The Impact of Post-Fire Smoke on Plant Communities: A Global Approach
Abstract
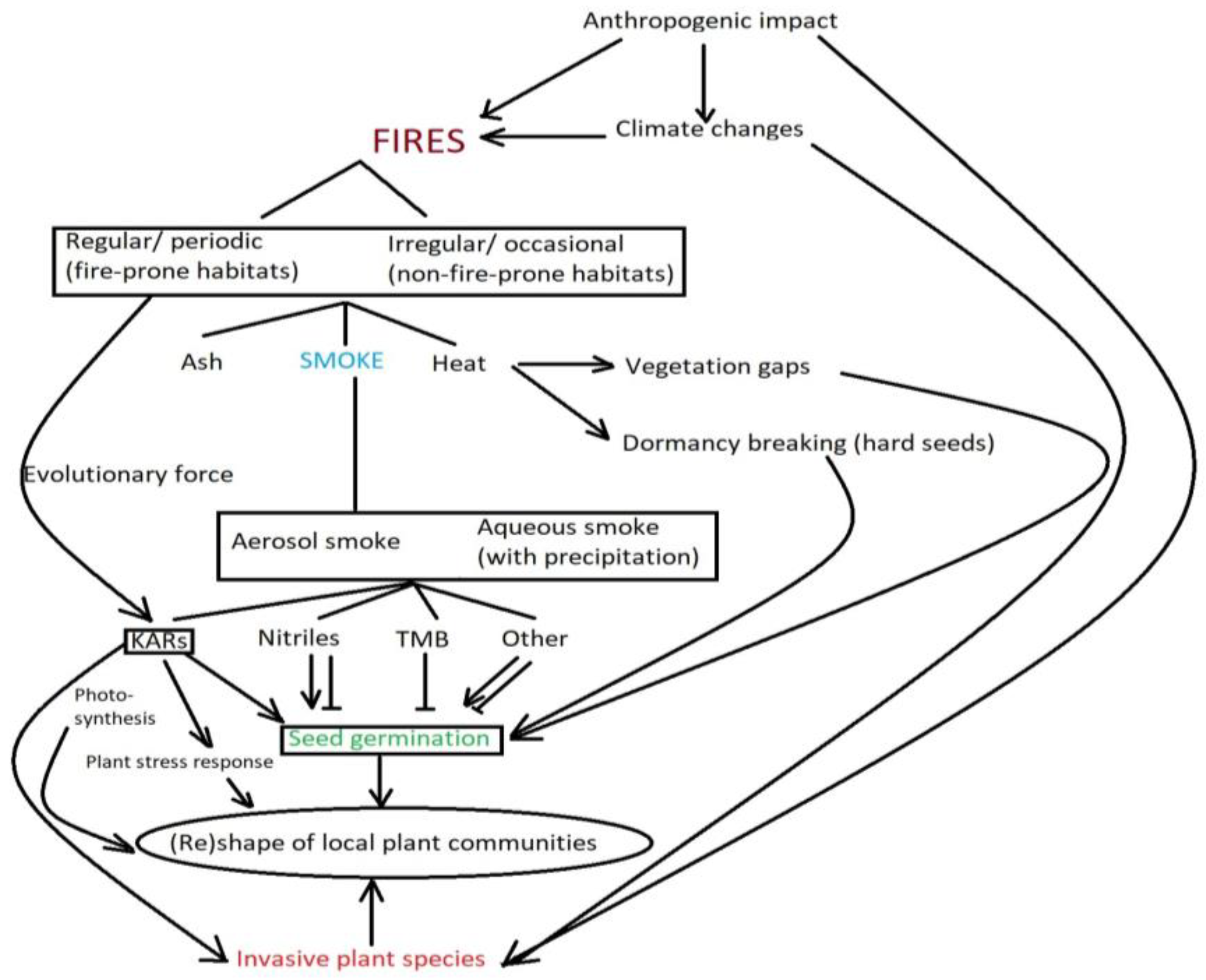
:1. Introduction
2. The Impact of Post-Fire Smoke on Plant Communities
2.1. Fire-Prone Ecosystems
2.1.1. Ecosystems with Mediterranean-Type Climates
2.1.2. Tropical- and Subtropical-Climate Ecosystems
2.2. Non-Fire-Prone Ecosystems
2.2.1. South American Mediterranean Matorral
2.2.2. Tropical Monsoon Ecosystems
2.2.3. Boreal Forests
2.2.4. European Habitats of Moderate Climate
2.2.5. Asian Habitats
3. General Mechanisms for Perception of Smoke by Plants at the Molecular Level
4. The Impact of Smoke and Its Isolated Compounds on Seed Germination and Photosynthesis
5. Karrikins as Protective Agents against Abiotic Stress
6. Smoke as an Evolutionary Force?
7. Large-Scale Testing of Smoke from Burnt Vegetation
7.1. Ground- and Aircraft Monitoring and Modeling
7.2. Satellite Observations
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pausas, J.G.; Verdú, M. Fire reduces morphospace occupation in plant communities. Ecology 2008, 89, 2181–2186. [Google Scholar] [CrossRef] [PubMed]
- Archibald, S.; Lehmann, C.E.; Belcher, C.M.; Bond, W.J.; Bradstock, R.A.; Daniau, A.-L.; Dexter, K.G.; Forrestel, E.J.; Greve, M.; He, T. Biological and geophysical feedbacks with fire in the Earth system. Environ. Res. Lett. 2018, 13, 033003. [Google Scholar] [CrossRef]
- Fox, S.; Sikes, B.A.; Brown, S.P.; Cripps, C.L.; Glassman, S.I.; Hughes, K.; Semenova-Nelsen, T.; Jumpponen, A. Fire as a driver of fungal diversity—A synthesis of current knowledge. Mycology 2022, 114, 215–241. [Google Scholar] [CrossRef] [PubMed]
- Kelly, L.T.; Giljohann, K.M.; Duane, A.; Aquilué, N.; Archibald, S.; Batllori, E.; Bennett, A.F.; Buckland, S.T.; Canelles, Q.; Clarke, M.F. Fire and biodiversity in the Anthropocene. Science 2020, 370, eabb0355. [Google Scholar] [CrossRef] [PubMed]
- Pausas, J.G.; Keeley, J.E. Evolutionary ecology of resprouting and seeding in fire-prone ecosystems. New Phytol. 2014, 204, 55–65. [Google Scholar] [CrossRef] [PubMed]
- King, R.A.; Menges, E.S. Effects of heat and smoke on the germination of six Florida scrub species. S. Afr. J. Bot. 2018, 115, 223–230. [Google Scholar] [CrossRef]
- He, T.; Lamont, B.B.; Pausas, J.G. Fire as a key driver of Earth’s biodiversity. Biol. Rev. 2019, 94, 1983–2010. [Google Scholar] [CrossRef]
- Dayamba, S.D.; Tigabu, M.; Sawadogo, L.; Oden, P.C. Seed germination of herbaceous and woody species of the Sudanian savanna-woodland in response to heat shock and smoke. For. Ecol. Manag. 2008, 256, 462–470. [Google Scholar] [CrossRef]
- Penman, T.D.; Binns, D.; Allen, R.; Shiels, R.; Plummer, S. Germination responses of a dry sclerophyll forest soil-stored seedbank to fire related cues. Cunninghamia 2008, 10, 547–555. [Google Scholar]
- Zirondi, H.L.; Silveira, F.A.; Fidelis, A. Fire effects on seed germination: Heat shock and smoke on permeable vs impermeable seed coats. Flora 2019, 253, 98–106. [Google Scholar] [CrossRef]
- Hodges, J.A.; Price, J.N.; Nicotra, A.B.; Neeman, T.; Guja, L.K. Smoke and heat accelerate and increase germination in fire-prone temperate grassy ecosystems. Ecosphere 2021, 12, e03851. [Google Scholar] [CrossRef]
- Khatoon, A.; Rehman, S.U.; Aslam, M.M.; Jamil, M.; Komatsu, S. Plant-derived smoke affects biochemical mechanism on plant growth and seed germination. Int. J. Mol. Sci. 2020, 21, 7760. [Google Scholar] [CrossRef] [PubMed]
- Chiwocha, S.D.; Dixon, K.W.; Flematti, G.R.; Ghisalberti, E.L.; Merritt, D.J.; Nelson, D.C.; Riseborough, J.-A.M.; Smith, S.M.; Stevens, J.C. Karrikins: A new family of plant growth regulators in smoke. Plant Sci. 2009, 177, 252–256. [Google Scholar] [CrossRef]
- Waters, M.T.; Nelson, D.C. Karrikin perception and signalling. New Phytol. 2023, 237, 1525–1541. [Google Scholar] [CrossRef]
- Flematti, G.R.; Merritt, D.J.; Piggott, M.J.; Trengove, R.D.; Smith, S.M.; Dixon, K.W.; Ghisalberti, E.L. Burning vegetation produces cyanohydrins that liberate cyanide and stimulate seed germination. Nat. Commun. 2011, 2, 360. [Google Scholar] [CrossRef] [PubMed]
- Tavşanoğlu, Ç.; Ergan, G.; Çatav, Ş.S.; Zare, G.; Küçükakyüz, K.; Özüdoğru, B. Multiple fire-related cues stimulate germination in Chaenorhinum rubrifolium (Plantaginaceae), a rare annual in the Mediterranean Basin. Seed Sci. Res. 2017, 27, 26–38. [Google Scholar] [CrossRef]
- Çatav, Ş.S.; Küçükakyüz, K.; Tavşanoğlu, Ç.; Pausas, J.G. Effect of fire-derived chemicals on germination and seedling growth in Mediterranean plant species. Basic Appl. Ecol. 2018, 30, 65–75. [Google Scholar] [CrossRef]
- Pošta, M.; Papenfus, H.B.; Light, M.E.; Beier, P.; Van Staden, J. Structure–activity relationships of N-and S-analogs of the seed germination inhibitor (3,4,5-trimethylfuran-2(5H)-one) for mode of action elucidation. Plant Growth Regul. 2017, 82, 47–53. [Google Scholar] [CrossRef]
- Gómez-González, S.; Sierra-Almeida, A.; Cavieres, L. Does plant-derived smoke affect seed germination in dominant woody species of the Mediterranean matorral of central Chile? For. Ecol. Manag. 2008, 255, 1510–1515. [Google Scholar] [CrossRef]
- Bączek-Kwinta, R.; Markowicz, M. The Impact of Plant-Derived Smoke on Seed Germination in the Context of Swailing. In Plant Functioning under Environmental Stress; Grzesiak, M.T., Rzepka, A., Hura, T., Grzesiak, S., Eds.; The Franciszek Górski Institute of Plant Physiology, Polish Academy of Sciences: Cracow, Poland, 2013; pp. 233–239. [Google Scholar]
- Moreira, B.; Pausas, J. Shedding light through the smoke on the germination of Mediterranean Basin flora. S. Afr. J. Bot. 2018, 115, 244–250. [Google Scholar] [CrossRef]
- Wilson, C.R.; Lusk, C.H.; Campbell, D.I. The role of the peat seed bank in plant community dynamics of a fire-prone New Zealand restiad bog. Austral. Ecol. 2022, 47, 1515–1527. [Google Scholar] [CrossRef]
- Bączek-Kwinta, R. Swailing affects seed germination of plants of European bio-and agricenosis in a different way. Open Life Sci. 2017, 12, 62–75. [Google Scholar] [CrossRef]
- Available online: https://www.who.int/health-topics/wildfires (accessed on 9 November 2023).
- Carthey, A.J.; Tims, A.; Geedicke, I.; Leishman, M.R. Broad-scale patterns in smoke-responsive germination from the south-eastern Australian flora. J. Veg. Sci. 2018, 29, 737–745. [Google Scholar] [CrossRef]
- Alahakoon, A.; Perera, G.; Merritt, D.; Turner, S.R.; Gama-Arachchige, N. Species-specific smoke effects on seed germination of plants from different habitats from Sri Lanka. Flora 2020, 263, 151530. [Google Scholar] [CrossRef]
- Dayamba, S.D.; Sawadogo, L.; Tigabu, M.; Savadogo, P.; Zida, D.; Tiveau, D.; Oden, P.C. Effects of aqueous smoke solutions and heat on seed germination of herbaceous species of the Sudanian savanna-woodland in Burkina Faso. Flora 2010, 205, 319–325. [Google Scholar] [CrossRef]
- Zuloaga-Aguilar, S.; Briones, O.; Orozco-Segovia, A. Seed germination of montane forest species in response to ash, smoke and heat shock in Mexico. Acta Oecol. 2011, 37, 256–262. [Google Scholar] [CrossRef]
- Zhou, J.; Teixeira da Silva, J.A.; Ma, G. Effects of smoke water and karrikin on seed germination of 13 species growing in China. Centr. Eur. J. Biol. 2014, 9, 1108–1116. [Google Scholar] [CrossRef]
- Mojzes, A.; Kalapos, T. Plant-derived smoke stimulates germination of four herbaceous species common in temperate regions of Europe. Plant Ecol. 2014, 215, 411–415. [Google Scholar] [CrossRef]
- Mackenzie, D.D.; Naeth, M.A. Effect of plant-derived smoke water and potassium nitrate on germination of understory boreal forest plants. Can. J. For. Res. 2019, 49, 1540–1547. [Google Scholar] [CrossRef]
- Li, S.; Ma, H.; Ooi, M.K. Fire-related cues significantly promote seed germination of some salt-tolerant species from non-fire-prone saline-alkaline grasslands in Northeast China. Plants 2021, 10, 2675. [Google Scholar] [CrossRef]
- Yusup, S.; Sundberg, S.; Ooi, M.K.; Zhang, M.; Sun, Z.; Rydin, H.; Wang, M.; Feng, L.; Chen, X.; Bu, Z.-J. Smoke promotes germination of peatland bryophyte spores. J. Exp. Bot. 2023, 74, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Ghebrehiwot, H.; Kulkarni, M.; Kirkman, K.; Van Staden, J. Smoke-water and a smoke-isolated butenolide improve germination and seedling vigour of Eragrostis Tef (Zucc.) Trotter under high temperature and low osmotic potential. J. Agron. Crop Sci. 2008, 194, 270–277. [Google Scholar] [CrossRef]
- Jain, N.; Kulkarni, M.G.; van Staden, J. A butenolide, isolated from smoke, can overcome the detrimental effects of extreme temperatures during tomato seed germination. Plant Growth Regul. 2006, 49, 263–267. [Google Scholar] [CrossRef]
- Flematti, G.R.; Waters, M.T.; Scaffidi, A.; Merritt, D.J.; Ghisalberti, E.L.; Dixon, K.W.; Smith, S.M. Karrikin and cyanohydrin smoke signals provide clues to new endogenous plant signaling compounds. Mol. Plant 2013, 6, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Keeley, J.E. Fire in Mediterranean climate ecosystems—A comparative overview. Isr. J. Ecol. Evol. 2012, 58, 123–135. [Google Scholar]
- Huerta, S.; Fernández-García, V.; Marcos, E.; Suarez-Seoane, S.; Calvo, L. Physiological and regenerative plant traits explain vegetation regeneration under different severity levels in Mediterranean fire-prone ecosystems. Forests 2021, 12, 149. [Google Scholar] [CrossRef]
- Lamont, B.B.; He, T.; Yan, Z. Evolutionary history of fire-stimulated resprouting, flowering, seed release and germination. Biol. Rev. 2019, 94, 903–928. [Google Scholar] [CrossRef]
- Daibes, L.F.; Martins, A.R.; Silveira, F.A.; Fidelis, A. Seed tolerance to post-fire temperature fluctuation of Cerrado legume shrubs with micromorphological implications. Flora 2021, 275, 151761. [Google Scholar] [CrossRef]
- Li, T.; Jeřábek, J.; Winkler, J.; Vaverková, M.D.; Zumr, D. Effects of prescribed fire on topsoil properties: A small-scale straw burning experiment. J. Hydrol. Hydromech. 2022, 70, 450–461. [Google Scholar] [CrossRef]
- Harper, A.R.; Doerr, S.H.; Santin, C.; Froyd, C.A.; Sinnadurai, P. Prescribed fire and its impacts on ecosystem services in the UK. Sci. Total Environ. 2018, 624, 691–703. [Google Scholar] [CrossRef]
- Wójcik, T.; Kostrakiewicz-Gierałt, K.; Makuch-Pietraś, I. The effect of accidental burning on habitat conditions and species composition of Molinion caeruleae meadows. J. Nat. Conserv. 2022, 70, 126294. [Google Scholar] [CrossRef]
- Wójcik, T.; Janicka, M. Current state and changes in Molinion meadows from Kostrze environs in Kraków. Ecol. Quest. 2016, 23, 15–27. [Google Scholar] [CrossRef]
- Çatav, Ş.S.; Elgin, E.S.; Dağ, Ç.; Stark, J.L.; Küçükakyüz, K. NMR-based metabolomics reveals that plant-derived smoke stimulates root growth via affecting carbohydrate and energy metabolism in maize. Metabolomics 2018, 14, 1–11. [Google Scholar] [CrossRef] [PubMed]
- De Lange, J.H.; Boucher, C. Autecological studies on Audouinia capitata (Bruniaceae). I. Plant-derived smoke as a seed germination cue. S. Afr. J. Bot. 1990, 56, 700–703. [Google Scholar] [CrossRef]
- Singh, S.; Uddin, M.; Khan, M.M.A.; Chishti, A.S.; Singh, S.; Bhat, U.H. The role of plant-derived smoke and karrikinolide in abiotic stress mitigation: An Omic approach. Plant Stress 2023, 7, 100147. [Google Scholar] [CrossRef]
- Wang, L.; Waters, M.T.; Smith, S.M. Karrikin-KAI2 signalling provides Arabidopsis seeds with tolerance to abiotic stress and inhibits germination under conditions unfavourable to seedling establishment. New Phytol. 2018, 219, 605–618. [Google Scholar] [CrossRef]
- Meng, Y.; Shuai, H.; Luo, X.; Chen, F.; Zhou, W.; Yang, W.; Shu, K. Karrikins: Regulators involved in phytohormone signaling networks during seed germination and seedling development. Front. Plant Sci. 2017, 7, 2021. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Lian, Y.; Wang, C. Comparing and contrasting the multiple roles of butenolide plant growth regulators: Strigolactones and karrikins in plant development and adaptation to abiotic stresses. Int. J. Mol. Sci. 2019, 20, 6270. [Google Scholar] [CrossRef] [PubMed]
- Stanga, J.P.; Smith, S.M.; Briggs, W.R.; Nelson, D.C. Suppressor of MORE AXILLARY GROWTH2 1 controls seed germination and seedling development in Arabidopsis. Plant Physiol. 2013, 163, 318–330. [Google Scholar] [CrossRef]
- Pošta, M.; Light, M.E.; Papenfus, H.B.; Van Staden, J.; Kohout, L. Structure–activity relationships of analogs of 3,4,5-trimethylfuran-2 (5H)-one with germination inhibitory activities. J. Plant Physiol. 2013, 170, 1235–1242. [Google Scholar] [CrossRef]
- Papenfus, H.B.; Naidoo, D.; Pošta, M.; Finnie, J.F.; Van Staden, J. The effects of smoke derivatives on in vitro seed germination and development of the leopard orchid Ansellia africana. Plant Biol. 2016, 18, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Baldos, O.C.; DeFrank, J.; Sakamoto, G.S. Germination response of dormant tanglehead (Heteropogon contortus) seeds to smoke-infused water and the smoke-associated stimulatory compounds, karrikinolide and cyanide. Hortic. Sci. 2015, 50, 421–429. [Google Scholar] [CrossRef]
- Cao, D.; Schöttner, M.; Halitschke, R.; Li, D.; Baldwin, G.; Rocha, C.; Baldwin, I.T. Syringaldehyde is a novel smoke-derived germination cue for the native fire-chasing tobacco, Nicotiana attenuata. Seed Sci. Res. 2021, 31, 292–299. [Google Scholar] [CrossRef]
- Demir, I.; Ozden, E.; Yıldırım, K.; Sahin, O.; Van Staden, J. Priming with smoke-derived karrikinolide enhances germination and transplant quality of immature and mature pepper seed lots. S. Afr. J. Bot. 2018, 115, 264–268. [Google Scholar] [CrossRef]
- Akeel, A.; Khan, M.M.A.; Jaleel, H.; Uddin, M. Smoke-saturated water and karrikinolide modulate germination, growth, photosynthesis and nutritional values of carrot (Daucus carota L.). J. Plant Growth Regul. 2019, 38, 1387–1401. [Google Scholar] [CrossRef]
- Bączek-Kwinta, R. An Interplay of Light and Smoke Compounds in Photoblastic Seeds. Plants 2022, 11, 1773. [Google Scholar] [CrossRef] [PubMed]
- Jamil, M.; Jahangir, M.; Rehman, S.U. Smoke induced physiological, biochemical and molecular changes in germinating rice seeds. Pak. J. Bot. 2020, 52, 865–871. [Google Scholar] [CrossRef]
- Noroozi Shahri, F.; Jalali Honarmand, S.; Saeidi, M.; Mondani, F. Evaluation of some biochemical characteristics of medicinal plant basil (Ocimum basilicum L.) under the application of growth phytohormones and phytohormones-like. J. Plant Proc. Func. 2021, 10, 189–210. [Google Scholar]
- Shah, F.A.; Wei, X.; Wang, Q.; Liu, W.; Wang, D.; Yao, Y.; Hu, H.; Chen, X.; Huang, S.; Hou, J. Karrikin improves osmotic and salt stress tolerance via the regulation of the redox homeostasis in the oil plant Sapium sebiferum. Front. Plant Sci. 2020, 11, 216. [Google Scholar] [CrossRef]
- Tan, Z.-Z.; Wang, Y.-T.; Zhang, X.-X.; Jiang, H.-Y.; Li, Y.; Zhuang, L.-L.; Yu, J.-J.; Yang, Z.-M. Karrikin1 Enhances Drought Tolerance in Creeping Bentgrass in Association with Antioxidative Protection and Regulation of Stress-Responsive Gene Expression. Agronomy 2023, 13, 675. [Google Scholar] [CrossRef]
- Ahmad, B.; Qadir, S.U.; Dar, T.A.; Alam, P.; Yousuf, P.Y.; Ahmad, P. Karrikins: Smoke-derived phytohormones from stress alleviation to signaling. J. Plant Growth Regul. 2022, 42, 4784–4796. [Google Scholar] [CrossRef]
- Obata, T.; Fernie, A.R. The use of metabolomics to dissect plant responses to abiotic stresses. Cell. Mol. Life Sci. 2012, 69, 3225–3243. [Google Scholar] [CrossRef] [PubMed]
- Dolferus, R. To grow or not to grow: A stressful decision for plants. Plant Sci. 2014, 229, 247–261. [Google Scholar] [CrossRef] [PubMed]
- Leuendorf, J.E.; Frank, M.; Schmülling, T. Acclimation, priming and memory in the response of Arabidopsis thaliana seedlings to cold stress. Sci. Rep. 2020, 10, 689. [Google Scholar] [CrossRef] [PubMed]
- Keeley, J.E.; Pausas, J.G.; Rundel, P.W.; Bond, W.J.; Bradstock, R.A. Fire as an evolutionary pressure shaping plant traits. Trends Plant Sci. 2011, 16, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Lamont, B.B.; He, T. Fire-proneness as a prerequisite for the evolution of fire-adapted traits. Trends Plant Sci. 2017, 22, 278–288. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Lamont, B.B. Baptism by fire: The pivotal role of ancient conflagrations in evolution of the Earth’s flora. Nat. Sci. Rev. 2018, 5, 237–254. [Google Scholar] [CrossRef]
- Keeley, J.E.; Pausas, J.G. Evolutionary ecology of fire. Annu. Rev. Ecol. Evol. Syst. 2022, 53, 203–225. [Google Scholar] [CrossRef]
- Mallia, D.V.; Kochanski, A.K.; Kelly, K.E.; Whitaker, R.; Xing, W.; Mitchell, L.E.; Jacques, A.; Farguell, A.; Mandel, J.; Gaillardon, P.E.; et al. Evaluating wildfire smoke transport within a coupled fire-atmosphere model using a high-density observation network for an episodic smoke event along Utah’s Wasatch Front. J. Geophys. Res. Atmos. 2020, 125, e2020JD032712. [Google Scholar] [CrossRef]
- Garofalo, L.A.; Pothier, M.A.; Levin, E.J.; Campos, T.; Kreidenweis, S.M.; Farmer, D. Emission and evolution of submicron organic aerosol in smoke from wildfires in the Western United States. ACS Earth Space Chem. 2019, 3, 1237–1247. [Google Scholar] [CrossRef]
- Ottmar, R.D.K.; Hiers, H.; Clements, C.B.; Butler, B.; Dickinson, M.B.; Potter, B.; Rowell, E.M.; Strand, T.M.; Zajkowski, T.J. Measurements, datasets and preliminary result from the RxCADRE project-2008, 2011 and 2012. Int. J. Wildland Fire 2016, 25, 1–9. [Google Scholar] [CrossRef]
- Prichard, S.; Larkin, N.S.; Ottmar, R.; French, N.H.F.; Baker, K.; Brown, T.; Clements, C.; Dickinson, M.; Hudak, A.; Kochanski, A.; et al. The Fire and Smoke Model Evaluation Experiment—A Plan for Integrated, Large Fire—Atmosphere Field Campaigns. Atmosphere 2019, 10, 66. [Google Scholar] [CrossRef] [PubMed]
- Mallia, D.V.; Kochanski, A.K.; Urbanski, S.P.; Mandel, J.; Farguell, A.; Krueger, S.K. Incorporating a Canopy Parameterization within a Coupled Fire-Atmosphere Model to Improve a Smoke Simulation for a Prescribed Burn. Atmosphere 2020, 11, 832. [Google Scholar] [CrossRef]
- Strand, T.; Gullet, B.; Urbanski, S.; O’Neill, S.; Potter, B.; Aurell, J.; Holder, A.; Larkin, N.; Moore, M.; Rorig, M. Grassland and forest understory biomass emissions from prescribed fires in southeastern United States—RxCADRE 2012. Int. J. Wildland Fire 2015, 25, 102–113. [Google Scholar] [CrossRef]
- Sokolik, I.N.; Soja, A.J.; DeMott, P.J.; Winker, D. Progress and challenges in quantifying wildfire smoke emissions, their properties, transport, and atmospheric impacts. J. Geophys. Res. Atmos. 2019, 124, 13005–13025. [Google Scholar] [CrossRef]
- Achtemeier, G.L. Planned Burn-Piedmont. A local operational numerical meteorological model for tracking smoke on the ground at night: Model development and sensitivity tests. Int. J. Wildland Fire 2005, 14, 85–98. [Google Scholar] [CrossRef]
- Sun, Y.; Jiang, L.; Pan, J.; Sheng, S.; Hao, L. A satellite imagery smoke detection framework based on the Mahalanobis distance for early fire identification and positioning. Int. J. Appl. Earth Obs. Geoinf. 2023, 118, 103257. [Google Scholar] [CrossRef]
- Guede-Fernández, F.; Martins, L.; de Almeida, R.V.; Gamboa, H.; Vieira, P. A Deep Learning Based Object Identification System for Forest Fire Detection. Fire 2021, 4, 75. [Google Scholar] [CrossRef]
- Ba, R.; Chen, C.; Yuan, J.; Song, W.; Lo, S. SmokeNet: Satellite Smoke Scene Detection Using Convolutional Neural Network with Spatial and Channel-Wise Attention. Remote Sens. 2019, 11, 1702. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, X.; Li, F.; Cochrane, M.A. Improved estimation of fire particulate emissions using a combination of VIIRS and AHI data for Indonesia during 2015–2020. Remote Sens. Environ. 2022, 281, 113238. [Google Scholar] [CrossRef]
| Region | Vegetation Type | Germination Stimulation with Application of Smoke Formulations | Reference | |
|---|---|---|---|---|
| Smoke | Smoke Water | |||
| Fire-prone ecosystems | ||||
| South Africa and Australia, MTC | Fynbos | Poaceae sp. | [25] | |
| Eurasia, Turkey, Mediterranean Basin, MTC | Chaparral | Annual herbaceous species | [17] | |
| South America (Brasil) | Cerrado | Mimosa somnians | [10] | |
| Cambessedesia hilariana | ||||
| Microlicia sp. | ||||
| Africa, Burkina Faso | Sudanian Savanna–Woodland | Pteleopsis suberosa | [8] | |
| Terminalia avicennioides | ||||
| Borreria scabra | [27] | |||
| Asia, Sri Lanka | Savanna–woodland | Flueggea leucopyrus | [26] | |
| Maesa indica | ||||
| Phyllanthus emblica L. | ||||
| Chromolaena odorata L. | ||||
| Hyptis suaveolens L. | ||||
| North America, Florida | Scrub species | Chrysopsis highlandsensis | [6] | |
| Eryngium cuneifolium | ||||
| Lechea cernua | ||||
| North America, Mexico | Montane forest | Fuchsia encliandra | [28] | |
| Pinus douglasiana | ||||
| Non-fire-prone ecosystems | ||||
| South America, Central Chile, MTC | Matorral | Acacia caven | [19] | |
| Baccharis vernalis | ||||
| Trevoa quinquenervia | ||||
| Asia, South of China | Monsoon climate | Aristolochia debilis Siebold and Zucc. | [29] | |
| Central Europe, Hungary | Not specified | Camelina microcarpa Andrz. ex DC. | [30] | |
| Capsella bursa-pastoris (L.) Medik | ||||
| Descurainia sophia (L.) Webb ex Prant | ||||
| Plantago lanceolata L. | ||||
| North America | Boreal forest | Vaccinium myrtilloides Michx. | [31] | |
| Central Europe, Poland | Not specified/local bio- and agricenoses | Matricaria chamomilla L. | [23] | |
| Solidago gigantea Aiton (alien, invasive) | ||||
| Trifolium repens L. | ||||
| Artemisia absinthium L. | ||||
| Plantago major L. | ||||
| Asia, Northeast China | Saline–alkaline grasslands | Setaria viridis (L.) P.Beauv. | [32] | |
| Kochia scoparia (L.). var. sieversiana (Pall.) Ulbr. ex Aschers. et Graebn | ||||
| Asia, Northeast China | Northern peatland | Sphagnum flexuosum Dozy and Molk | [33] | |
| S. subnitens Russow and Warnst | ||||
| S.imbricatum Hornschuch ex. Russow | ||||
| S. magellanicum Brid. | ||||
| S. fuscum (Schimp.) H.Klinggr | ||||
| S. squarrosum Crome | ||||
| Polytrichum strictum. | ||||
| Drepanocladus aduncus (Hedw.) Warnst. | ||||
| Physcomitrium sphaericum Brid | ||||
| Hypnum callichroum Hedw. | ||||
| Plant Species | Physiologically Active Smoke Compound | Mode of Action | Reference |
|---|---|---|---|
| Lactuca sativa | KAR1 | Stimulates seed germination | [52] |
| Chaenorhinum rubrifolium Robill. and Castagne ex DC | Mix (aqueous smoke), nitrate | Breakdown of physiological dormancy | [16] |
| KAR1, MAN | Stimulates seed germination | ||
| Ansellia africana Lindl. | TMB | Reduces the germination rate index and the development rate index | [53] |
| Heteropogon contortus (L.) P.Beauv. ex Roem. and Schult. | Benzaldehyde, cyanide, potassium cyanide | Stimulates seed germination | [54] |
| Lactuca sativa L. | MAN | Inhibits seed germination | [18] |
| Nicotiana attenuata Torr. ex S.Watson | SLA | Stimulates seed germination | [55] |
| 32 plant species belonging to Apiaceae, Asteracea, Boraginaceae, Caryophyllaceae, Cistaceae, Hypericaceae, Lamiaceae, Malvaceae, apaveracea, Poaceae, Polygonaceae and Rosaceae | Glyceronitrile and smoke/butanolide solution | Seed germination and seedling length are enhanced | [17] |
| Capsicum annuum L. | KAR1 | Stimulates germination and seedling emergence | [56] |
| Daucus carota L. | KAR1 | Positively affects seed germination and plant height | [57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahed, M.; Bączek-Kwinta, R. The Impact of Post-Fire Smoke on Plant Communities: A Global Approach. Plants 2023, 12, 3835. https://doi.org/10.3390/plants12223835
Zahed M, Bączek-Kwinta R. The Impact of Post-Fire Smoke on Plant Communities: A Global Approach. Plants. 2023; 12(22):3835. https://doi.org/10.3390/plants12223835
Chicago/Turabian StyleZahed, Mahboube, and Renata Bączek-Kwinta. 2023. "The Impact of Post-Fire Smoke on Plant Communities: A Global Approach" Plants 12, no. 22: 3835. https://doi.org/10.3390/plants12223835






