43% and 30.82% of the total composition, respectively. Monoterpenes and their oxygenated derivatives made an appreciable contribution to the total composition as well, with the OM being detected at higher concentration levels as compared to MH (16.31%, 7.54%, respectively). The main components detected in this FR-EO sample were γ-muurolene (11.20%), terpinen-4-ol (5.47%), (E)-caryophyllene (3.51%), (E)-β-farnesene (3.20%), sylvestrene (3.19%), and α-zingiberene (3.10%). In the HD-EO obtained from plant samples dried in shade (ShD), OM were the main contributors, amounting to 33.57% of the total composition followed by SH (26.61%). This variation in composition was attributed to the detection of higher concentration levels of terpinen-4-ol (16.94%) as compared to its content in the FR-EO sample. This variation in concentration levels was also observed for γ-muurolene (6.82%), α-zingiberene (5.46%), and (Z)-β-farnesene (5.11%).
The SD-HD-EO was characterized by high content of sesquiterpenes and their oxygenated derivatives, both accounting in total for 69.29% of the total composition. The content of OM detected in this sample was almost similar to the one obtained from FR-EO (16.74%). The main individual representatives of SD-EO included terpinen-4-ol (9.35%), spathulenol (7.96%), α-cedrene epoxide (6.65%), γ-muurolene (6.00%), and (Z)-β-farnesene (5.91%).
The HD-EOs obtained from O40D, O60D, and MWD were characterized by high content of SH (29.04%; 53.69%; and 59.38%, respectively). Again, qualitative and quantitative variations among the individual constituents were observed. The main constituents detected in the O40D-EO included each of terpinen-4-ol (12.91%), γ-muurolene (7.95%), and (E)-caryophyllene (5.42%). The main constituents in O60D-EO were γ-muurolene (18.69%), (E)-β-farnesene (10.92%), α-zingiberene (8.60%), and caryophyllene oxide (6.60%). It was observed that the main constituents in the MWD-EO were like those detected in the O60D-EO, but with variable concentration levels. The main constituents in the MWD-EO were γ-muurolene (18.73%), α-zingiberene (14.70%), (Z)-β-farnesene (9.26%), and α-(E, E)-farnesene (5.12%).
In our current study, it was noticed that upon increasing the temperature during the drying process (specifically, oven drying at 40 °C and 60 °C), terpenoidal content varied. Drying at high temperature lowered the total content of monoterpenes but increased the total content of sesquiterpenes. This was in total agreement with the previous work [19,40,41], mainly being attributed to the higher volatility of monoterpenes as compared to sesquiterpenes.
2.3. Principal Component Analysis and Cluster Analysis
In the current study, principal component analysis (PCA) and cluster analysis (CA) were applied to the data shown in Table 1, in order to decipher the underlying patterns in the concentrations of the different constituents detected in the oils extracted from A. palestina aerial parts subjected to different drying methods.
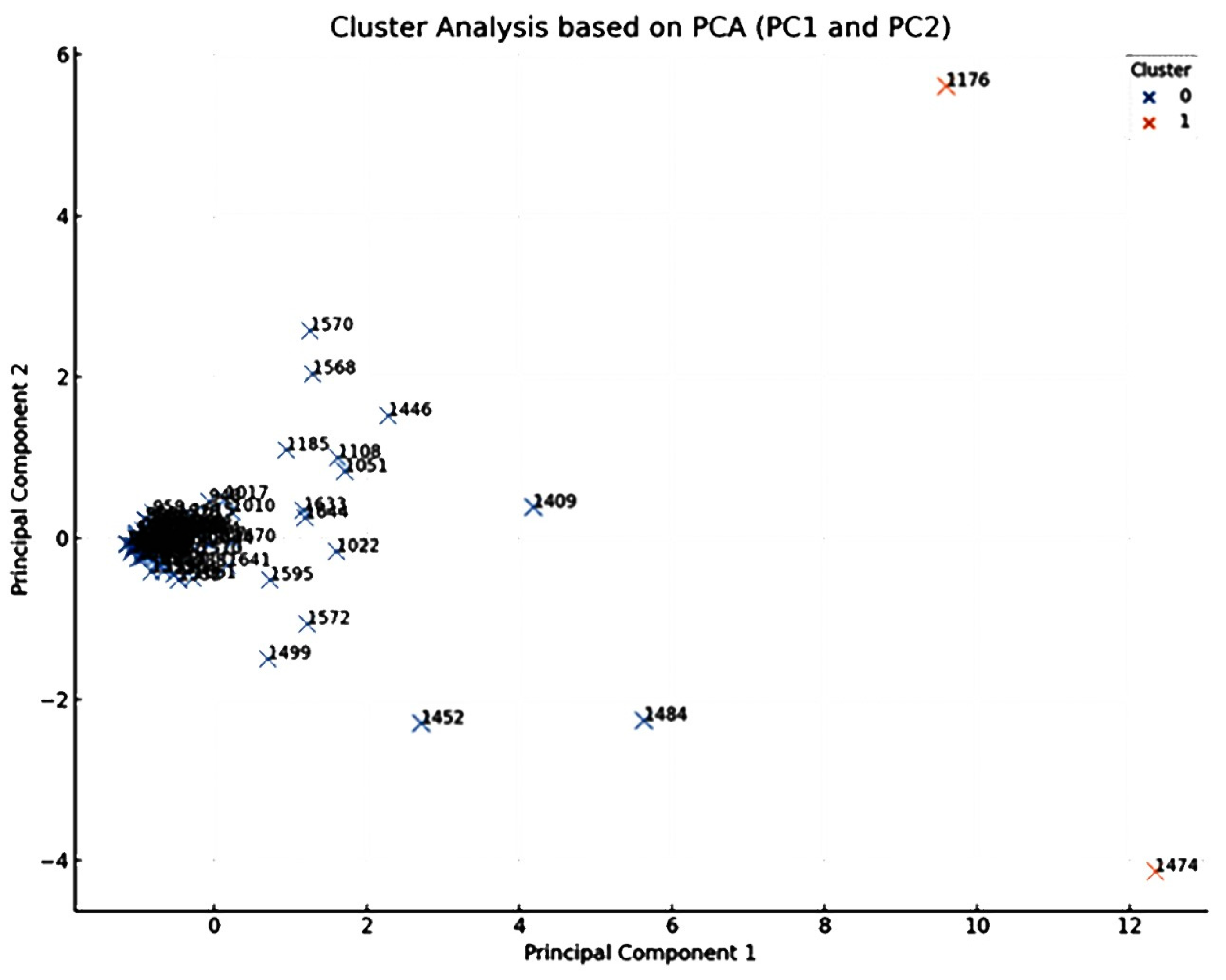
The obtained PCA scatter plot, showing the scores of individual chemical components on the primary two principal components (PC1 and PC2) is shown in Figure 2. Each point within this plot corresponds to a distinct chemical component, identified by its KI value as listed in Table 1. The distribution of these points encapsulates their concentration profiles across an array of the different drying techniques employed in the current study including the FR, ShD, SD, O40D, O60D, and MWD methods.
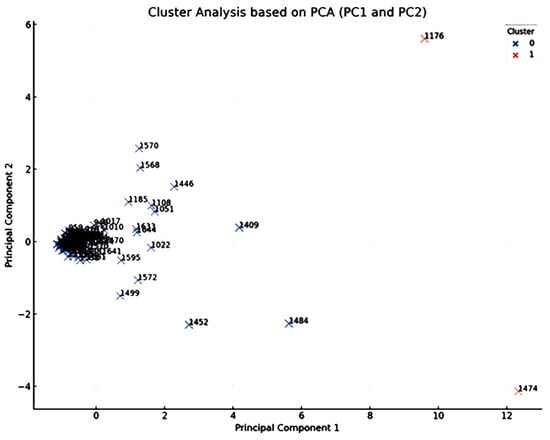
Figure 2.
PCA loadings plot, based on the first two principal components PC1 and PC2 of the HDEOs constituents (identified by their Kovat Index KI). Each point represents a chemical constituent identified by its Kovats index, detected in the oils obtained from A. palestina subjected to different drying methods (p value > 0.05).
Close and careful inspection of this scatter plot revealed an interesting observation related to the emergence of two main clusters that signified chemical components with identical concentration profiles across the different drying techniques. Components located proximally on the plot share analogous profiles, signifying similar responses to the drying methods, while those distantly placed exhibit divergent concentration profiles, implying differential responses to the drying processes.
Components that are prominently positioned on the extremities of the plot, especially along PC1 and PC2 axes, require special attention. The strategic position of these components underscores their distinct concentration profiles, deviating markedly from the average response. These components, particularly γ-muurolene (1474), terpinen-4-ol (KI 1176), and α-zingiberene (1484), could be significant as potential biomarkers for this genus/species.
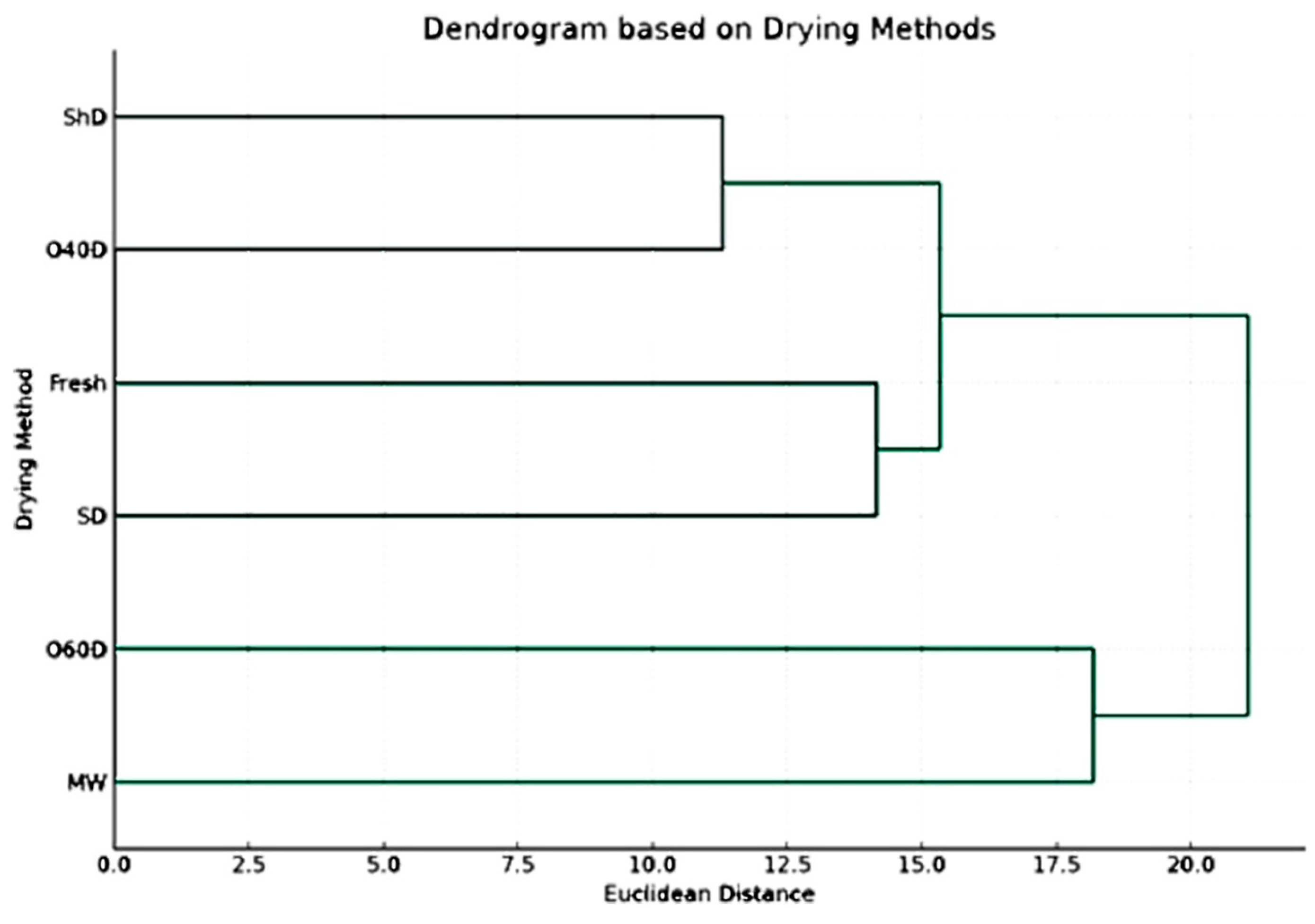
To confirm and better understand the obtained results, a dendrogram based on drying methods was established (Figure 3) in which the different drying methods are grouped based on their impact on the concentration of chemical components, revealing a hierarchy of which drying methods resulted in similar chemical profiles. Based on the results shown in this dendrogram, it is clear that the chemical profiles of the HD-EO obtained from the plant material subjected to drying in shade (ShD) and oven drying at 40 °C (O40D) were most similar as compared to other profiles resulting from other drying techniques. The second group of similar chemical profiles corresponded to FR and SD EO-samples.
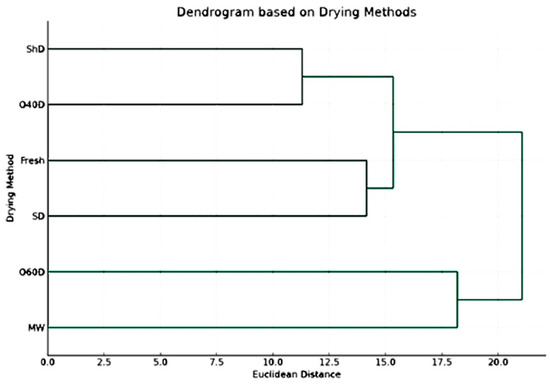
Figure 3.
The dendrogram is based on drying methods, including untreated fresh plant material (p value > 0.05).
Understanding the effect of drying methods on the EO composition and which could produce similar chemical profiles can be very helpful in taking the correct informative decisions during extraction and preservation processes.
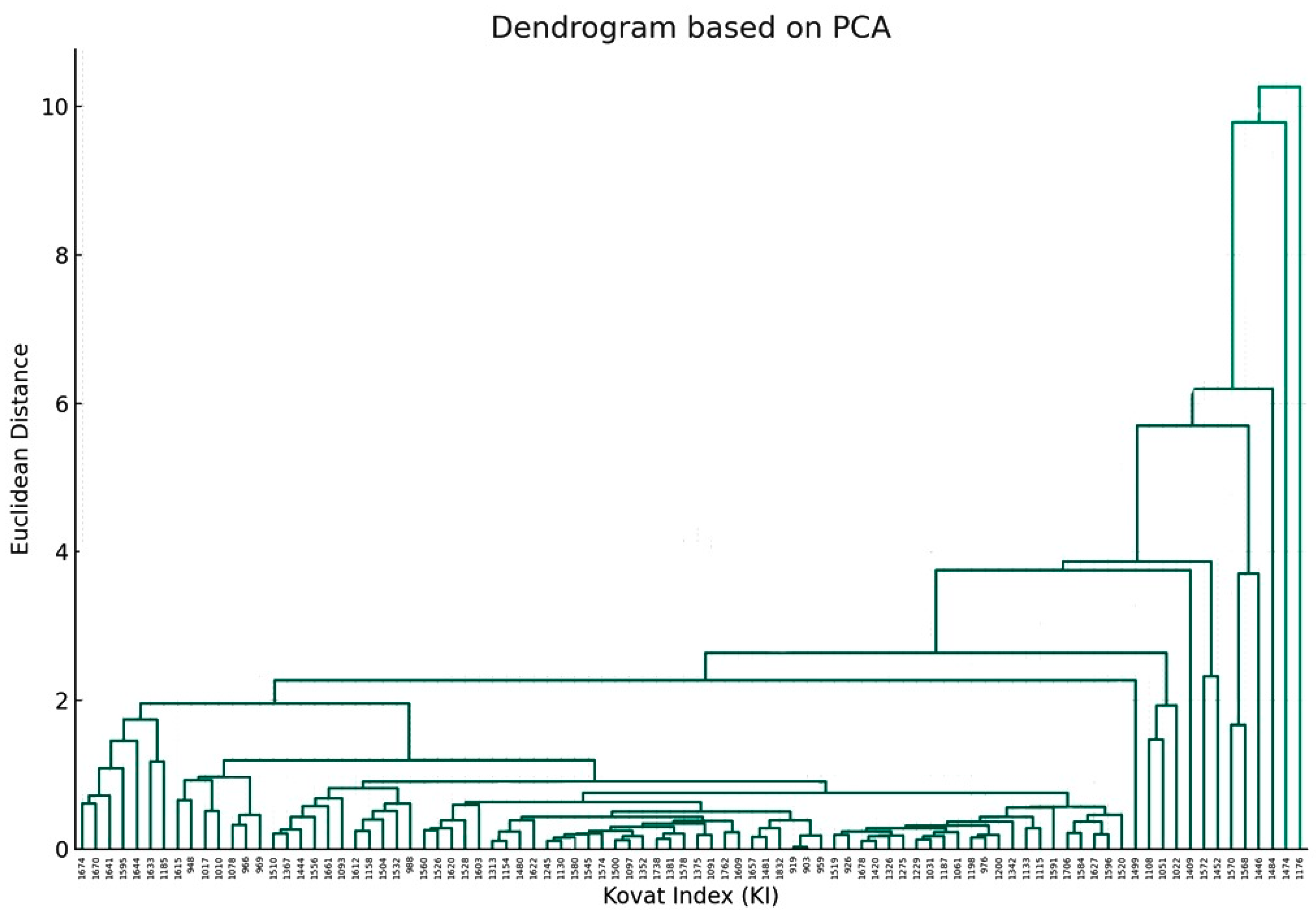
Furthermore, another dendrogram, based on PCA, that focuses on the relationship between individual components was also obtained (Figure 4).
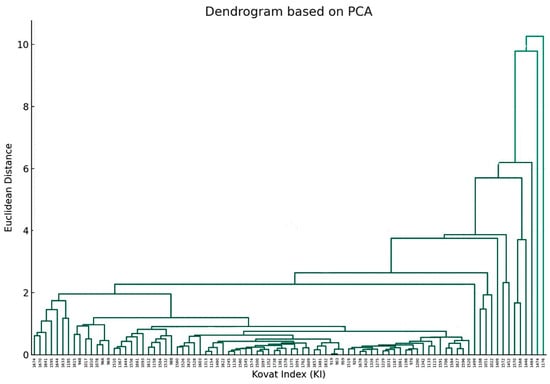
Figure 4.
The dendrogram visualizes the hierarchical clustering of the chemical components based on their PCA results. The x-axis represents the Kovat Index (KI) for each chemical component (as mentioned in Table 1), and the y-axis represents the Euclidean distance, which indicates similarity (the closer the distance is to zero, the more similar the components are) (p value > 0.05).
The fusion of branches at varying heights indicates the degree of similarity between components, with lower heights indicating greater similarity. Components that merge at shorter distances on the y-axis exhibited similar concentration profiles across drying methods. The farther up the y-axis these merges occur, the more dissimilar the components are.
Joining information obtained from the two dendrograms, one can recognize which drying method is optimal for enhancing or diminishing specific chemical components. If a particular cluster of chemical components (Figure 4) is considered beneficial, the drying method dendrogram (Figure 3) can guide towards the method that maximizes these components.
2.4. Antioxidant Activities of HD-EOs
In the present investigation, the antioxidant activities of HD-EOs extracted from the aerial parts of A. palestina subjected to different drying methods were determined and compared to the two positive controls, ascorbic acid and α-tocopherol (Table 2 and Figure S2).

Table 2.
Antioxidant IC50 values (determined by DPPH and ABTS assay methods) of the HD-EOs obtained from aerial parts of A. palestina subjected to different drying methods.
The HD-EO obtained from FR plant material had the highest DPPH radical scavenging activity as compared to other HD-EOs (IC50: (1.00 ± 0.03) ×10−2 μg/mL). The EOs obtained from plant material subjected to SD and O40D were comparable ((1.31 ± 0.03) × 10−2; (1.66 ± 0.06) × 10−2 μg/mL, respectively). These two EOs had also the highest ABTS scavenging activity ((1.34 ± 0.01) × 10−2; (1.91 ± 0.01) × 10−2 μg/mL, respectively). Results in Table 2 clearly demonstrate the effect of drying method on the antioxidant activity. These findings are in total agreement not only with those listed in the literature [42] but also with the results of statistical analysis performed in our current investigation, that indicated the effect of the different drying methods on the chemical composition. Again, combining the information obtained from dendrograms in Figure 3 and Figure 4 together can offer guidance in choosing the proper drying method that maximizes the beneficial effect (in this case, the antioxidant activity).
2.5. Total Phenol Content (TPC), Total Flavonoid Content (TFC) and Antioxidant Activity of APM Extracts
The different A. palestina methanolic extracts (APM) extracts were tested for their TPC and TFC according to the procedure described in the literature [1,2,3,7]. The obtained results are listed in Table 2.
As could be deduced from the results obtained in Table 3, APM extract obtained from the SD plant material had the highest TPC (105.37 ± 0.19 mg GA/g DE) and TFC (305.16 ± 3.93 mg Q/g DE). The extract obtained from the plant material subjected to O60D had the lowest TPC and TFC (43.49 ± 0.57 mg GA/g DE, 52.94 ± 0.90 mg of Q/g DE, respectively).

Table 3.
TPC, TFC, and antioxidant IC50 values (determined by DPPH and ABTS assay methods) of the APM extracts obtained from aerial parts of A. palestina subjected to different drying methods.
The results demonstrated that extracts obtained from the plants dried at the low temperatures (ShD and SD) had the highest TPC when compared to those obtained at higher temperatures (O40D, O60D, and MWV), thus confirming the effect of drying methods on the composition (especially the phenolics content) [43]. The high phenolic content of the extract obtained from MW-dried plant material could be attributed to the effect of microwave radiation and its elevated temperature on the plant tissue, especially the cell wall. This drying method could have enabled the release of cell wall phenolics, and consequently increased their measured content [44].
Most extracts showed moderate to high TFC, except for the extract obtained from the plant dried in the oven at 60 ºC, which had the lowest content (52.94 ± 0.90 mg Q/g DE).
In our study, DPPH and ABTS radical scavenging methods were used to evaluate the antioxidant activity of the different APM extracts; the results are summarized in Table 3 and Figure S3. Most tested extracts had interesting scavenging activities, in the two methods, with the APM-SD extract having the highest DPPH and ABTS methods ((4.42 ± 0.02) × 10−2; (3.87 ± 0.02) × 10−2 mg/mL, respectively). The observed strong antioxidant activity of this extract could be correlated with its high TPC and TFC. The lowest DPPH and ABTS activities were recorded for APM-O60D (IC50 of (17.51 ± 1.72) × 10−2 and (23.99 ± 1.62) × 10−2 μg/mL, respectively) which was characterized with the lowest TPC and TFC. Our findings strongly support the effect of different drying methods on the observed antioxidant activity of plant extracts [45,46].
2.6. Correlation Studies: Antioxidant Activities, TPC, and TFC
In the current study, the obtained data (TPC, TFC, DPPH, and ABTS) were evaluated to calculate the correlation matrix of the different investigated variables (phenolic and flavonoid contents with antioxidant activities). The calculated correlation matrices are displayed in Table 4 (Figures S6 and S7).

Table 4.
Correlation matrix of the variables for the APM extracts of A. palestina (p value > 0.05).
As could be deduced from the obtained correlation results, there is a very strong correlation (0.99027) between TPC and TFC, suggesting that these two variables are positively related.
The data revealed also strong negative correlations observed between each of TPC and DPPH (−0.95541); TPC and ABTS (−0.95987); TFC and DPPH (−0.95703); and TFC and ABTS (−0.94990). These strong negative correlations indicate an inverse relationship between each pair, confirming the effect of higher phenolic and flavonoid contents on the observed DPPH and ABTS antioxidant activities; as the content increases, IC50 value decreases, indicating stronger antioxidant activity. There was also a positive correlation observed between the DPPH and ABTS (0.84878).
2.7. LC-MS Analysis of Phytochemicals
In the current investigation, the presence of a selected set of constituents in the APM extracts obtained from aerial parts of A. palaestina, dried as described previously, was determined by LC-MS/MS using both the positive and negative ionization modes. The total ion chromatograms (TICs) of the four APM extracts corresponding to the different drying methods employed in the current study are shown in Figure S4; results of the LC-Ms/MS analysis are summarized in Table 5. A total of 43 compounds were detected and identified, including 23 flavonoids, 5 organic acids, 6 phenolic acids, and 10 other compounds. Several compounds were common to all analyzed extracts and were mostly reported to occur in the plants belonging to the same family. The findings of this analysis are in total agreement with those of TPC and TFC, and support the high antioxidant activity observed for the extracts, especially for the APM-SD extract that contained most of the detected compounds, including all phenolic acids and flavonoids.

Table 5.
Major compounds identified in the LC-MS chromatograms of the different APM extracts obtained from A. palaestina subjected to different drying methods.
3. Materials and Methods
3.1. Plant Material
The aerial parts of A. Palestina were collected from Samou region in Irbid, Jordan, during the full flowering stage (the spring of 2022). The identity of the plant was confirmed using characteristics related to growth habits and morphological attributes in regional floras [24] and was further confirmed by Prof. Dr Jamil Al-Lahham, Department of Biological Sciences—Faculty of Science, Yarmouk University, Irbid, Jordan. A voucher specimen was deposited in the herbarium of the Department of Biological Sciences-Yarmouk University, Irbid, Jordan (YU/09/ AA/1002).
3.2. Drying Conditions
Fresh aerial parts of A. Palestina were subjected to different drying methods, immediately after collection. The different drying methods included drying in shade (ShD), sun drying (SD), oven drying at 40 °C (O40D), oven drying at 60 °C (O60D), and microwave drying (MWD). In the ShD method, the aerial parts of the plant (250 g) were dried in a dark dry room with appropriate ventilation until constant weight was achieved (4 weeks, at 25 ± 2 °C). In SD, the aerial parts (250 g) were dried on paper trays under direct sunlight at temperatures ranging between 19 °C and 36 °C for 7 days until constant weight was achieved. In the oven drying (OD) method, the aerial parts of the plant (250 g) were dried in a ventilated oven at two selected temperatures separately, 40 °C and 60 °C, for 3 days to assure constant weight achievement. In MWD (100 W), same amount of aerial plant material was dried for 3 min.
3.3. Extraction of Essential Oil
Each dried sample (250 g) was subjected to hydrodistillation for 4 h using a Clevenger-type apparatus. The obtained oils were collected, dried over anhydrous MgSO4, and then stored in sealed amber vials and kept at 4 °C until analysis was performed.
3.4. Preparation of the Methanolic Extract
Air-dried (ShD) and grinded aerial parts of A. palestina were subjected to Soxhlet extraction with petroleum ether to get rid of fatty material and waxes. Then, the dried defatted plant residue was extracted in the same apparatus with methanol three times. The combined methanolic extract was evaporated under reduced pressure and the obtained residue (APM) was then assayed for its total phenol content (TPC), total flavonoid content (TFC), and antioxidant activity using DPPH and ABTS assay methods. The extract was then further assayed for its chemical constituents by HPLC-MS/MS analysis.
3.5. GC and GC-MS Analysis
A sample of 1 μL of each oil was diluted to 3.0 μL with GC grade n-hexane, and then analyzed by GC-MS (Chromatec Crystal GC-MSD, Yoshkar-Ola, Russia) equipped with a CR-5 MS column (5% diphenyl, 95% dimethyl polysiloxane, 30 m × 0.25 mm, 0.25-μm film thicknesses). In the MS detector, an electron ionization mode of 70 eV was used. The temperature in the MS source was set at 300 °C and transfer line temperature at 230 °C. The temperature column was programmed from 40 °C for 1 min (isothermal) to 280 °C at a constant rate of 3 °C/min, with the lower and upper temperatures being held constant for 3 min. Helium was used as a carrier gas (1.0 mL/min). The relative peak areas were used to calculate the relative percentage concentrations of the detected compounds. A standard solution of C8–C30 n-alkanes mixture was analyzed under the same chromatographic conditions. The chemical constituents of the essential oils were identified by comparing their calculated Kovats retention index (KI) (relative to n-alkanes C8–C30), matching their recorded mass spectra with the built-in library spectra and by comparing their mass spectra to those of authentic standards.
3.6. Total Phenolic and Total Flavonoid Contents
The total phenolic and total flavonoid contents (TPC, TFC, respectively) of the APM extracts obtained from each drying method were determined by two assay methods, including the Folin–Ciocalteu and aluminum chloride methods, respectively, as previously described [1,2].
3.7. Antioxidant Activity of HDEOs and Methanolic Extracts
The antioxidant activity of the different APM extracts and HDEOs was evaluated by the DPPH and ABTS methods as described in [1,2,3,7]. The scavenging ability was calculated based on the following equation:
where Ac is the absorbance of the control, and As is the absorbance in the presence of extracts or standards.
% Activity = [(Ac − As)/Ac] × 100,
3.8. LC-MS Analysis of Phytochemicals
Secondary metabolites profiling of the crude methanolic extract (APM) was done using a Bruker Daltonik Elute UHPLC system equipped with Impact II ESI-Q-TOF System (Bremen, Germany), in both the positive [M + H]+ and negative [M − H]− electrospray ionization modes. Isothermal chromatographic separation was performed on a C-18 reversed phase column (100 × 2.1 mm, 1.8 µm, 120 Å, Bruker Daltonik, Germany) at 30 °C with a total elution time of 20 min. The autosampler temperature was kept constant at 8 °C. Plant samples were dissolved with 2.0 mL DMSO, and then the total volume of the sample was completed to 50 mL using acetonitrile (HPLC grade). The sample was centrifuged at 4000 rpm for 2 min before injection (injection volume was 3.0 µL). Secondary metabolite constituents in the studied extract were identified based on their m/z ratio with reference to the retention time of the used standards.
3.9. Statistical Analysis
Data analysis was carried out with SAS software version 9.4 (2013). TPC, TFC, DPPH, ABTS were analyzed by Pearson Correlation and one-way ANOVA model with Drying methods. Before ANOVA, descriptive statistics for all the measurements were made in order to observe the distribution of the data and check the normality by general linear model (p value > 0.05). Means were separated using LSD test at a significance level of 0.05.
4. Conclusions
The current study revealed the impact of employing different drying methods on the phytochemical composition and antioxidant activities of essential oils and methanolic extracts obtained from the aerial parts of A. palestina growing wild in Jordan. Indeed, the employed drying methods affected the chemical composition of the HF-EOs qualitatively and quantitatively. Principal components analysis and cluster analysis grouped the different drying methods according to their impact on the chemical composition. The EO obtained from the sun-dried plant material had the highest antioxidant activity due to its chemical profile. This was also in total agreement with chemical profiling and antioxidant activity tests observed for the different APM extracts. Again, the extract obtained from the SD plant material had the highest TPC and TFC, and accordingly demonstrated the highest antioxidant activity in the two assay methods. Correlation studies confirmed these findings and were all in agreement with the LC-MS/MS results, which indicated the richness of the SD-APM extract in phenolic compounds and flavonoids. Consequently, this HD-EO and APM extract obtained from SD method showed the highest favorable antioxidant activity. This study underscores the importance of recognizing the impact of drying methods on the chemical profiles of EOs and extracts. Such studies can help in taking the correct decision in employing the correct drying technique for obtaining the best-desired extract with optimal composition and activity. In the current investigation, of the different drying methods used, sun drying of plant material afforded methanolic extract with favorable composition and antioxidant activity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants12223914/s1, Figure S1: Representative gas chromatograms for oils extracted from A. palaestina obtained by hydro-distillation at different drying methods in comparison to the fresh sample. Figure S2: Antioxidant activity (DPPH and ABTS) of the essential oils (EO) from A. Palestina obtained by hydro-distillation at different drying methods in comparison to the fresh sample. Figure S3: Antioxidant activity (DPPH and ABTS) of the methanol extract from A. palestina (APM) obtained by different drying methods. Figure S4: LC-MS chromatograms (positive and negative modes) of methanol extract from A. palestina (APM) obtained at different drying methods. Figure S5: standards. Figure S6. Distribution of TPC and TFC among the different APM extracts obtained using different drying methods. Figure S7. Distribution of DPPH and ABTS antioxidant activities among the different APM extracts obtained using different drying methods.
Author Contributions
Conceptualization, M.A.A.-Q., S.T.A.-O. and A.M.M.R.; methodology, A.K.A. and T.T.B.; software, H.I.A.-J., H.S.H. and F.M.A.O.; validation, M.A.A.-Q., A.G.A. and A.I.A.; formal analysis, H.S.H. and A.I.A.; investigation, M.A.A.-Q.; resources, A.K.A.; data curation, H.S.H.; writing—original draft preparation, M.A.A.-Q. and A.K.A.; writing—review and editing, H.I.A.-J., A.G.A. and S.T.A.-O.; visualization, M.A.A.-Q.; supervision, M.A.A.-Q.; project administration, M.A.A.-Q.; funding acquisition, M.A.A.-Q. and F.M.A.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia through the project number IFP-IMSIU-2023048.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research through the project number IFP-IMSIU-2023048. The authors also appreciate the Deanship of Scientific Research at Imam Mohammad Ibn Saud Islamic University (IMSIU) for supporting and supervising this project. Also, this work has been carried out during a sabbatical leave granted to the corresponding author (Mahmoud A. Al-Qudah) from Yarmouk University, Jordan during the academic year 2022/2023.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Al-Bataineh, N.; Algethami, F.K.; Al-Jaber, H.I.; Alhamzani, A.G.; Bataineh, R.M.; Al-Dalahmeh, Y.; Bataineh, T.T.; Abu-Orabi, S.T.; Al-Qudah, M.A. Ballota saxatilis from Jordan: Evaluation of essential oil composition and phytochemical profiling of crude extracts and their in-vitro antioxidant Activity. Separations 2023, 10, 114. [Google Scholar] [CrossRef]
- Al-Qudah, M.A.; Al-Smadi, Z.M.; Al-Jaber, H.I.; Tashtoush, H.I.; Alkhatib, R.Q.; Bataineh, T.T.; Dalahmeh, Y.; Orabi, S.T.A. GC/MS and LC-MS/MS phytochemical evaluation of the essential oil and selected secondary metabolites of Ajuga orientalis from Jordan and its antioxidant activity. Arab. J. Chem. 2023, 16, 104641. [Google Scholar] [CrossRef]
- Al-Qudah, M.A. Antioxidant acitvity and chemical composition of essential oils of fresh and air-dried Jordanian Nepeta curviflora Boiss. J. Biol. Act. Prod. Nat. 2016, 6, 101–111. [Google Scholar]
- Diniz do Nascimento, L.; Moraes, A.A.; Costa, K.S.; Pereira Galúcio, J.M.; Taube, P.S.; Costa, C.M.; Neves Cruz, J.; de Aguiar Andrade, E.H.; Faria, L.J. Bioactive natural compounds and antioxidant activity of essential oils from spice plants: New findings and potential applications. Biomolecules 2020, 10, 988. [Google Scholar] [CrossRef]
- Al-Shuneigat, J.M.; Al-Tarawneh, I.N.; Al-Qudah, M.A.; Al-Sarayreh, S.A.; Al-Saraireh, Y.M.; Alsharafa, K.Y. The chemical composition and the antibacterial properties of Ruta graveolens L. essential oil grown in Northern Jordan. Jordan J. Biol. Sci. 2015, 8, 139–143. [Google Scholar] [CrossRef]
- Boukhatem, M.N.; Kameli, A.; Ferhat, M.A.; Saidi, F.; Tayebi, K. The food preservative potential of essential oils: Is lemongrass the answer? J. Verbraucherschutz Leb. 2013, 9, 13–21. [Google Scholar] [CrossRef]
- Al-Momani, L.A.; Abu-Orabi, S.T.; Hlail, H.M.; Alkhatib, R.Q.; Al-Dalahmeh, Y.; Al-Qudah, M.A. Anthemis cotula L. from Jordan: Essential oil composition, LC-ESI-MS/MS profiling of phenolic acids-flavonoids and in vitro antioxidant activity. Arab. J. Chem. 2023, 16, 104470. [Google Scholar] [CrossRef]
- Abu Zarga, M.H.; Al-Jaber, H.I.; Baba Amer, Z.Y.; Sakhrib, L.; Al-Qudah, M.A.; Al-humaidi, J.Y.; Abaza, I.F.; Afifi, F.U. Chemical composition, antimicrobial and antitumor activities of essential oil of Ammodaucus leucotrichus growing in Algeria. J. Biol. Act. Prod. Nat. 2013, 3, 224–231. [Google Scholar]
- da Costa, K.; Galúcio, J.; da Costa, C.; Santana, A.; dos Santos Carvalho, V.; do Nascimento, L. Exploring the potentiality of natural products from essential oils as inhibitors of odorant-binding proteins: A structure- and ligand-based virtual screening approach to find novel mosquito repellents. ACS Omega 2019, 4, 22475–22486. [Google Scholar] [CrossRef]
- Liang, J.-Y.; Guo, S.-S.; Zhang, W.-J.; Geng, Z.-F.; Deng, Z.-W.; Du, S.-S.; Zhang, J. Fumigant and repellent activities of essential oil extracted from Artemisia dubia and its main compounds against two stored product pests. Nat. Prod. Res. 2017, 32, 1234–1238. [Google Scholar] [CrossRef]
- Bertoli, A.; Conti, B.; Mazzoni, V.; Meini, L.; Pistelli, L. Volatile chemical composition and bioactivity of six essential oils against the stored food insect sitophilus zeamais motsch. (Coleoptera Dryophthoridae). Nat. Prod. Res. 2011, 26, 2063–2071. [Google Scholar] [PubMed]
- Boutebouhart, H.; Didaoui, L.; Tata, S.; Sabaou, N. Effect of extraction and drying method on chemical composition, and evaluation of antioxidant and antimicrobial activities of essential oils from Salvia officinalis. J. Essent. Oil Bear. Plants 2019, 22, 717–727. [Google Scholar] [CrossRef]
- Prusinowska, R.; Śmigielski, K. Losses of essential oils and antioxidants during the drying of herbs and spices. A review. Eng. Sci. Technol. 2015, 2, 49–62. [Google Scholar] [CrossRef]
- Figiel, A.; Szumny, A.; Gutiérrez-Ortíz, A.; Carbonell-Barrachina, Á.A. Composition of oregano essential oil (Origanum vulgare) as affected by drying method. J. Food Eng. 2010, 98, 240–247. [Google Scholar] [CrossRef]
- Sellami, I.H.; Wannes, W.A.; Bettaieb, I.; Berrima, S.; Chahed, T.; Marzouk, B.; Limam, F. Qualitative and quantitative changes in the essential oil of laurus nobilis L. leaves as affected by different drying methods. Food Chem. 2011, 126, 691–697. [Google Scholar] [CrossRef]
- Al-Qudah, M.A.; Onizat, M.A.; Alshamari, A.K.; Al-Jaber, H.I.; Bdair, O.M.; Muhaidat, R.; Al-Bataineh, N. Chemical composition and antioxidant activity of Jordanian Artemisia judaica L. as affected by different drying methods. Int. J. Food Prop. 2021, 24, 482–492. [Google Scholar] [CrossRef]
- Rohloff, J.; Dragland, S.; Mordal, R.; Iversen, T. Effect of harvest time and drying method on biomass production, essential oil yield, and quality of peppermint (Mentha piperita L.). J. Agric. Food Chem. 2005, 53, 4143–4148. [Google Scholar] [CrossRef]
- Asekun, O.T.; Grierson, D.S.; Afolayan, A.J. Effects of drying methods on the quality and quantity of the essential oil of Mentha longifolia L. subsp. capensis. Food Chem. 2007, 101, 995–998. [Google Scholar] [CrossRef]
- Rahimmalek, M.; Goli, S.A.H. Evaluation of six drying treatments with respect to essential oil yield, composition, and color characteristics of Thymys daenensis subsp. daenensis. Celak leaves. Ind. Crops Prod. 2013, 42, 613–619. [Google Scholar] [CrossRef]
- Sárosi, S.Z.; Sipos, L.; Kókai, Z.; Pluhár, Z.S.; Szilvássy, B.; Novák, I. Effect of different drying techniques on the aroma profile of Thymus vulgaris analyzed by GC-MS and sensory profile method. Ind. Crops Prod. 2013, 46, 210–216. [Google Scholar] [CrossRef]
- Europaea, F.; Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, A. (Eds.) Fernandes R: Genus Anthemis L. In Flora Europaea; Cambridge University Press: Cambridge, MA, USA; London, UK, 1976; Volume 4, pp. 145–159. [Google Scholar]
- Bardaweel, S.K.; Tawaha, K.A.; Hudaib, M.M. Antioxidant, antimicrobial and antiproliferative activities of Anthemis palestina essential oil. BMC Complement. Altern. Med. 2014, 14, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Bremer, K. Asteraceae, Cladistics and Classification; Timber Press: Portland, OR, USA, 1994. [Google Scholar]
- Al-Eisawi, D. List of Jordan vascular plants. Mitt. Bot. Munchen. 1982, 18, 79–182. [Google Scholar]
- Javidnia, K.; Miri, R.; Kamalinejad, M.; Sarkarzadeh, H.; Jamalian, A. Chemical composition of the essential oils of Anthemis altissima L. grown in Iran. Flavour Fragr. J. 2004, 19, 213–216. [Google Scholar] [CrossRef]
- Saroglou, V.; Dorizas, N.; Kypriotakis, Z.; Skaltsa, H.D. Analysis of the essential oil composition of eight Anthemis species from Greece. J. Chromatogr. A 2006, 1104, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Staneva, J.D.; Todorova, M.N.; Evstatieva, L.N. Sesquiterpene lactones as chemotaxonomic markers in genus Anthemis. Phytochemistry 2008, 69, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Bohlmann, F.; Arndt, C.; Bornowski, H.; Kleine, K.M. Polyacetylenverbindung XLVIII. Die Ployine der Gattung Anthemis L. Chem. Berichte 1963, 96, 1485–1494. [Google Scholar] [CrossRef]
- Bohlmann, F.; Kleine, K.M.; Arndt, C.; Köhn, S. Polyacetylenverbindungen, LXXVIII: Neue Inhaltsstoffe der Gattung Anthemis L. Chem. Berichte 1965, 98, 1616–1622. [Google Scholar] [CrossRef]
- Bohlmann, F.; Bohm, D.; Rybak, C. Polyacetylenverbindungen, LXXXVII: Über die Struktur und Biogenese eines aus Anthemis-Arten isolierten Thioäthers. Chem. Berichte 1965, 98, 3087–3091. [Google Scholar] [CrossRef]
- Bohlmann, F.; Kleine, K.M. Polyacetylenverbindungen, CVI. Über einige neue Acetylenverbindungen aus der Gattung Anthemis L. Chem. Berichte 1966, 99, 2096–2103. [Google Scholar] [CrossRef]
- Williams, C.A.; Greenham, J.; Harborne, J.B. The role of lipophilic and polar flavonoids in the classification of temperate members of the Anthemideae. Biochem. Syst. Ecol. 2001, 29, 929–945. [Google Scholar] [CrossRef]
- Bulatovic, V.M.; Menkovic, N.R.; Vajs, V.E.; Milosavijevic, S.M.; Djokovic, D.D. Essential oil of Anthemis carpatica. J. Essent. Oil Res. 1997, 9, 397–400. [Google Scholar] [CrossRef]
- Bulatovic, V.M.; Menkovic, N.R.; Vajs, V.E.; Milosavljevic, S.M.; Djokovic, D.D. Essential oil of Anthemis montana. J. Essent. Oil Res. 1998, 10, 223–226. [Google Scholar] [CrossRef]
- Grace, M.H. Chemical composition and biological activity of the volatiles of Anthemis melampodina and Pluchea dioscoridis. Phytother. Res. 2002, 16, 183–185. [Google Scholar] [CrossRef] [PubMed]
- Bassols, F.; Thomas, A.F. The occurrence of 3-phenylpropyl isobutyrate in Roman Camomile oil. J. Essent. Oil Res. 1991, 3, 309–312. [Google Scholar] [CrossRef]
- Klimes, I.; Lamparsky, D.; Scholz, E. Vorkommen neuer bifunktioneller. Ester im Römisch-Kamillenöl (Anthemis nobilis L.). Helv. Chim. Acta 1981, 64, 2338–2349. [Google Scholar] [CrossRef]
- Thomas, A.F. The Occurrence of some novel diesters in Roman camomile oil. Helv. Chim. Acta 1981, 64, 2397–2400. [Google Scholar] [CrossRef]
- Khleifat, K.M.; Matar, S.A.; Jaafreh, M.; Qaralleh, H.; Al-limoun, M.O.; Alsharafa, K.Y. Essential oil of Centaurea damascena aerial parts, antibacterial and synergistic effect. J. Essent. Oil Bear. Plants 2019, 22, 356–367. [Google Scholar] [CrossRef]
- Khangholi, S.; Rezaeinodehi, A. Effect of drying temperature on essential oil content and composition of sweet wormwood (Artemisia annua) growing wild in Iran. Pak. J. Biol. Sci. 2008, 11, 934–937. [Google Scholar] [CrossRef]
- Hamrouni-Sellami, I.; Rahali, F.Z.; Rebey, I.B.; Bourgou, S.; Limam, F.; Marzouk, B. Total phenolics, flavonoids, and antioxidant activity of sage (Salvia officinalis L.) plants as affected by different drying methods. Food Bioprocess Technol. 2012, 6, 806–817. [Google Scholar] [CrossRef]
- Tomaino, A.; Cimino, F.; Zimbalatti, V.; Venuti, V.; Sulfaro, V.; De Pasquale, A.; Saija, A. Influence of heating on antioxidant activity and the chemical composition of some spice essential oils. Food Chem. 2005, 89, 549–554. [Google Scholar] [CrossRef]
- Martínez-Las Heras, R.; Heredia, A.; Castelló, M.L.; Andrés, A. Influence of drying method and extraction variables on the antioxidant properties of persimmon leaves. Food Biosci. 2014, 6, 1–8. [Google Scholar] [CrossRef]
- Capecka, E.; Marecczek, A.; Leja, M. Antioxidant activity of fresh and dry herbs of some Lamiaceae species. Food Chem. 2005, 93, 223–226. [Google Scholar] [CrossRef]
- Inchuen, S.; Narkrugsa, W.; Pornchaloempong, P. Effect of drying methods on chemical composition, color and antioxidant properties of Thai red curry powder. Kasetsart J. Nat. Sci. 2010, 44, 142–151. [Google Scholar]
- Annamalai, A. Effect of drying treatment on the contents of antioxidants in Cardiospermum halicacabum Linn. Int. J. Pharm. Biol. Sci. 2011, 2, 304–313. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).