Phytotoxicity of Extracts of Argemone mexicana and Crotalaria longirostrata on Tomato Seedling Physiology
Abstract
1. Introduction
2. Results
2.1. Effect of Extracts on Tomato Seed Germination
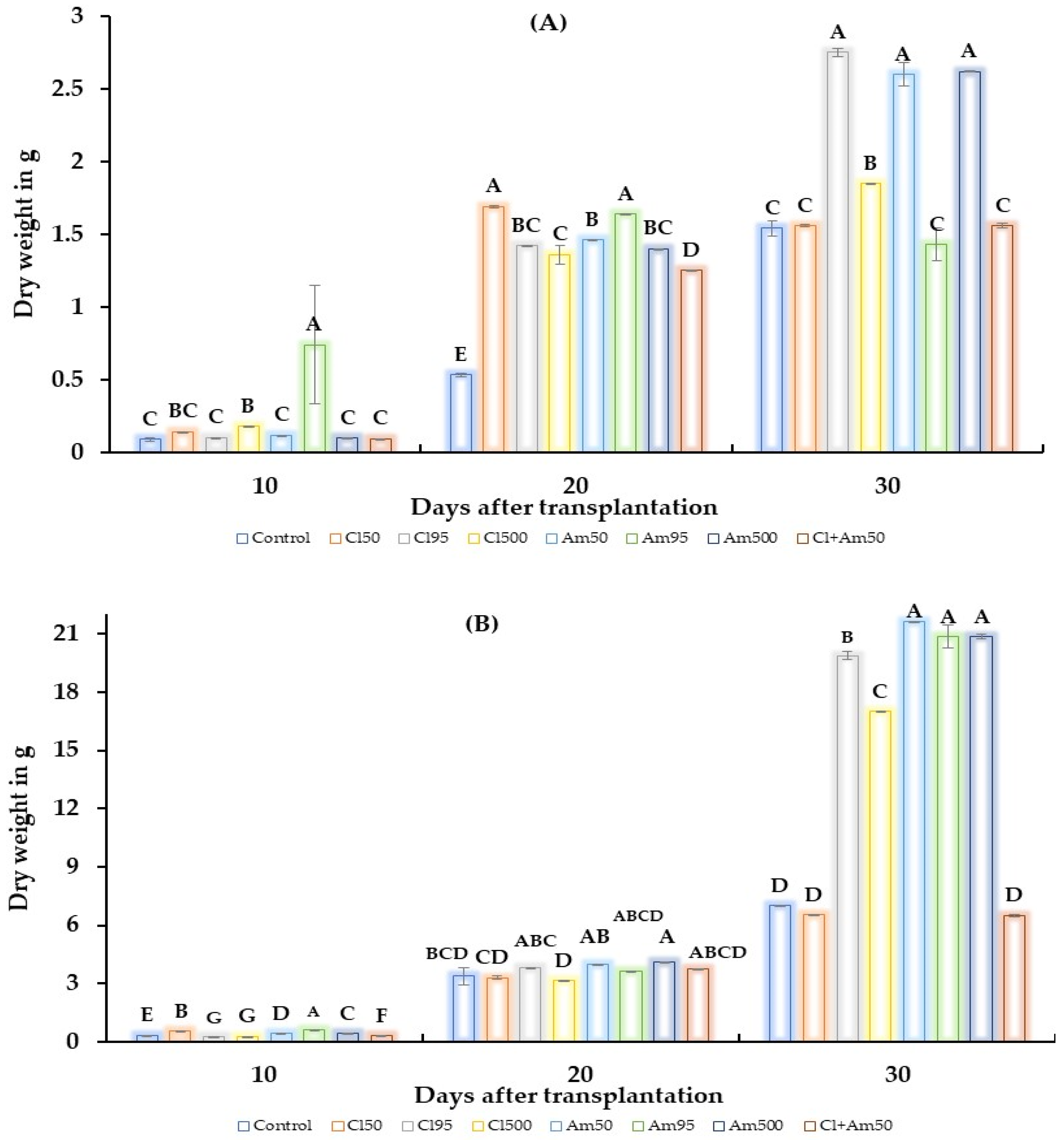
2.2. Greenhouse Test
3. Discussion
4. Materials and Methods
4.1. Sampling of Plants and Preparation of Extracts
4.2. Plant Material
4.3. Effect of Extracts on Tomato Seed Germination
4.4. Greenhouse Assay
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shennan, C.; Krupnik, T.J.; Baird, G.; Cohen, H.; Forbush, K.; Lovell, R.J.; Olimpi, E.M. Organic and conventional agriculture: A useful framing? Annu. Rev. Environ. Resour. 2017, 42, 317–346. [Google Scholar] [CrossRef]
- Gomiero, T. Food quality assessment in organic vs. conventional agricultural produce: Findings and issues. Appl. Soil Ecol. 2018, 123, 714–728. [Google Scholar] [CrossRef]
- Brzozowski, L.; Mazourek, M. A sustainable agricultural future relies on the transition to organic agroecological pest management. Sustainability 2018, 10, 2023. [Google Scholar] [CrossRef]
- Altieri, M.A.; Nicholls, C.I. Agroecology and the reconstruction of a post-COVID-19 agriculture. J. Peasant Stud. 2020, 47, 881–898. [Google Scholar] [CrossRef]
- Jeanneret, P.; Aviron, S.; Alignier, A.; Lavigne, C.; Helfenstein, J.; Herzog, F.; Kay, S.; Petit, S. Agroecology landscapes. Landsc. Ecol. 2021, 36, 2235–2257. [Google Scholar] [CrossRef]
- Khan, S.; Basra, S.M.A.; Nawaz, M.; Hussain, I.; Foidl, N. Combined application of moringa leaf extract and chemical growth-promoters enhances the plant growth and productivity of wheat crop (Triticum aestivum L.). S. Afr. J. Bot. 2020, 129, 74–81. [Google Scholar] [CrossRef]
- Anza Cruz, H.G.; Ramírez González, S.I.; López Báez, O.; Espinoza Zaragoza, S. Fitotoxicidad de extractos vegetales en la germinación de semillas y desarrollo inicial de plantas mono y dicotiledóneas. Espac. I+D Innovación Más Desarro. 2023, 12, 119–140. [Google Scholar] [CrossRef]
- Geilfus, C.-M. Plant secondary compounds. In Controlled Environment Horticulture: Improving Quality of Vegetables and Medicinal Plants; Springer International Publishing: Cham, Switzerland, 2019; pp. 19–33. [Google Scholar] [CrossRef]
- Nxumalo, K.A.; Aremu, A.O.; Fawole, O.A. Potentials of medicinal plant extracts as an alternative to synthetic chemicals in postharvest protection and preservation of horticultural crops: A review. Sustainability 2021, 13, 5897. [Google Scholar] [CrossRef]
- Tembo, Y.; Mkindi, A.G.; Mkenda, P.A.; Mpumi, N.; Mwanauta, R.; Stevenson, P.C.; Ndakidemi, P.A.; Belmain, S.R. Pesticidal plant extracts improve yield and reduce insect pests on legume crops without harming beneficial arthropods. Front. Plant Sci. 2018, 9, 1425. [Google Scholar] [CrossRef]
- Camarillo-Castillo, F.; Mangan, F.X. Biological nitrogen fixation in chipilin (Crotalaria longirostrata Hook. & Arn.), a sustainable nitrogen source for commercial production. Rev. Chapingo Ser. Hortic. 2020, 26, 125–141. [Google Scholar] [CrossRef]
- Lynch, M.J.; Mulvaney, M.J.; Hodges, S.C.; Thompson, T.L.; Thomason, W.E. Decomposition, nitrogen and carbon mineralization from food and cover crop residues in the central plateau of Haiti. Springer Plus 2016, 5, 973. [Google Scholar] [CrossRef] [PubMed]
- Schinzoumka, P.; Jean, A.N.; Valère, T. Effects of Acacia albida and Crotalaria retusa on the Growth and Development of Tomato. J. Agric. Ecol. Res. Int. 2016, 8, 1–9. [Google Scholar] [CrossRef]
- Ogunsusi, M.; Akinlalu, A.O.; Komolafe, I.J.; Oyedapo, O.O. Allelopathic effects of alkaloid fraction of Crotalaria retusa Linn on growth and some biochemical parameters of bean seedlings (Phaseolus vulgaris). Int. J. Plant Physiol. Biochem. 2018, 10, 1–9. [Google Scholar] [CrossRef]
- Andleeb, S.; Alsalme, A.; Al-Zaqri, N.; Warad, I.; Alkahtani, J.; Bukhari, S.M. In-vitro antibacterial and antifungal properties of the organic solvent extract of Argemone mexicana L. J. King Saud Univ. -Sci. 2020, 32, 2053–2058. [Google Scholar] [CrossRef]
- Singh, R.; Chaubey, N.; Mishra, R.K. Evaluation of Anti-Asthmatic Activity of Ethanolic Extract of Argemone mexicana Stems. Saudi J. Med. Pharm. Sci. 2021, 7, 39–44. [Google Scholar] [CrossRef]
- Mistry, J.; Mukhopadhyay, A.P.; Baur, G.N. Status of N P K in vermicompost prepared from two common weed and two medicinal plants. Int. J. Appl. Sci. Biotechnol. 2015, 3, 193–196. [Google Scholar] [CrossRef]
- Miranda-Arámbula, M.; Reyes-Chilpa, R.; Anaya, L.A.L. Phytotoxic activity of aqueous extracts of ruderal plants and its potential application to tomato crop. Bot. Sci. 2021, 99, 487–498. [Google Scholar] [CrossRef]
- Goyal, S.; Lambert, C.; Cluzet, S.; Mérillon, J.M.; Ramawat, K.G. Secondary Metabolites and Plant Defence. In Plant Defence: Biological Control; Mérillon, J., Ramawat, K., Eds.; Progress in Biological Control; Springer: Dordrecht, The Netherlands, 2012; pp. 109–138. [Google Scholar] [CrossRef]
- Tahir, N.A.; Lateef, D.D.; Mustafa, K.M.; Rasul, K.S. Under Natural Field Conditions, Exogenous Application of Moringa Organ Water Extract Enhanced the Growth- and Yield-Related Traits of Barley Accessions. Agriculture 2022, 12, 1502. [Google Scholar] [CrossRef]
- Tahir, N.A.; Qader, K.O.; Azeez, H.A.; Rashid, J.S. Inhibitory allelopathic effects of Moringa oleifera Lamk plant extracts on wheat and Sinapis arvensis L. Allelopath. J. 2018, 44, 35–48. [Google Scholar] [CrossRef]
- Ferrández-Gómez, B.; Jordá, J.D.; Cerdán, M.; Sánchez, A. Valorization of Posidonia oceanica biomass: Role on germination of cucumber and tomato seeds. Waste Manag. 2023, 171, 634–641. [Google Scholar] [CrossRef]
- López-López, H.; Beltrán-Beache, M.; Ochoa-Fuentes, Y.M.; Castro-del Ángel, E.; Cerna-Chávez, E.; Delgado-Ortiz, J.C. Extracto metanólico de Crotalaria longirostrata: Identificación de metabolitos secundarios y su efecto insecticida. Sci. Agropecu. 2022, 13, 71–78. [Google Scholar] [CrossRef]
- Delgado-Ortiz, J.C.; López-López, H.; Beltrán-Beache, M.; Ochoa-Fuentes, Y.M.; Cerna-Chávez, E.; Castro del Ángel, E. Efecto insecticida del extracto metanólico de Argemone mexicana para el control de Bactericera cockerelli (Sulc.) (Hemípteros: Triozidae). Rev. Bio Cienc. 2023, 10, e1404. [Google Scholar] [CrossRef]
- Siddiqui, I.A.; Shaukat, S.S.; Khan, G.H.; Zaki, M.J. Evaluation of Argemone mexicana for control of root-infecting fungi in tomato. J. Phytopathol. 2002, 150, 321–329. [Google Scholar] [CrossRef]
- Khan, A.; Tariq, M.; Ahmad, F.; Mennan, S.; Khan, F.; Asif, M.; Nadeem, H.; Ansari, T.; Shariq, M.; Siddiqui, M.A. Assessment of nematicidal efficacy of chitosan in combination with botanicals against Meloidogyne incognita on carrot. Acta Agric. Scand. Sect. B Soil Plant Sci. 2021, 71, 225–236. [Google Scholar] [CrossRef]
- Jourand, P.; Rapior, S.; Fargette, M.; Mateille, T. Nematostatic effects of a leaf extract from Crotalaria virgulata subsp. grantiana on Meloidogyne incognita and its use to protect tomato roots. Nematology 2004, 6, 79–84. [Google Scholar] [CrossRef]
- Lewerenz, L.; Abouzeid, S.; Yahyazadeh, M.; Hijazin, T.; Selmar, D. Novel cognitions in allelopathy: Implications from the “horizontal natural product transfer”. Plants 2022, 11, 3264. [Google Scholar] [CrossRef]
- Skinner, E.M.; Díaz-Pérez, J.C.; Phatak, S.C.; Schomberg, H.H.; Vencill, W. Allelopathic effects of sunnhemp (Crotalaria juncea L.) on germination of vegetables and weeds. HortScience 2012, 47, 138–142. [Google Scholar] [CrossRef]
- Samuel, P.N.K.J.; Kumar, R.S.A.S. Antioxidant, antimicrobial, haemolytic, germination and growth promoting properties of Crotalaria juncea L. Plant Sci. Today 2020, 7, 201–205. [Google Scholar] [CrossRef]
- da Cruz-Silva, C.T.A.; Matiazzo, E.B.; Pacheco, F.; Nóbrega, L.H.P. Allelopathy of Crotalaria juncea L. aqueous extracts on germination and initial development of maize. Idesia 2015, 33, 27–32. Available online: https://www.scielo.cl/pdf/idesia/v33n1/art03.pdf (accessed on 12 March 2023). [CrossRef]
- Alagesaboopathi, C. Allelopathic effect of different concentration of water extract of Argemone mexicana L. on seed germination and seedling growth of Sorghum bicolor (L.) Moench. J. Pharm. Biol. Sci. 2013, 5, 52–55. [Google Scholar] [CrossRef]
- Namkeleja, H.S.; Tarimo, M.T.; Ndakidemi, P.A. Allelopathic effect of aqueous extract of Argemone mexicana L. on germination and growth of Brachiaria dictyoneura L. and Clitoria ternatea L. Am. J. Plant Sci. 2013, 4, 2138–2147. [Google Scholar] [CrossRef]
- Ohdan, H.; Daimon, H.; Mimoto, H. Evaluation of allelopathy in Crotalaria plants by the growth pouch method. J. Crop Sci. Soc. Jpn. 1995, 64, 644–649. [Google Scholar] [CrossRef][Green Version]
- Bundit, A.; Ostlie, M.; Prom-U-Thai, C. Sunn hemp (Crotalaria juncea) weed suppression and allelopathy at different timings. Biocontrol Sci. Technol. 2021, 31, 694–704. [Google Scholar] [CrossRef]
- Zhao, X.; Joo, J.C.; Kim, D.; Lee, J.K.; Kim, J.Y. Estimation of the seedling vigor index of sunflowers treated with various heavy metals. J. Bioremediat. Biodegrad. 2016, 7, 353. [Google Scholar] [CrossRef]
- Wen, D.; Hou, H.; Meng, A.; Meng, J.; Xie, L.; Zhang, C. Rapid evaluation of seed vigor by the absolute content of protein in seed within the same crop. Sci. Rep. 2018, 8, 5569. [Google Scholar] [CrossRef]
- Sangakkara, U.R.; Liedgens, M.; Soldati, A.; Stamp, P. Root and shoot growth of maize (Zea mays) as affected by incorporation of Crotalaria juncea and Tithonia diversifolia as green manures. J. Agron. Crop Sci. 2004, 190, 339–346. [Google Scholar] [CrossRef]
- Subaedah, S.; Aladin, A. Fertilization of nitrogen, phosphor, and application of green manure of Crotalaria juncea in increasing yield of maize in marginal dry land. Agric. Agric. Sci. Procedia 2016, 9, 20–25. [Google Scholar] [CrossRef][Green Version]
- Islam, M.M.; Urmi, T.A.; Rana, M.S.; Alam, M.S.; Haque, M.M. Green manuring effects on crop morpho-physiological characters, rice yield and soil properties. Physiol. Mol. Biol. Plants 2019, 25, 303–312. [Google Scholar] [CrossRef]
- Bonaventure, G.; Salas, J.J.; Pollard, M.R.; Ohlrogge, J.B. Disruption of the FATB gene in Arabidopsis demonstrates an essential role of saturated fatty acids in plant growth. Plant Cell 2003, 15, 1020–1033. [Google Scholar] [CrossRef]
- Liu, S.; Ruan, W.; Li, J.; Xu, H.; Wang, J.; Gao, Y.; Wang, J. Biological control of phytopathogenic fungi by fatty acids. Mycopathologia 2008, 166, 93–102. [Google Scholar] [CrossRef]
- Zhang, Y.; Fernie, A.R. On the role of the tricarboxylic acid cycle in plant productivity. J. Integr. Plant Biol. 2018, 60, 1199–1216. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.P.; Chen, Z.H.; Famiani, F. Gluconeogenesis in plants: A key interface between organic acid/amino acid/lipid and sugar metabolism. Molecules 2021, 26, 5129. [Google Scholar] [CrossRef] [PubMed]
- Kachroo, A.; Kachroo, P. Fatty acid-derived signals in plant defense. Annu. Rev. Phytopathol. 2009, 47, 153–176. [Google Scholar] [CrossRef]
- Jang, G.; Yoon, Y.; Choi, Y.D. Crosstalk with jasmonic acid integrates multiple responses in plant development. Int. J. Mol. Sci. 2020, 21, 305. [Google Scholar] [CrossRef] [PubMed]
- Bhambhani, S.; Kondhare, K.R.; Giri, A.P. Diversity in chemical structures and biological properties of plant alkaloids. Molecules 2021, 26, 3374. [Google Scholar] [CrossRef]
- Dey, P.; Kundu, A.; Kumar, A.; Gupta, M.; Lee, B.M.; Bhakta, T.; Dash, S.; Kim, H.S. Analysis of alkaloids (indole alkaloids, isoquinoline alkaloids, tropane alkaloids). Recent Adv. Nat. Prod. Anal. 2020, 5, 505–567. [Google Scholar] [CrossRef]
- Agathokleous, E.; Calabrese, E.J. Hormesis: The dose response for the 21st century: The future has arrived. Toxicology 2019, 425, 152249. [Google Scholar] [CrossRef]
- Jalal, A.; de Oliveira-Junior, J.C.; Ribeiro, J.S.; Fernandes, G.C.; Mariano, G.G.; Trindade, V.D.R.; Dos-Reis, A.R. Hormesis in plants: Physiological and biochemical responses. Ecotoxicol. Environ. Saf. 2021, 207, 111225. [Google Scholar] [CrossRef]
- Erofeeva, E.A. Hormesis in plants: Its common occurrence across stresses. Curr. Opin. Toxicol. 2022, 30, 100333. [Google Scholar] [CrossRef]
- Parejo-Farnés, C.; Aparicio, A.; Albaladejo, R.G. Una aproximación a la ecología epigenética en plantas. Ecosistemas 2019, 28, 69–74. [Google Scholar] [CrossRef]
- Moustakas, M.; Moustaka, J.; Sperdouli, I. Hormesis in photosystem II: A mechanistic understanding. Curr. Opin. Toxicol. 2022, 29, 57–64. [Google Scholar] [CrossRef]
- Agathokleous, E.; Feng, Z.; Peñuelas, J. Chlorophyll hormesis: Are chlorophylls major components of stress biology in higher plants? Sci. Total Environ. 2020, 726, 138637. [Google Scholar] [CrossRef]
- Abbas, T.; Nadeem, M.A.; Tanveer, A.; Chauhan, B.S. Can hormesis of plant-released phytotoxins be used to boost and sustain crop production? Crop Prot. 2017, 93, 69–76. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Blain, R.B. Hormesis and plant biology. Environ. Pollut. 2009, 157, 42–48. [Google Scholar] [CrossRef]
- Perveen, S.; Mushtaq, M.N.; Yousaf, M.; Sarwar, N. Allelopathic hormesis and potent allelochemicals from multipurpose tree Moringa oleifera leaf extract. Plant Biosyst. -Int. J. Deal. All Asp. Plant Biol. 2021, 155, 154–158. [Google Scholar] [CrossRef]
- Belz, R.G.; Duke, S.O. Modelling biphasic hormetic dose responses to predict sub-NOAEL effects using plant biology as an example. Curr. Opin. Toxicol. 2022, 29, 36–42. [Google Scholar] [CrossRef]
- Miranda-Granados, J.; Chacón, C.; Ruiz-Lau, N.; Vargas-Díaz, M.E.; Zepeda, L.G.; Alvarez-Gutiérrez, P.; Meza-Gordillo, R.; Lagunas-Rivera, S. Alternative use of extracts of Chipilín leaves (Crotalaria longirostrata Hook. & Arn) as antimicrobial. Sustainability 2018, 10, 883. [Google Scholar] [CrossRef]
- Bobi, A.H.; Bandiya, M.H.; Suleiman, M.; Usman, M. Evaluation of Insecticidal Efficacy of Some Selected Plants Leaf- Ethanol Extracts against Musca domestica L. [Diptera: Muscidae]. Entomol. Appl. Sci. Lett. 2015, 2, 23–28. Available online: https://easletters.com/article/zxkd-evaluation-of-insecticidal-efficacy-of-some-selected-plants-leaf-ethanol-extracts-against-musca-domestica-l-diptera-muscidae (accessed on 23 April 2023).
- Ray, J.; Bordolui, S.K. Effect of seed priming as pre-treatment factors on germination and seedling vigour of tomato. Int. J. Plant Soil Sci. 2022, 34, 302–311. [Google Scholar] [CrossRef]
- Steiner, A.A. A universal method for preparing nutrient solutions of a certain desired composition. Plant Soil 1961, 15, 134–154. [Google Scholar] [CrossRef]
- López-López, H.; Ruiz-Lau, N.; Meza-Gordillo, R.; Ruiz-Valdiviezo, V.M.; Robledo-Luchetti, J.G.; Lecona-Guzmán, C.A.; Villalobos-Maldonado, J.J.; Dendooven, L.; Montes-Molina, J.A. Antifungal potential of Beauveria bassiana on Solanum lycopersicum L. infected with Fusarium oxysporum f. sp. lycopersici. Phyton-Int. J. Exp. Bot. 2023, 92, 1235–1255. [Google Scholar] [CrossRef]

| Treatments | % Germination |
|---|---|
| Control | 88.9 ± 3.81 bc |
| Clong50 | 91.1 ± 3.81 ab |
| Clong95 | 86.7 ± 0.15 bc |
| Clong500 | 80.0 ± 6.70 c |
| Amex50 | 86.7 ± 0.10 bc |
| Amex95 | 86.7 ± 0.15 bc |
| Amex500 | 84.47 ± 3.87 bc |
| Cl50 + Am50 | 100 ± 0 a |
| p-value | 0.0001 |
| Treatments | Height (cm) Days after Transplantation | ||
|---|---|---|---|
| 10 | 20 | 30 | |
| Control | 11.25 ± 1.75 a | 18.83 ± 1.44 ab | 22.30 ± 0.96 b |
| Clong50 | 10.08 ± 1.32 ab | 19.80 ± 2.02 a | 23.67 ± 1.26 ab |
| Clong95 | 8.42 ± 0.86 b | 18.67 ± 0.58 ab | 22.60 ± 0.36 ab |
| Clong500 | 9.70 ± 1.64 ab | 20.00 ± 1.73 a | 25.73 ± 2.16 a |
| Amex50 | 9.75 ± 1.69 ab | 19.16 ± 1.62 ab | 25.30 ± 0.87 ab |
| Amex95 | 11.08 ± 0.58 a | 20.17 ± 1.44 a | 24.40 ± 0.53 ab |
| Amex500 | 10.27 ± 0.98 ab | 17.83 ± 0.29 ab | 24.60 ± 0.36 ab |
| Cl50 + Am50 | 9.08 ± 0.66 ab | 15.70 ± 0.17 b | 25.63 ± 1.56 a |
| p-value | 0.0066 | 0.0162 | 0.0122 |
| Treatments | Plant Vigor Index Days after Transplantation | ||
|---|---|---|---|
| 10 | 20 | 30 | |
| Control | 996.7 ± 114.7 a | 1672.2 ± 108.6 a | 1979.8 ± 13.1 b |
| Clong50 | 918.3 ± 74.6 abc | 1812.2 ± 255.9 a | 2156.7 ± 156.2 ab |
| Clong95 | 729.4 ± 62.6 c | 1617.8 ± 50.1 a | 1958.7 ± 31.2 b |
| Clong500 | 774.0 ± 87.7 bc | 1606.7 ± 257.9 a | 2068.6 ± 345.2 b |
| Amex50 | 845.0 ± 86.7 abc | 1652.4 ± 140.1 a | 2192.6 ± 75.1 ab |
| Amex95 | 960.6 ± 33.1 ab | 1747.8 ± 125.1 a | 2114.6 ± 45.9 b |
| Amex500 | 867.2 ± 69.1 abc | 1505.6 ± 60.8 a | 2076.4 ± 66.8 b |
| Cl50 + Am50 | 908.3 ± 38.2 abc | 1570.0 ± 17.3 a | 2563.3 ± 155.7 a |
| p-value | 0.007 | 0.034 | 0.005 |
| Treatments | Root Length (mm) Days after Transplantation | ||
|---|---|---|---|
| 10 | 20 | 30 | |
| Control | 6.7 ± 0.35 c | 15.2 ± 1.63 b | 24.1 ± 0.10 b |
| Clong50 | 9.2 ± 0.49 ab | 15.5 ± 0.23 ab | 26.5 ±2.12 ab |
| Clong95 | 7.7 ± 0.35 bc | 15.0 ± 0.52 b | 26.7 ± 1.06 ab |
| Clong500 | 9.0 ± 0.71 ab | 17.6 ± 0.31 a | 28.6 ± 0.49 ab |
| Amex50 | 9.9 ± 0.85 ab | 14.5 ± 0.10 b | 24.7 ± 0.35 ab |
| Amex95 | 10.7 ± 0.35 a | 17.5 ± 0.20 a | 29.5 ± 2.12 a |
| Amex500 | 8.0 ± 0.71 bc | 16.5 ± 0.10 ab | 29.3 ± 0.92 a |
| Cl50 + Am50 | 8.3 ± 0.35 bc | 15.0 ± 0.24 b | 25.0 ± 1.41 ab |
| p-value | 0.0018 | 0.0037 | 0.0136 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López, H.L.; Beltrán Beache, M.; Ochoa Fuentes, Y.M.; Cerna Chavez, E.; Ángel, E.C.d.; Delgado Ortiz, J.C. Phytotoxicity of Extracts of Argemone mexicana and Crotalaria longirostrata on Tomato Seedling Physiology. Plants 2023, 12, 3856. https://doi.org/10.3390/plants12223856
López HL, Beltrán Beache M, Ochoa Fuentes YM, Cerna Chavez E, Ángel ECd, Delgado Ortiz JC. Phytotoxicity of Extracts of Argemone mexicana and Crotalaria longirostrata on Tomato Seedling Physiology. Plants. 2023; 12(22):3856. https://doi.org/10.3390/plants12223856
Chicago/Turabian StyleLópez, Henry López, Mariana Beltrán Beache, Yisa María Ochoa Fuentes, Ernesto Cerna Chavez, Epifanio Castro del Ángel, and Juan Carlos Delgado Ortiz. 2023. "Phytotoxicity of Extracts of Argemone mexicana and Crotalaria longirostrata on Tomato Seedling Physiology" Plants 12, no. 22: 3856. https://doi.org/10.3390/plants12223856
APA StyleLópez, H. L., Beltrán Beache, M., Ochoa Fuentes, Y. M., Cerna Chavez, E., Ángel, E. C. d., & Delgado Ortiz, J. C. (2023). Phytotoxicity of Extracts of Argemone mexicana and Crotalaria longirostrata on Tomato Seedling Physiology. Plants, 12(22), 3856. https://doi.org/10.3390/plants12223856