Therapeutics to Treat Psychiatric and Neurological Disorders: A Promising Perspective from Algerian Traditional Medicine
Abstract
:1. Introduction
2. Medicinal Plants Used for Mental Illnesses
3. Traditional Treatment of Mental Disorders in Algeria
4. Results
4.1. Medicinal Plant Diversity
4.2. Most Frequently Cited Plant Species
4.3. Used Parts
4.4. Method of Preparation
4.5. Pharmacognostic Investigations on the CNS System
4.6. In Vitro and In Vivo Pharmacological Evidence


4.7. Clinical Trials and Therapeutic Applications
4.8. Toxicological Evidence
5. Materials and Methods
6. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quezel, P.; Santa, S. New Flora of Algeria and Southern Desert Regions; Centre National de la Recherche Scientifique: Paris, France, 1962. [Google Scholar]
- Ozenda, P. Flore du Sahara Septentrional et Central; Centre National de la Recherche Scientifique: Paris, France, 1958. [Google Scholar]
- Benchelah, A.-C.; Bouziane, H.; Maka, M. Fleurs du Sahara, arbres et arbustes, voyage au coeur de leurs usages avec les Touaregs du Tassili. Phytothérapie 2004, 2, 191–197. [Google Scholar] [CrossRef]
- Bellakhdar, J. Plantes Médicinales au Maghreb et Soins de Base. Précis de Phytothérapie Moderne; Editions le Fennec: Casablanca, Morocco, 2006; 386p. [Google Scholar]
- Baba Aissa, F. Encyclopédie des Plantes Utiles; EDAS: Leonia, NJ, USA, 1999. [Google Scholar]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.T. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Amrani, S.; Seaward, M.R.; Sipman, H.J.; Feuerer, T. Lichenological exploration of Algeria II: Checklist of lichenized, lichenicolous and allied fungi. Herzogia 2018, 31, 817–892. [Google Scholar] [CrossRef]
- Cercleux, A.-L.; Comãnescu, L.; Costachie, S.; Demeter, T.; Dobre, R.; Grecu, F.; Hachemi, K.; Cristian, I.; Kanapyanov, T.; Mihai, B. Cinq Continents Revue Roumaine de Géographie. 2018, Volume 17, pp. 2247–2290. Available online: http://cinqcontinents.geo.unibuc.ro/ (accessed on 5 November 2023).
- Tahri, D.; Elhouiti, F.; Ouinten, M.; Yousfi, M. Historical perspective of Algerian pharmacological knowledge. Adv. Tradit. Med. 2020, 20, 279–290. [Google Scholar] [CrossRef]
- Yoeli-Tlalim, R. Reorienting Histories of Medicine: Encounters Along the Silk Roads; Bloomsbury Publishing: London, UK, 2021. [Google Scholar]
- Bustinza, V.P.d. How Early Islamic Science Advanced Medicine. National Geographic HISTORY. 2016. Available online: https://www.nationalgeographic.com/history/history-magazine/article/muslim-medicine-scientific-discovery-islam (accessed on 23 October 2023).
- Al-Khalili, J. The greatest scientific advances from the Muslim world. The Guardian. 2010. Available online: https://www.theguardian.com/science/2010/feb/01/islamic-science (accessed on 23 October 2023).
- Abdekhoda, M.; Ranjbaran, F. The Holy Quran and Treatment of Mental and Physical Diseases. Pastor. Psychol. 2022, 71, 423–435. [Google Scholar] [CrossRef]
- Awaad, R.; Elsayed, D.; Helal, H. Holistic healing: Islam’s legacy of mental health. Yaqeen Inst. Islam. Res. 2021. Available online: https://yaqeeninstitute.ca/read/paper/holistic-healing-islams-legacy-ofmental-health. (accessed on 23 October 2023).
- AlRawi, S.N.; Khidir, A.; Elnashar, M.S.; Abdelrahim, H.A.; Killawi, A.K.; Hammoud, M.M.; Fetters, M.D. Traditional Arabic & Islamic medicine: Validation and empirical assessment of a conceptual model in Qatar. BMC Complement. Altern. Med. 2017, 17, 1–10. [Google Scholar]
- Available online: https://www.ixl.com/social-studies/grade-7/the-ancient-silk-road-geography-and-transportation (accessed on 5 November 2023).
- Feigin, V.L.; Nichols, E.; Alam, T.; Bannick, M.S.; Beghi, E.; Blake, N.; Culpepper, W.J.; Dorsey, E.R.; Elbaz, A.; Ellenbogen, R.G. Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef]
- WHO. Mental Disorders. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/mental-disorders (accessed on 23 October 2023).
- Ding, C.; Wu, Y.; Chen, X.; Chen, Y.; Wu, Z.; Lin, Z.; Kang, D.; Fang, W.; Chen, F. Global, regional, and national burden and attributable risk factors of neurological disorders: The Global Burden of Disease study 1990–2019. Front. Public Health 2022, 10, 952161. [Google Scholar] [CrossRef]
- Metzner, R. Hallucinogenic drugs and plants in psychotherapy and shamanism. J. Psychoact. Drugs 1998, 30, 333–341. [Google Scholar] [CrossRef]
- Moore, J.; Martin, E. Trial of reserpine in treatment of schizophrenia. Br. Med. J. 1957, 1, 8. [Google Scholar] [CrossRef] [PubMed]
- Lobay, D. Rauwolfia in the treatment of hypertension. Integr. Med. A Clin. J. 2015, 14, 40. [Google Scholar]
- Prisinzano, T. Natural products as tools for neuroscience: Discovery and development of novel agents to treat drug abuse. J. Nat. Prod. 2009, 72, 581–587. [Google Scholar] [CrossRef]
- Blaylock, R.L.; Maroon, J. Natural plant products and extracts that reduce immunoexcitotoxicity-associated neurodegeneration and promote repair within the central nervous system. Surg. Neurol. Int. 2012, 3, 19. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.T.; Nguyen, T.H. Natural product for the treatment of Alzheimer’s disease. J. Basic Clin. Physiol. Pharmacol. 2017, 28, 413–423. [Google Scholar] [CrossRef]
- Hritcu, L.; Ionita, R.; Postu, P.A.; Gupta, G.K.; Turkez, H.; Lima, T.C.; Carvalho, C.U.S.; de Sousa, D.P. Antidepressant Flavonoids and Their Relationship with Oxidative Stress. Oxidat. Med. Cell. Longev. 2017, 2017, 5762172. [Google Scholar] [CrossRef]
- Oliveira, A.I.; Pinho, C.; Sarmento, B.; Dias, A.C. Neuroprotective activity of Hypericum perforatum and its major components. Front. Plant Sci. 2016, 7, 1004. [Google Scholar] [CrossRef]
- Taqui, R.; Debnath, M.; Ahmed, S.; Ghosh, A. Advances on plant extracts and phytocompounds with acetylcholinesterase inhibition activity for possible treatment of Alzheimer’s disease. Phytomed. Plus 2022, 2, 100184. [Google Scholar] [CrossRef]
- WHO. Mental Health Atlas 2020 Country Profile: Algeria. Available online: https://www.who.int/publications/m/item/mental-health-atlas-dza-2020-country-profile (accessed on 23 October 2023).
- Kacha, F. La psychiatrie en Algérie. l’Inf. Psychiatr. 2005, 81, 145–148. [Google Scholar]
- Iserin, P.; Masson, M.; Restellini, J.-P. LAROUSSE des Plantes Médicinales; Larousse: Paris, France, 2007. [Google Scholar]
- Al-Issa, I. psychiatry in Algeria. Psychiatr. Bull. 1989, 13, 240–245. [Google Scholar] [CrossRef]
- Dein, S. Traditional Healers and Global Mental Health. In Innovations in Global Mental Health; Springer: Cham, Switzerland, 2021; pp. 807–817. [Google Scholar]
- Bouasla, A.; Bouasla, I. Ethnobotanical survey of medicinal plants in northeastern of Algeria. Phytomedicine 2017, 36, 68–81. [Google Scholar] [CrossRef]
- Hassaïne, S.; Benmalek, S. Medicinal plants traditionally used in the Algerian Sahara: An ethnobotanical study. Vegetos 2023, 36, 400–426. [Google Scholar] [CrossRef]
- Belhouala, K.; Benarba, B. Medicinal plants used by traditional healers in Algeria: A multiregional ethnobotanical study. Front. Pharmacol. 2021, 12, 760492. [Google Scholar] [CrossRef] [PubMed]
- Mann, J. Magic, Murder and Medicine; Oxford University Press: Oxford, UK, 1994. [Google Scholar]
- Mulas, M. Traditional uses of Labiatae in the Mediterranean area. Acta Hortic. 2006, 723, 25–32. [Google Scholar] [CrossRef]
- Cornara, L.; La Rocca, A.; Marsili, S.; Mariotti, M. Traditional uses of plants in the Eastern Riviera (Liguria, Italy). J. Ethnopharmacol. 2009, 125, 16–30. [Google Scholar] [CrossRef]
- EAGL. Related Expert Assessment Group: Maghreb Algeria. Available online: https://iucngreenlist.org/country/algeria/ (accessed on 24 October 2023).
- Debbache-Benaida, N.; Berboucha, M.; Ayouni, K.; Atmani, D.; Nassima, C.; Boudaoud, H.; Djebli, N.; Atmani, D. Anti-hyperuricemic and neuroprotective effects of Populus nigra L. (Saliacaceae) flower buds used in Algerian folk medicine. J. Pharm. Pharmacogn. Res. 2018, 6, 471–482. [Google Scholar]
- Do Rego, J.-C.; Benkiki, N.; Chosson, E.; Kabouche, Z.; Seguin, E.; Costentin, J. Antidepressant-like effect of hyperfoliatin, a polyisoprenylated phloroglucinol derivative from Hypericum perfoliatum (Clusiaceae) is associated with an inhibition of neuronal monoamines uptake. Eur. J. Pharmacol. 2007, 569, 197–203. [Google Scholar] [CrossRef]
- Larit, F.; Elokely, K.M.; Chaurasiya, N.D.; Benyahia, S.; Nael, M.A.; León, F.; Abu-Darwish, M.S.; Efferth, T.; Wang, Y.-H.; Belouahem-Abed, D. Inhibition of human monoamine oxidase A and B by flavonoids isolated from two Algerian medicinal plants. Phytomedicine 2018, 40, 27–36. [Google Scholar] [CrossRef]
- Sakhri, F.Z.; Adachi, N.; Zerizer, S.; Ohashi, Y.; Ikemoto, H.; Tsukada, M.; Kabouche, Z.; Hisamitsu, T.; Sunagawa, M. Behavioral and neurological improvement by Cydonia oblonga fruit extract in chronic immobilization stress rats. Phytother. Res. 2021, 35, 2074–2084. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. Constituents and pharmacology of Narcissus tazetta. IOSR J. Pharm. 2020, 10, 44–53. [Google Scholar]
- Choukry, K.T. Neuf Espèces Végétales Anti-Epileptiques de la Flore d’Algérie. Alger. J. Nat. Prod. 2019, 7, 706–713. [Google Scholar]
- López, S.; Bastida, J.; Viladomat, F.; Codina, C. Acetylcholinesterase inhibitory activity of some Amaryllidaceae alkaloids and Narcissus extracts. Life Sci. 2002, 71, 2521–2529. [Google Scholar] [CrossRef]
- Azib, L.; Debbache-Benaida, N.; Da Costa, G.; Atmani-Kilani, D.; Saidene, N.; Ayouni, K.; Richard, T.; Atmani, D. Pistacia lentiscus L. leaves extract and its major phenolic compounds reverse aluminium-induced neurotoxicity in mice. Ind. Crops Prod. 2019, 137, 576–584. [Google Scholar] [CrossRef]
- Taïbi, K.; Abderrahim, L.A.; Ferhat, K.; Betta, S.; Taïbi, F.; Bouraada, F.; Boussaid, M. Ethnopharmacological study of natural products used for traditional cancer therapy in Algeria. Saudi Pharm. J. 2020, 28, 1451–1465. [Google Scholar] [CrossRef]
- Hammiche, V.; Merad, R.; Azzouz, M.; Goetz, P. Plantes Toxiques à Usage Médicinal du Pourtour Méditerranéen; Springer: Paris, France, 2013. [Google Scholar]
- Küpeli Akkol, E.; Tatlı Çankaya, I.; Şeker Karatoprak, G.; Carpar, E.; Sobarzo-Sánchez, E.; Capasso, R. Natural compounds as medical strategies in the prevention and treatment of psychiatric disorders seen in neurological diseases. Front. Pharmacol. 2021, 12, 669638. [Google Scholar] [CrossRef]
- Tagliapietra, S.; Aragno, M.; Ugazio, G.; Nano, G. Experimental studies on the toxicity of some compounds isolated from Ferula communis in the rat. Res. Commun. Chem. Pathol. Pharmacol. 1989, 66, 333–336. [Google Scholar] [PubMed]
- Mahendra, P.; Bisht, S. Ferula asafoetida: Traditional uses and pharmacological activity. Pharmacogn. Rev. 2012, 6, 141. [Google Scholar] [CrossRef]
- Chevallier, A. The Encyclopedia of Medicinal Plants; Dorling Kindersley Limited: London, UK, 2001. [Google Scholar]
- Motti, R.; de Falco, B. Traditional herbal remedies used for managing anxiety and insomnia in Italy: An ethnopharmacological overview. Horticulturae 2021, 7, 523. [Google Scholar] [CrossRef]
- Elyebdri, N.; Boumediou, A.; Addoun, S. Ethnobotanical study on the usage of toxic plants in traditional medicine in the city center of Tlemcen, Algeria. Int. J. Pharmacol. Pharm. Sci. 2017, 11, 642–646. [Google Scholar]
- Boudjelal, A.; Henchiri, C.; Sari, M.; Sarri, D.; Hendel, N.; Benkhaled, A.; Ruberto, G. Herbalists and wild medicinal plants in M’Sila (North Algeria): An ethnopharmacology survey. J. Ethnopharmacol. 2013, 148, 395–402. [Google Scholar] [CrossRef]
- Desai, C. Meyler’s side effects of drugs: The international encyclopedia of adverse drug reactions and interactions. Indian J. Pharmacol. 2016, 48, 224. [Google Scholar]
- Bellakhdar, J. Contribution à l’Étude de la Pharmacopée Traditionnelle au Maroc: La Situation Actuelle, les Produits, les Sources du Savoir (Enquête Ethnopharmacologique de Terrain Réalisée de 1969 à 1992); Université Paul Verlaine-Metz: Metz, France, 1997. [Google Scholar]
- Lefranc, M.E. Étude Botanique, Chimique et Toxicologique sur l’Atractylis Gummifera. Bull. Soc. Bot. Fr. 1866, 13, 146–157. [Google Scholar] [CrossRef]
- Bouabid, K.; Lamchouri, F.; Toufik, H.; Faouzi, M.E.A. Phytochemical investigation, in vitro and in vivo antioxidant properties of aqueous and organic extracts of toxic plant: Atractylis gummifera L. J. Ethnopharmacol. 2020, 253, 112640. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, M.; El-Hagrassi, A.M.; Osman, A.F.; Soltan, M.M. Bioactive compounds from Matricaria chamomilla: Structure identification, in vitro antiproliferative, antimigratory, antiangiogenic, and antiadenoviral activities. Z. Für Nat. C 2022, 77, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Hamel, T.; Sadou, S.; Seridi, R.; Boukhdir, S.; Boulemtafes, A. Pratique traditionnelle d’utilisation des plantes médicinales dans la population de la péninsule de l’edough (nord-est algérien). Ethnopharmacologia 2018, 59, 65–70. [Google Scholar]
- Baziz, K.; Maougal, R.A.A. An ethnobotanical survey of spontaneous plants used in traditional medicine in the region of Aures, Algeria. Eur. J. Ecol. 2021, 6, 14669. [Google Scholar] [CrossRef]
- Meddour, R.; Sahar, O.; Ouyessad, M. Ethnobotanical survey on medicinal plants in the Djurdjura National Park and its influence area, Algeria. Ethnobot. Res. Appl. 2020, 20, 1–25. [Google Scholar]
- Tavares, L.; McDougall, G.J.; Fortalezas, S.; Stewart, D.; Ferreira, R.B.; Santos, C.N. The neuroprotective potential of phenolic-enriched fractions from four Juniperus species found in Portugal. Food Chem. 2012, 135, 562–570. [Google Scholar] [CrossRef]
- Dabaghzadeh, F.; Sharififar, F.; Ahmadzadeh, A.-M.; Karami-Mohajeri, S. The effects of Berberis vulgaris L. root extract on the opiate withdrawal syndrome and psychological factors: A randomized double-blind clinical trial. J. Basic Clin. Physiol. Pharmacol. 2021, 34, 465–472. [Google Scholar] [CrossRef]
- Imenshahidi, M.; Qaredashi, R.; Hashemzaei, M.; Hosseinzadeh, H. Inhibitory effect of Berberis vulgaris aqueous extract on acquisition and reinstatement effects of morphine in conditioned place preferences (CPP) in mice. Jundishapur J. Nat. Pharm. Prod. 2014, 9, e16145. [Google Scholar] [CrossRef]
- Meliani, N.; Dib, M.E.A.; Allali, H.; Tabti, B. Hypoglycaemic effect of Berberis vulgaris L. in normal and streptozotocin-induced diabetic rats. Asian Pac. J. Trop. Biomed. 2011, 1, 468–471. [Google Scholar] [CrossRef] [PubMed]
- Rahimi-Madiseh, M.; Lorigoini, Z.; Zamani-Gharaghoshi, H.; Rafieian-Kopaei, M. Berberis vulgaris: Specifications and traditional uses. Iran. J. Basic Med. Sci. 2017, 20, 569. [Google Scholar]
- Merzouki, A.; Ed-Derfoufi, F.; Mesa, J.M. Contribution to the knowledge of Rifian traditional medicine. II: Folk medicine in Ksar Lakbir district (NW Morocco). Fitoterapia 2000, 71, 278–307. [Google Scholar] [CrossRef] [PubMed]
- Ouelbani, R.; Bensari, S.; Mouas, T.N.; Khelifi, D. Ethnobotanical investigations on plants used in folk medicine in the regions of Constantine and Mila (North-East of Algeria). J. Ethnopharmacol. 2016, 194, 196–218. [Google Scholar] [CrossRef] [PubMed]
- Azzi, R.; Djaziri, R.; Lahfa, F.; Sekkal, F.Z.; Benmehdi, H.; Belkacem, N. Ethnopharmacological survey of medicinal plants used in the traditional treatment of diabetes mellitus in the North Western and South Western Algeria. J. Med. Plants Res. 2012, 6, 2041–2050. [Google Scholar]
- Madia, V.N.; De Angelis, M.; De Vita, D.; Messore, A.; De Leo, A.; Ialongo, D.; Tudino, V.; Saccoliti, F.; De Chiara, G.; Garzoli, S. Investigation of Commiphora myrrha (Nees) Engl. oil and its main components for antiviral activity. Pharmaceuticals 2021, 14, 243. [Google Scholar] [CrossRef]
- Akkol, E.K.; Ilhan, M.; Karpuz, B.; Genç, Y.; Sobarzo-Sánchez, E. Sedative and anxiolytic activities of Opuntia ficus indica (L.) Mill.: An experimental assessment in mice. Molecules 2020, 25, 1844. [Google Scholar] [CrossRef]
- Sharma, V.; Katiyar, A.; Agrawal, R. Glycyrrhiza glabra: Chemistry and pharmacological activity. In Sweeteners; Springer: Cham, Switzerland, 2016; pp. 1–14. [Google Scholar]
- Lemus-Mondaca, R.; Marin, J.; Rivas, J.; Sanhueza, L.; Soto, Y.; Vera, N.; Puente-Díaz, L. Pumpkin seeds (Cucurbita maxima). A review of functional attributes and by-products. Rev. Chil. Nutr 2019, 46, 783–791. [Google Scholar] [CrossRef]
- Benítez, G.; González-Tejero, M.; Molero-Mesa, J. Pharmaceutical ethnobotany in the western part of Granada province (southern Spain): Ethnopharmacological synthesis. J. Ethnopharmacol. 2010, 129, 87–105. [Google Scholar] [CrossRef]
- Pistelli, L.; D’angiolillo, F.; Morelli, E.; Basso, B.; Rosellini, I.; Posarelli, M.; Barbafieri, M. Response of spontaneous plants from an ex-mining site of Elba island (Tuscany, Italy) to metal (loid) contamination. Environ. Sci. Pollut. Res. 2017, 24, 7809–7820. [Google Scholar] [CrossRef]
- Gürağaç Dereli, F.T.; Khan, H.; Sobarzo-Sánchez, E.; Akkol, E.K. Antidepressant Potential of Lotus corniculatus L. subsp. corniculatus: An Ethnobotany Based Approach. Molecules 2020, 25, 1299. [Google Scholar] [CrossRef]
- Morris, J.B.; Tonnis, B.D.; Wang, M.L. Variability for Sennoside A and B concentrations in eight Senna species. Ind. Crops Prod. 2019, 139, 111489. [Google Scholar] [CrossRef]
- Rosmalena, R.; Senlia, A.O.; Hanafi, M.; Artanti, N.; Eldafira, E.; Handayani, S.I.; Lotulung, P.D.; Hartati, S.; Elya, B.; Zulfa, A. Phytochemical, Antioxidant and Antidiabetic properties of Senna alexandrina Leaf Extract. Res. J. Pharm. Technol. 2022, 15, 5835–5840. [Google Scholar] [CrossRef]
- Bouyahya, A.; El Omari, N.; Elmenyiy, N.; Guaouguaou, F.-E.; Balahbib, A.; El-Shazly, M.; Chamkhi, I. Ethnomedicinal use, phytochemistry, pharmacology, and toxicology of Ajuga iva (L.) schreb. J. Ethnopharmacol. 2020, 258, 112875. [Google Scholar] [CrossRef]
- Wang, X.Q. Operational guidance: Information needed to support clinical trials of herbal products. Chin. J. Clin. Pharmacol. Ther. 2005, 12, 582. [Google Scholar]
- Wei, H.; Kong, S.; Jayaraman, V.; Selvaraj, D.; Soundararajan, P.; Manivannan, A. Mentha arvensis and Mentha × piperita-Vital Herbs with Myriads of Pharmaceutical Benefits. Horticulturae 2023, 9, 224. [Google Scholar] [CrossRef]
- Arnold, H.-J.; Gulumian, M. Pharmacopoeia of traditional medicine in Venda. J. Ethnopharmacol. 1984, 12, 35–74. [Google Scholar] [CrossRef]
- Pooley, R.A. Fundamental physics of MR imaging. Radiographics 2005, 25, 1087–1099. [Google Scholar] [CrossRef]
- Boualam, K.; Bouhaddou, N.; Sobeh, M.; Tabyaoui, M.; Taghzouti, K. Mentha rotundifolia (L.) Huds. aqueous extract attenuates H2O2 induced oxidative stress and neurotoxicity. Front. Neurosci. 2023, 17, 1121029. [Google Scholar] [CrossRef]
- González-Tejero, M.; Casares-Porcel, M.; Sánchez-Rojas, C.; Ramiro-Gutiérrez, J.; Molero-Mesa, J.; Pieroni, A.; Giusti, M.; Censorii, E.; De Pasquale, C.; Della, A. Medicinal plants in the Mediterranean area: Synthesis of the results of the project Rubia. J. Ethnopharmacol. 2008, 116, 341–357. [Google Scholar] [CrossRef]
- Benarba, B.; Belabid, L.; Righi, K.; amine Bekkar, A.; Elouissi, M.; Khaldi, A.; Hamimed, A. Ethnobotanical study of medicinal plants used by traditional healers in Mascara (North West of Algeria). J. Ethnopharmacol. 2015, 175, 626–637. [Google Scholar] [CrossRef] [PubMed]
- Farida, S.H.M.; Ghorbani, A.; Ajani, Y.; Sadr, M.; Mozaffarian, V. Ethnobotanical applications and their correspondence with phylogeny in Apiaceae-Apioideae. Res. J. Pharmacogn. 2018, 5, 79–97. [Google Scholar]
- Piozzi, F.; Bruno, M. Diterpenoids from roots and aerial parts of the genus Stachys. Rec. Nat. Prod. 2011, 5, 1. [Google Scholar]
- Tomou, E.-M.; Barda, C.; Skaltsa, H. Genus Stachys: A review of traditional uses, phytochemistry and bioactivity. Medicines 2020, 7, 63. [Google Scholar] [CrossRef] [PubMed]
- Uritu, C.M.; Mihai, C.T.; Stanciu, G.-D.; Dodi, G.; Alexa-Stratulat, T.; Luca, A.; Leon-Constantin, M.-M.; Stefanescu, R.; Bild, V.; Melnic, S. Medicinal plants of the family Lamiaceae in pain therapy: A review. Pain Res. Manag. 2018, 2018, 7801543. [Google Scholar] [CrossRef]
- Kemassi, A.; Darem, S.; Cherif, R.; Boual, Z.; Sadine, S.E.; Aggoune, M.S.; Ould El Hadj-Khelil, A.; Ould El Hadj, M. Recherche et identification de quelques plantes médicinales à caractère hypoglycémiant de la pharmacopée traditionnelle des communautés de la vallée du M’Zab (Sahara septentrional Est Algérien). J. Adv. Res. Sci. Technol. 2014, 1, 1–5. [Google Scholar]
- Telli, A.; Esnault, M.-A.; Khelil, A.O.E.H. An ethnopharmacological survey of plants used in traditional diabetes treatment in south-eastern Algeria (Ouargla province). J. Arid Environ. 2016, 127, 82–92. [Google Scholar] [CrossRef]
- Mehdi, A.; Lamiae, B.; Samira, B.; Ramchoun, M.; Abdelouahed, K.; Tamas, F.; Hicham, B. Pomegranate (Punica granatum L.) Attenuates Neuroinflammation Involved in Neurodegenerative Diseases. Foods 2022, 11, 2570. [Google Scholar] [CrossRef]
- Belwal, T.; Nabavi, S.M.; Nabavi, S.F.; Dehpour, A.R.; Shirooie, S. Naturally Occurring Chemicals against Alzheimer’s Disease; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Fatma, B.; Fatiha, M.; Elattafia, B.; Noureddine, D. Phytochemical and antimicrobial study of the seeds and leaves of Peganum harmala L. against urinary tract infection pathogens. Asian Pac. J. Trop. Dis. 2016, 6, 822–826. [Google Scholar] [CrossRef]
- Belkhir, M.; Rebai, O.; Dhaouadi, K.; Sioud, B.; Amri, M.; Fattouch, S. Antioxidant and antimicrobial activities of Tunisian azarole (Crataegus azarolus L.) leaves and fruit pulp/peel polyphenolic extracts. Int. J. Food Prop. 2013, 16, 1380–1393. [Google Scholar] [CrossRef]
- Ampofo, O. Plants That Heal; World Health Organization: Geneva, Switzerland, 1977; pp. 26–30. [Google Scholar]
- El Omari, N.; Guaouguaou, F.E.; El Menyiy, N.; Benali, T.; Aanniz, T.; Chamkhi, I.; Balahbib, A.; Taha, D.; Shariati, M.A.; Zengin, G. Phytochemical and biological activities of Pinus halepensis mill., and their ethnomedicinal use. J. Ethnopharmacol. 2021, 268, 113661. [Google Scholar] [CrossRef]
- Mitra, S.; Anjum, J.; Muni, M.; Das, R.; Rauf, A.; Islam, F.; Emran, T.B.; Semwal, P.; Hemeg, H.A.; Alhumaydhi, F.A. Exploring the journey of emodin as a potential neuroprotective agent: Novel therapeutic insights with molecular mechanism of action. Biomed. Pharmacother. 2022, 149, 112877. [Google Scholar] [CrossRef]
- Chen, J.; Liu, X.; Li, Z.; Qi, A.; Yao, P.; Zhou, Z.; Dong, T.T.; Tsim, K.W. A review of dietary Ziziphus jujuba fruit (Jujube): Developing health food supplements for brain protection. Evid.-Based Complement. Altern. Med. 2017, 2017, 3019568. [Google Scholar] [CrossRef]
- Seong, N.-W.; Oh, W.-J.; Kim, I.-S.; Kim, S.-J.; Seo, J.-E.; Park, C.-E.; Kim, D.-Y.; Ko, J.-W.; Kim, J.-C. Efficacy and local irritation evaluation of Eriobotrya japonica leaf ethanol extract. Lab. Anim. Res. 2019, 35, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sagar, N.; Pareek, S.; Bhardwaj, R.; Vyas, N. Bioactive compounds of loquat (Eriobotrya japonica (Thunb.) L.). In Bioactive Compounds in Underutilized Fruits and Nuts; Springer: Cham, Switzerland, 2020; pp. 123–143. [Google Scholar]
- Szewczyk, A.; Grabowski, M.; Zych, D. Ruta chalepensis L. In Vitro Cultures as a Source of Bioactive Furanocoumarins and Furoquinoline Alkaloids. Life 2023, 13, 457. [Google Scholar] [CrossRef]
- Al-Said, M.S.; Tariq, M.; Al-Yahya, M.; Rafatullah, S.; Ginnawi, O.; Ageel, A. Studies on Ruta chalepensis, an ancient medicinal herb still used in traditional medicine. J. Ethnopharmacol. 1990, 28, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Debbache, N.; Atmani, D.; Atmani, D. Chemical analysis and biological activities of Populus nigra, flower buds extracts as source of propolis in Algeria. Ind. Crops Prod. 2014, 53, 85–92. [Google Scholar] [CrossRef]
- Ali, M.; Alhazmi, H.A.; Ansari, S.; Hussain, A.; Ahmad, S.; Alam, M.S.; Ali, M.S.; El-Sharkawy, K.A.; Hakeem, K.R. Tamarix aphylla (L.) Karst. Phytochemical and bioactive profile compilations of less discussed but effective naturally growing Saudi plant. In Plant and Human Health, Volume 3: Pharmacology and Therapeutic Uses; Springer: Cham, Switzerland, 2019; pp. 343–352. [Google Scholar]
- Bano, S.; Sharif, A.; Akhtar, B.; Abdel-Daim, M.M.; Akhtar, M.F.; Ali, F.L. Mechanistic insights on the possible protective role of polyphenols extracted from Tamarix aphylla aerial parts against sodium arsenite-induced hepatotoxicity in rats. Environ. Sci. Pollut. Res. 2023, 30, 16565–16578. [Google Scholar] [CrossRef]
- Khan, A.W.; Khan, A.-u.; Ahmed, T. Anticonvulsant, anxiolytic, and sedative activities of Verbena officinalis. Front. Pharmacol. 2016, 7, 499. [Google Scholar] [CrossRef] [PubMed]
- Kubica, P.; Szopa, A.; Dominiak, J.; Luczkiewicz, M.; Ekiert, H. Verbena officinalis (Common Vervain)—A review on the investigations of this medicinally important plant species. Planta Med. 2020, 86, 1241–1257. [Google Scholar] [CrossRef]
- Bekara, A.; Amazouz, A.; Douma, T.B. Evaluating the antidepressant Effect of Verbena officinalis L. (Vervain) aqueous extract in adult rats. Basic Clin. Neurosci. 2020, 11, 91–98. [Google Scholar] [CrossRef]
- Zhao, H.; Ren, S.; Yang, H.; Tang, S.; Guo, C.; Liu, M.; Tao, Q.; Ming, T.; Xu, H. Peppermint essential oil: Its phytochemistry, biological activity, pharmacological effect and application. Biomed. Pharmacother. 2022, 154, 113559. [Google Scholar] [CrossRef]
- Abdelhalim, A.R. The effect of Mentha piperita L. on the mental health issues of university students: A pilot study. J. Pharm. Pharmacogn. Res. 2021, 9, 49–57. [Google Scholar] [CrossRef]
- Mahdavikian, S.; Rezaei, M.; Modarresi, M.; Khatony, A. Comparing the effect of aromatherapy with peppermint and lavender on the sleep quality of cardiac patients: A randomized controlled trial. Sleep Sci. Pract. 2020, 4, 1–8. [Google Scholar] [CrossRef]
- Gumisiriza, H.; Birungi, G.; Olet, E.A.; Sesaazi, C.D. Medicinal plant species used by local communities around queen elizabeth national park, maramagambo central forest reserve and ihimbo central forest reserve, south western Uganda. J. Ethnopharmacol. 2019, 239, 111926. [Google Scholar] [CrossRef]
- Tsobou, R.; Mapongmetsem, P.M.; Van Damme, P. Medicinal plants used for treating reproductive health care problems in Cameroon, Central Africa. Econ. Bot. 2016, 70, 145–159. [Google Scholar] [CrossRef]
- Das, G.; Shin, H.-S.; Tundis, R.; Gonçalves, S.; Tantengco, O.A.G.; Campos, M.G.; Acquaviva, R.; Malfa, G.A.; Romano, A.; Robles, J.A.H. Plant species of sub-family Valerianaceae—A review on its effect on the central nervous system. Plants 2021, 10, 846. [Google Scholar] [CrossRef]
- Ortiz, Y.T.; McMahon, L.R.; Wilkerson, J.L. Medicinal cannabis and central nervous system disorders. Front. Pharmacol. 2022, 13, 881810. [Google Scholar] [CrossRef]
- Perera, P.K.; Meedeniya, A.C.B.; Chamikara, N.H.A. Traditional Medicinal Plants of Sri Lanka and Their Derivatives of Benefit to the Nervous System. In Medicinal Herbs and Fungi Neurotoxicity vs. Neuroprotection; Springer: Singapore, 2021; pp. 315–346. [Google Scholar]
- Morris, G.; Gamage, E.; Travica, N.; Berk, M.; Jacka, F.N.; O’Neil, A.; Puri, B.K.; Carvalho, A.F.; Bortolasci, C.C.; Walder, K. Polyphenols as adjunctive treatments in psychiatric and neurodegenerative disorders: Efficacy, mechanisms of action, and factors influencing inter-individual response. Free Radic. Biol. Med. 2021, 172, 101–122. [Google Scholar] [CrossRef]
- Weston-Green, K.; Clunas, H.; Jimenez Naranjo, C. A review of the potential use of pinene and linalool as terpene-based medicines for brain health: Discovering novel therapeutics in the flavours and fragrances of cannabis. Front. Psychiatry 2021, 12, 583211. [Google Scholar] [CrossRef]
- Trebaticka, J.; Ďuračková, Z. Psychiatric disorders and polyphenols: Can they be helpful in therapy? Oxidative Med. Cell. Longev. 2015, 2015, 248529. [Google Scholar] [CrossRef] [PubMed]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Robards, K.; Prenzler, P.D.; Tucker, G.; Swatsitang, P.; Glover, W. Phenolic compounds and their role in oxidative processes in fruits. Food Chem. 1999, 66, 401–436. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, T.; Shi, M.; Wei, Y.; Huang, X.; Shen, J.; Zhang, X.; Xie, Z.; Huang, P.; Yuan, K. Polyphenols: Natural food grade biomolecules for treating neurodegenerative diseases from a multi-target perspective. Front. Nutr. 2023, 10, 1139558. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative stress and inflammation: What polyphenols can do for us? Oxidative Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef]
- Cháirez-Ramírez, M.H.; de la Cruz-López, K.G.; García-Carrancá, A. Polyphenols as antitumor agents targeting key players in cancer-driving signaling pathways. Front. Pharmacol. 2021, 12, 710304. [Google Scholar] [CrossRef]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, M.S. Role of phenolic compounds in human disease: Current knowledge and future prospects. Molecules 2021, 27, 233. [Google Scholar] [CrossRef]
- Zhang, X.-L.; Guo, Y.-S.; Wang, C.-H.; Li, G.-Q.; Xu, J.-J.; Chung, H.Y.; Ye, W.-C.; Li, Y.-L.; Wang, G.-C. Phenolic compounds from Origanum vulgare and their antioxidant and antiviral activities. Food Chem. 2014, 152, 300–306. [Google Scholar] [CrossRef]
- Gutiérrez-Grijalva, E.; Picos-Salas, M.; Leyva-López, N.; Criollo-Mendoza, M.; Vazquez-Olivo, G.; Heredia, J.J.P. Flavonoids and phenolic acids from oregano: Occurrence, biological activity and health benefits. Plants 2018, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Calis, Z.; Mogulkoc, R.; Baltaci, A.K. The roles of flavonols/flavonoids in neurodegeneration and neuroinflammation. Mini Rev. Med. Chem. 2020, 20, 1475–1488. [Google Scholar] [CrossRef]
- Macready, A.L.; Kennedy, O.B.; Ellis, J.A.; Williams, C.M.; Spencer, J.P.; Butler, L.T. Flavonoids and cognitive function: A review of human randomized controlled trial studies and recommendations for future studies. Genes Nutr. 2009, 4, 227–242. [Google Scholar] [CrossRef] [PubMed]
- Socci, V.; Tempesta, D.; Desideri, G.; De Gennaro, L.; Ferrara, M. Enhancing human cognition with cocoa flavonoids. Front. Nutr. 2017, 4, 19. [Google Scholar] [CrossRef]
- Bakoyiannis, I.; Daskalopoulou, A.; Pergialiotis, V.; Perrea, D. Phytochemicals and cognitive health: Are flavonoids doing the trick? Biomed. Pharmacother. 2019, 109, 1488–1497. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.P. The impact of fruit flavonoids on memory and cognition. Br. J. Nutr. 2010, 104, S40–S47. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O. Biological activities of essential oils: From plant chemoecology to traditional healing systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef] [PubMed]
- Sattayakhom, A.; Wichit, S.; Koomhin, P. The Effects of Essential Oils on the Nervous System: A Scoping Review. Molecules 2023, 28, 3771. [Google Scholar] [CrossRef]
- Pai, S.R.; Sonkamble, V.V.; Wagh, N.S. Essential oils as effective agents against neurological disorders. In Plant-Derived Bioactives Production, Properties and Therapeutic Applications; Springer: Singapore, 2020; pp. 409–433. [Google Scholar]
- Lehrner, J.; Marwinski, G.; Lehr, S.; Johren, P.; Deecke, L. Ambient odors of orange and lavender reduce anxiety and improve mood in a dental office. Physiol. Behav. 2005, 86, 92–95. [Google Scholar] [CrossRef]
- Moss, M.; Cook, J.; Wesnes, K.; Duckett, P. Aromas of rosemary and lavender essential oils differentially affect cognition and mood in healthy adults. Int. J. Neurosci. 2003, 113, 15–38. [Google Scholar] [CrossRef]
- Bikmoradi, A.; Seifi, Z.; Poorolajal, J.; Araghchian, M.; Safiaryan, R.; Oshvandi, K. Effect of inhalation aromatherapy with lavender essential oil on stress and vital signs in patients undergoing coronary artery bypass surgery: A single-blinded randomized clinical trial. Complement. Ther. Med. 2015, 23, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Lundstrom, K.; Pham, H.T.; Dinh, L.D. Interaction of plant extracts with central nervous system receptors. Medicines 2017, 4, 12. [Google Scholar] [CrossRef]
- Du Sert, N.P.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; Emerson, M. Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 2020, 18, e30004112020. [Google Scholar]
- Prior, H.; Haworth, R.; Labram, B.; Roberts, R.; Wolfreys, A.; Sewell, F. Justification for species selection for pharmaceutical toxicity studies. Toxicol. Res. 2020, 9, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E. The therapeutic potential of apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef] [PubMed]
- Avallone, R.; Zanoli, P.; Corsi, L.; Cannazza, G.; Baraldi, M. Benzodiazepine-like compounds and GABA in flower heads of Matricaria chamomilla. Phytother. Res. 1996, 10, 177–179. [Google Scholar]
- Bisht, D.; Kumar, D.; Kumar, D.; Dua, K.; Chellappan, D.K. Phytochemistry and pharmacological activity of the genus artemisia. Arch. Pharmacal. Res. 2021, 44, 439–474. [Google Scholar] [CrossRef] [PubMed]
- Zámboriné Németh, É.; Thi Nguyen, H. Thujone, a widely debated volatile compound: What do we know about it? Phytochem. Rev. 2020, 19, 405–423. [Google Scholar] [CrossRef]
- Rivera, E.; Cid, M.P.; Zunino, P.; Baiardi, G.; Salvatierra, N.A. Central α-and β-thujone: Similar anxiogenic-like effects and differential modulation on GABAA receptors in neonatal chicks. Brain Res. 2014, 1555, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Bent, S.; Padula, A.; Moore, D.; Patterson, M.; Mehling, W. Valerian for sleep: A systematic review and meta-analysis. Am. J. Med. 2006, 119, 1005–1012. [Google Scholar] [CrossRef]
- Khalfa, H.; Rebbas, K.; Miara, M.; Bendif, H.; Boufissiou, A.; Souilah, N.; Daoud, N.; Peroni, A. Diversity and Traditional Use Value of Medicinal Plants in Bou Saada District of M’Sila Province, South East Algeria. J. Biodivers. Conserv. Bioresour. Manag. 2022, 8, 61–78. [Google Scholar] [CrossRef]
- Valle-Mojica, D.; Lisa, M.; Cordero-Hernández, J.M.; González-Medina, G.; Ramos-Vélez, I.; Berríos-Cartagena, N.; Torres-Hernández, B.A.; Ortíz, J.G. Aqueous and ethanolic Valeriana officinalis extracts change the binding of ligands to glutamate receptors. Evid.-Based Complement. Altern. Med. 2011, 2011, 891819. [Google Scholar]
- Becker, A.; Felgentreff, F.; Schröder, H.; Meier, B.; Brattström, A. The anxiolytic effects of a Valerian extract is based on valerenic acid. BMC Complement. Altern. Med. 2014, 14, 1–5. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, R.A. Secondary Metabolites of Medicinal Plants, 4 Volume Set: Ethnopharmacological Properties, Biological Activity and Production Strategies; John Wiley & Sons: Hoboken, NJ, USA, 2020. [Google Scholar]
- Valle-Mojica, D.; Lisa, M.; Ayala-Marín, Y.M.; Ortiz-Sanchez, C.M.; Torres-Hernández, B.A.; Abdalla-Mukhaimer, S.; Ortiz, J.G. Selective interactions of Valeriana officinalis extracts and valerenic acid with [3H] glutamate binding to rat synaptic membranes. Evid.-Based Complement. Altern. Med. 2011, 2011, 403591. [Google Scholar]
- Wang, W.; Wang, Y.; Guo, Q.; Li, H.; Wang, Z.; Li, J.; Li, T.; Tang, T.; Wang, Y.; Jia, Y. Valerian essential oil for treating insomnia via the serotonergic synapse pathway. Front. Nutr. 2022, 9, 927434. [Google Scholar] [CrossRef] [PubMed]
- Simmen, U.; Higelin, J.; Berger-Büter, K.; Schaffner, W.; Lundstrom, K. Neurochemical studies with St. John’s wort in vitro. Pharmacopsychiatry 2001, 34, 137–142. [Google Scholar] [CrossRef]
- Simmen, U.; Burkard, W.; Berger, K.; Schaffner, W.; Lundstrom, K. Extracts and constituents of Hypericum perforatum inhibit the binding of various ligands to recombinant receptors expressed with the Semliki Forest virus system. J. Recept. Signal Transduct. 1999, 19, 59–74. [Google Scholar] [CrossRef]
- Butterweck, V.; Nahrstedt, A.; Evans, J.; Hufeisen, S.; Rauser, L.; Savage, J.; Popadak, B.; Ernsberger, P.; Roth, B.L. In vitro receptor screening of pure constituents of St. John’s wort reveals novel interactions with a number of GPCRs. Psychopharmacology 2002, 162, 193–202. [Google Scholar] [CrossRef]
- Caldeira, G.I.; Gouveia, L.P.; Serrano, R.; Silva, O.D. Hypericum Genus as a Natural Source for Biologically Active Compounds. Plants 2022, 11, 2509. [Google Scholar] [CrossRef]
- Marrelli, M.; Statti, G.; Conforti, F.; Menichini, F. New potential pharmaceutical applications of hypericum species. Mini Rev. Med. Chem. 2016, 16, 710–720. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, Q.; Huang, Y. Species diversity and distribution of Salvia (Lamiaceae). Biodivers. Sci. 2015, 23, 3. [Google Scholar]
- Petrisor, G.; Motelica, L.; Craciun, L.N.; Oprea, O.C.; Ficai, D.; Ficai, A. Melissa officinalis: Composition, pharmacological effects and derived release systems—A review. Int. J. Mol. Sci. 2022, 23, 3591. [Google Scholar] [CrossRef]
- Porres-Martínez, M.; González-Burgos, E.; Emilia Carretero, M.; Pilar Gómez-Serranillos, M. Salvia spp.: An Updated on Antioxidant Activity and Pharmacological Uses. In Salvia Biotechnology; Springer: Cham, Switzerland, 2017; pp. 151–177. [Google Scholar]
- Kabouche, A.; Kabouche, Z.; Öztürk, M.; Kolak, U.; Topçu, G. Antioxidant abietane diterpenoids from Salvia barrelieri. Food Chem. 2007, 102, 1281–1287. [Google Scholar] [CrossRef]
- Kabouche, A.; Boutaghane, N.; Kabouche, Z.; Seguin, E.; Tillequin, F.; Benlabed, K. Components and antibacterial activity of the roots of Salvia jaminiana. Fitoterapia 2005, 76, 450–452. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Ozcelik, B.; Altın, G.; Daşkaya-Dikmen, C.; Martorell, M.; Ramírez-Alarcón, K.; Alarcón-Zapata, P.; Morais-Braga, M.F.B.; Carneiro, J.N.; Leal, A.L.A.B. Salvia spp. plants-from farm to food applications and phytopharmacotherapy. Trends Food Sci. Technol. 2018, 80, 242–263. [Google Scholar] [CrossRef]
- Quézel, P.; Santa, S. Nouvelle Flore de l’Algérie et des Régions Désertiques Méridionales; Éditions du Centre National de la Recherche Scientifique: Paris, France, 1962. [Google Scholar]
- Ghorbani, A.; Esmaeilizadeh, M. Pharmacological properties of Salvia officinalis and its components. J. Tradit. Complement. Med. 2017, 7, 433–440. [Google Scholar] [CrossRef]
- Boussadia, A.; Kharoubi, O.; Lahouel, Z.; Benglia, A.; Aoues, A. Effect of aqueous Salvia officinalis extract on Aluminum chloride-induced neurotoxicity in female rats. Int. J. Pharm. Res. Allied Sci. 2020, 9, 139–150. [Google Scholar]
- Chouit, H.; Touafek, O.; Brada, M.; Benssouici, C.; Fauconnier, M.-L.; Hattab, M.E. GC-MS analysis and biological activities of Algerian Salvia microphylla essential oils. J. Mex. Chem. Soc. 2021, 65, 582–601. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Herrera-Bravo, J.; Akram, M.; Abbaass, W.; Semwal, P.; Painuli, S.; Konovalov, D.A.; Alfred, M.A.; Kumar, N.V.A. Phytochemical constituents, biological activities, and health-promoting effects of the Melissa officinalis. Oxidative Med. Cell. Longev. 2021, 2021, 1–20. [Google Scholar] [CrossRef]
- Wake, G.; Court, J.; Pickering, A.; Lewis, R.; Wilkins, R.; Perry, E. CNS acetylcholine receptor activity in European medicinal plants traditionally used to improve failing memory. J. Ethnopharmacol. 2000, 69, 105–114. [Google Scholar] [CrossRef]
- Iranshahi, M.; Javadi, B. Neurological and Neuroprotective effects of Melissa officinalis L. Navid No 2019, 22, 60–71. [Google Scholar]
- Capatina, L.; Todirascu-Ciornea, E.; Napoli, E.M.; Ruberto, G.; Hritcu, L.; Dumitru, G. Thymus vulgaris essential oil protects zebrafish against cognitive dysfunction by regulating cholinergic and antioxidants systems. Antioxidants 2020, 9, 1083. [Google Scholar] [CrossRef] [PubMed]
- Javed, H.; Azimullah, S.; Meeran, M.N.; Ansari, S.A.; Ojha, S. Neuroprotective effects of thymol, a dietary monoterpene against dopaminergic neurodegeneration in rotenone-induced rat model of Parkinson’s disease. Int. J. Mol. Sci. 2019, 20, 1538. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.-Y.; Li, H.-Y.; Chen, J.-J.; Li, R.-P.; Qu, R.; Fu, Q.; Ma, S.-P. Thymol produces an antidepressant-like effect in a chronic unpredictable mild stress model of depression in mice. Behav. Brain Res. 2015, 291, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Azizi, A.; Yan, F.; Honermeier, B. Herbage yield, essential oil content and composition of three oregano (Origanum vulgare L.) populations as affected by soil moisture regimes and nitrogen supply. Ind. Crops Prod. 2009, 29, 554–561. [Google Scholar] [CrossRef]
- Lee, B.H.; Nam, T.G.; Park, W.J.; Kang, H.; Heo, H.J.; Chung, D.K.; Kim, G.H.; Kim, D.-O. Antioxidative and neuroprotective effects of volatile components in essential oils from Chrysanthemum indicum Linné flowers. Food Sci. 2015, 24, 717–723. [Google Scholar] [CrossRef]
- Sammi, S.R.; Trivedi, S.; Rath, S.K.; Nagar, A.; Tandon, S.; Kalra, A.; Pandey, R. 1-Methyl-4-propan-2-ylbenzene from Thymus vulgaris attenuates cholinergic dysfunction. Mol. Neurobiol. 2017, 54, 5468–5481. [Google Scholar] [CrossRef]
- Zotti, M.; Colaianna, M.; Morgese, M.G.; Tucci, P.; Schiavone, S.; Avato, P.; Trabace, L. Carvacrol: From Ancient Flavoring to Neuromodulatory Agent. Molecules 2013, 18, 6161. [Google Scholar] [CrossRef]
- Peters, M.; Trembovler, V.; Alexandrovich, A.; Parnas, M.; Birnbaumer, L.; Minke, B.; Shohami, E. Carvacrol together with TRPC1 elimination improve functional recovery after traumatic brain injury in mice. J. Neurotrauma 2012, 29, 2831–2834. [Google Scholar] [CrossRef]
- Ghannadi, A.; Sajjadi, S.E.; Kabouche, A. Thymus fontanesii Boiss. & Reut.-A potential source of thymol-rich essential oil in North Africa. Z. Für Nat. C 2004, 59, 187–189. [Google Scholar]
- Kabouche, A.; Kabouche, Z.; Bruneau, C. Analysis of the essential oil of Thymus numidicus (Poiret) from Algeria. Flavour Fragr. J. 2005, 20, 235–236. [Google Scholar] [CrossRef]
- Rahbardar, M.G.; Hosseinzadeh, H. Therapeutic effects of rosemary (Rosmarinus officinalis L.) and its active constituents on nervous system disorders. Iran. J. Basic Med. Sci. 2020, 23, 1100. [Google Scholar]
- Enwright, P.; Blank, S.; Wells, B.M.; Nightingale, L.M.; Torgerud, S. Effect of lavender and rosemary aromatherapy on test anxiety in chiropractic students. J. Chiropr. Educ. 2023, 37, 26–32. [Google Scholar] [CrossRef]
- Coelho, V.R.; Vieira, C.G.; de Souza, L.P.; Moysés, F.; Basso, C.; Papke, D.K.M.; Pires, T.R.; Siqueira, I.R.; Picada, J.N.; Pereira, P. Antiepileptogenic, antioxidant and genotoxic evaluation of rosmarinic acid and its metabolite caffeic acid in mice. Life Sci. 2015, 122, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Kondo, S.; El Omri, A.; Han, J.; Isoda, H. Antidepressant-like effects of rosmarinic acid through mitogen-activated protein kinase phosphatase-1 and brain-derived neurotrophic factor modulation. J. Funct. Foods 2015, 14, 758–766. [Google Scholar] [CrossRef]
- Shan, Y.; Wang, D.-D.; Xu, Y.-X.; Wang, C.; Cao, L.; Liu, Y.-S.; Zhu, C.-Q. Aging as a precipitating factor in chronic restraint stress-induced tau aggregation pathology, and the protective effects of rosmarinic acid. J. Alzheimer’s Dis. 2016, 49, 829–844. [Google Scholar] [CrossRef]
- Crișan, I.; Ona, A.; Vârban, D.; Muntean, L.; Vârban, R.; Stoie, A.; Mihăiescu, T.; Morea, A. Current trends for lavender (Lavandula angustifolia Mill.) crops and products with emphasis on essential oil quality. Plants 2023, 12, 357. [Google Scholar] [CrossRef]
- Batiha, G.E.-S.; Teibo, J.O.; Wasef, L.; Shaheen, H.M.; Akomolafe, A.P.; Teibo, T.K.A.; Al-Kuraishy, H.M.; Al-Garbeeb, A.I.; Alexiou, A.; Papadakis, M. A review of the bioactive components and pharmacological properties of Lavandula species. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2023, 396, 877–900. [Google Scholar] [CrossRef] [PubMed]
- Linck, V.M.; da Silva, A.L.; Figueiro, M.; Caramao, E.B.; Moreno, P.R.H.; Elisabetsky, E. Effects of inhaled Linalool in anxiety, social interaction and aggressive behavior in mice. Phytomedicine 2010, 17, 679–683. [Google Scholar] [CrossRef]
- Harada, H.; Kashiwadani, H.; Kanmura, Y.; Kuwaki, T. Linalool odor-induced anxiolytic effects in mice. Front. Behav. Neurosci. 2018, 12, 241. [Google Scholar] [CrossRef]
- Boughendjioua, H. Composition chimique et activité antibactérienne de l’huile essentielle de Lavandula officinalis cultivées dans la région de Skikda-Algérie. Chemical composition and antibacterial activity of essential oil of Lavandula officinalis grown in the region of Skikda-Algeria. Bull. Soc. R. Sci. Liège 2017, 86, 88–95. [Google Scholar]
- Ferhat, M.; Erol, E.; Beladjila, K.A.; Çetintaş, Y.; Duru, M.E.; Öztürk, M.; Kabouche, A.; Kabouche, Z. Antioxidant, anticholinesterase and antibacterial activities of Stachys guyoniana and Mentha aquatica. Pharm. Biol. 2017, 55, 324–329. [Google Scholar] [CrossRef]
- Bahadori, M.B.; Maggi, F.; Zengin, G.; Asghari, B.; Eskandani, M. Essential oils of hedgenettles (Stachys inflata, S. lavandulifolia, and S. byzantina) have antioxidant, anti-Alzheimer, antidiabetic, and anti-obesity potential: A comparative study. Ind. Crops Prod. 2020, 145, 112089. [Google Scholar] [CrossRef]
- Rabbani, M.; Sajjadi, S.; Zarei, H. Anxiolytic effects of Stachys lavandulifolia Vahl on the elevated plus-maze model of anxiety in mice. J. Ethnopharmacol. 2003, 89, 271–276. [Google Scholar] [CrossRef]
- Laggoune, S.; Kabouche, A.; Kabouche, Z.; El-Azzouny, A. Analysis of the Essential Oil of Stachys circinnata l’Her. from Algeria. J. Essential Oil Res. 2009, 21, 67–68. [Google Scholar] [CrossRef]
- Mamadalieva, N.; Ashour, M.; Mamedov, N. Peganum harmala: Phytochemistry, traditional uses, and biological activities. In Biodiversity, Conservation and Sustainability in Asia: Volume 2: Prospects and Challenges in South and Middle Asia; Springer: Cham, Switzerland, 2022; pp. 721–744. [Google Scholar]
- Li, L.-N. Peganum harmala L.: A Review of Botany, Traditional Use, Phytochemistry, Pharmacology, Quality marker, and Toxicity. Comb. Chem. HighThroughput Screen. 2023; e-pub ahead of print. [Google Scholar]
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. Improving health benefits with considering traditional and modern health benefits of Peganum harmala. Clin. Phytosci. 2021, 7, 1–9. [Google Scholar] [CrossRef]
- Filban, F.; Ravanbakhsh, M.; Poormohammadi, A.; Khaghani, S.; Sadeghi-Nejad, B.; Neisi, A.; Goudarzi, G. Antimicrobial properties of Peganum harmala L. seeds’ smoke in indoors: Applications and prospects. Environ. Monit. Assess. 2022, 194, 1–13. [Google Scholar] [CrossRef]
- Apostolico, I.; Aliberti, L.; Caputo, L.; De Feo, V.; Fratianni, F.; Nazzaro, F.; Souza, L.F.; Khadhr, M. Chemical composition, antibacterial and phytotoxic activities of Peganum harmala seed essential oils from five different localities in Northern Africa. Molecules 2016, 21, 1235. [Google Scholar] [CrossRef] [PubMed]
- Ayipo, Y.O.; Mordi, M.N.; Mustapha, M.; Damodaran, T. Neuropharmacological potentials of β-carboline alkaloids for neuropsychiatric disorders. Eur. J. Pharmacol. 2021, 893, 173837. [Google Scholar] [CrossRef]
- Shatarat, A.T.; Abuhamdah, S.; Alefishat, E.; Al-Essa, M.K.; Altaweel, R.; Mohammed, F.; Badran, D.; Jafar, H. Effects of beta-carboline alkaloids of Peganum harmala on induced rat ileum contractions. Pharmacogn. J. 2020, 12, 260–265. [Google Scholar] [CrossRef]
- Herraiz, T.; González, D.; Ancín-Azpilicueta, C.; Arán, V.J.; Guillén, H. β-Carboline alkaloids in Peganum harmala and inhibition of human monoamine oxidase (MAO). Food Chem. Toxicol. 2010, 48, 839–845. [Google Scholar] [CrossRef]
- Nasehi, M.; Hasanvand, S.; Khakpai, F.; Zarrindast, M.-R. The effect of CA1 dopaminergic system on amnesia induced by harmane in mice. Acta Neurol. Belg. 2019, 119, 369–377. [Google Scholar] [CrossRef]
- Saleh, R.A.; Eissa, T.F.; Abdallah, D.M.; Saad, M.A.; El-Abhar, H.S. Peganum harmala enhanced GLP-1 and restored insulin signaling to alleviate AlCl3-induced Alzheimer-like pathology model. Sci. Rep. 2021, 11, 12040. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira-Silva, C.B.M.; Jesus, O.N.; Santos, E.S.; Corrêa, R.X.; Souza, A.P. Genetic breeding and diversity of the genus Passiflora: Progress and perspectives in molecular and genetic studies. Int. J. Mol. Sci. 2014, 15, 14122–14152. [Google Scholar] [CrossRef]
- Castañeda, R.; Cáceres, A.; Velásquez, D.; Rodríguez, C.; Morales, D.; Castillo, A. Medicinal plants used in traditional Mayan medicine for the treatment of central nervous system disorders: An overview. J. Ethnopharmacol. 2022, 283, 114746. [Google Scholar] [CrossRef] [PubMed]
- Souilah, N.; Miara, M.D.; Bendif, H.; Medjroubi, K.; Snorek, J. Traditional Ethnobotanical Knowledge on Medicinal Plants Used by the Populations in Central Russikada (Northeastern Algeria). J. Herbs Spices Med. Plants 2022, 28, 15–35. [Google Scholar] [CrossRef]
- He, X.; Luan, F.; Yang, Y.; Wang, Z.; Zhao, Z.; Fang, J.; Wang, M.; Zuo, M.; Li, Y. Passiflora edulis: An insight into current researches on phytochemistry and pharmacology. Front. Pharmacol. 2020, 11, 617. [Google Scholar] [CrossRef] [PubMed]
- Akhondzadeh, S.; Naghavi, H.; Vazirian, M.; Shayeganpour, A.; Rashidi, H.; Khani, M. Passionflower in the treatment of generalized anxiety: A pilot double-blind randomized controlled trial with oxazepam. J. Clin. Pharm. Ther. 2001, 26, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Akhondzadeh, S.; Kashani, L.; Mobaseri, M.; Hosseini, S.; Nikzad, S.; Khani, M. Passionflower in the treatment of opiates withdrawal: A double-blind randomized controlled trial. J. Clin. Pharm. Ther. 2001, 26, 369–373. [Google Scholar] [CrossRef]
- Reginatto, F.H.; De-Paris, F.; Petry, R.D.; Quevedo, J.; Ortega, G.G.; Gosmann, G.; Schenkel, E.P. Evaluation of anxiolytic activity of spray dried powders of two South Brazilian Passiflora species. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2006, 20, 348–351. [Google Scholar]
- Wheatley, D. Medicinal plants for insomnia: A review of their pharmacology, efficacy and tolerability. J. Psychopharmacol. 2005, 19, 414–421. [Google Scholar] [CrossRef]
- Singh, B.; Singh, D.; Goel, R.K. Dual protective effect of Passiflora incarnata in epilepsy and associated postictal depression. J. Ethnopharmacol. 2012, 139, 273–279. [Google Scholar] [CrossRef]
- Ożarowski, M.; Karpiński, T.M. Extracts and flavonoids of Passiflora species as promising anti-inflammatory and antioxidant substances. Curr. Pharm. Des. 2021, 27, 2582–2604. [Google Scholar] [CrossRef] [PubMed]
- Frye, A.; Haustein, C. Extraction, identification, and quantification of harmala alkaloids in three species of Passiflora. Am. J. Undergrad. Res. 2007, 6, 19–26. [Google Scholar] [CrossRef]
- Fonseca, L.R.d.; Rodrigues, R.d.A.; Ramos, A.d.S.; da Cruz, J.D.; Ferreira, J.L.P.; Silva, J.R.d.A.; Amaral, A.C.F. Herbal medicinal products from Passiflora for anxiety: An unexploited potential. Sci. World J. 2020, 2020, 6598434. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Verma, N.; Gauthaman, K. Passiflora incarnata Linn: A review on morphology, phytochemistry and pharmacological aspects. Pharmacogn. Rev. 2009, 3, 186. [Google Scholar]
- Dhawan, K.; Kumar, S.; Sharma, A. Suppression of alcohol-cessation-oriented hyper-anxiety by the benzoflavone moiety of Passiflora incarnata Linneaus in mice. J. Ethnopharmacol. 2002, 81, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.; Hurd, N.S.; McCall, S.; Ceremuga, T.E. Evaluation of the anxiolytic effects of chrysin, a Passiflora incarnata extract, in the laboratory rat. AANA J. 2007, 75, 333–337. [Google Scholar]
- Bouzabata, A.; Boukhari, A. Variation in the Traditional Knowledge of Curcuma longa L. in North-Eastern Algeria. Int. J. Pharmacol. Pharm. Sci. 2014, 8, 1227–1231. [Google Scholar]
- El-Saadony, M.T.; Yang, T.; Korma, S.A.; Sitohy, M.; El-Mageed, A.; Taia, A.; Selim, S.; Al Jaouni, S.K.; Salem, H.M.; Mahmmod, Y. Impacts of turmeric and its principal bioactive curcumin on human health: Pharmaceutical, medicinal, and food applications: A comprehensive review. Front. Nutr. 2023, 9, 1040259. [Google Scholar] [CrossRef]
- Farooqui, A.A. Curcumin in Neurological Disorders. In Curcumin for Neurological and Psychiatric Disorders; Elsevier: Amsterdam, The Netherlands, 2019; pp. 45–62. [Google Scholar]
- Fan, F.; Lei, M. Mechanisms underlying curcumin-induced neuroprotection in cerebral ischemia. Front. Pharmacol. 2022, 13, 893118. [Google Scholar] [CrossRef]
- Ramaholimihaso, T.; Bouazzaoui, F.; Kaladjian, A. Curcumin in depression: Potential mechanisms of action and current evidence—A narrative review. Front. Psychiatry 2020, 11, 572533. [Google Scholar] [CrossRef]
- den Haan, J.; Morrema, T.H.; Rozemuller, A.J.; Bouwman, F.H.; Hoozemans, J.J. Different curcumin forms selectively bind fibrillar amyloid beta in post mortem Alzheimer’s disease brains: Implications for in-vivo diagnostics. Acta Neuropathol. Commun. 2018, 6, 1–12. [Google Scholar] [CrossRef]
- Tang, M.; Taghibiglou, C. The mechanisms of action of curcumin in Alzheimer’s disease. J. Alzheimer’s Dis. 2017, 58, 1003–1016. [Google Scholar] [CrossRef]
- Abate, L.; Tadesse, M.G.; Bachheti, A.; Bachheti, R.K. Traditional and phytochemical bases of herbs, shrubs, climbers, and trees from Ethiopia for their anticancer response. BioMed Res. Int. 2022, 2022, 1589877. [Google Scholar] [CrossRef]
- Malík, M.; Tlustoš, P. Nootropic Herbs, Shrubs, and Trees as Potential Cognitive Enhancers. Plants 2023, 12, 1364. [Google Scholar] [CrossRef]
- Häne, K.; Dobbertin, M.K. Le frêne, arbre aux mille vertus. La Forêt 2006, 59, 20–21. [Google Scholar]
- Beloued, A. Plantes médicinales d’Algérie; Office des Publications Universitaires: Algiers, Algeria, 1998; 277p. [Google Scholar]
- Kis, B.; Avram, S.; Pavel, I.Z.; Lombrea, A.; Buda, V.; Dehelean, C.A.; Soica, C.; Yerer, M.B.; Bojin, F.; Folescu, R. Recent advances regarding the phytochemical and therapeutic uses of Populus nigra L. buds. Plants 2020, 9, 1464. [Google Scholar] [CrossRef]
- Zouaghi, N.; Houda Bensiradj, N.E.; Cavaleiro, C.; Nadjemi, B.; Trari, M. Phytochemical Study and Antibacterial Effects of Fraxinus angustifolia Vahl (Algeria): Experimental and Computational Investigations. Waste Biomass Valorizat. 2021, 12, 3605–3616. [Google Scholar] [CrossRef]
- Bouguellid, G.; Russo, C.; Lavorgna, M.; Piscitelli, C.; Ayouni, K.; Wilson, E.; Kim, H.K.; Verpoorte, R.; Choi, Y.H.; Kilani-Atmani, D. Antimutagenic, antigenotoxic and antiproliferative activities of Fraxinus angustifolia Vahl. leaves and stem bark extracts and their phytochemical composition. PLoS ONE 2020, 15, e02306902020. [Google Scholar] [CrossRef]
- Sarfraz, I.; Rasul, A.; Jabeen, F.; Younis, T.; Zahoor, M.K.; Arshad, M.; Ali, M. Fraxinus: A plant with versatile pharmacological and biological activities. Evid.-Based Complement. Altern. Med. 2017, 2017, 4269868. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, W.; Liu, H.; Wu, G.; Song, M.; Yang, B.; Yang, D.; Wang, Q.; Kuang, H. Simultaneous determination of aesculin, aesculetin, fraxetin, fraxin and polydatin in beagle dog plasma by UPLC-ESI-MS/MS and its application in a pharmacokinetic study after oral administration extracts of Ledum palustre L. Molecules 2018, 23, 2285. [Google Scholar] [CrossRef]
- Liang, C.; Ju, W.; Pei, S.; Tang, Y.; Xiao, Y. Pharmacological activities and synthesis of esculetin and its derivatives: A mini-review. Molecules 2017, 22, 387. [Google Scholar] [CrossRef] [PubMed]
- Azib, L.; Debbache-Benaida, N.; Da Costa, G.; Atmani-Kilani, D.; Saidene, N.; Bouguellid, G.; Ourabah, A.; Krisa, S.; Richard, T.; Atmani, D. Neuroprotective effects of Fraxinus angustifolia Vahl. bark extract against Alzheimer’s disease. J. Chem. Neuroanat. 2020, 109, 101848. [Google Scholar] [CrossRef] [PubMed]
- Tavares, W.R.; Seca, A.M. The current status of the pharmaceutical potential of Juniperus L. metabolites. Medicines 2018, 5, 81. [Google Scholar] [CrossRef] [PubMed]
- Bais, S.; Gill, N.S.; Rana, N.; Shandil, S. A phytopharmacological review on a medicinal plant: Juniperus communis. Int. Sch. Res. Not. 2014, 2014, 634723. [Google Scholar] [CrossRef]
- Gao, Y.; Xu, X.; Chang, S.; Wang, Y.; Xu, Y.; Ran, S.; Huang, Z.; Li, P.; Li, J.; Zhang, L. Totarol prevents neuronal injury in vitro and ameliorates brain ischemic stroke: Potential roles of Akt activation and HO-1 induction. Toxicol. Appl. Pharmacol. 2015, 289, 142–154. [Google Scholar] [CrossRef]
- Seca, A.M.; Pinto, D.; Silva, A.M. The current status of bioactive metabolites from the genus Juniperus. Bioact. Phytochem. Perspect. Mod. Med. 2015, 3, 365–407. [Google Scholar]
- Mohan, V.; Doss, A.; Tresina, P. Ethnomedicinal Plants with Therapeutic Properties; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Goyal, M.R.; Suleria, H.A.R.; Ayeleso, A.O.; Joel, T.J.; Panda, S.K. The Therapeutic Properties of Medicinal Plants: Health-Rejuvenating Bioactive Compounds of Native Flora; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Karbwang, J.; Crawley, F.P.; Na-Bangchang, K.; Maramba-Lazarte, C. Herbal medicine development: Methodologies, challenges, and issues. Evid.-Based Complement. Altern. Med. 2019, 2019, 4935786. [Google Scholar] [CrossRef]
- Singh, D.B.; Pathak, R.K.; Rai, D. From traditional herbal medicine to rational drug discovery: Strategies, challenges, and future perspectives. Rev. Bras. Farmacogn. 2022, 32, 147–159. [Google Scholar] [CrossRef]
- Kashte, S.; Gulbake, A.; El-Amin, S.F., III; Gupta, A. COVID-19 vaccines: Rapid development, implications, challenges and future prospects. Hum. Cell 2021, 34, 711–733. [Google Scholar] [CrossRef]
- Kuter, B.J.; Offit, P.A.; Poland, G.A. The development of COVID-19 vaccines in the United States: Why and how so fast? Vaccine 2021, 39, 2491. [Google Scholar] [CrossRef]
- Ministère. Arrêté Fixant les Éléments du Dossier de Demande d’Agrément de l’Établissement Pharmaceutique d’Éxploitation. Available online: www.miph.gov.dz (accessed on 25 October 2023).
- Laakmann, G.; Schüle, C.; Baghai, T.; St. Kieser, M. John’s wort in mild to moderate depression: The relevance of hyperforin for the clinical efficacy. Pharmacopsychiatry 1998, 31, 54–59. [Google Scholar] [CrossRef]
- Henderson, L.; Yue, Q.; Bergquist, C.; Gerden, B.; Arlett, P. St John’s wort (Hypericum perforatum): Drug interactions and clinical outcomes. Br. J. Clin. Pharmacol. 2002, 54, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Dimpfel, W.; Todorova, A.; Vonderheid-Guth, B. Pharmacodynamic properties of St. John’s wort—A single blind neurophysiological study in healthy subjects comparing two commercial preparations. Eur. J. Med. Res. 1999, 4, 303–312. [Google Scholar]
- Iserin, P.; Masson, M.; Restellini, J.; Ybert, E.; De Laage de Meux, A.; Moulard, F.; Zha, E.; De la Roque, R.; De la Roque, O.; Vican, P. Larousse des Plantes Médicinales Identification, Préparation, Soins; Larousse: Paris, France, 2001; Volume 15. [Google Scholar]
- Safari, M.; Asadi, A.; Aryaeian, N.; Huseini, H.F.; Jazayeri, S.; Malek, M.; Hosseini, A.F. The effects of Melissa officinalis on depression and anxiety in type 2 diabetes patients with depression: A randomized double-blinded placebo-controlled clinical trial. BMC Complement. Med. Ther. 2023, 23, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.O.; Wake, G.; Savelev, S.; Tildesley, N.T.; Perry, E.K.; Wesnes, K.A.; Scholey, A. Modulation of mood and cognitive performance following acute administration of single doses of Melissa officinalis (Lemon balm) with human CNS nicotinic and muscarinic receptor-binding properties. Neuropsychopharmacology 2003, 28, 1871–1881. [Google Scholar] [CrossRef] [PubMed]
- Araj-Khodaei, M.; Noorbala, A.A.; Yarani, R.; Emadi, F.; Emaratkar, E.; Faghihzadeh, S.; Parsian, Z.; Alijaniha, F.; Kamalinejad, M.; Naseri, M. A double-blind, randomized pilot study for comparison of Melissa officinalis L. and Lavandula angustifolia Mill. with Fluoxetine for the treatment of depression. BMC Complement. Med. Ther. 2020, 20, 1–9. [Google Scholar] [CrossRef]
- Kennedy, D.O.; Pace, S.; Haskell, C.; Okello, E.J.; Milne, A.; Scholey, A.B. Effects of cholinesterase inhibiting sage (Salvia officinalis) on mood, anxiety and performance on a psychological stressor battery. Neuropsychopharmacology 2006, 31, 845–852. [Google Scholar] [CrossRef]
- Movafegh, A.; Alizadeh, R.; Hajimohamadi, F.; Esfehani, F.; Nejatfar, M. Preoperative oral Passiflora incarnata reduces anxiety in ambulatory surgery patients: A double-blind, placebo-controlled study. Anesth. Analg. 2008, 106, 1728–1732. [Google Scholar] [CrossRef]
- Akaberi, M.; Iranshahy, M.; Iranshahi, M. Review of the traditional uses, phytochemistry, pharmacology and toxicology of giant fennel (Ferula communis L. subsp. communis). Iran. J. Basic Med. Sci. 2015, 18, 1050. [Google Scholar]
- Appendino, G.; Jakupovic, J.; Alloatti, S.; Ballero, M. Daucane esters from Ferula arrigonii. Phytochemistry 1997, 45, 1639–1643. [Google Scholar] [CrossRef]
- Louvet, M.-S.; Gault, G.; Lefebvre, S.; Popowycz, F.; Boulven, M.; Besse, S.; Benoit, E.; Lattard, V.; Grancher, D. Comparative inhibitory effect of prenylated coumarins, ferulenol and ferprenin, contained in the ‘poisonous chemotype’of Ferula communis on mammal liver microsomal VKORC1 activity. Phytochemistry 2015, 118, 124–130. [Google Scholar] [CrossRef]
- Lahmar, R.; Mahjoub, T.; Fourel, I.; Gault, G.; Grancher, D. Giant fennel (Ferula communis L.) intoxication in goats in Tunisia. Vet. Rec. Case Rep. 2018, 6, e000738. [Google Scholar] [CrossRef]
- Philbey, A.; Hawker, A.; Evers, J. A neurological locomotor disorder in sheep grazing Stachys arvensis. Aust. Vet. J. 2001, 79, 427–430. [Google Scholar] [CrossRef]
- Berdai, M.A.; Labib, S.; Harandou, M. Peganum harmala L. intoxication in a pregnant woman. Case Rep. Emerg. Med. 2014, 2014, 783236. [Google Scholar] [PubMed]
- Dehiri, M.; Diafat, A.; Fatmi, W.; Bouaziz, F.; Khalil, R.; Bahloul, A. Toxicity evaluation of Algerian Peganum harmala seed hydromethanolic extract. Toxicol. Environ. Health Sci. 2022, 14, 351–359. [Google Scholar] [CrossRef]
- Djafer, R.; Akil Dahdouh, S.; Boukachabia, R.; Megueddem, M. À propos d’un cas d’intoxication mortelle par le harmel (Peganum harmala L.). Phytothérapie 2017, 15, 288–289. [Google Scholar] [CrossRef]
- Simão, A.Y.; Gonçalves, J.; Gradillas, A.; García, A.; Restolho, J.; Fernández, N.; Rodilla, J.M.; Barroso, M.; Duarte, A.P.; Cristóvão, A.C. Evaluation of the cytotoxicity of ayahuasca beverages. Molecules 2020, 25, 5594. [Google Scholar] [CrossRef]
- Hamill, J.; Hallak, J.; Dursun, S.M.; Baker, G. Ayahuasca: Psychological and physiologic effects, pharmacology and potential uses in addiction and mental illness. Curr. Neuropharmacol. 2019, 17, 108–128. [Google Scholar] [CrossRef]
- Brito-da-Costa, A.M.; Dias-da-Silva, D.; Gomes, N.G.; Dinis-Oliveira, R.J.; Madureira-Carvalho, Á. Toxicokinetics and toxicodynamics of ayahuasca alkaloids N, N-dimethyltryptamine (DMT), harmine, harmaline and tetrahydroharmine: Clinical and forensic impact. Pharmaceuticals 2020, 13, 334. [Google Scholar] [CrossRef]
- Fatur, K. “Hexing herbs” in ethnobotanical perspective: A historical review of the uses of anticholinergic Solanaceae plants in Europe. Econ. Bot. 2020, 74, 140–158. [Google Scholar] [CrossRef]
- Houmani, Z.; Cosson, L.; Corbineau, F.; Côme, D. Etude de la teneur en hyoscyamine et scopolamine d’une population sauvage de Datura stramonium L. en Algérie. Acta Bot. Gall. 1994, 141, 61–66. [Google Scholar] [CrossRef]
- Grynkiewicz, G.; Gadzikowska, M. Tropane alkaloids as medicinally useful natural products and their synthetic derivatives as new drugs. Pharmacol. Rep. 2008, 60, 439. [Google Scholar] [PubMed]
- Chang, S.-S.; Wu, M.-L.; Deng, J.-F.; Lee, C.-C.; Chin, T.-F.; Liao, S.-J. Poisoning by Datura leaves used as edible wild vegetables. Vet. Hum. Toxicol. 1999, 41, 242–245. [Google Scholar] [PubMed]
- Kwakye, G.F.; Jiménez, J.; Jiménez, J.A.; Aschner, M. Atropa belladonna neurotoxicity: Implications to neurological disorders. Food Chem. Toxicol. 2018, 116, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Meggs, W.; Weisman, R. Anticholinergic poisoning associated with an herbal tea—New York City, 1994. MMWR Morb. Mortal. Wkly. Rep. 1995, 44, 193–195. [Google Scholar]
- Control, C.f.D.; Prevention. Scopolamine poisoning among heroin users—New York City, Newark, Philadelphia, and Baltimore, 1995 and 1996. MMWR Morb. Mortal. Wkly. Rep. 1996, 45, 457–460. [Google Scholar]
- Bouzidi, A.; Mahdeb, N.; Kara, N. Toxicity studies of alkaloids of seeds of Datura stramonium and synthesis alkaloids in male rats. J. Med. Plants Res. 2011, 5, 3421–3431. [Google Scholar]
- Benouadah, Z.; Mahdeb, N.; Bouzidi, A. Evaluation of acute and sub-acute toxicity of alkaloids from Datura stramonium sp. in mice. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 1759–1766. [Google Scholar]
- Nath, S.S.; Pandey, C.; Roy, D. A near fatal case of high dose peppermint oil ingestion-Lessons learnt. Indian J. Anaesth. 2012, 56, 582. [Google Scholar] [CrossRef]
- Singletary, K. Oregano: Overview of the literature on health benefits. Nutr. Today 2010, 45, 129–138. [Google Scholar] [CrossRef]
- Llana-Ruiz-Cabello, M.; Gutiérrez-Praena, D.; Puerto, M.; Pichardo, S.; Jos, Á.; Cameán, A.M. In vitro pro-oxidant/antioxidant role of carvacrol, thymol and their mixture in the intestinal Caco-2 cell line. Toxicol. Vitr. 2015, 29, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Azirak, S.; Rencuzogullari, E. The in vivo genotoxic effects of carvacrol and thymol in rat bone marrow cells. Environ. Toxicol. Int. J. 2008, 23, 728–735. [Google Scholar] [CrossRef]
- Achour, S.; Abourazzak, S.; Mokhtari, A.; Soulaymani, A.; Soulaymani, R.; Hida, M. Juniper tar (cade oil) poisoning in new born after a cutaneous application. Case Rep. 2011, 2011, bcr0720114427. [Google Scholar] [CrossRef]
- Azizi, M.; El Kaouini, A.; Lafkih, M.A.; Bouayed, M.Z.; Bkiyar, H.; Housni, B. A rare case of cade oil poisoning complicated by acute pancreatitis and acute tubular necrosis. Ann. Med. Surg. 2022, 76, 103562. [Google Scholar] [CrossRef] [PubMed]
- Skalli, S.; Chebat, A.; Badrane, N.; Bencheikh, R.S. Side effects of cade oil in Morocco: An analysis of reports in the Moroccan herbal products database from 2004 to 2012. Food Chem. Toxicol. 2014, 64, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-R.; Perumalsamy, H.; Shin, H.M.; Lee, S.-G.; Ahn, Y.-J. Toxicity of Juniperus oxycedrus oil constituents and related compounds and the efficacy of oil spray formulations to Dermatophagoides farinae (Acari: Pyroglyphidae). Exp. Appl. Acarol. 2017, 73, 385–399. [Google Scholar] [CrossRef]











| S/No | Plant Name | Family | Common Name | Local Algerian Name(s) | Use for Mental Disorders | Plant Part/s Used | Traditional Preparation and Administration Methods | Identified Active Constituent(s) | Other Relevant Phytoconstituents Identified in the Plant | Interaction/Toxicity | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Narcissus tazetta L. | Amaryllidaceae | Narcisse à bouquet | Nardjes | Epilepsy, memorigenic, hysteria, AD. | Roots, flowers, bulbs. | Infusion. | Alkaloids (galanthamine, lycorine, homolycorine, tazettine, narciclasine). | Flavonoids, saponins, tannins, cardiac glycosides, essential oil, steroids, terpenoids, anthraquinones. | [45,46,47] | |
| 2 | Pistacia lentiscus L. | Anacardiaceae | Pistachier lentisque | Dharw | Memory. | Leaves, fruits, resin, essential oil. | Decoction, infusion, fruits can be naturally eaten raw. | Tannins, essential oil (monoterpenes), triterpenes. | Polyphenols, phytosterols, flavonoids, triglycerides, tocopherols, carotenoids. | Toxic at higher doses. | [34,48,49,50] |
| 3 | Coriandrum sativum L. | Apiaceae | Coriandre | Kousbor | Epilepsy, nervous tension, tranquilizer, migraine. | Essential oil, leaves, seeds. | Decoction, infusion, can be naturally chewed. | Essential oil (coriandrol, pinenes, terpinenes, borneol, linalool, geraniol). | Aromatic acids, Isocoumarins, polyphenols. | [46,51] | |
| 4 | Ferula communis L. | Apiaceae | La Férule | Fessoukh, Kelekh | Anxiety, anti-hysteria. | Gum resin (latex), roots, leaves, stems | Decoction infusion powder cataplasm fumigation. | Coumarins, sesquiterpene prenylated coumarins (ferulenol). | Daucane esters, phenylpropanoids, phenolic compounds. | Toxic (ferulenol, 4-hydroxycoumarin derivatives, ferprenin). | [5,50,52] |
| 5 | Ferula assa-foetida L. | Apiaceae | Férule persique | Hantit | Epilepsy, tranquilizer, stimulant to the brain and nerves. | Oleo-gum resin, aerial parts, seeds, roots, young shoots and leaves. | Decoction, powder. | Essential oil, sesquiterpene, coumarins (foetidin). | Disulfides, ferulic acid, valeric acid. | [53,54] | |
| 6 | Pimpinella anisum L. | Apiaceae | L’anis vert | Habet h’lawa, Yansoune | Insomnia. | Essential oil, seeds. | Infusion. | Essential oil (anethol, methyl chavicol) furanocoumarins, flavonoids. | Sterols, proteins, fatty acid, terpenes. | May be toxic under certain conditions. | [55,56] |
| 7 | Anacyclus pyrethrum (L.) Lag. | Asteraceae | Pyrèthre d’Afrique | Agargarha, Kantass | Epilepsy, paralysis, seizures, depression, anxiety. | Roots, essential oil. | Decoction, chew, lozenge, powder. | Essential oil, anacycline, inulin. | Pellitorine. | [5,54] | |
| 8 | Artemisia absinthium L. | Asteraceae | L’absinthe | Chadjrat meriem | Insomnia, slightly antidepressant. | Aerial parts, leaves. | Infusion, maceration, decoction. | Essential oil, terpenes, azulenes, thujone, sesquiterpene lactones (artabasine, absinthin). | Phenolic compounds, lignans. | Essential oil constituents are highly toxic (alpha-thujone and beta-thujone). | [34,54,57,58] |
| 9 | Artemisia herba alba Asso. | Asteraceae | L’armoise | Chih | AD, epilepsy, depression, neuroinflammation. | Aerial parts. | Infusion, decoction. | Essential oil, herbalbin, cis-chryanthenyl acetate, flavonoids (hispidulin and cirsilineol), monoterpenes, sesquiterpene lactones. | Coumarins, tannins. | Toxic at over dose. | [5,34,50,57] |
| 10 | Carlina gummifera (L.) Less. Syn. Atractylis gummifera L. | Asteraceae | Le chardon à glu | Addad | Epilepsy, seizure management, mania. | Capitulum, leaves, roots. | Decoction. | Polyphenols, tannins. | Diterpenoid glucosides. | Roots are highly toxic (mortal). | [36,50,59,60,61] |
| 11 | Chamaemelum Nobile syn. Anthemis nobilis L. | Asteraceae. | La Camomille romaine | Babounej | Anxiety. | Flowers, essential oil. | Infusion, decoction. | Essential oil (angelic acid esters, chamazulenes), sesquiterpene lactones (nobilin), flavonoids, coumarins. | Polyphenols. | [5,34,54] | |
| 12 | Matricaria chamomilla L. | Asteraceae | La Camomille sauvage | Babounej | Migraines, insomnia. | Capitulum. | Infusion. | Essential oil (alpha-bisabolol, chamazulene), flavonoids, coumarins, tannins. | Polyphenols. | [36,62] | |
| 13 | Scolymus hispanicus L. | Asteraceae | Chardon d’ Espagne | Zernich, Guernina | Different neurological conditions. | Roots, stems, leaves, flowers. | Infusion. | Flavonoids, tannins. | Phenolics. | [63,64,65,66] | |
| 14 | Silybum marianum (L.) Gaertner | Asteraceae | Chardon marie | Chouk | Depression. | Seeds. | Dried seeds, decoction, tincture. | Silymarin, silybin A, silybin B, isosilybin A, isosilybin B, silychristin A, silydianin, taxifolin. | Polyphenols, essential oil, tannins. | [5,63] | |
| 15 | Berberis vulgaris L. | Berberidaceae | L’épine-vinette | Oud ghriss | Sedative, morphine addiction. | Leaves, fruits, stem bark, roots. | Raw, decoction, infusion. | Berberine. | Alkaloids (berbamine, jateorrhizine, palmatine, oxycanthine). | Toxic in higher doses. | [54,67,68,69,70] |
| 16 | Lepidium sativum L. | Brassicaceae | Cresson alénois | Hab err-chad | Insomnia, memory. | Seeds. | Powder. | Tannins, vitamins, minerals. | Flavonoids, carbohydrates, phenolics, alkaloids, proteins, saponins, lipids. | [71,72,73] | |
| 17 | Commiphora myrrha (Nees) Engl. | Burseraceae | Myrrh | El-morra | Memory impairment, tranquilizer, anxiety. | Gum resin, seeds. | Infusion, powder. | Essential oil, sesquiterpenes, furanosesquiterpenes, polysaccharides, tannins. | Proteins and long-chain aliphatic derivatives, steroids, sterols, terpenes. | [5,74] | |
| 18 | Boswellia sacra Flueck. | Burseraceae | Oliban | Loubene | CNS disorders, AD, depression, mental fatigue, stress. | Gum resin, stems. | Powder, infusion, maceration, mastication, decoction, fumigation. | Boswellic acids. | Essential oil, phenols, terpenoids, uronic acids, steroids, tannins. | [5,54] | |
| 19 | Opuntia ficus-indica (L.) Mill. | Cactaceae | Figuier de Barbarie | Tine chawki, El-hendi | Headache, dizziness. | Leaves. | Decoction. | Flavonoids, polyphenols. | Polysaccharides, sterols, omega-3 fatty acid. | [31,36,75] | |
| 20 | Humulus lupulus L. | Cannabaceae | Houblon | Jenjel | Headache. | Leaves. | Raw: topical. | Flavonoids, polyphenols. | Sesquiterpenoids, diterpenoids, triterpenoids. | [36,76] | |
| 21 | Cucurbita maxima Duchesne | Cucurbitaceae | Potiron | Elkaraea | Migraine. | Seeds. | Decoction: inhalation. | Phytosterols (cucurbitacin), vitamins (tocopherols and carotenoids), unsaturated fatty acids. | Mineral salts (zinc, selenium). | [31,36,77] | |
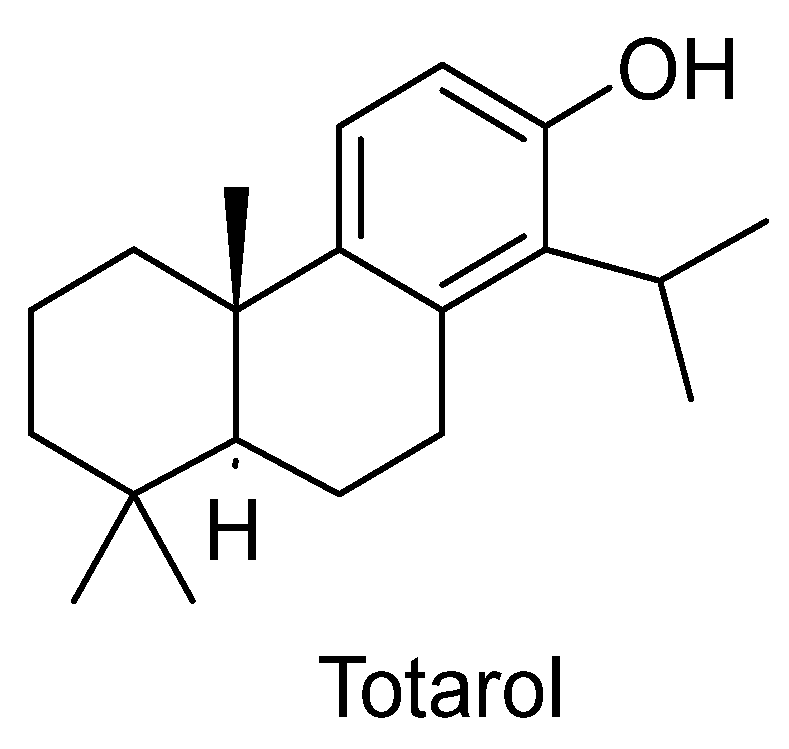
| 22 | Juniperus phoenicea L. | Cupressaceae | Genévrier de Phénicie | Aaraar | Different neurological conditions. | Aerial parts, berries. | Decoction, infusion, tablet. | Essential oil, flavonoids, terpenoids, diterpenes (totarol), lignans, tannins. | Phenyl propanoids and furanone glycosides, sugars, fatty acids, organic acids, sterols. | Toxic in higher doses. | [5,57] |
| 23 | Juniperus oxycedrus L. | Cupressaceae | Genévrier cade | Ttaga | Different neurological conditions. | Aerial parts, berries. | Infusion, decoction, ext. essential oil, tablet. | Cade oil. | Essential oil, phenolic compounds, sesquiterpenes, cresol. | Cade oil is toxic in excessive amounts. | [5,78] |
| 24 | Bituminaria bituminosa (L.) C. H. Stirt. | Fabaceae | Herbe au bitume, Trèfle bitumineux | Adna, Menita | Epilepsy. | Whole plant. | Infusion. | Phenylpropanoids, coumarins, furanocoumarins, pterocarpans, flavonoids, isoflavones (daidzein and genistein), meroterpenes, sesquiterpenes. | Chalcones, phenols, phenolic cinnamates, phenylpropenes, sterols, terpenes, tocopherols, benzofurans, fatty acids. | Toxic in excessive amounts. | [66,79] |
| 25 | Glycyrrhiza glabra L. | Fabaceae | La réglisse | Ark-essous | Head problems, psychosis. | Roots. | Decoction: oral/topical. | Triterpene saponins (glycyrrhizin), isoflavones, phytosterols, coumarins, polysaccharides, asparagine. | Pectins, simple sugars, amino acids, mineral salts, essential oil, gum, protein, resin, volatile oils, tannins, glycosides. | [31,36,76] | |
| 26 | Lotus corniculatus L. | Fabaceae | Lotier corniculé | Lotus el karni | Insomnia, depression, tranquilizer, neurological and psychological disorders. | Leaves, aerial parts. | Infusion, decoction. | Flavonoids, tannins. | Alkaloids, terpenes, fatty acids. | [63,66,80] | |
| 27 | Senna alexandrina Mill. | Fabaceae | Le séné | Sena-mekki | Head problems, psychosis. | Leaves. | Decoction, topical/oral. | Sennosides A and B, dianthrones, anthrone. | Polyphenols. | [36,81,82] | |
| 28 | Trigonella foenum-graecum L. | Fabaceae | Le fenugrec | Helba | Anxiety. | Seeds. | Infusion, raw, cataplasm. | Essential oil, alkaloids, flavonoids. | Saponins, proteins, vitamins, minerals, carbohydrates. | [34,36] | |
| 29 | Hypericum perforatum L. | Hypericaceae | St. John’s worth, Millepertuis | Mesmoun, Berslouna | Epilepsy, anxiety, depression, neurosedative, nervousness. | Flower heads. | Infusion. | Phenolic compounds (hyperforin), naphthodianthrones (hypericin), proanthocyanins, essential oil. | Flavonoids, amentoflavones, carotenoids, catechic tannins. | [46,54] | |
| 30 | Crocus sativus L. | Iridaceae | Safran cultivé | Zaafran | Memory and learning, insomnia, tranquilizer, neurodegenerative diseases, mild-to-moderate depression, anxiety, headache. | Stamen. | Infusion, powder, raw. | Essential oil, crocetin glucosides (crocins), carotenoids, vitamins (B1, B2). | Flavonoids. | Toxic at higher doses. | [5,54,71,72,78] |
| 31 | Ajuga iva (L.) Schreb | Lamiaceae | Bugle ivette | Chengoura | CNS diseases, memory, mental nervousness. | Whole plant, aerial parts, leaves, flowers, roots. | Infusion, decoction, powder, cataplasm. | Iridoids, diterpenes, phytoecdysone, caffeic acids. | Steroids, terpenoids, flavonoids, fatty acids. | [63,72,83] | |
| 32 | Lavandula stoechas L. | Lamiaceae | Lavande papillon, lavande à toupet, lavande stéchade | Helhal | Anxiety, depression. | Leaves, stems, flowers. | Decoction, infusion, ext. essential oil. | Essential oil (terpenes, linalyl acetate, linalool, cineole, limonene), tannins, coumarins, flavonoids. | Triterpenes, alcohols, ketones, polyphenols. | [31] | |
| 33 | Lavandula officinalis Chaix syn. Lavandula angustifolia Mill. | Lamiaceae | Lavande vraie, lavande à feuilles étroites | Khzama | Stress, anxiety, nervousness, depression, insomnia, tranquilizer. | Leaves, stems, flowers. | Decoction, infusion, ext. essential oil. | Essential oil (linalyl acetate, cineol, linalool, borneol), flavonoids, tannins, coumarins. | Polyphenols. | [31] | |
| 34 | Melissa officinalis L. | Lamiaceae | Melisse | Melissa | Depression, stress, anxiety, nervousness, insomnia, nervous tonic. | Aerial parts. | Decoction, infusion, ext. essential oil, cataplasm, ointment. | Essential oil (citral, caryophyllene, linalool, citronellal), flavonoids, triterpenes, tannins. | Terpenoids, polyphenols. | The essential oil has moderate toxicity at higher doses. | [34,36,54,84] |
| 35 | Mentha arvensis L. | Lamiaceae | Menthe des champs | Naana | Head problems, anxiety, psychosis, insomnia. | Aerial parts. | Raw. | Menthol, menthone. | Isomenthone, neomenthol, limonene, methyl acetate, piperitone, beta-caryophyllene, alpha-pinene, beta-pinene, tannins, flavonoids. | [54,85] | |
| 36 | Mentha aquatica L. | Lamiaceae | Menthe aquatique | Hbak elmaa | Anxiety, hypochondria. | Aerial part. | Raw. | Menthol, menthone. | Flavonoids (luteolin, menthoside), phenols, triterpenes. | [36,54] | |
| 37 | Mentha rotundifolia (L.) Huds. | Lamiaceae | Menthe à feuilles rondes, Menthe du Nil | Timarssat | Pain, mental illnesses, removal of “curses” and protect from “evil spirits”. | Aerial part. | Decoction. | Menthol, menthone, pulegone. | Phenolic acids, flavonoids. | [86,87,88] | |
| 38 | Mentha pulegium L. | Lamiaceae | Menthe pouliot | Fliou | Insomnia, headache, migraine. | Aerial part. | Decoction. | Pulegone, isopulegone, menthol. | Terpenes, tannins. | The essential oil is highly toxic. | [31,54] |
| 39 | Mentha x piperita L. | Lamiaceae | La Menthe | Naana | Tranquilizer, anxiety. | Aerial parts. | Infusion, capsules, essential oil: topical. | Essential oil (menthol, menthone), flavonoids (luteolin, menthoside). | Phenols, triterpenes. | [31] | |
| 40 | Ocimum basilicum L. | Lamiaceae | Basilic | Hbak | Epilepsy, depression, anxiety, sleeping disorders, insomnia. mental exhaustion, migraine. | Leaves, flowering tops. | Decoction, powder, ext. essential oil. | Essential oil (linalool, methyl-chavicol, methyl cinnamate, cineol). | Terpenes. | [54,72,89,90] | |
| 41 | Origanum majorana L. | Lamiaceae | Marjolaine | Merdakouche | Anxiety, insomnia, nervousness. | Aerial parts, ext. Essential oil. | Infusion. | Essential oil (sabinene hydrate, linalool, carvacrol), caffeic acid, rosmarinic acid, flavonoids. | Triterpenes. | [31] | |
| 42 | Origanum vulgare L. | Lamiaceae | Origan | Zaatar | Anxiety, sleeping disorders. | Aerial parts, ext. essential oil. | Infusion. | Essential oil (thymol, carvacrol, borneol, linalool, beta-bisabolene, caryophyllene), flavonoids. | Phenolic acids, tannins. | [31] | |
| 43 | Rosmarinus officinalis L. | Lamiaceae | Romarin | Leklil | Memory, slightly anti-depressive, psychical stimulant, nervousness. | Leaves, flowers, aerial parts. | Infusion, decoction, maceration. | Essential oil (borneol, camphor, camphene, cineol), flavonoids (apigenin, diosmin), rosmarinic acid, alkaloids(rosmaricin). | Tannins, diterpenes. | Toxic at higher doses. | [34,54,72] |
| 44 | Salvia Officinalis L. | Lamiaceae | Sauge | Miramia | Stress, tranquilizer, nervous tonic, nervousness, memory, insomnia, agitation in AD. | Leaves, stems. | Raw leaves, infusion, Decoction, maceration. | Essential oil, phenolic compounds (coumarins, flavonoids, tannins). | Alkaloids, carbohydrate, fatty acids, glycosidic derivatives, poly acetylenes, steroids, terpenoids. | Toxic in case of prolonged use or at high doses. | [34,54,72,91] |
| 45 | Stachys arvensis (L.) L. | Lamiaceae | Épiaire des champs | Chatra | Antidepressant, anxiety. | Capitulum. | Decoction. | Hydroxyflavone-allosylglucosides. | Flavonoids, iridoids, fatty acids, phenolic acids, diterpenoids. | [38,63,92,93] | |
| 46 | Stachys officinalis L. Syn. Betonica officinalis L. | Lamiaceae | la Bétoine | Chatra | Nervous system stimulant, stress, insomnia, anxiety, tranquilizer, memory deficit, lightly sedative. | Aerial parts. | Tea of dried leaves, extract of flowers. | Stachydrine, betonicine, betaine, choline. | Iridoids, flavonoid, tannins, fatty acids, phenolic acids, diterpenoids, phenols, hydroxycinnamic acid derivatives, sesquiterpenes. | [54,92,93,94] | |
| 47 | Thymus vulgaris L. | Lamiaceae | Thym | Zaatar | Anxiety, phobia. | Aerial parts, ext. essential oil. | Infusion, decoction. | Essential oil (thymol, carvacrol, linalool, cineol), flavonoids. | Phenolic acids, saponins, tannins. | [34,54,95,96] | |
| 48 | Cinnamomum camphora (L.) | Lauraceae | Camphrier | Kafour | Migraine. | Wax. | Infusion: topical. | Camphor. | Essential oil (camphor, safrole, eugenol, terpineol), lignans. | Should be used externally only. | [31,36] |
| 49 | Cinnamomum verum J. Presl | Lauraceae | Cannelier | Karfa | Migraine. | Bark. | Raw: oral/topical. | Essential oil (cinnamaldehyde: 65–75%). | Polyphenols (vanillic acid, caffeic acid, gallic acid, p-coumaric acid, ferulic acid, cinnamic acid, proanthocyanidins A and B, kaempferol,), tannins (phlobatannins), coumarins, mucilages, eugenol. | [31] | |
| 50 | Lawsonia inermis L. Syn. Lawsonia alba | Lythraceae | Henné | Henna | Anxiety, hypochondria. | Leaves | Raw: topical. | Naphtoquinones (lawsone). | Polyphenols, coumarins, flavonoids, tannins, alkaloids, terpenoids, sterols, carbohydrates, proteins, fatty acids. | [31] | |
| 51 | Punica granatum L. | Lythraceae | Grenadier | Rommane | Headache. | Fruit peels, fruits. | Decoction: topical. | Flavonoids, ellagitannins, alcaloids. | Triterpens, polyphenols. | Peels may be toxic if consumed in excess. | [31,97] |
| 52 | Myristica fragrans Houtt. | Myristicaceae | Muscadier | Djouz-ettib | Head problems, psychosis. | Seeds. | Raw: topical. | Essential oil (α-pinene, β-pinene, alpha terpinene, beta terpinene, myristicin, elemicin, safrole). | Fixed oil (myristicin). | Toxic overdose may cause CNS excitation with anxiety/fear. | [98] |
| 53 | Syzygium aromaticum (L.) Merr. & L.M.Perry. syn. Eugenia caryophyllata | Myrtaceae | Girofle | K’rounfel | Mental asthenia, loss of memory, migraine. | Flower buds, leaves, stems. | Infusion, essential oil: topical. | Essential oil (eugenol, eugenol acetyl, methyl salicylate, pinene, vanillin). | Gum, tannins. | [54] | |
| 54 | Myrtus communis L. | Myrtaceae | Le Myrte commun | Rihane | Anxiety, tranquilizer. | Leaves and flowers, essential oil, fruits may be eaten raw or dried. | Infusion, decoction. | Essential oil (alpha pinene, cineol, myrtenol), flavonoids, tannins. | Phenolic compounds. | [34,54] | |
| 55 | Peganum harmala L. | Nitrariaceae | Harmel | Harmal | Anxiety, memory, nervous system disorders, Parkinson’s agitation. | Seeds, roots. | Infusion, decoction, fumigation. | Alkaloids (harmine, harmaline, harmalol). | Saponins, tannins, glycosides, terpenoids, steroids. | Toxic. | [34,54,99] |
| 56 | Fraxinus angustifolia Vahl. | Oleaceae | Frêne à feuilles étroites | Dardar | AD, different nervous system conditions. | Barks, leaves, grains. | Infusion. | Tannins, flavonoids, coumarins, essential oil, resin, malic acid. | Saccharides, minerals, vitamins. | [5,63,66,100] | |
| 57 | Olea europaea L. | Oleaceae | Olive | Zitoun | Memory, anxiety, head problems, psychosis, depression. | Leaves, fruits, fruit oil. | Infusion, raw. | Oleuropein, oleuropeoside, monounsaturated fatty acids, tyrosol fatty acid esters, hydroxytyrosols, oleic acid, tocopherols. | Flavonoids, tannins, saponines, saccharides, triterpenes, vitamins, minerals. | [5,54] | |
| 58 | Papaver rhoeas L. | Papaveraceae | Coquelicot | Khashkhash, thekouche | Insomnia, stress; tranquilizer, nervousness. | Flowers. | Infusion. | Alkaloids (papaverine, rheadine, isorheadine, rhoeagenine). | Anthocyanins, mucilage, tannins. | Toxic. | [54,72] |
| 59 | Passiflora incarnata L. | Passifloraceae | Passiflore | Nouar al saa | Epilepsy, depression, insomnia, anxiety, nervousness, hysteria, stress, tranquilizer. | Leaves, flowers. | Infusion, compressed. | Indole alkaloids (harmane), glycosidic flavonoids (vitexin, isovitexin, orientin), flavonoids (luteolin, chrysin, kaempferol, apigenin), glycosides (passiflorine), alkaloids (harmine, harmaline), palmitic acid, myristic acid. | Maltol, cyanogenetic glucosides (gynocardin). | [54,72,101] | |
| 60 | Pinus halepensis Mill. | Pinaceae | Pin maritime | Snoubar, Z’koukou (fruits) | Memory deficits, tranquilizer, strengthen the nervous system. | Leaves, buds, bark seeds, resin. | Decoction, infusion, chewed, ext. ointment, cataplasm, powder, essential oil. | Essential oil. | Terpenoids, phenolic acids, flavonoids, fatty acids, steroids, aldehydes, ketones. | [34,66,72,102] | |
| 61 | Avena sativa L. | Poaceae | Avoine | Chofan | Depression, asthenia, stress, insomnia, nervous fatigue, stimulates the nervous system. | Seeds, dried stems. | Infusion, tincture. | Saponins, alkaloids, trigonelline, silicic acid. | Proteins, vitamins B, minerals. | [54] | |
| 62 | Nigella sativa L. | Ranunculaceae | Nigelle | Sanouj, Zrarâ | Anxiety. | Seeds. | Decoction, infusion, ext. powder, essential oil. | Fatty acids (linoleic acid, oleic acid), saponins, essential oil. | Alkaloids (Nigellimine N-oxide, nigellidine, nigellicine), carbohydrates, proteins, minerals, tannins. | Toxic (melanthin). | [34,54] |
| 63 | Rhamnus alaternus L. | Rhamnaceae | Le Nerprun alaterne | Melilesse | Neuroprotective. | Barks, leaves, fruits. | Powder, decoction, infusion. | Polyphenols, anthraquinones (emodin), anthrone, anthranols. | Tannins, anthocyanins, alkaloids. | [54,63,66,72,89,103] | |
| 64 | Zizyphus lotus L. | Rhamnaceae | Le jujubier sauvage | Sedra | Neuroprotective, promoting memory and learning, insomnia, forgetfulness, hypnotic sedative, anxiolytic. | Leaves, fruits, roots. | Infusion, decoction, powder, fruits may be eaten raw or dried. | Tannins, vitamins, flavonoids, polyphenols, polysaccarides. | Alkaloids, minerals. | [54,57,72,104] | |
| 65 | Crataegus oxyacantha L. | Rosaceae | Aubepine | Boukhrourou, Bou m’kherry | Epilepsy, loss of memory, insomnia, neurosedative, sleep disorders. | Flowers, fruits. | Infusion decoction, tincture, compressed. | Flavonoids (rutin, quercetin), flavones (vitexin, orientin, rhamnosylvitexin), triterpenes, proanthocyanes, polyphenols, tannins, coumarins, saponins, alkaloids (nicotine). | Amines, anthocyans, phenolic acids, triterpenic acids, sitosterols, purines. | [5,54,72] | |
| 66 | Crataegus azarolus L. | Rosaceae | Azerolier | Zaarour | Anxiety, stress, psychical disorders, insomnia, nervous tonic. | Leaves, flowers, fruits. | Extracts, tincture, fruit can be eaten raw, cooked, or as preserves. | Essential oil, tannins, amino acids, proanthocyanidins, flavonoids. | Sugars (fructose, glucose et rhamnose), vitamins. | [5,72,89,100] | |
| 67 | Eriobotrya japonica (Thunb.) Lindl | Rosaceae | Le néflier du Japon | Nifla | Headache, dizziness. | Leaves | Decoction | Quercetin, ursolic acid, oleanolic acid, tannins, chlorogenic acid, caffeoylquinic acid. | Polyphenols, flavonoids, carotenoids, triterpenoids. | [105,106] | |
| 68 | Rosa canina L. | Rosaceae | Églantier | Nasrine, Ouardzeroub | Tranquilizer, anxiety, depression. | Fruits (rose hips), leaves, flowers. | Tea infusion. | Vitamins (C, A, B1, B2, P, K), flavonoids, tannins, citric acid, carotenoids, essential oil, D-sorbitol. | Polyphenols, carotenoids, carbohydrates, fatty acids. | [54,72,89] | |
| 69 | Galium verum L. | Rubiaceae | Caille-lait | Fouaoua | Epilepsy. | Aerial parts, flowers. | Infusion. | Iridoids (asperulosides), flavonoids, anthraquinones, alcanes. | Phenolic compounds, tannins, saponins, triterpenes, essential oil, wax, pigments, vitamin C. | [54,66] | |
| 70 | Citrus limon (L.) Osbeck. | Rutaceae | Citronnier | Laymoune | Dizziness. | Fruits. | Decoction. | Essential oil, terpenes (limonene), flavonoids (hesperidin), vitamins, mucilage. | Sesquiterpenes, aldehydes (citral), coumarins. | [31,36] | |
| 71 | Ruta chalepensis L. | Rutaceae | Rue de Chalep | Fidjel | Headache, mental disorders. | Aerial parts | Decoction | Coumarins (furanocoumarins, dihydrofuranocoumarins), furoquinoline alkaloids. | Flavonoids, essential oil. | [107,108] | |
| 72 | Populus Nigra L. | Salicaceae | Peuplier noir | Safsaf | Neurodegenerative disorders. | Leaves, flower buds, barks. | Infusion, tincture, powder, ointment. | Tannins, flavonoids, saccharides, essential oil. | Phenolic compounds, terpenoids. | [5,73,109] | |
| 73 | Santalum album L. | Santalaceae | Santal blanc | Sandel | Migraine. | Bark, fruits. | Decoction: topical/oral. | Essential oil, tannins. | Resin. | [31] | |
| 74 | Atropa belladonna L. | Solanaceae | Belladone | Sitt Elhossn | Parkinson’s disease, neurological disorders, tranquilizer. | Leaves, roots. | Tincture, decoction. | Tropane alkaloids. | Nicotine, flavonoids, coumarins. | Toxic (effects includes pupil dilatation, confusion, hallucination). | [34,54,72] |
| 75 | Datura stramonium L. | Solanaceae | Stramoine | Tatura, Mendj | Parkinson’s disease, stress. | Leaves, capitulum, seeds. | Tincture, decoction, fumigation, powder. | Tropane alkaloids (hyoscyamine, hyoscine). | Flavonoids, withanolides, coumarins, tannins, minerals. | Highly toxic. | [54,72] |
| 76 | Tamarix aphylla (L.) H.Karst | Tamaricaceae | Tamarix aphylla | Tahtah | Headache. | Leaves. | Decoction. | Flavonoids, polyphenols, tannins. | Catechins, triterpenoids. | [110,111] | |
| 77 | Tilia cordata Mill. | Tiliaceae | Tilleul | Zayzafoune | Epilepsy, neurosedative, sleep disorders, stress, anxiety. | Flowers. | Infusion. | Flavonoids (quercetin, rhamnoside, kaempferol). | Tannins, essential oil, mucilage. | [54,72,89] | |
| 78 | Valeriana tuberosa L. | Valerianaceae | Valériane | Senbel, nardine | Epilepsy, stress, anxiety, insomnia. | Roots and rhizome. | Infusion, decoction. | Essential oil (bornyl acetate, beta-caryophyllene), iridoids (valepotriate), alkaloids | Tannins, flavonoids. | [5,46] | |
| 79 | Valeriana officinalis L. | Valerianaceae | Valériane | Senbel | Epilepsy, stress, anxiety, insomnia, nervousness. | Roots and rhizome. | Infusion, decoction, tincture, powder, compressed. | Essential oil (bornyl acetate, beta-caryophyllene), iridoids (valepotriate), alkaloids. | Tannins, flavonoids. | [5,54,72,90] | |
| 80 | Verbena officinalis L. | Verbenaceae | Verveine | Louisa | Tranquilizer, lightly sedative, anxiolytic, anticonvulsant, lightly antidepressive, insomnia, anxiety, mental fatigue. | Aerial parts. | Tincture, infusion, powder. | Iridoids (verbenone, verbenaline), Essential oil. | Mucilage, tannins, flavonoids, phenolic acids. | [54,112,113,114] | |
| 81 | Zingiber officinale L. | Zigiberaceae | Gingembre | Zanjabil | Anxiety, head problems, psychosis. | Rhizome. | Infusion, maceration, capsules, tincture, ext. essential oil. | Essential oil, sesquiterpenes, oleoresin, phenols (gingerol). | Phenolic compounds. | [34,54] | |
| 82 | Curcuma longa L. | Zigiberaceae | Curcuma | Korkoum | Anxiety, hypochondria. | Rhizome. | Decoction, powder, cataplasm, tincture. | Essential oil, zingiberene, turmerone, curcuminoids (curcumin), resin. | Phenolic compounds, caffeic acid derivatives. | [31,34,36] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Larit, F.; León, F. Therapeutics to Treat Psychiatric and Neurological Disorders: A Promising Perspective from Algerian Traditional Medicine. Plants 2023, 12, 3860. https://doi.org/10.3390/plants12223860
Larit F, León F. Therapeutics to Treat Psychiatric and Neurological Disorders: A Promising Perspective from Algerian Traditional Medicine. Plants. 2023; 12(22):3860. https://doi.org/10.3390/plants12223860
Chicago/Turabian StyleLarit, Farida, and Francisco León. 2023. "Therapeutics to Treat Psychiatric and Neurological Disorders: A Promising Perspective from Algerian Traditional Medicine" Plants 12, no. 22: 3860. https://doi.org/10.3390/plants12223860
APA StyleLarit, F., & León, F. (2023). Therapeutics to Treat Psychiatric and Neurological Disorders: A Promising Perspective from Algerian Traditional Medicine. Plants, 12(22), 3860. https://doi.org/10.3390/plants12223860









