Abstract
Quercus species have been widely used in traditional medicine, and recently, researchers’ attention has focused on galls of the genus Quercus as a source of health-promoting phytochemicals. This review presents a summary of the most recent findings on the phytochemistry and bioactivity of oak galls, following the screening of scientific papers published in two relevant databases, PubMed and Embase, between January 2018 and June 2023. The oak galls are rich in active compounds, mostly gallotannins and phenolic acids. Due to these secondary metabolites, the reviewed studies have demonstrated a wide range of biological activities, including antioxidant and anti-inflammatory actions, antimicrobial properties, tissue-protective effects, and antitumor, anti-aging, and hypoglycemic potential. Thus, oak galls are a promising natural matrix, to be considered in obtaining pharmaceutical and cosmetic preparations used in anti-aging strategies and, together with medications, in the management of age-related diseases. In further evaluations, the valuable functional properties of oak galls, reported mostly in preclinical studies, should be confirmed with clinical studies that would also take into account the potential health risks of their use.
1. Introduction
Oak is a plant belonging to the genus Quercus of the family Fagaceae and it includes over 200 species, which differ in morphology, from tremendous trees to shrubs [1,2]. Regarding the topic of our study, two essential members of this genus are Q. infectoria G. Olivier, also known as gall oak [3] or Aleppo oak [4], a small tree or shrub about 2.5 m high [5], growing in countries such as Cyprus, Greece, Turkey, Egypt, Iraq, Iran, Saudi Arabia, Syria, Malaysia, and some parts of India [3,5,6,7], as well as Q. brantii Lindl., the most common species in Iran [1].
An important source of polyphenols of Quercus sp. is represented by galls (called “oak galls”, “Turkish galls”, “gallnuts”, “nutgalls”, “Mecca galls”, “Aleppo galls”, or “Galla Turcica”), abnormal outgrowths of plant tissue, round in shape, and formed on the leaves, buds, flowers, and young branches, as a result of the sting and laying of eggs by the female gall wasps, Cynips gallae tinctoriae and Adleria gallae-tinctoria [2,6,7,8].
Historically, Quercus sp. galls have been used for millennia in both Western and Eastern cultures as traditional remedies to treat inflammatory conditions, including diarrhea and dysentery, stomach aches, toothaches, and tooth decay, as well as in postpartum care, in combating metabolic abnormalities and oxidative stress-related diseases [1,6,9,10]. In addition to medicinal use, the industrial use of Quercus sp. galls dates back to ancient times. Thus, the importance of these species of Quercus throughout history is confirmed in medieval manuscripts, galls being used for tanning leather, as dyeing agents for paintings, and natural dyes for carpet yarns, respectively, as a component of ink [1,4,5].
Pharmacologically, Quercus sp. galls have been reported to possess strong antibacterial, antioxidant, and anti-inflammatory activities, and also antitumor, antifungal, antiviral, antiprotozoal, antiamoebic, antiulcer, larvicidal, tooth and gum tonic, antipyretic, analgesic/local anesthetic, antidiabetic, cardioprotective, hepatoprotective, antiparkinsonian, antitremor, and accelerated wound healing effects [1,3,4,8,9,11,12]. Despite the multiple therapeutic properties, the long-term intake of gallnuts in high doses is not recommended. Due to the astringent effect of hydrolyzable tannins, they can cause adverse effects, such as irritation of the gastric mucosa, nausea, and vomiting [13]. Also, galls can aggravate lung and throat disorders, such as hoarseness and cough, and can cause anemia and dyspepsia, through the chelation of metal ions, respectively, and the inhibition of digestive enzymes, by tannins [5,13].
In recent years, the search for natural products to prevent or treat diseases is increasing. Among these products, oak galls have attracted the attention of researchers through the biological activities demonstrated both in vitro and in vivo, which are related to the chemical composition rich in antioxidant phenolic compounds [10,12,14,15,16,17]. Compared to other galls, the galls of the genus Quercus stand out for the highest level of tannins (50–70%) [1,4]. In addition to tannins, the diverse phenolic profile mainly includes numerous flavonoids and simple phenolic compounds, such as phenolic acids, hydroxyphenols and coumarins, and in a smaller number, representatives from other groups of phenolic compounds, i.e., phenolic aldehydes, naphthodianthrones, acyl-phloroglucinols, phenolic alcohols, and stilbenes [9,10,18,19,20,21].
Through the valuable antioxidant phytochemicals, oak galls could exert their actions and potential effectiveness with fewer side effects and adverse reactions, and lower costs for the population, compared to drugs. Although some articles have been published in this field, to the best of our knowledge, there is no comprehensive and up-to-date analysis of data regarding the Quercus sp. galls, with particular focus on the phytochemical profile and biological activities. In this context, the present systematic review integrates in vitro and in vivo studies, published in the last 5 years, and provides an insight into the potential of Quercus sp. galls as a source of bioactive secondary metabolites and the relevance of their use in the treatment of various pathologies.
2. Methods and Materials
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [22] (Figure 1), and the design was registered in INPLASY on 4 October 2023. The registration code is INPLASY2023100012, with DOI 10.37766/inplasy2023.10.0012, https://inplasy.com/inplasy-2023-10-0012/ (accessed on 4 October 2023).
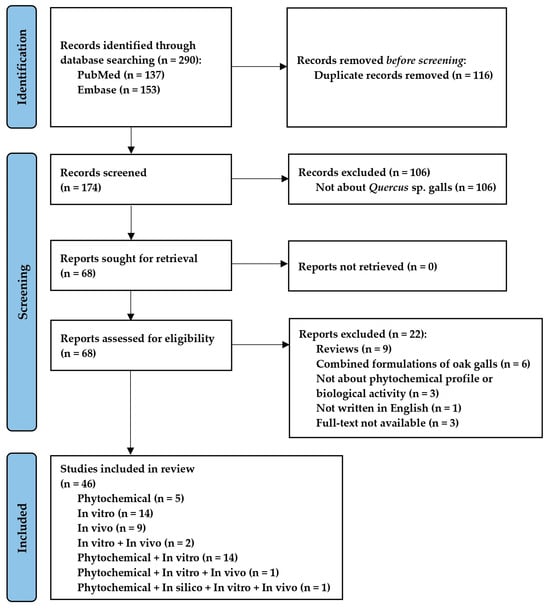
Figure 1.
PRISMA flow diagram. Synthesis of the bibliographic analysis.
2.1. Focus Question
The question to be answered in this systematic review is the following: what are the biological activities shown by the bioactive metabolites of Quercus sp. galls in the in vitro and in vivo studies of the last 5 years?
2.2. Information Sources
A bibliographic investigation was carried out in PubMed and Embase databases, searching for articles describing the phytochemical profile and biological activity of oak galls published from 1 January 2018 to 30 June 2023. In order to conduct exhaustive research, the bibliographies of the included studies and recent reviews were also examined.
2.3. Search Strategy
For the purpose of searching the databases, we made use of a combination of free-text words, as well as their synonyms, singular and plural versions, and thesaurus words (Medical Subject Headings for PubMed: (“quercus” [MeSH Terms] OR “quercus” [All Fields] OR “oak” [All Fields] OR “quercus infectoria” [All Fields] OR “quercus brantii” [All Fields]) AND (“gall” [All Fields] OR “galls” [All Fields] OR “oak gall” [All Fields] OR “gallnuts” [All Fields] OR “nutgall” [All Fields]), and Emtree for Embase: (‘quercus’/exp OR ‘quercus’ OR ‘oak’ OR ‘quercus infectoria’ OR ‘quercus brantii’) AND (‘gall’ OR ‘galls’ OR ‘oak gall’ OR ‘gallnuts’ OR ‘nutgall’)).
2.4. Eligibility Criteria
After the preliminary screening and the elimination of duplicates, the full texts of possibly relevant articles were retrieved and then evaluated to determine whether or not they qualified for inclusion in the review.
The inclusion criteria were (1) experimental studies to identify and/or quantify phytochemical compounds; (2) in vitro and in vivo biological activity studies.
The exclusion criteria were (1) reviews and meta-analyses; (2) secondary studies (i.e., editorials, commentaries, letters to the editor, conference abstracts, or any other publications without original data); (3) studies investigating other types of galls than those collected from Quercus sp.; (4) duplicate studies or databases; (5) studies not written in English; and (6) publications with full text not available and the corresponding author could not be contacted.
2.5. Selection Process
Three of the authors performed the literature search, removed duplicate articles, and examined titles and abstracts according to eligibility criteria. Two independent reviewers (R.B. and M.E.R.) conducted the literature search, removed duplicate articles, and carried out the screening of articles according to eligibility criteria. After the titles and abstracts of the extracted references were checked for relevance, the full texts of all potentially eligible articles were screened against the inclusion/exclusion criteria. In case of discrepancies, the disagreements were resolved between them or by a third reviewer (D.-S.P.) who decided whether the study met the inclusion criteria. Furthermore, if essential data for the review were missing, the corresponding author was contacted to obtain the complete information.
2.6. Data Collection
Using structured tables, the key data from each study were extracted according to the following descriptive indices: (1) publication characteristics—authors, year of publication, country; (2) study purpose; (3) study type—phytochemical composition study, in vitro study/biological systems analysis, and in vivo study/animal models/with humans; (4) information about the oak gall treatment—plant species used, plant material/extract/formulation type, dose, frequency of administration and treatment in the control group, route of administration; (5) study outcomes. Data from included studies were collected by one reviewer (R.B.) and cross-checked by two others (M.E.R. and D.-S.P.) to ensure content integrity.
3. Results and Discussion
3.1. PRISMA Guideline
The initial search in PubMed and Embase databases identified 290 records from the last 5 years, out of which 116 were duplicates and were excluded. A total of 106 studies with inadequate thematics were excluded after reading the title and abstract. Of the 68 remaining studies, 22 articles were excluded after reading the full text for not meeting the eligibility criteria. Following these exclusions, 46 were suitable for inclusion in the systematic review. The reference list includes five phytochemical studies; fourteen in vitro studies; nine in vivo studies; two studies both in vitro and in vivo; fourteen studies both phytochemical and in vitro; a phytochemical, in vitro, and in vivo study; and, respectively, a phytochemical, in silico, in vitro, and in vivo study. The flowchart of the review and each step performed in the selection process are shown in Figure 1. Table 1 shows the characteristics and the main findings of the studies included in the systematic review.

Table 1.
Characteristics of the selected studies.
3.2. Publication Characteristics
Among the studies included in this review, 10.87% of the studies evaluated the phytochemical composition of oak galls (n = 5); 30.43% of the studies evaluated the effects of oak galls in vitro (n = 14), 19.57% in animal models or humans (n = 9), 4.35% both in vitro and in vivo (n = 2); 30.43% evaluated both the phytochemical composition and the in vitro effects (n = 14); 2.17% evaluated both the phytochemical composition and the in vitro and in vivo effects (n = 1), respectively; and 2.17% evaluated both the phytochemical composition and the in silico, in vitro, and in vivo effects (n = 1), respectively.
Regarding the country where the studies in this review were conducted, Malaysia dominated with 21.74% of the studies conducted on oak galls in the last 5 years (n = 10), followed by Iraq with 17.39% (n = 8), Iran with 15.22% (n = 7), China with 13.04% (n = 6), India with 8.70% (n = 4), Egypt and Turkey with 6.52% (n = 3), Saudi Arabia with 4.35% (n = 2), and Indonesia, Pakistan, and Thailand, respectively, with 2.17% (n = 1).
3.3. Phytochemicals Found in Quercus sp. Galls
The articles analyzed in our review revealed that the positive effect of oak galls, consisting of their numerous biological activities, can be attributed to the presence of various bioactive substances.
According to the findings, the composition of the metabolites found in the Quercus sp. galls showed great variation, both quantitatively and qualitatively, despite the fact that they all came from the same species.
Among the articles selected for this review, 39.13% of the studies examined the phytochemical composition of oak galls (n = 18), and 6.52% of the studies performed only the phytochemical screening (n = 3), while 8.70% of the studies targeted both the phytochemical screening and the study of the phytochemical composition (n = 4). Some studies analyzed the effects of oak galls but did not perform a phytochemical characterization (54.35%; n = 25).
Of all the studies included in the review, 36.96% investigated the main compounds responsible for the biological activities of oak galls, i.e., phenolic constituents, including phenolic acids and their esters, phenolic alcohols, hydroxyphenols, and dihydroxyphenols, respectively, and their derivatives, flavonoids, naphthodianthrones, prenylated phloroglucinol derivatives, coumarins and stilbenes, and also hydrolysable tannins—gallotannins and ellagitannins (n = 17). Only 13.04% of the studies examined other types of non-phenolic compounds present in oak galls, including lipid compounds, hydrocarbons, alcohols, carboxylic acids, ethers, esters, proteins, and elements (n = 6).
3.3.1. Sample Preparation and Phenolic Compound Extraction
For phenolic identification research, it was essential to take into consideration the sample preparation. Due to the intricate nature of the majority of samples, the method employed for their preparation typically exerted a discernible influence on the outcomes of the entire extraction process. Several standard sample preparation methods, such as drying, homogenization, filtration, and grinding, were commonly employed prior to the extraction process [54].
Regarding the oak gall samples, their preparation before the extraction of phenolic compounds consisted of cleaning, drying, and grinding. The cleaning of the gall samples was performed with washing [8,43], some studies using tap water [19], and others boiling water [9]. The galls were air-dried at room temperature [19,43], in the shade [8,20] or in an oven at 40–45 °C for approximately 24 h [9,21]. For the coarse grinding of the plant material, either grinding the galls in a disc mill [51] or crushing the galls in a mortar with a pestle [8,43] was used. Grinding into fine particles was carried out using an electric grinder [21] or a vibrating-type ultrafine grinder [51]. To obtain a uniform powder, grinding was followed by sieving through sieves of different diameters [8,21,51]. The particle size of the powders subjected to extraction varied from 0.5 mm to <50 μm [9,21,43,51].
The solvent extraction method was commonly used to prepare crude extracts [54]. Phenolic compounds were extracted through the utilization of solvents with varying degrees of polarity, including methanol, ethanol, water, ethyl acetate, acetone, and/or their combinations [55]. In the present review, methanol was the solvent used for extraction in most of the phenolic composition studies (n = 7), followed by ethanol (n = 6), water (n = 5), and ethyl acetate (n = 2). Other solvents used were acetone (n = 1), n-butanol (n = 1), and mixtures, water/diethyl ether/ethyl acetate (n = 1) and diethyl ether/ethanol/water (n = 1). Among the conventional extraction techniques, maceration extraction [14,19,21,34,47], decoction technique [15], digestion technique [8,9,10,40,47], exhaustive serial extraction [15], soxhlet extraction [33], and reflux extraction were used [17,18,51].
Although the aim of an extraction process should be to ensure a maximum yield of active substances and of the highest quality, only a few of the studies included in the review aimed to optimize some parameters of the solvent extraction process, among the investigated variables being sample pre-treatment (particle size reduction), type of solvent, extraction method or extraction time, and temperature.
Reducing the particle size should increase the surface area available for mass transfer and increase the extraction yield [56]. Lu et al. [51] investigated the influence of a vibratory ultrafine grinding treatment on the physical and chemical properties and antioxidant activity of Turkish gall powder (TGP) with particle sizes >450, 400–250, 250–100, 100–50, and <50 μm, and they concluded that for the TGP extract with the smallest particle size (<50 μm), the highest gallic acid content (9.47 mg/g), methyl gallate content (34.78 mg/g), and ellagic acid content (0.79 mg/g) were obtained. Thus, reducing the particle size with ultrafine grinding facilitated the release of the three components from Turkish galls and consequently contributed to the increased DPPH, hydroxyl radical, and superoxide radical scavenging activities.
Regarding the type of solvent used to extract phenolic substances from natural sources, alcoholic solvents have been commonly used because they lead to a high yield of the total extract, although they are not highly selective for phenolics. In contrast, mixtures of alcohols and water were found to be more efficient in the extraction of phenolic constituents than the corresponding mono-component solvent system [56]. Thus, a study that aimed to evaluate the content of tannic acid, a well-known gallotannin, in different extracts of Quercus sp. galls using an HPLC analysis used for the extraction four different mixtures of solvents and water (96% ethanol, 80% ethanol, 70% acetone, and diethylether/ethanol/water mixture (25:3:1)), and two extraction techniques (maceration extraction and digestion technique), establishing that the highest amount of tannic acid (127.683 mg/g) was obtained in the 80% ethanolic extract obtained with maceration [47].
Comparing the effect of different solvents on the extraction efficiency of polyphenolic compounds, it was observed that the number of identified gallotannins varied from seven compounds identified in the case of extraction with ethyl acetate [16] or a mixture of solvents (water/diethyl ether/ethyl acetate) [17] to nine compounds in the extraction with ethanol [14], respectively, and thirteen compounds in the case of aqueous extraction [18], in all cases mass spectrometry (MS) was being used as the identification method.
In the case of phenolic acids, the number of representatives identified was higher when alcoholic solvents were used, than when the extraction solvent used was water. Thus, the extraction in methanol led to the identification of 11 phenolic acids by each of the two research teams led by Kılınçarslan Aksoy et al. [10,40]; the ethanolic extracts allowed the identification of 11 [14] and, respectively, 14 phenolic acids [21], while only 4 [18] and, respectively, 5 representatives [43] were identified in the aqueous extracts. The lowest extraction efficiency was observed when the solvents used were ethyl acetate [16] and, respectively, a mixture of solvents, water/diethyl ether/ethyl acetate [17], which led to the identification of only two phenolic acids.
The efficiency of solvents in the extraction of phenolic compounds varies, on the one hand, depending on the matrix, whether it is grassy or lignified (e.g., for the extraction of phenolics from hazelnut skin, maximum efficiency was obtained with 80% acetone [57] and 50% acetone for the walnut septum [58]), and, on the other hand, on the type of phenolic compounds, their polarity, and antioxidant activity being different, depending on the class of phenolic compounds. Some phenolic compounds are more polar, and they are more easily extracted in water, e.g., flavonoids; others are more easily extracted in alcohols or other less polar solvents. Depending on the phenolic compound composition of the plant, the extraction yields differ in different solvents.
Extraction temperature is another extraction parameter that plays a significant role in achieving an optimal quality of the extracted bioactive compounds because a high temperature can either increase the amount of extracted active compounds or cause their degradation. Using a conventional extraction method that was not used in the studies of this review, namely, the aqueous decoction method, a recent study investigated the effects of extraction temperatures (50, 75, and 100 °C) on the extracted tannin (tannic acid) content from the galls of Q. infectoria and on the antioxidant activity. The outcomes showed that the extraction temperatures had significant effects on the response variables (tannin content and antioxidant activity), the highest tannin concentration (2233.82 ± 1.311 mg/g) and the highest antioxidant activity (93.422 ± 0.256%) being obtained at the extraction temperature of 75 °C, this temperature being optimal for the hydrolysis of condensed tannins and the release of more active monomers [59].
In recent years, in addition to the conventional techniques used for the extraction of phenolic compounds from plant materials, unconventional extraction techniques, i.e., assisted extraction methods, such as those involving ultrasounds, microwaves, and pressurized/supercritical fluids, have also begun to be commonly used [54,60]. The innovative extraction techniques including supercritical fluid extraction [9] and ultrasound-assisted extraction via two types of the system, either classical ultrasonic-bath assisted extraction (CUBAE) or ultrasonic-probe assisted extraction (UPAE) [43], were also used in the reviewed studies.
Thus, in research that aimed to extract phenolic acids from oak galls using the UPAE method in the presence of ionic liquid, several variables were investigated on which the efficiency of the extraction of these compounds depends, namely, sonication time, extraction methods, solid-to-solvent ratio, type of solvent, and its concentration. The UPAE method was compared with the CUBAE method and the conventional aqueous extraction (CAE) method, with and without the presence of ionic liquid [43]. In contrast to the results obtained with the conventional extraction techniques (maceration and digestion) [47], the maximum amount of tannic acid (2430.48 mg/g) was extracted when the innovative UPAE extraction technique was used, in the presence of the ionic liquid [Bmim][Tf2N] [43].
A previous study investigated the effect of sonication time (from 1 to 12 h), solvent types (water and Hexadecyltrimethylammonium bromide (CTAB)), and solvent concentration (from 0.05 M to 0.2 M) on the extraction yield of gallic and tannic acids, extracted from Q. infectoria galls, using two ultrasound extraction systems (UPAE and CUBAE), and the results were compared with the conventional extraction system. The results showed that the UPAE extraction technique with 0.1 M CTAB as a solvent led to the maximum extraction yield of gallic acid and tannic acid (2155.77 mg/kg and 15,236.83 mg/kg, respectively) and shortened the extraction time (8 h), being more efficient than the CUBAE method and conventional extraction [61].
In the experiment by Purbowati et al. [9], the supercritical CO2 extraction method using methanol as a co-solvent led in the LC–MS/MS analysis of the extract to a more complete phenolic composition (27 phenolic compounds) and a higher amount compared to the extraction method without using a co-solvent (12 phenolic compounds). Methanol, with its polarity, favors the solubilization and extraction of more polar phenolic compounds, such as phenolic acids and flavonoids (Table 2), and thus improves the extraction yield. By changing the co-solvent concentration, the selectivity of the extraction process can be modulated. In another recent study, which also used the supercritical CO2 method with the addition of methanol as a co-solvent for the extraction of hydrolyzable tannins from Q. infectoria galls, the optimization of the extraction conditions was aimed at the extraction yield and the content of tannic acid and gallic acid. Thus, at the optimal values of the parameters (pressure, temperature, and mean particle size), optimal responses were achieved, such as an increased concentration of gallic acid (96.85 mg/g sample), but also of tannic acid (6149.71 mg/g sample), the latter being higher compared to the tannic acid concentrations reported in the studies selected from this review and obtained with other extraction methods (solvent extraction or UPAE method) [62].

Table 2.
Phenolic compounds identified and quantified in galls of Quercus species.
Another work that aimed to identify and quantify the phenolic compounds from the extracts of the nutgall of Iraqian Aleppo oak (Q. infectoria) with LC-MS/MS used three different solvents and two extraction methods to obtain the extracts. The results showed that the extraction yield was strictly dependent on the nature of the solvents and extraction methods, methanol being the solvent that extracted the most components from the plant, followed by ethanol and water, respectively, and the microwave extraction technique proved to be much more efficient than the conventional one, considering the extraction yield [63].
Literature evidence suggests that these innovative extraction methods are preferred over conventional methods due to their numerous advantages, such as reduction in extraction time, temperature, organic solvent consumption, or reduction in toxic residues, as well as higher yields and improved experimental reproducibility [43,54].
3.3.2. Separation and Characterization of Phenolic Compounds in Quercus sp. Galls
The extracts obtained by using the previously mentioned extraction techniques were complex products that needed to be separated because they contained a variety of natural components, as well as impurities. Separation is a purification technique, and it is frequently combined with characterization techniques to identify various molecules. The methods applied in phenolic compound separation include centrifugation, ultrafiltration, concentration of extracts, solvent separation methods, and chromatographic methods [60]. In this review, centrifugation [47,51] and concentration of the extracts [17,19,33,34,47], but also chromatographic methods [15,33,43], were used to separate the phenolic compounds present in the oak gall extracts.
Regarding the characterization of phenolic compounds, the identification of individual phenolic classes is usually performed with liquid chromatography (LC), gas chromatography (GC), or high-performance liquid chromatography (HPLC) and their detection using sensitive detectors [64].
LC assisted with mass spectrometry (MS) detection is an advanced analytical technique that, in recent years, has been used for the analysis of phenolic compounds due to its high sensitivity and selectivity [64]. Liquid chromatography with tandem mass spectrometry (LC–MS/MS) is considered one of the most reliable techniques for characterizing phenolic compounds [60]. In this review, LC-MS (n = 2) [16,17], LC-MS/MS (n = 3) [9,14,20], and liquid chromatography–electrospray ionization–tandem mass spectrometry (LC-ESI-MS/MS) techniques (n = 1) [18] were employed to assess the phytochemical profiles.
LC-MS is a useful tool in the metabolic profiling of plant samples, which has demonstrated its significant role in the identification, purification, and characterization of phenolic acids and flavonoids from the richest source of phenolic compounds with excellent antioxidant properties, namely, green leafy vegetables [65]. The LC-MS/MS method was also used in a study for the quantitative estimation of five phenolic acids, i.e., gallic acid, ellagic acid, corilaginic acid, caffeic acid, and syringic acid, and three flavonoids, respectively, i.e., rutin hydrate, quercetin, and morin hydrate, in the aqueous and hydroalcoholic extracts of Q. infectoria galls [66].
HPLC is a separation and characterization method, which can be combined with different detectors, such as an ultraviolet–visible (UV) and photodiode-array detector (PDA), to examine phenolic compounds [60]. HPLC coupled with PDA, also known as a diode-array detector (DAD), is the most useful and common method for analyzing the phenolic compounds in plants [64]. Even though LC-MS or LC-MS/MS are useful methods, the HPLC technique with UV detection is more accessible and successfully used in the quantification of phenolic compounds in plant extracts. Indeed, most of the reports in this review employed the HPLC-DAD/HPLC-PDA technique to identify phenolic compounds (n = 10) [8,10,15,21,33,34,40,43,47,51].
HPLC-DAD chromatographic separation was also used in a previous study to separate 13 phenolic acids and derivatives from galls, including hydroxybenzoic acids and hydroxycinnamic acids, among them gallic acid, 3,4-dihydroxybenzoic acid, syringic acid, and ellagic acid [67]. Phenolic acids were mainly detected using UV–visible, DAD, or fluorescence detectors [65]. The HPLC-UV technique was also utilized to detect the compounds gallic acid and 1, 2, 3, 4, 6-O-pentagalloyl glucose in a study that aimed to identify anticancer compounds through a PCA-constructed secondary metabolite map in Galla Chinensis and Galla Turcica gallnuts [68].
GC is considered an ideal method for the separation, identification, and quantification of some phenolic compounds in plants, such as tannins, flavonoids, and anthocyanins [64]. In recent years, due to its great selectivity and sensitivity in quantification, GC coupled with an MS detector has become increasingly common for analyzing complex compounds [60,64]. GC-MS was used for phytochemical characterization of Q. infectoria galls in one of the studies in this review [19]. In a previous study, Hussein et al. [69] also conducted the phytochemical screening of the methanolic dried galls’ extract of Q. infectoria, and the GC-MS analysis of the methanolic extract showed a highly complex profile containing twelve phytochemical compounds.
Thin-layer chromatography (TLC) is a relatively inexpensive chromatographic technique that can separate phenolic compounds in crude plant extracts and detect several substances on the same TLC plate in a relatively short amount of time [64]. This method was also applied in some works of the present review (n = 2) [15,33]. This technique was also employed by Ou et al., who developed a simple, rapid, and efficient TLC chromatographic method for the analysis and quantitative determination of ellagic acid, gallic acid, and methyl gallate in the galls of Q. infectoria Olivier. The conclusions showed that methyl gallate possessed the highest antioxidant efficacy, followed by gallic acid and ellagic acid, and the TLC-DPPH method could be exercised for the screening of antioxidant components [70].
Table 2 summarizes the studies that investigated the presence of phenolic compounds in oak galls and the related methodology.
3.3.3. Phenolic Compounds of Quercus sp. Galls
Both simple phenolic compounds and polyphenols were identified in the galls collected from Quercus sp. In total, 67 phenolic compounds and their isomers or derivatives were described in the eligible studies.
Among the simple phenolic compounds, three subclasses were found in oak galls: simple phenolics, i.e., hydroxyphenols (catechol) and derivatives (2-allyl-5-t-butylhydroquinone), and dihydroxyphenols (pyrogallol) and derivatives (pyrocatechol); coumarins (coumarin); and phenolic acids.
In a previous study, pyrogallol was the major component extracted from Q. infectoria galls that displayed significant anti-Candida activity; however, due to the synergistic effect, the whole plant extract had a more potent antimicrobial activity compared to isolated phytomolecules [71].
From the subclass of phenolic acids, hydroxybenzoic acids (salicylic acid, p-hydroxybenzoic acid), dihydroxybenzoic acids (protocatechuic acid, gentisic acid) and their derivatives (vanillic acid), trihydroxybenzoic acids (gallic acid) and gallic acid derivatives (m-digallic acid, p-digallic acid, digallic methyl ester, digallic dimethyl ester, trigallic dimethyl ester, ellagic acid, syringic acid, 2-O-galloyl hydroxymalonic acid), tetrahydroxybenzoic acids (quinic acid), as well as hydroxycinnamic acids (cinnamic acid, p-coumaric acid, caffeic acid, ferulic acid, isoferulic acid) and their derivatives (chlorogenic acid, rosmarinic acid) were identified.
A survey that aimed to investigate the additional effects of active constituents from a Q. infectoria extract on staphylococcal cytoplasmic membrane function concluded that among the major components of the extract included in the study (ellagic, gallic, syringic, and tannic acids), only gallic acid and tannic acid, respectively, demonstrated good MIC⁄MBC values at the test concentrations and showed activity against methicillin-resistant Staphylococcus aureus [72]. Recent research demonstrated the anti-proliferative effects of two anticancer active compounds, tannic acid and gallic acid, extracted from Q. infectoria galls, on the human glioblastoma multiforme cell line (DBTRG-05MG) [73]. A previous experiment revealed that the ellagic acid glycoside, quercoside, isolated from the ethanolic extract of Q. infectoria Olivier galls, possessed nitric oxide and superoxide inhibiting activity in murine macrophages [74].
The structures of the representative phenolic acids are presented in Figure 2.
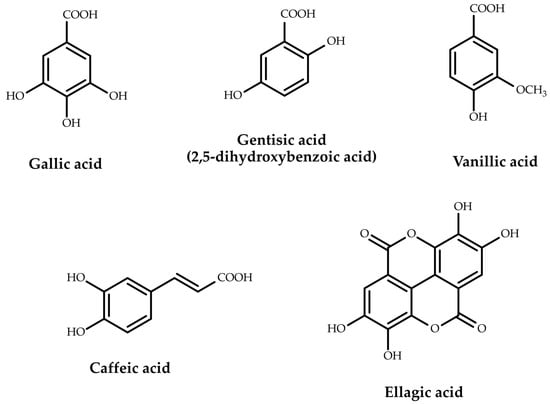
Figure 2.
Representative phenolic acids found in oak galls.
The polyphenolic compounds analyzed in the studies belong to two large polyphenol subclasses, flavonoids and tannins. Four types of flavonoids were identified, including flavones (luteolin, chrysin, apigenin, 7-hydroxyflavone) and their derivatives (apigetrin, lucenin 2), flavonols (kaempferol, myricetin, quercetin, fisetin) and their derivatives (quercitrin, rhamnetin, hyperoside, astragalin, rutin), flavanones (naringenin, hesperetin) and their derivatives (hesperidin, luteolin-7-glucoside, naringin), and flavan-3-ols (catechin, epicatechin). Among the tannins, only the hydrolyzable ones were identified, i.e., gallotannins and ellagitannins. The gallotannins were mainly represented by tannic acid (syn. gallotannin), but also by methyl gallate and mono-, di-, tri-, tetra-, penta-, hexa-, and hepta-galloyl-glucose (Figure 3).
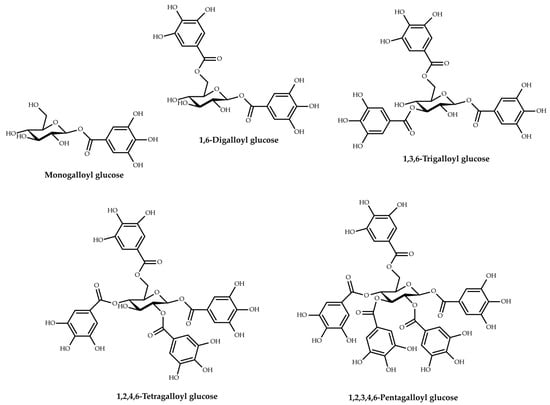
Figure 3.
Key gallotannins reported in oak galls.
A recent investigation that tested the antioxidant activity of Q. infectoria galls, on both chemical and biological models, argued that the strong antioxidant activity of the ethanolic extract (scavenging of DPPH and •OH radicals, Fe2+ chelation, inhibition of lipid peroxidation, and protection of macrophages from oxidative damage induced with tertiary butyl hydroperoxide) could be attributed at least partially to the tannic acid that constituted a major proportion of the extract (19.925%), but also to gallic acid (8.75%) [75]. A former work exposed the inhibitory activity of hexagalloyl glucose isolated from a Q. infectoria gall methanol extract against alpha-glycosidases, which was comparable to the hypoglycemic agent acarbose [76]. Another investigation that studied the inhibition effectiveness and specificity of Aleppo tannin (gallotannin), isolated from the gallnut of the Aleppo oak, on human salivary amylase confirmed that it was a more efficient amylase inhibitor than tannin with a quinic acid core [77].
The ellagitannins were identified in a single study, being represented by galloyl-HHDP-glucose and pedunculagin.
Other phenolic compounds were less analyzed in the included studies, such as benzaldehydes (vanillin), naphthodianthrones (pseudohypericin, hypericin), and prenylated phloroglucinol derivatives (hyperforin), which were identified in one study, and phenolic alcohols (3-hydroxytyrosol) and stilbenes (resveratrol), which were quantified also only in one experiment among the forty-six selected.
Despite the great diversity of structural classes and subclasses assessed in oak galls, the predominant phenolic subclasses were represented by phenolic acids, gallotannins, and flavonoids. Of the analyzed studies, over 28% identified phenolic acids (n = 13), while gallotannins and flavonoids were found in 26.09% (n = 12) and 17.39% (n = 8), respectively.
In quantitative analyses, gallic acid was evaluated in most of the phenolic composition studies (n = 7), followed by ellagic acid (n = 6) and caffeic acid (n = 5), these three representatives also proving to be the most abundant in oak galls compared to the rest of the quantified phenolic acids.
Among the phenolic acids, gallic acid is recognized as the most prevalent hydroxybenzoic acid, being abundant both in natural sources (oak gallnuts/leaves/bark/acorns, pomegranate root bark, berry/tea leaves, many fruits and vegetables), as well as in processed beverages (red wine and green tea) [78,79,80]. Significant amounts of gallic acid were reported in oak galls, both in the case of extraction with a conventional technique (291 mg/g dry weight (dw)) [8] and with ultrasonic-probe assisted extraction (UPAE) (130.76 mg/g dw) [43].
Ellagic acid is a dimeric gallic acid derivative, widely present in fruits (pomegranate, mango, grapes), berries (blackberry, raspberry, blueberry, cranberry, and strawberry), nuts (walnuts, pecans, chestnuts, almonds), seeds, dry fruits, and some types of honey, but also in herbs, roots, and alcoholic beverages matured in oak wooden barrels [78,81]. In oak galls, the highest amounts of ellagic acid (261,997.718 and 187,696.132 μg/g dw, respectively) were reported in two studies by Kılınçarslan Aksoy et al. [10,40]. In the study conducted by Shendge and Kamalapurkar [8], the concentration of ellagic acid (131 mg/g dw) was also much higher than in three other studies (0.64–33.44 mg/g dw).
The results of previous reports confirmed that phenolic acids were widely distributed in all oak matrices, gallic acid being found in leaves and acorns, ellagic acid in leaves, bark, seeds, and wood, and caffeic acid in wood of several species of Quercus [79]. In a study that evaluated the phenolic composition of oak galls, gallic acid and ellagic acid were found to be the most abundant phenolic components in aqueous and hydroalcoholic extracts. For both, gallic acid and ellagic acid, the concentration in the aqueous extract (106,711.25 ± 951.25 μg/g dw and, respectively, 5105.03 ± 102.34 μg/g dw) was higher than in the hydroalcoholic extract (84,613.34 ± 589.12 μg/g dw and 3522.31 ± 82.36 μg/g dw, respectively) [66]. In contrast, another experiment reported a much lower content for gallic acid (3724.12 μg/g dw) in the methanolic extract of Q. infectoria nutgalls [63].
Caffeic acid, a hydroxycinnamic acid derivative, is found in various natural sources including olives, berries, potatoes, and carrots, with coffee beans being particularly rich in this compound [78]. Its concentration in oak galls varied widely among the five studies included in the review, between 0.50 mg/g dw and 589.041 mg/g dw [10,21,34,40,43]. A recent work reported a lower caffeic acid content in oak galls, 0.07 ± 0.01 μg/g dw in the aqueous extract and 1.70 ± 0.16 μg/g dw in the hydroalcoholic extract [66].
Gallotannins, considered the simplest hydrolyzable tannins, are formed by gallic acid molecules bound to a central d-glucose with ester bonds (Figure 3) [82]. Tannic acid (penta-m-digalloyl glucose) is composed of a central glucose esterified to all five hydroxyl moieties with two molecules of gallic acid, totaling ten galloyl groups [83]. The concentration of tannic acid in oak galls was determined in five studies and varied depending on the type of solvent and extraction method used. The lowest amounts were reported in the case of maceration with a mixture of solvents (diethylether/ethanol/water (25:3:1)) (0.016–0.112 mg/g dw) [47], while the highest amounts were obtained using the UPAE method, 2287.90 mg/g dw in the presence of ionic liquid and 776.75–1556.26 mg/g dw in the absence of ionic liquid [43]. Another recent survey [59] reported a concentration of tannic acid (2233.82 ± 1.311 mg/g) in the aqueous decoction of Q. infectoria (Manjakani) galls, which was much higher than the amounts obtained with conventional extraction methods in the works of this review. The same was true for Mohd-Nasir et al. [62], who obtained a higher amount of tannic acid (6149.69 mg/g) in Q. infectoria galls’ extracts in the case of supercritical CO2 extraction versus the results mentioned in our review for unconventional extraction techniques. Methyl gallate was quantified in a single study, with reported concentrations varying between 26.07 and 34.78 mg/g, depending on the size of the Turkish gall powder particles [51].
Flavonoids are bioactive polyphenolic phytochemicals consisting of a 15-carbon (C6–C3–C6) skeleton that is composed of two benzene rings (C6) and a 3-carbon (C3) linking chain [54,84]. They are abundant compounds in nature, being present in most plants and in numerous foods, such as fruits, vegetables, legumes, nuts, medicinal plants, tea, chocolate, or red wine [54,85,86]. In oak galls, the most prevalent flavonoid compound was quercetin, quantified in five phenolic composition studies [10,21,34,40,43], with the highest amount reported in the work of Mohammadzadeh et al. (5.00 mg/g dw) [34]. Other assays reported lower concentrations for quercetin, namely, an amount of 6.36 ± 0.81 μg/g dw in the aqueous extract, 0.38 ± 0.05 μg/g dw in the hydroalcoholic extract [66], and, respectively, 3.7597 μg/g dw in the methanolic extract [63]. Among the flavonoids detected in significant amounts, the highest concentrations were reported for the two flavan-3-ols, epicatechin (171,497.57 μg/g dw) [10] and catechin (15,622.42 μg/g dw) [21], respectively, but also for naringin (19,097.058 μg/g dw) [10] and rutin (10.72 mg/g dw μg/g dw) [34]. Lower concentrations were reported for five other flavonoids, i.e., myricetin (0.05–0.55 mg/g dw), apigenin (0.01–0.09 mg/g dw) [43], quercitrin (89.82 μg/g dw), hesperetin (4.66 μg/g dw), and 7-hydroxyflavone (3.5 μg/g dw) [21], each of them being quantified in a single phenolic composition study. A previous experiment performed the quantitative analysis of several flavonoid compounds in the methanolic extract of Q. infectoria nutgalls, compounds that in the studies of this review were only identified. Thus, the authors quantified hyperoside (44,534 μg/g dw), hesperidin (24.788 μg/g dw), kaempferol (0.6318 μg/g dw), luteolin (0.1357 μg/g dw), naringenin (0.110 μg/g dw), rhamnetin (0.0639 μg/g dw), and fisetin (0.00957 μg/g dw). On the other hand, for the rest of the flavonoids quantified, namely, rutin (2.4745 μg/g dw), myricetin (0.54704 μg/g dw), apigenin (0.0701 μg/g dw), and hesperetin (0.0374 μg/g dw), the results were lower compared to those reported in our review [63].
Despite such a diverse and rich phenolic profile of this plant matrix, studies have shown that the positive activity results could be attributed to the main constituents of Q. infectoria galls, including tannic acid constituents (50–70%), especially tannic acid and gallotannins containing mixtures of polygalloyl groups, and gallic acid, which represents 2–4% of the total compounds, but also to other minor components, such as ellagic acid and syringic acid [7,72,73,75,77].
3.3.4. Non-Phenolic Compounds of Quercus sp. Galls
The largest diversity of non-phenolic molecules, i.e., a total of 34 compounds and nine elements, was reported in the study of Jalill [50], while five other studies identified 16 other non-phenolic compounds [9,19,21,34,43]. Terpenes and terpenoids were present in a larger number, i.e., 17 compounds, followed by lipid compounds, hydrocarbons, and carboxylic acids. The presence of lipid compounds, i.e., fatty acids, fatty amides, and fatty aldehydes, was reported in two of the studies included in the review. The same studies identified aliphatic alcohols in the oak galls [19,50].
Among the six reports that investigated the presence of non-phenolic compounds in oak galls, only three studies performed their quantitative analysis. Carboxylic acids were detected in significant amounts, the highest concentration being reported for malic acid (79.28 mg/g dw) [43], followed by aconitic acid (20.37 mg/g dw) [43] and benzoic acid (9.25 mg/g dw) [34]. Also, Tayel et al. [21] obtained a significant concentration of caffeine (21.676 mg/g dw).
Other surveys that analyzed the non-phenolic phytochemical composition of Q. infectoria galls mainly investigated the volatile and lipid composition, with GC-MS. Thus, a recent study characterized 29 substances in the volatile essential oil of Q. infectoria, the majority component being (Z)-anethole (28.55%) [87]. This was also identified in Jalill’s experiment [50], along with three other main components, pentadecanolide (26.44%), diethyl phthalate (6.46%), and acetoin (5.66%) [87]. In another study, Hussein et al. [69] identified 12 bioactive compounds in the methanolic dried galls’ extract of Q. infectoria, including phytosterols, monoterpenes, or pteridines, some of them being known for their antimicrobial, anti-inflammatory, and antitumor activities; anti-psychotic, mood-stabilizer, and anti-parasite actions; as well as estrogenic, progesterogenic, and anti-infective effects.
Table 3 shows the non-phenolic compounds identified and/or quantified in the oak galls.

Table 3.
Other compounds identified and quantified in galls of Quercus species.
3.4. Biological Activities
3.4.1. In Vitro Activity
Antioxidant and Anti-Inflammatory Activities
The antioxidant properties of oak galls demonstrated in both in vitro and in vivo studies [10,12,15,27,30,33,34,40] could be attributed to their phytochemical profile. Many of their components, such as phenolic acids, flavonoids, and hydrolyzable tannins, but also hydroxyphenols, coumarins, phenolic aldehydes, naphthodianthrones, acyl-phloroglucinols, and phenolic alcohols, showed antioxidant effects through direct free-radical scavenging action or indirect action. Previously, Kaur et al. [75] reported that the polyphenols present in a Q. infectoria gall extract possessed a potent reducing power, scavenging free radicals, such as DPPH (IC50~0.5 μg/mL), ABTS (IC50~1 μg/mL), and hydroxyl (*OH) radicals (IC50~6 μg/mL).
The mechanisms were by complexing some metals involved in the oxidative stress induction or by activating cellular signaling pathways associated with cytoprotective mechanisms: up-regulation of the Nrf2/ARE pathway and down-regulation of the NF-κB transcription factor pathway, followed by reduction in inflammatory processes [88]. Some of these metabolites including flavonoids could induce antioxidant and anti-inflammatory responses through scavenging free radicals, up-regulating HO-1 expression, inhibiting the COX-2 and 5-LOX proinflammatory signaling pathways, or modulating the function stabilization of the intestinal barrier, thus contributing to the intestinal wall and blood–brain barrier integrity via the gut–brain axis [81].
Zang et al. [17] identified nine category constituents including phenolic acids and gallotannins in Turkish galls. Among them, methyl gallate, digallic acid, di-O-galloyl-β-d-glucose, and tri-O-galloyl-β-d-glucose mainly contributed to the anti-inflammatory activity via suppressing the NO, IL-6, and TNF-α production. Similar compounds including phenolic acids (cinnamic acid, p-coumaric acid, ferulic acid) and gallotannins (digalloylglucose), found in other plant matrices, demonstrated active roles against oxidative stress and type 2 diabetes [89]. However, the presence of galls on leaves of Q. robur had a negative effect on cell membrane integrity and the antioxidant potential of the host plant [90].
Recently, the aqueous extract of Q. infectoria galls was suggested to have the potential for augmenting immunomodulatory activity and modulate the innate immune response through cellular-mediated mechanisms [36]. Thus, in gall-extract-treated murine macrophage (J774A.1) cells compared to untreated cells, the phagocytosis increased, while the NO production decreased in a dose-dependent manner. Moreover, the extract lowered IL-4, IL-6, and IL-12 gene expression and improved the output of anti-inflammatory cytokine IL-13, which can inhibit proinflammatory cytokine production in vitro. Previous studies have shown that a Q. infectoria gall extract could suppress oxidative stress and inflammation in murine bone-marrow-derived macrophages by inhibiting the Set-7/NF-κB pathway, therefore controlling chronic inflammation associated with several disorders including age-related diseases [91].
Antimicrobial Activity
Extracts of the Q. infectoria gall were revealed to have broad-spectrum in vitro antimicrobial activity. Our review assayed various studies (Table 1) that determined the antimicrobial activity of a Q. infectoria gall extract against pathogenic organisms and evaluated the morphological changes of extract-treated cells.
A Q. infectoria gall extract possessed efficient antimicrobial activity against Streptococcus mutans, S. sobrinus, and Candida albicans [11]. As this activity was synergistically enhanced in the presence of a Scrophularia striata extract, the two extracts may be used together for preparing dental products with anticariogenic potential.
A further analysis revealed that a Q. infectoria gall extract showed antimicrobial activity against Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Salmonella enterica serovar Typhimurium, and C. albicans. Immersion in a 1% gall extract solution sharply reduced eggshell microbial contamination, while E. coli and S. aureus were completely suppressed after 60 min of immersion. The investigation revealed that gall extracts might be suggested as natural and effective disinfectants [21].
Similarly, the hydroalcoholic extract of Q. infectoria galls manifested high antimicrobial activity against E. coli, S. aureus, S. epidermidis, and Klebsiella pneumonia, as well as good antioxidant capacity due to the presence of polyphenols, especially gallic acid, rutin, quercetin, benzoic acid, and caffeic acid [34]. In addition, an ethanolic extract of Q. infectoria galls exhibited inhibitory and bactericidal effects on strains of E. coli with related antibacterial mechanisms from disruption of the outer wall and cytoplasmic membranes to loss of bacterial cellular integrity [92].
Additionally, Nair et al. [19] corroborated the antibacterial effect of Q. infectoria galls. At a dose rate of 50 mg/mL, the methanolic extract manifested a complete bactericidal effect on S. enterica ser. Typhi and S. enterica ser. Enteritidis, while at lower concentrations, had a significant bacteriostatic effect. At the same time, the antibacterial effect obtained from the combination of a Q. infectoria gall extract and methanolic extract from fruits of Phyllanthus emblica, rich in ellagitannins, was synergic, greater than the sum of the individual effects (p < 0.001) [19].
Yet another study revealed that the ethanol extract of Q. infectoria galls inhibited the growth of all the bacterial strains at a concentration of 1000 μg/mL and, in combination with ceftazidime, exhibited a strong synergistic activity on P. aeruginosa and E. coli [93].
A degree of novelty presented in our research was the use of oak galls’ extracts to prepare metal nanoparticles. Based on the fact that metal nanoparticles have good antimicrobial activity, several studies analyzed the effects of such treatments. The production of these metal nanoparticles employed green synthesis methods using an extract of Q. infectoria galls as a reducing and capping agent. Silver nanoparticles (AgNPs) and a Q. infectoria gall extract inhibited the growth of P. aeruginosa [49]. Silver nanoparticles are well known for antibacterial and immunostimulant activities [94]. In addition, a thermosensitive antibacterial gel from a Q. infectoria gall aqueous extract and AgNPs for the treatment of mouth ulcers and gum disorder were developed [8]. Bioactive compounds of oak galls, such as tannic, gallic, and ellagic acids, in addition to the nanoparticles that can penetrate the cell membrane and prevent the replication via interfering with bacterial DNA, showed in vitro activity against P. aeruginosa, S. aureus, and E. coli. The formulated gel, with antibacterial activity greater than the commercial gel containing only tannic acid, may be used as an internal topical in oral infectious disorders.
These results were consistent with other findings showing that the gall extract and AgNPs revealed excellent antioxidant capacity and antibacterial activity against Klebsiella pneumonia and Enterococcus faecalis, besides P. aeruginosa and S. aureus. Still, AgNPs significantly exhibited more antibacterial activities compared to the galls’ extract, with the highest antibacterial activity against K. pneumonia. Furthermore, both treatments exposed anticancer activity against human breast cancer cells (MCF-7); yet again, AgNPs exhibited stronger cytotoxic activity [27].
The antibacterial activity of a Q. infectoria gall extract and copper oxide nanoparticles (CuONPs) was evaluated against two Gram-positive bacteria and four Gram-negative bacteria, including P. aeruginosa and E. coli. This study, in which Q. infectoria galls were used for the first time to synthesize CuONPs, concluded that both treatments showed good antibacterial activity against Gram-positive and Gram-negative bacteria, but CuONPs significantly displayed more antibacterial activity compared to the oak gall extract [26]. Moreover, the extract of the Q. infectoria gall combined with a Calendula officinalis flower extract and CuONPs demonstrated considerable antibacterial function and significant wound-healing potentials [24].
P. aeruginosa, one of the most virulent Gram-negative bacterial pathogens in humans, causes many acute and chronic infections through a plethora of cytotoxins [95]. Ahmed and Salih confirmed the antibacterial activity of Q. infectoria gall extracts against P. aeruginosa [20]. This activity involved two mechanisms, either a direct growth inhibitory effect or the down-regulation of virulence-regulator genes. The potential ability to reduce the expression of these genes could be a valuable prophylactic and therapeutic use of oak gall extracts.
The pathogenicity of Helicobacter pylori can also be altered with Q. infectoria gall extracts. This pathogenic bacterium may be found in human gastric mucosa and can cause chronic stomach inflammation, peptic ulcer, or gastric adenocarcinoma. Attia et al. [14] evaluated the action of an oak gall extract and zinc oxide nanoparticles based on a Q. infectoria gall extract (Qi-ZnONPs) against H. pylori. Although both treatments exhibited moderate antibacterial activity, the Qi-ZnONPs displayed greater inhibition (98.4%) compared to amoxicillin (93.2%) and clarithromycin (90.7%). Moreover, the study concludes that the combination of Qi-ZnONPs and amoxicillin (4:1) is a potential candidate for an effective anti-H. pylori drug.
Ethanol and water extracts of Q. infectoria galls also demonstrated strong bacteriostatic activity against Vibrio parahaemolyticus and antibacterial efficacy against all bacterial strains. Besides these effects, an herbal formulation containing Nigella sativa seeds, Piper retrofractum fruit, Punica granatum pericarp, and Q. infectoria galls reduced the swarming motility of E. coli and inhibited biofilm production by S. aureus [41]. Another experiment proved that a methanolic extract of oak galls was more effective than a water extract against S. sanguis, S. aureus, S. mutans, and S. salivarius [96].
Moreover, a Q. infectoria gall extract combined with cetrimonium bromide displayed efficacy in the removal of S. enterica ser. Typhimurium biofilm, suggesting an alternative to remove biofilm from food contact surfaces in the household and food industry [97]. Biofilm defends bacteria from the surrounding environment, including antibiotic, antiseptic, and chemotherapeutic treatments. Periodontal diseases and dental caries are biofilm-mediated and are major public health concerns [98]. Q. infectoria gall extracts disclosed significant antibiofilm (92%) and antibacterial (19.00 ± 7.07 mm) activities against Rothia dentocariosa, a Gram-positive bacterial pathogen responsible for causing dental caries through biofilm formation [46]. Likewise, oak gall extracts showed antimicrobial activity against other oral bacteria. Among tested bacteria, the extract showed good antibacterial activity and ability to reduce biofilm against S. aureus and S. mutans, respectively [39]. An early report also exposed that Q. infectoria gall extracts had significant (p < 0.05) biofilm removal activity and antibacterial effects against S. mutans [99]. Thus, the oak galls may be considered preventing therapeutic agents of biofilm formation by oral pathogens.
The hydroalcoholic extract of Q. infectoria galls was also evaluated on Aggregatibacter actinomycetemcomitans, a bacterium associated with aggressive forms of periodontitis [100]. This in vitro study concluded that a hydroalcoholic extract of Q. infectoria galls may be used in mouthwashes to alter periodontal biofilm. Similarly, methanol and acetone extracts of Q. infectoria galls exhibited antibacterial activity against two Gram-positive (S. mutans and S. salivarius) and two Gram-negative bacteria (Porphyromonas gingivalis and Fusobacterium nucleatum) known to cause dental caries and periodontitis [101].
In a recent review, Taib et al. [102] stated that Q. infectoria galls possessed astringent, antiseptic, anti-inflammatory, and cicatrizing properties. Indeed, due to the fact that Q. infectoria galls contain large amounts of gallotannins and other bioactive components that have an astringent action on vessels and tissues, an oak gall extract could be used in preparations used to inhibit the growth of oral bacteria, with therapeutic effects in patients with gingivitis and bacterial plaque [8,37], including in the treatment of periodontitis, a pathology that frequently affects the elderly and/or patients with aging-related pathologies [103].
The antimicrobial action of a Q. infectoria gall extract was applied against skin pathogens with S. aureus strains being more sensitive than C. albicans strains [53]. Previously, the extracts of the Q. infectoria gall exhibited promising in vitro antibacterial activities, especially against Gram-positive bacteria including S. aureus [104], and displayed anti-Candida activity and could treat yeast infections caused by Candida species [71].
The bioactive compounds obtained from Q. infectoria galls also demonstrated antifungal activity against Penicillium expansum and Aspergillus flavus [25]. Both these pathogenic fungi produce mycotoxins, which can be toxic to humans. P. expansum produces patulin, a neurotoxic metabolite particularly for children [105], while Aspergillus sp. initiates aspergillosis, an infection usually of the lungs that may compromise the immune system and cause complications in the respiratory disorder population [106].
The results of a broth microdilution assay confirmed that the aqueous Q. infectoria gall extract displayed antimicrobial inhibition and killing activity against two pathogenic Leptospira interrogans isolates, therefore showing potential in the treatment of leptospirosis [52].
The reduced efficacy of the antimalarial medicines requires the need to develop new drugs that can target Plasmodium falciparum, the parasite causing malaria, one of the leading causes of death worldwide. Two experiments reported interesting in vitro antimalarial effects of oak gall extracts. Thus, the acetone and methanol extracts of Q. infectoria galls displayed promising antimalarial activity (IC50 = 5.85 ± 1.64 and 10.31 ± 1.90 μg/mL, respectively), while ethanol and aqueous extracts showed low activity [42]. Furthermore, acetone extract treatment significantly (p < 0.001) changed the pH of the digestive vacuole of the malaria parasite, P. falciparum [35]. New findings confirmed these results. Hence, ellagic acid, the phenolic compound found in oak galls, presented strong antimalarial activity similar to a standard drug, artemisinin, while the pH of the digestive vacuole of ellagic-acid-treated parasites was significantly altered (pH = 6.11 to 6.74, p < 0.001) in a concentration-dependent manner versus untreated parasites [107].
Considering these outcomes, the extract of Q. infectoria galls is a promising antimalarial treatment and could be used as a primary substance in treating different microbial infections and oxidative-stress-related diseases.
Anticancer Activity
Despite advances in treatment strategies, cancer statistic data show that the prevalence of cancer continues to rise worldwide. Due to the fact that conventional cancer treatments manifest low cure rates and numerous adverse effects, many cancer therapy strategies have lately included natural products, usually well tolerated even at high dosages, that can sensitize cancer cells, inhibit tumor growth and proliferation, and induce cell cycle arrest and apoptosis, thus representing a promising approach in the therapy of cancer. Several studies that assessed gall extracts reached outcomes consistent with these findings (Table 1).
Bioactive compounds from a water extract of Q. infectoria galls produced by Cynips gallae tinctoriae wasps contributed to the cytotoxic effect on colorectal cancer (CRC) cells. This cytotoxicity was related to the intracellular ROS accumulation, which triggered cancer cell growth limitation and autophagic cell death via inhibiting the AKT/mTOR signaling pathway. In addition, the gall extract significantly suppressed the epithelial mesenchymal transition (EMT) process known to be involved in tumorigenesis and migration of cancer cells. In addition, the activated extracellular signal-regulated kinase (Erk) signaling pathway promoted the autophagic CRC cell death [45].
A recent survey also explored the cytotoxic effects of galls of Q. brantii. The results showed that the extract at a concentration of 0.05 mg/mL significantly (p < 0.001) increased cytotoxicity, ROS formation, lipid peroxidation, and cytochrome-c release in A375 and SK-MEL-3 melanoma versus AGO-1522 normal human fibroblast cell lines [108].
A further analysis revealed the potent cytotoxic activity of a Q. infectoria gall extract against cervical cancer (HeLa) cells (IC50 = 6.33 ± 0.33 μg/mL) regulated with apoptotic cell death characterized by chromatin and nuclear condensation, DNA fragmentation, as well as apoptotic body formation [44]. Moreover, the Q. infectoria gall extract was shown to induce HeLa cell apoptosis via activation of caspase-8 and caspase-9 [28]. Ismail et al. [32] also demonstrated the cytotoxicity of Q. infectoria gall extracts on HeLa cells. The cancerous cells experienced apoptosis in response to the treatment, which was noticed in annexin V/PI staining and in acridine orange and propidium iodide (AO/PI) stained cells compared to the control (p < 0.05). These studies indicated that oak gall extracts significantly inhibited HeLa cell growth via apoptosis induction.
In the study of Jalill [50], all concentrations of Q. infectoria gall extracts decreased the mouse mammary carcinoma cell line, with IC50 = 0.2 mg/mL. Volatile compounds, such as eucalyptol and eugenol, found in gall extracts could be responsible for this activity. Eucalyptol and eugenol, known antioxidants [109], could suppress production of α-TNF, interleukin-1β, and leukotrienes, and inhibit human cancer cell proliferation through cell cycle arrest and autophagic and apoptotic effects [110,111].
Kilincarslan Aksoy’s research team analyzed the gall of Andricus, a genus of oak gall wasps. According to the outcomes, both A. tomentosus and A. sternlichti gall extracts contained important amounts of phenolics, flavonoids, and tannins associated with antioxidant, cytotoxic, and antiproliferative activities [10,40].
The regulation of the immune system is essential for prevention and treatment of infection, autoimmune diseases, and cancer.
Concomitantly, Kamarudin et al. [15] reported that specific active constituents of Q. infectoria galls have the potential to inhibit glioblastoma multiforme (GBM), a highly invasive stage IV malignant brain tumor. In this experiment, a two-phase system consisting of aqueous soxhlet extraction and methanolic enrichment fractionation was utilized to extract gallotannin, an anticancer component. This optimized system successfully produced a powerful fraction (F4) with around 71% gallotannin that had significantly higher antioxidant activities compared to its crude extract and to a commercial synthetic pure gallotannin. Related to its content and higher antioxidant property, the F4 was also established to better suppress GBM cell growth compared to the gall crude extract and pure gallotannin. Interestingly, the inhibitory capacity exerted with the F4 fraction on GBM cells was comparable to the effects of two clinically used chemo-drugs, Tamoxifen and Temozolomide, signaling the high efficiency of an enriched fraction of a Q. infectoria gall extract in fighting cancer cells in vitro.
3.4.2. In Vivo Activity
Recent results revealed that the phytochemical bioactive molecules (gallotannins, gallic and elagic acids) of oak galls might be responsible for the diverse biological in vivo activities including anti-inflammatory, antioxidant, and antimicrobial properties, or anticancer potential (Table 1).
Ulcerative colitis (UC) is an inflammatory disease that belongs to the inflammatory bowel disease group describing chronic inflammatory conditions of the gastrointestinal tract and occurring from the proximal to the distal ends of the colon. Its etiology is not well defined, one possible cause being the proinflammatory cytokines that initiate an inflammatory event. Since the recommended anti-UC medications have only modest therapeutic effects, which could be associated with serious side effects, alternative therapeutic strategies with no toxicity have recently been explored.
According to the outcomes of an animal investigation, a rich fraction of a Q. infectoria gall extract, which included methyl gallate, digallic acid, di-O-galloyl-β-d-glucose, and tri-O-galloyl-β-d-glucose, had protective effects on the colon length of UC mice and ameliorated colon shortening, one of the parameters in the assessment of colonic inflammation [17]. The treatment exposed antioxidant and anti-inflammatory potential, significantly decreasing IL-1β, IL-6, TNF-α, ICAM-1, and TLR4 levels and inhibiting the NF-κB signaling pathway. Previously, Khanavi et al. [112] showed that the extract of the Q. brantii gall exerted an antioxidant effect by lowering the levels of cellular lipid peroxidation, and anti-inflammatory capacity via decreasing TNF-α and IL-1β levels, all biochemical and pathological biomarkers of UC.
A further analysis revealed that microcapsules of gallotannins isolated from Q. infectoria galls combined with iron (III) displayed anti-inflammatory effects in Kunming mice with induced UC [16]. The bioactive components were prone to attach to the surface of the inflamed colon epithelium, inhibit the plasma levels of TNF-α and IL-1β, and alleviate UC symptoms.
The gut microbiota and the balance between beneficial and pathogenic bacteria have a strong influence in many disease processes. The dysbiosis of the gut microbiome is a key pathogenetic mechanism, and the pathogenic bacteria in the UC animal intestinal tract were correlated with proinflammatory factors, while beneficial bacteria were linked with anti-inflammatory markers [113]. Several studies exposed that plant bioactive components could modulate the composition of intestinal microorganisms by stimulating beneficial bacteria and reducing pathogenic bacteria, thus promoting the expression of tight junction proteins, such as occludin and zonula occludens-1, in order to conserve the intestinal mucosal barrier function and prevent UC [114]. The experiment of Yu et al. [48], based on the idea that plant extracts might treat UC via intestinal flora modulation, was in line with the above studies. The treatment with extracts of Q. infectoria galls in UC mice reduced harmful bacteria, such as Helicobacter, Bilophila, and Acinetobacter, while the levels of SCFA-producing bacteria (e.g., Bacteriodes, Allobaculum, Blautia, Butyricimonas) and anti-inflammatory bacteria, Lactococcus and Bifidobacterium, were significantly increased, these results emphasizing the modulation of intestinal flora as another mechanism of Q. infectoria galls in treating UC.
Taken together, the preceding outcomes highlight that oak galls could efficiently modify UC inflammatory mediators and pathological markers, and, hence, might be promising natural agents in the management of UC.
Diabetes mellitus, an age-related chronic metabolic disorder characterized by hyperglycemia, polyuria, and polyphagia, leads to secondary pathophysiological dysfunction in various tissues [115]. Diabetes mellitus and thyroid diseases are two endocrine metabolic disorders that tend to coexist in humans, since thyroid hormones regulate the insulin secretion of pancreatic beta cells and regulate glucose homeostasis [116]. Furthermore, diabetes mellitus creates male infertility via an increase in ROS levels and a cellular antioxidant activity decrease [117].
It has been exposed that numerous plant extracts, due to their phytochemical compounds, have antioxidant action and roles in the antihyperglycemic activity and diabetes management [118]. Interestingly, depending on the gall-inducing species and the host plant species, it was noticed that some galls have higher carotenoid and polyphenol concentrations, which might be mechanisms to maintain oxidative homeostasis [119]. The oak galls manifested promising in vivo results against diabetic complications in thyroid gland functions [31]. The treatment with a Q. infectoria gall extract (500 mg and 1000 mg/kg bw for 15 days) significantly ameliorated the concentrations of both thyroid hormones, triiodothyronine (T3) and thyroxine (T4), demonstrating positive outcomes in the function of the thyroid gland usually impaired in diabetes. Also, the treatment induced an antihyperglycemic effect in diabetic rats, significantly decreasing serum blood glucose almost to normal levels. Lower intestinal glucose absorption or increased insulin secretion could be the mechanisms involved in this action [120].
Furthermore, oak galls showed wound healing beneficial effects in diabetic animals [30]. The administration of an ointment prepared from a hydroethanolic extract of Q. infectoria galls activated open wound healing in a diabetic mouse model by increasing collagen deposition, antioxidant capacity, and cellular proliferation, while the concentrations of malondialdehyde and proinflammatory IL-6 and TNF-α cytokines were decreased. A past study also revealed that a pharmaceutically formulated topical agent based on the antibacterial and antioxidant activities of a Q. infectoria gall extract enhanced the wound healing process in diabetic rats [121].
Non-steroidal anti-inflammatory drugs (NSAIDs) are commonly used to treat pain and fever via inhibiting the activity of cyclooxygenase enzymes (COX-1 and COX-2). Paracetamol (acetaminophen) is an NSAID that, for an acute overdose, could cause hepatotoxicity manifested with hepatic glutathione depletion, significant oxidative stress, and inflammatory effects [122]. Recent findings revealed that various plant extracts reduced paracetamol-induced toxicity through hepatoprotective and antioxidant activity mechanisms [123]. Likewise, oak galls showed promising results. Thus, the treatment with a Q. infectoria gall extract, 250 mg/kg/day for 3 consecutive days, significantly defended against paracetamol-induced toxicity via reducing oxidative stress and inflammatory and tissue-damaging effects (p < 0.001) in mice [4]. Moreover, the same doses of an oak gall extract lowered serum cholesterol and triglycerides, and restored serum albumin, denoting cellular preventive and tissue-protective effects [23]. Additionally, hyperlipidemic rabbits fed Q. infectoria gall extracts had significantly (p < 0.001) decreased plasma levels of TC, LDL, and TG, revealing the atherogenic and hypolipidemic activities of oak galls [124].
These findings are in agreement with recent studies reporting that natural flavonoids, such as naringenin and kaempferol, or flavonoid glycosides, identified and quantified in galls of Quercus species (Table 2), presented hepatoprotective effects in several animal species due to the antioxidant, anti-inflammatory, and anti-apoptotic activities [125,126].
Carcinogenic substances associated with environmental pollution could lead to uncontrolled growth of cutaneous cells into squamous cell carcinoma (SCC), a common type of epidermal neoplasia [127]. A new study disclosed that in mice orally treated with 2 g/kg of a Q. infectoria gall extract, the mouse skin induced tumorigenesis showed a significant reduction in tumor incidence and yield, as well as the number of papilloma, besides a significant increase in the average latent period as compared to the control group [29]. The antitumor activity showed with the Q. infectoria gall extract could be due to its phytochemical profile. Hence, roburic acid, a tetracyclic triterpene acid isolated from oak galls, exhibited anti-inflammatory activity and antitumor effects through inhibition of the TNF-induced NF-κB signaling pathway. Moreover, it displayed antitumor activity both in vitro and in vivo by stimulating G0/G1 cell cycle arrest and apoptosis in colorectal cancer cells [128].
Tannic acid, another bioactive compound found in oak galls in the form of gallotannins as previously discussed, was demonstrated to exert antioxidant and anti-inflammatory activity via its many hydroxyl groups, as well as anticancer action by inducing apoptosis in several cancer cell types [129]. Moreover, the cumulative concentrations of polyphenols from crude extracts of Q. floribunda galls in various solvents exhibited in vivo anti-inflammatory, analgesic, and antipyretic activities, as well as in vitro antioxidant capacity [130].
A recent study performed on human skin showed that an emulsion enriched with a Q. infectoria gall extract had potent antioxidant capacity and improved the mechanical properties of skin, including moisture and elasticity improvement, and reduced pores and sebum levels, which could have anti-aging effects [12]. In addition, the topical administration of a gall-extract-enriched emulsion possessed an anti-inflammatory effect and wound healing activity due to the capacity to modify the energy metabolism and protein production in bacteria, such as S. aureus [131]. These findings are in line with the results of Kaur et al. [132], which indicated in vivo anti-inflammatory activity of Q. infectoria gall extracts; the topical application controlled ear inflammation, while oral extract administration significantly suppressed carrageenan, histamine, and prostaglandin E2 induced paw edemas.
ROS and the inflammatory process associated with venous injury affecting the valves and venous wall induce senescence in various cell populations, including keratinocytes and fibroblasts, impeding wound healing in patients with varicose ulcers [133]. Through the excellent antimicrobial, antioxidant, and anti-inflammatory properties and through the ability to promote wound healing [24], Q. infectoria gall extracts could be included in preparations intended for the topical treatment of varicose ulcers, a pathology often encountered in the elderly with peripheral venous circulation problems. The wound-healing potential of topical treatment with Q. infectoria galls has also been demonstrated previously in an animal model of a diabetic foot [30,121].
It is encouraging that the aqueous extracts of oak galls did not induce lethality and acute toxic effects in mice (the maximum tolerance dose > 10 g/kg bw for rectal administration). Also, the extracts did not induce local mucosal irritation at the level of the colon and anal tissues in rabbits in the doses tested (the rabbit being the most sensitive species for this test) and there was no chronic toxicity or mortality in the groups of exposed Wistar rats compared to the control group [134].
However, due to the fact that higher doses (500 and 1000 mg/kg bw) of oak gall extracts were found to cause microscopic lesions in some rat tissues, including the liver, kidneys, heart, or lungs, after daily repeated exposure (28 days), the maximum dosage level should be limited [1].
4. Conclusions
In the last decades, plant-derived extracts have received increased attention, the existing scientific evidence underlining their important contribution in the prevention and/or treatment of various diseases, many of these diseases having oxidative stress as the basis of their etiology.
In this sense, the present systematic review summarized the literature available from the last 5 years that reported data on the phytochemical profile and pharmacological effects of the extracts obtained from the galls of Quercus sp. This literature review resulted in a comprehensive report on the phytochemical composition of this plant matrix and its health benefits, with a view of exploiting it as an important natural source in phytotherapy and pharmacotherapy. As highlighted in our analysis, the biological properties of oak galls can be attributed to their diverse and rich phytochemical profile, as the predominant representatives are phenolic acids, flavonoids, and hydrolyzable tannins, followed by other phenolic constituents, i.e., hydroxyphenols, coumarins, phenolic aldehydes, naphthodianthrones, acyl-phloroglucinols, and phenolic alcohols, but also non-phenolic constituents, i.e., terpenes and terpenoids, lipid compounds, carboxylic acids, and minerals. In vitro experiments have highlighted the strong antioxidant capacity and anti-inflammatory effects and antibacterial, antifungal, antimalarial, and antitumor activities of oak gall extracts, as well as the anti-aging skin properties. Promising results have been obtained in the last 5 years with metal nanoparticles (silver, zinc, copper) prepared with green methods using oak gall extracts, particularly antibacterial efficacy, increased efficacy in wound healing, as well as anticancer and anti-aging potential. In vivo experiments confirmed the outcomes obtained with in vitro studies, such as antioxidant and anti-inflammatory capacity or the anticarcinogenic activity, and also revealed the hypoglycemic potential. Furthermore, oak gall extracts exposed in vivo antibacterial activity with wound healing and skin protection effects and even improved the retention of gum tissue in human patients diagnosed with gingivitis by inhibiting the growth of oral bacteria and by having an anti-inflammatory effect. Notably, the aqueous extract of Q. infectoria galls did not show evident toxicity signs and mortality in acute and chronic treatment in rodents.
Further pharmaceutical, in vivo, and clinical scientific investigations are needed to incorporate the oak gall extracts into proper pharmaceutical forms and exploit them for therapeutic or cosmetic purposes. Based on the present systematic review, oak galls can be considered a likely candidate for the management of various pathologies, mainly associated with oxidative stress and chronic inflammation. Future human research should confirm the preclinical evidence and find causality between bioactive compounds from Quercus galls and prevention or eventual treatment of some age-related diseases including cardiovascular pathologies, type 2 diabetes, or cancer.
Author Contributions
Conceptualization, R.B., M.E.R. and D.-S.P.; methodology, R.B.; investigation, R.B., M.E.R. and D.-S.P.; writing—original draft preparation, R.B., M.E.R. and D.-S.P.; writing—review and editing, R.B., L.F., M.E.R. and D.-S.P.; supervision, M.E.R. and D.-S.P.; project administration, R.B. and L.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shourmij, M.; Khalili Fard, J.; Najafizadeh, P.; Mousavi, Z. Safety assessment of the Quercus brantii gall hydroalcoholic extract: Single and repeated oral dose toxicity studies. Iran. J. Basic. Med. Sci. 2022, 25, 1389–1395. [Google Scholar] [CrossRef]
- Mahboubi, M. Quercus infectoria fruit hulls and galls and female genital disorders. Clin. Phytosci. 2020, 6, 44. [Google Scholar] [CrossRef]
- Jain, M.; Chahar, P.; Jain, V.; Sharma, A.; Yadav, N.R. Role of Quercus infectoria in health and oral health—A Review. Int. J. Green Pharm. 2019, 13, 180. [Google Scholar]
- Abdallah, A.; Bafail, R.; Zaman, A.; Aldhafiri, A.; Alalawi, A.; Omran, F.; Baghdadi, H.H.; A Abdellah, W.; Alsharif, A.M.; Al Thagfan, S.S.; et al. Acute paracetamol toxicity-induced inflammatory and oxidative effects are relieved by Aleppo galls: A novel experimental study. Int. J. Physiol. Pathophysiol. Pharmacol. 2023, 15, 1–11. [Google Scholar]
- Askari, S.F.; Azadi, A.; Namavar Jahromi, B.; Tansaz, M.; MirzapourNasiri, A.; Mohagheghzadeh, A.; Badr, P. A comprehensive review about Quercus infectoria G. Olivier gall. Res. J. Pharmacogn. (RJP) 2020, 7, 67–75. [Google Scholar]
- Gao, J.; Yang, X.; Yin, W.; Li, M. Gallnuts: A Potential Treasure in Anticancer Drug Discovery. Evid. Based Complement. Altern. Med. 2018, 2018, 4930371. [Google Scholar] [CrossRef]
- Elham, A.; Arken, M.; Kalimanjan, G.; Arkin, A.; Iminjan, M. A review of the phytochemical, pharmacological, pharmacokinetic, and toxicological evaluation of Quercus Infectoria galls. J. Ethnopharmacol. 2021, 273, 113592. [Google Scholar] [CrossRef]
- Shendge, A.R.; Kamalapurkar, K.A. Design and characterization of topical antibacterial formulation containing extract of Quercus Infectoria galls. Int. J. Pharm. Sci. Res. 2023, 14, 1220–1241. [Google Scholar]
- Purbowati, R.; Taufikurohmah, T.; Syahrani, A. Green extraction of Quercus infectoria gall with supercritical CO2 and methanol co-solvent. Environ. Sci. Pollut. Res. 2023. [Google Scholar] [CrossRef]
- Kılınçarslan Aksoy, Ö.; Seçme, M.; Mammadov, R. Antioxidant, Cytotoxicity, Apoptotic Properties of Extracts of Andricus sternlichti galls and Their Phenolic Characterisation by HPLC. Chem Biodivers 2023, 20, e202200742. [Google Scholar] [CrossRef]
- Falakdin, P.; Dastan, D.; Pourmoslemi, S. Combined Antimicrobial Activity of Extracts from Quercus infectoria Galls and Scrophularia striata Aerial Parts for an Anticariogenic Herbal Mouthwash. J. Pharmacopunct. 2023, 26, 44–52. [Google Scholar] [CrossRef]
- Choudhry, A.; Akhtar, N. Formulation, characterization of Quercus infectoria (Olivier) emulsions, and in vitro, in vivo evaluation as cosmeceutical formulation. J. Cosmet. Dermatol. 2023, 1–11. [Google Scholar] [CrossRef]
- Sariozlu, N.Y.; Kivanc, M. Gallnuts (Quercus infectoria Oliv. and Rhus chinensis Mill.) and Their Usage in Health. In Nuts and Seeds in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2011; pp. 505–511. [Google Scholar] [CrossRef]
- Attia, H.G.; Albarqi, H.A.; Said, I.G.; Alqahtani, O.; Raey, M.A.E.I. Synergistic Effect between Amoxicillin and Zinc Oxide Nanoparticles Reduced by Oak Gall Extract against Helicobacter pylori. Molecules 2022, 27, 4559. [Google Scholar] [CrossRef]
- Kamarudin, N.A.; Nik Salleh, N.N.H.; Tan, S.C. Gallotannin-enriched fraction from quercus infectoria galls as an antioxidant and inhibitory agent against human glioblastoma multiforme. Plants 2021, 10, 2581. [Google Scholar] [CrossRef]
- Zhang, X.; Zang, J.; Ma, S.; Yu, W.; Long, F.; Qi, R.; Guo, G.; Zhou, L.; Han, B. Hollow Microcapsules with Ulcerative Colitis Therapeutic Effects Made of Multifunctional Turkish Galls Extraction. ACS Appl. Mater. Interfaces 2019, 11, 25054–25065. [Google Scholar] [CrossRef]
- Zang, J.; Ma, S.; Wang, C.; Guo, G.; Zhou, L.; Tian, X.; Lv, M.; Zhang, J.; Han, B. Screening for active constituents in Turkish galls against ulcerative colitis by mass spectrometry guided preparative chromatography strategy: In silico, in vitro and in vivo study. Food Funct. 2018, 9, 5124–5138. [Google Scholar] [CrossRef]
- Ma, S.; Qin, H.; Jiang, M.; Wang, J.; Wang, W.; Guo, G.; Zhou, L.; Chen, W.; Han, B. Identification and Comparison of Tannins in Gall of Rhus chinensis Mill. and Gall of Quercus infectoria Oliv. by High-Performance Liquid Chromatography-Electrospray Mass Spectrometry. J. Chromatogr. Sci. 2020, 58, 403–410. [Google Scholar] [CrossRef]
- Nair, A.; Balasaravanan, T.; Jadhav, S.; Mohan, V.; Kumar, C. Harnessing the antibacterial activity of Quercus infectoria and Phyllanthus emblica against antibiotic-resistant Salmonella Typhi and Salmonella Enteritidis of poultry origin. Vet. World 2020, 13, 1388–1396. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Salih, F.A. Quercus infectoria gall extracts reduce quorum sensing-controlled virulence factors production and biofilm formation in Pseudomonas aeruginosa recovered from burn wounds. BMC Complement. Altern. Med. 2019, 19, 177. [Google Scholar] [CrossRef]
- Tayel, A.A.; El-Sedfy, M.A.; Ibrahim, A.I.; Moussa, S.H. Application of Quercus infectoria extract as a natural antimicrobial agent for chicken egg decontamination. Rev. Argent. Microbiol. 2018, 50, 391–397. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, A.A.M.; Albadawi, E.A.; Aboonq, M.S.; Desouky, M.K.; Ahmed, A.R.; Bafail, R.; Abdel-Halim, O.B.; AbdElmoniem, M.M.; Aldhafiri, A.J.; Alalawi, A.; et al. Aleppo galls alleviate paracetamol-induced hepatotoxicity and tissue damage: An experimental study. Int. J. Biochem. Mol. Biol. 2023, 14, 1–9. [Google Scholar] [PubMed]
- Fahimirad, S.; Satei, P.; Ganji, A.; Abtahi, H. Wound healing performance of PVA/PCL based electrospun nanofiber incorporated green synthetized CuNPs and Quercus infectoria extracts. J. Biomater. Sci. Polym. Ed. 2023, 34, 277–301. [Google Scholar] [CrossRef] [PubMed]
- Kadium, S.; Abd Al-Raouf Ammar Semysim, E.; Sahib, R.A. Antifungal Activity of Phenols Compound Separated from Quercus infectoria and Citrullus colocynthis against Toxic Fungi. Arch. Razi. Inst. 2023, 78, 297–303. [Google Scholar]
- Khatamifar, M.; Fatemi, S.J. Green synthesis of pure copper oxide nanoparticles using Quercus infectoria galls extract, thermal behavior and their antimicrobial effects. Part. Sci. Technol. 2022, 40, 18–26. [Google Scholar] [CrossRef]
- Khatamifar, M.; Fatemi, S.J.; Torkzadeh-Mahani, M.; Mohammadi, M.; Hassanshahian, M. Green and eco-friendly synthesis of silver nanoparticles by Quercus infectoria galls extract: Thermal behavior, antibacterial, antioxidant and anticancer properties. Part. Sci. Technol. 2022, 40, 281–289. [Google Scholar] [CrossRef]
- Abdullah, H.; Ismail, I.; Suppian, R. Induction of apoptosis in hela cervical cancer cells treated with aqueous and supercritical fluid extracts of quercus infectoria. Res. J. Pharmacogn. 2021, 8, 63–77. [Google Scholar]
- Amedi, S.I.; Mohammad, B.M. Chemopreventive effect of Quercus infectoria galls on DMBA induced mouse skin tumorigenesis. Iraqi J. Vet. Sci. 2021, 35, 57–64. [Google Scholar]
- Dardmah, F.; Farahpour, M.R. Quercus infectoria gall extract aids wound healing in a streptozocin-induced diabetic mouse model. J. Wound Care 2021, 30, 618–625. [Google Scholar] [CrossRef]
- Ibrahim, S.H. Impact of Quercus infectoria Galls Extract on Thyroid Gland and Testicular Functions in Diabetic Rats. Iraqi J. Vet. Med. 2021, 45, 51–59. [Google Scholar] [CrossRef]
- Ismail, I.; Suppian, R.; Mohamad, H.; Mohd Radzi, S.A.; Abdullah, H. In vitro cytotoxicity and apoptosis-inducing activity of quercus infectoria extracts in HeLa cells. Pharmacogn. J. 2021, 13, 401–410. [Google Scholar] [CrossRef]
- Kamarudin, N.A.; Muhamad, N.; Salleh, N.N.H.N.; Tan, S.C. Impact of solvent selection on phytochemical content, recovery of tannin and antioxidant activity of quercus infectoria galls. Pharmacogn. J. 2021, 13, 1195–1204. [Google Scholar] [CrossRef]
- Mohammadzadeh, N.; Ghiasian, M.; Faradmal, J.; Dastan, D. Quantitative and qualitative analyses of the constituents of the hydroalcoholic extract of Quercus infectoria gall from Kermanshah and evaluation of its antioxidant and antibacterial activities. J. Rep. Pharm. Sci. 2021, 10, 287–293. [Google Scholar]
- Nik Mat Zin, N.N.I.; Ibrahim, N.; Zakaria, Y.; Abu-Bakar, N. The acetone crude extract of quercus infectoria (Olivier) galls alters ph of the digestive vacuole of the malaria parasite, plasmodium falciparum. Trop. Biomed. 2021, 38, 40–47. [Google Scholar]
- Wan-Nor-Amilah, W.A.W.; Syifaa’-Liyana, M.L.; Azlina, Y.; Shafizol, Z.; Nurul, A.A. In vitro immunomodulatory activity of aqueous quercus infectoria gall extract. Oman. Med. J. 2021, 36, e265. [Google Scholar] [CrossRef] [PubMed]
- Alhamadani, A.H.; Saeed, H.A.; Khayoon, H.A. Clinical study on the therapeutic effects of Quercus infectoria galls as oral powder in gingivitis and plaque patients. Indian J. Forensic Med. Toxicol. 2020, 14, 744–748. [Google Scholar]
- Amedi, S.I.; Mohammed, B.M. Anticlastogenic properties of Quercus infectoria galls extract against DMBA induced genotoxicity in bone marrow cells of mice in vivo. Iraqi J. Vet. Sci. 2020, 34, 279–285. [Google Scholar] [CrossRef]
- Dsouza, M.R.; Aishwarya, B.S.; Supriya, S.S. Anticariogenic activity of galls of Quercus Infectoria Olivier against oral pathogens causing dental caries. Int. J. Pharm. Sci. Res. 2020, 11, 1711–1718. [Google Scholar]
- Kılınçarslan Aksoy, Ö.; Mammadov, R.; Seçme, M. Antioxidant activity, phytochemical composition of Andricus tomentosus and its antiproliferative effect on Mia-Paca2 cell line. Mol. Biol. Rep. 2020, 47, 7633–7641. [Google Scholar] [CrossRef]
- Limsuwan, S.; Jarukitsakul, S.; Issuriya, A.; Chusri, S.; Joycharat, N.; Jaisamut, P.; Saising, J.; Jetwanna, K.W.N.; Voravuthikunchai, S.P. Thai herbal formulation ‘Ya-Pit-Samut-Noi’: Its antibacterial activities, effects on bacterial virulence factors and in vivo acute toxicity. J. Ethnopharmacol. 2020, 259, 112975. [Google Scholar] [CrossRef]
- Zin, N.N.I.N.M.; Mohamad, M.N.; Roslan, K.; Sazeli, A.W.; Moin, N.I.A.; Alias, A.; Zakaria, Y.; Abu-Bakar, N. In vitro antimalarial and toxicological activities of quercus infectoria (Olivier) gall extracts. Malays. J. Med. Sci. 2020, 27, 36–50. [Google Scholar] [PubMed]
- Sukor, N.F.; Jusoh, R.; Kamarudin, N.S.; Abdul Halim, N.A.; Sulaiman, A.Z.; Abdullah, S.B. Synergistic effect of probe sonication and ionic liquid for extraction of phenolic acids from oak galls. Ultrason Sonochem 2020, 62, 104876. [Google Scholar] [CrossRef] [PubMed]
- Yusof, W.N.S.W.; Abdullah, H. Phytochemicals and cytotoxicity of Quercus infectoria ethyl acetate extracts on human cancer cells. Trop. Life Sci. Res. 2020, 31, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Y.; Liu, J.; Kuerban, K.; Li, J.; Iminjan, M.; Ye, L. Traditional Uyghur medicine Quercus infectoria galls water extract triggers apoptosis and autophagic cell death in colorectal cancer cells. BMC Complement. Med. Ther. 2020, 20, 371. [Google Scholar] [CrossRef]
- Ambulkar, S.; Tale, V.; Khilari, S.; Pawar, J. Antibacterial and antibiofilm activity of Quercus Infectoria galls on Rothia dentocariosa isolated from dental caries. Asian J. Pharm. Clin. Res. 2019, 12, 159–162. [Google Scholar] [CrossRef]
- Saltan, F.Z.; Canbay, H.S.; Üvez, A.; Konak, M.; Armutak, E.İ. Quantitative Determination of Tannic Acid in Quercus Species by High Performance Liquid Chromatography. Fabad J. Pharm. Sci. 2019, 44, 197–203. [Google Scholar]
- Yu, W.; Su, X.; Chen, W.; Tian, X.; Zhang, K.; Guo, G.; Zhou, L.; Zeng, T.; Han, B. Three types of gut bacteria collaborating to improve Kui Jie’an enema treat DSS-induced colitis in mice. Biomed. Pharmacother. 2019, 113, 108751. [Google Scholar] [CrossRef]
- Hussein, N.N. Antihaemolytic and antimicrobial activity of three types of local plants. Biochem. Cell Arch. 2018, 18, 1721–1726. [Google Scholar]
- Jalill, R.D.A. Chemical analysis and anticancer effects of Juniperus polycarpos and oak gall plants extracts. Res. J. Pharm. Technol. 2018, 11, 2372–2387. [Google Scholar] [CrossRef]
- Lu, M.; Yan, L.; Wang, B.; Tian, S. Effect of vibrating-type ultrafine grinding on the physicochemical and antioxidant properties of Turkish galls in Uyghur medicine. Powder Technol. 2018, 339, 560–568. [Google Scholar] [CrossRef]
- Mustafa, H.; Ismail, N.; Wahab, W.N.A.W.A. Anti-microbial activity of aqueous Quercus infectoria gall extract against pathogenic Leptospira. Malays. J. Med. Sci. 2018, 25, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Tayel, A.A.; Ghanem, R.A.; Moussa, S.H.; Fahmi, M.; Tarjam, H.M.; Ismail, N. Skin protectant textiles loaded with fish collagen, chitosan and oak galls extract composite. Int. J. Biol. Macromol. 2018, 117, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of phenolic compounds: A review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Gadioli, I.L.; da Cunha, M.d.S.B.; de Carvalho, M.V.O.; Costa, A.M.; de Lacerda de Oliveira Pineli, L. A systematic review on phenolic compounds in Passiflora plants: Exploring biodiversity for food, nutrition, and popular medicine. Crit. Rev. Food Sci. Nutr. 2018, 58, 785–807. [Google Scholar] [CrossRef]
- Spigno, G.; Tramelli, L.; De Faveri, D.M. Effects of extraction time, temperature and solvent on concentration and antioxidant activity of grape marc phenolics. J. Food Eng. 2007, 81, 200–208. [Google Scholar] [CrossRef]
- Alasalvar, C.; Karamać, M.; Kosińska, A.; Rybarczyk, A.; Shahidi, F.; Amarowicz, R. Antioxidant activity of hazelnut skin phenolics. J. Agric. Food Chem. 2009, 57, 4645–4650. [Google Scholar] [CrossRef]
- Rusu, M.E.; Georgiu, C.; Pop, A.; Mocan, A.; Kiss, B.; Vostinaru, O.; Fizesan, I.; Stefan, M.-G.; Gheldiu, A.-M.; Mates, L.; et al. Antioxidant effects of walnut (Juglans regia L.) Kernel and Walnut septum extract in a D-Galactose-induced aging model and in naturally aged rats. Antioxidants 2020, 9, 424. [Google Scholar] [CrossRef]
- Iylia Arina, M.Z.; Harisun, Y. Effect of extraction temperatures on tannin content and antioxidant activity of Quercus infectoria (Manjakani). Biocatal. Agric. Biotechnol. 2019, 19, 101104. [Google Scholar] [CrossRef]
- Shi, L.; Zhao, W.; Yang, Z.; Subbiah, V.; Suleria, H.A.R. Extraction and characterization of phenolic compounds and their potential antioxidant activities. Environ. Sci. Pollut. Res. 2022, 29, 81112–81129. [Google Scholar] [CrossRef]
- Sukor, N.; Jusoh, R.; Rahim, S.A.; Kamarudin, N. Ultrasound assisted methods for enhanced extraction of phenolic acids from Quercus infectoria. galls. Mater. Today Proc. 2018, 5, 21990–21999. [Google Scholar] [CrossRef]
- Mohd-Nasir, H.; Putra, N.R.; Chuo, S.C.; Daud, N.M.; Hartati, H.; Bakeri, N.; Ruslan, M.S.; Mohd-Setapar, S.H.; Ahmad, A.; Md Salleh, L. Optimization of the supercritical carbon dioxide extraction of Quercus infectoria galls extracts and its bioactivities. J. Food Process. Preserv. 2021, 45, e15156. [Google Scholar] [CrossRef]
- Hamad, H.O.; Alma, M.H.; Gulcin, İ.; Yılmaz, M.A.; Karaoğul, E. Evaluation of Phenolic Contents and Bioactivity of Root and Nutgall Extracts from Iraqian Quercus infectoria Olivier. Rec. Nat. Prod. 2017, 11, 205–210. [Google Scholar]
- Zhang, Y.; Cai, P.; Cheng, G.; Zhang, Y. A Brief Review of Phenolic Compounds Identified from Plants: Their Extraction, Analysis, and Biological Activity. Nat. Prod. Commun. 2022, 17, 1–14. [Google Scholar] [CrossRef]
- Kumar, B.R. Application of HPLC and ESI-MS techniques in the analysis of phenolic acids and flavonoids from green leafy vegetables (GLVs). J. Pharm. Anal. 2017, 7, 349–364. [Google Scholar] [CrossRef]
- Saini, A.K.; Sawant, L.; Zahiruddin, S.; Shrivastva, D.; Mitra, R.; Rai, R.K.; Ahmad, S. LC-MS/MS-based Targeted Metabolomic Profiling of Aqueous and Hydro-alcoholic Extracts of Pistacia integerrima Linn., Quercus infectoria Olivier and Terminalia chebula Retz. Pharmacogn. Mag. 2023, 19, 222–230. [Google Scholar] [CrossRef]
- Restivo, A.; Degano, I.; Ribechini, E.; Colombini, M.P. Development and optimisation of an HPLC-DAD-ESI-Q-ToF method for the determination of phenolic acids and derivatives. PLoS ONE 2014, 9, e88762. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Yang, X.; Hu, J.; Yin, W. Identification of Anticancer Compounds in Gallnuts Through PCA-constructed Secondary Metabolite Map. Int. J. Pharmacol. 2019, 15, 515–522. [Google Scholar] [CrossRef]
- Hussein, A.O.; Mohammed, G.J.; Hadi, M.Y.; Hameed, I.H. Phytochemical screening of methanolic dried galls extract of Quercus infectoria using gas chromatography-mass spectrometry (GC-MS) and Fourier transform-infrared (FT-IR). J. Pharmacogn. Phytother. 2016, 8, 49–59. [Google Scholar]
- Ou, L.; He, Q.; Ji, Z.; Li, K.; Tian, S. Quantitative high-performance thin-layer chromatographic analysis of three active compounds in gall of Quercus infectoria Olivier (fagaceae) and use of thin-layer chromatography-2,2-diphenyl-1-picrylhydrazyl to screen antioxidant component. J. Planar Chromatogr. Mod. TLC 2015, 28, 300–306. [Google Scholar] [CrossRef]
- Baharuddin, N.; Abdullah, H.; Abdul Wahab, W.N. Anti-Candida activity of Quercus infectoria gall extracts against Candida species. J. Pharm. Bioallied. Sci. 2015, 7, 15–20. [Google Scholar] [CrossRef]
- Chusri, S.; Voravuthikunchai, S.P. Damage of staphylococcal cytoplasmic membrane by Quercus infectoria G. Olivier and its components. Lett. Appl. Microbiol. 2011, 52, 565–572. [Google Scholar] [CrossRef]
- Tan, S.C.; Balachandran, L.; Mohamad, N.; Kang, I.N.; Nasarudin, A.; Zakaria, R.A.; Abdullah, H. The potential of neural stem cell as vehicle to deliver quercus infectoria extract to glioma cell in vitro. Sains Malays. 2018, 47, 1209–1219. [Google Scholar] [CrossRef]
- Hamid, H.; Kaur, G.; Abdullah, S.T.; Ali, M.; Athar, M.; Alam, M.S. Two new compounds from the galls of Quercus infectoria with nitric oxide and superoxide inhibiting ability. Pharm. Biol. 2005, 43, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Athar, M.; Alam, M.S. Quercus infectoria galls possess antioxidant activity and abrogates oxidative stress-induced functional alterations in murine macrophages. Chem. Biol. Interact. 2008, 171, 272–282. [Google Scholar] [CrossRef]
- Hwang, J.; Kong, T.; Baek, N.; Pyun, Y.R. alpha-Glycosidase inhibitory activity of hexagalloylglucose from the galls of Quercus infectoria. Planta Med. 2000, 66, 273–274. [Google Scholar] [CrossRef] [PubMed]
- Zajácz, Á.; Gyémánt, G.; Vittori, N.; Kandra, L. Aleppo tannin: Structural analysis and salivary amylase inhibition. Carbohydr. Res. 2007, 342, 717–723. [Google Scholar] [CrossRef]
- Nazam, N.; Jabir, N.R.; Ahmad, I.; Alharthy, S.A.; Khan, M.S.; Ayub, R.; Tabrez, S. Phenolic Acids-Mediated Regulation of Molecular Targets in Ovarian Cancer: Current Understanding and Future Perspectives. Pharmaceuticals 2023, 16, 274. [Google Scholar] [CrossRef]
- Othón-Díaz, E.D.; Fimbres-García, J.O.; Flores-Sauceda, M.; Silva-Espinoza, B.A.; López-Martínez, L.X.; Bernal-Mercado, A.T.; Ayala-Zavala, J.F. Antioxidants in Oak (Quercus sp.): Potential Application to Reduce Oxidative Rancidity in Foods. Antioxidants 2023, 12, 861. [Google Scholar] [CrossRef]
- Banc, R.; Socaciu, C.; Miere, D.; Filip, L.; Cozma, A.; Stanciu, O.; Loghin, F. Benefits of Wine Polyphenols on Human Health: A Review. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Food Sci. Technol. 2014, 71, 79–87. [Google Scholar] [CrossRef]
- Banc, R.; Rusu, M.E.; Filip, L.; Popa, D.S. The Impact of Ellagitannins and Their Metabolites through Gut Microbiome on the Gut Health and Brain Wellness within the Gut–Brain Axis. Foods 2023, 12, 270. [Google Scholar] [CrossRef]
- Wen, C.; Dechsupa, N.; Yu, Z.; Zhang, X.; Liang, S.; Lei, X.; Xu, T.; Gao, X.; Hu, Q.; Innuan, P.; et al. Pentagalloyl Glucose: A Review of Anticancer Properties, Molecular Targets, Mechanisms of Action, Pharmacokinetics, and Safety Profile. Molecules 2023, 28, 4856. [Google Scholar] [CrossRef] [PubMed]
- Daré, R.G.; Nakamura, C.V.; Ximenes, V.F.; Lautenschlager, S.O.S. Tannic acid, a promising anti-photoaging agent: Evidences of its antioxidant and anti-wrinkle potentials, and its ability to prevent photodamage and MMP-1 expression in L929 fibroblasts exposed to UVB. Free Radic. Biol. Med. 2020, 160, 342–355. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Qian, M.; Jiang, Q.; Tan, B.; Yin, Y.; Han, X. Evidence of Flavonoids on Disease Prevention. Antioxidants 2023, 12, 527. [Google Scholar] [CrossRef] [PubMed]
- Baroni, L.; Sarni, A.R.; Zuliani, C. Plant foods rich in antioxidants and human cognition: A systematic review. Antioxidants 2021, 10, 714. [Google Scholar] [CrossRef]
- Banc, R.; Popa, D.-S.; Cozma-Petruţ, A.; Filip, L.; Kiss, B.; Fărcaş, A.; Nagy, A.; Miere, D.; Loghin, F. Protective Effects of Wine Polyphenols on Oxidative Stress and Hepatotoxicity Induced by Acrylamide in Rats. Antioxidants 2022, 11, 1347. [Google Scholar] [CrossRef]
- Ceylan, Ş.; Yardımcı, Ş.S.; Camadan, Y.; Saral, Ö.; Batur, Ö.Ö. Chemical composition of essential oil by SPME and evaluation of antimicrobial, antioxidant activities of Quercus infectoria gall. Acta Sci. Pol. Hortorum Cultus 2021, 20, 93–103. [Google Scholar] [CrossRef]
- Rusu, M.E.; Mocan, A.; Ferreira, I.C.F.R.; Popa, D.S. Health benefits of nut consumption in middle-aged and elderly population. Antioxidants 2019, 8, 302. [Google Scholar] [CrossRef]
- Mourabit, Y.; El Hajjaji, S.; Taha, D.; Badaoui, B.; El Yadini, M.; Rusu, M.E.; Lee, L.-H.; Bouyahya, A.; Bourais, I. HPLC-DAD-ESI/MS phytochemical investigation, antioxidant, and antidiabetic activities of Moroccan Rosa canina L. extracts. Biocatal. Agric. Biotechnol. 2023, 52, 102817. [Google Scholar] [CrossRef]
- Kot, I.; Sempruch, C.; Rubinowska, K.; Michałek, W. Effect of Neuroterus quercusbaccarum (L.) galls on physiological and biochemical response of Quercus robur leaves. Bull. Entomol. Res. 2020, 110, 34–43. [Google Scholar] [CrossRef]
- Chokpaisarn, J.; Urao, N.; Voravuthikunchai, S.P.; Koh, T.J. Quercus infectoria inhibits Set7/NF-κB inflammatory pathway in macrophages exposed to a diabetic environment. Cytokine 2017, 94, 29–36. [Google Scholar] [CrossRef]
- Suwalak, S.; Voravuthikunchai, S.P. Morphological and ultrastructural changes in the cell structure of enterohaemorrhagic Escherichia coli O157:H7 following treatment with Quercus infectoria nut galls. J. Electron. Microsc. 2009, 58, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Isaei, E.; Mansouri, S.; Rahmani, M.; Sharififar, F.; Salary, A. Novel synergistic activity of quercus infectoria gall extract with ceftazidime against standard and multiple drug resistant pseudomonas aeruginosa and Escherichia coli isolates. Arch. Iran. Med. 2021, 24, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Vedeanu, N.; Voica, C.; Magdas, D.A.; Kiss, B.; Stefan, M.G.; Simedrea, R.; Georgiu, C.; Berce, C.; Vostinaru, O.; Boros, R.; et al. Subacute co-exposure to low doses of ruthenium(III) changes the distribution, excretion and biological effects of silver ions in rats. Environ. Chem. 2020, 17, 163–172. [Google Scholar] [CrossRef]
- Wood, S.J.; Goldufsky, J.W.; Seu, M.Y.; Dorafshar, A.H.; Shafikhani, S.H. Pseudomonas aeruginosa Cytotoxins: Mechanisms of Cytotoxicity and Impact on Inflammatory Responses. Cells 2023, 12, 195. [Google Scholar] [CrossRef] [PubMed]
- Vermani, A.; Navneet, P. Screening of Quercus infectoria gall extracts as anti-bacterial agents against dental pathogens. Indian J. Dent. Res. 2009, 20, 337–339. [Google Scholar] [CrossRef]
- Damrongsaktrakul, P.; Ruengvisesh, S.; Rahothan, A.; Sukhumrat, N.; Tuitemwong, P.; Phung-On, I. Removal of salmonella typhimurium biofilm from food contact surfaces using quercus infectoria gall extract in combination with a surfactant. J. Microbiol. Biotechnol. 2021, 31, 439–446. [Google Scholar] [CrossRef]
- Aizenbud, I.; Wilensky, A.; Almoznino, G. Periodontal Disease and Its Association with Metabolic Syndrome—A Comprehensive Review. Int. J. Mol. Sci. 2023, 24, 13011. [Google Scholar] [CrossRef]
- Mohammadi-Sichani, M.; Karbasizadeh, V.; Dokhaharani, S.C. Evaluation of biofilm removal activity of Quercus infectoria galls against Streptococcus mutans. Dent. Res. J. 2016, 13, 46–51. [Google Scholar] [CrossRef]
- Tabibzadeh Noori, Z.; Tabatabaei Rad, M.; Hakemi Vala, M.; Karimi, M.; Esmaeil Nejad, A. Evaluation of the antibacterial effect of hydroalcoholic extract of the galls of Quercus infectoria on Aggregatibacter actinomycetemcomitans. J. Adv. Periodontol. Implant. Dent. 2023, 15, 35–41. [Google Scholar] [CrossRef]
- Basri, D.F.; Tan, L.S.; Shafiei, Z.; Zin, N.M. In vitro antibacterial activity of galls of Quercus infectoria Olivier against oral pathogens. Evid.-Based Complement. Altern. Med. 2012, 2012, 632796. [Google Scholar] [CrossRef]
- Taib, M.; Rezzak, Y.; Bouyazza, L.; Lyoussi, B. Medicinal Uses, Phytochemistry, and Pharmacological Activities of Quercus Species. Evid.-Based Complement. Altern. Med. 2020, 2020, 1920683. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhou, C.; Chen, S.; Huang, D.; Jiang, Y.; Lan, Y.; Zou, S.; Li, Y. Osteoporosis and Alveolar Bone Health in Periodontitis Niche: A Predisposing Factors-Centered Review. Cells 2022, 11, 3380. [Google Scholar] [CrossRef] [PubMed]
- Wan Nor Amilah, W.; Masrah, M.; Hasmah, A.; Noor Izani, N. In vitro antibacterial activity of Quercus infectoria gall extracts against multidrug resistant bacteria. Trop. Biomed. 2014, 31, 680–688. [Google Scholar] [PubMed]
- Malir, F.; Pickova, D.; Toman, J.; Grosse, Y.; Ostry, V. Hazard characterisation for significant mycotoxins in food. Mycotoxin Res. 2023, 39, 81–93. [Google Scholar] [CrossRef]
- Ledoux, M.P.; Herbrecht, R. Invasive Pulmonary Aspergillosis. J. Fungi 2023, 9, 131. [Google Scholar] [CrossRef]
- Muchtar, N.H.; Nik Mat Zin, N.N.I.; Mohamad, F.S.; Abu-Bakar, N. Ellagic Acid Induces in vitro Alkalinisation of the Digestive Vacuole in Drug-Sensitive Plasmodium falciparum Strain. Malays. J. Med. Sci. 2022, 29, 43–52. [Google Scholar] [CrossRef]
- Yosefsani, B.S.; Mohajer, K.; Qobadi, A.; Aghazadeh, E.; Shirani, K.; Pourahmad, J. The Selective Cytotoxicity of Quercus Brantii Lindl. Galls on A375 and SK-MEL-3 Human Malignant Melanoma Cell Lines. Asian Pac. J. Cancer Prev. 2023, 24, 2383–2388. [Google Scholar] [CrossRef]
- Benali, T.; Lemhadri, A.; Harboul, K.; Chtibi, H.; Khabbach, A.; Jadouali, S.M.; Quesada-Romero, L.; Louahlia, S.; Hammani, K.; Ghaleb, A.; et al. Chemical Profiling and Biological Properties of Essential Oils of Lavandula stoechas L. Collected from Three Moroccan Sites: In Vitro and In Silico Investigations. Plants 2023, 12, 1413. [Google Scholar] [CrossRef]
- Blowman, K.; Magalhães, M.; Lemos, M.F.L.; Cabral, C.; Pires, I.M. Anticancer Properties of Essential Oils and Other Natural Products. Evid. Based Complement. Altern. Med. 2018, 2018, 3149362. [Google Scholar] [CrossRef]
- Begum, S.N.; Ray, A.S.; Rahaman, C.H. A comprehensive and systematic review on potential anticancer activities of eugenol: From pre-clinical evidence to molecular mechanisms of action. Phytomedicine 2022, 107, 154456. [Google Scholar] [CrossRef]
- Khanavi, M.; Sabbagh-Bani-Azad, M.; Abdolghaffari, A.H.; Vazirian, M.; Isazadeh, I.; Rezvanfar, M.A.; Baeeri, M.; Mohammadirad, A.; Rahimi, R.; Shams-Ardekani, M.R.; et al. On the beneft of galls of Quercus brantii Lindl. in murine colitis: The role of free gallic acid. Arch. Med. Sci. 2014, 10, 1225–1234. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Zhang, L.; Han, T.; Huang, H.; Chen, J. Dynamic Changes in Gut Microbiome of Ulcerative Colitis: Initial Study from Animal Model. J. Inflamm. Res. 2022, 15, 2631–2647. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Ma, Y.; Xiong, K.; Wang, Y.; Liu, Y.; Sun, Y.; Yang, Y.; Ma, A. Ameliorating Effects of Vitamin K2 on Dextran Sulfate Sodium-Induced Ulcerative Colitis in Mice. Int. J. Mol. Sci. 2023, 24, 2986. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Yang, Z.; Wang, L.; Han, Y.; Peng, C.; Yan, C.; Yan, D. Metabolite biomarkers of type 2 diabetes mellitus and pre-diabetes: A systematic review and meta-analysis. BMC Endocr. Disord. 2020, 20, 174. [Google Scholar] [CrossRef]
- Biondi, B.; Kahaly, G.J.; Robertson, R.P. Thyroid Dysfunction and Diabetes Mellitus: Two Closely Associated Disorders. Endocr. Rev. 2019, 40, 789–824. [Google Scholar] [CrossRef]
- Ayad, B.; Omolaoye, T.S.; Louw, N.; Ramsunder, Y.; Skosana, B.T.; Oyeipo, P.I.; Du Plessis, S.S. Oxidative Stress and Male Infertility: Evidence From a Research Perspective. Front. Reprod. Health 2022, 4, 822257. [Google Scholar] [CrossRef]
- El Menyiy, N.; Aboulaghras, S.; Bakrim, S.; Moubachir, R.; Taha, D.; Khalid, A.; Abdalla, A.N.; Algarni, A.S.; Hermansyah, A.; Ming, L.C.; et al. Genkwanin: An emerging natural compound with multifaceted pharmacological effects. Biomed. Pharmacother. 2023, 165, 115159. [Google Scholar] [CrossRef]
- Ferreira, B.G.; Oliveira, D.C.; Moreira, A.S.F.P.; Faria, A.P.; Guedes, L.M.; França, M.G.C.; Álvarez, R.; Isaias, R.M.S. Antioxidant metabolism in galls due to the extended phenotypes of the associated organisms. PLoS ONE 2018, 13, e0205364. [Google Scholar] [CrossRef]
- Tran, N.; Pham, B.; Le, L. Bioactive compounds in anti-diabetic plants: From herbal medicine to modern drug discovery. Biology 2020, 9, 252. [Google Scholar] [CrossRef]
- Chokpaisarn, J.; Chusri, S.; Amnuaikit, T.; Udomuksorn, W.; Voravuthikunchai, S.P. Potential wound healing activity of Quercus infectoria formulation in diabetic rats. PeerJ 2017, 5, e3608. [Google Scholar] [CrossRef]
- Ramachandran, A.; Jaeschke, H. Acetaminophen Hepatotoxicity. Semin. Liver Dis. 2019, 39, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Tedyanto, C.P.; Wihanto, L.; Hendrata, A.P. Hepatoprotective Effect of Dried Red Jujube Fruit Extract Against Acetaminophen-Induced Acute Hepatotoxicity. Cureus 2023, 15, e33272. [Google Scholar] [CrossRef]
- Gholamhoseinian, A.; Shahouzehi, B.; Joukar, S.; Iranpoor, M. Effect of Quercus infectoria and Rosa damascena on lipid profile and atherosclerotic plaque formation in rabbit model of hyperlipidemia. Pak. J. Biol. Sci. 2012, 15, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Yu, R.-J.; Yang, C.; Wang, Q.; Xuan, Y.; Wang, Z.; He, Z.; Xu, Y.; Kou, L.; Zhao, Y.-Z.; et al. Evaluation of the hepatoprotective effect of naringenin loaded nanoparticles against acetaminophen overdose toxicity. Drug Deliv. 2022, 29, 3256–3269. [Google Scholar] [CrossRef] [PubMed]
- Alkandahri, M.Y.; Pamungkas, B.T.; Oktoba, Z.; Shafirany, M.Z.; Sulastri, L.; Arfania, M.; Anggraeny, E.N.; Pratiwi, A.; Astuti, F.D.; Indriyani; et al. Hepatoprotective Effect of Kaempferol: A Review of the Dietary Sources, Bioavailability, Mechanisms of Action, and Safety. Adv. Pharmacol. Pharm. Sci. 2023, 2023, 1387665. [Google Scholar] [CrossRef]
- Halim, A.S.; Ramasenderan, N. High-risk cutaneous squamous cell carcinoma (CSCC): Challenges and emerging therapies. Asian J. Surg. 2023, 46, 47–51. [Google Scholar] [CrossRef]
- Xu, H.; Liu, T.; Li, J.; Chen, F.; Xu, J.; Hu, L.; Jiang, L.; Xiang, Z.; Wang, X.; Sheng, J. Roburic Acid Targets TNF to Inhibit the NF-κB Signaling Pathway and Suppress Human Colorectal Cancer Cell Growth. Front. Immunol. 2022, 13, 853165. [Google Scholar] [CrossRef]
- Baldwin, A.; Booth, B.W. Biomedical applications of tannic acid. J. Biomater. Appl. 2022, 36, 1503–1523. [Google Scholar] [CrossRef]
- Ahmad, F.M.; Zafar, A.; Ahmed, M.; Akhtar, N.; Hasan, M.M.U.; Abdel-Maksoude, M.A.; Aufy, M. Quercus floribunda Lindl. Ex A. Camus; a tremendous remedy against inflammation and associated symptoms. Fitoterapia 2023, 170, 105628. [Google Scholar] [CrossRef]
- Khairon, R.; Zin, N.M.; Abdul Rahman, M.; Basri, D.F. Comparative Proteomic Analysis of Differential Proteins in Response to Aqueous Extract of Quercus infectoria Gall in Methicillin-Resistant Staphylococcus aureus. Int. J. Proteomics. 2016, 2016, 4029172. [Google Scholar] [CrossRef]
- Kaur, G.; Hamid, H.; Ali, A.; Alam, M.S.; Athar, M. Antiinflammatory evaluation of alcoholic extract of galls of Quercus infectoria. J. Ethnopharmacol. 2004, 90, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Coelho, G.A.; Secretan, P.H.; Tortolano, L.; Charvet, L.; Yagoubi, N. Evolution of the Chronic Venous Leg Ulcer Microenvironment and Its Impact on Medical Devices and Wound Care Therapies. J. Clin. Med. 2023, 12, 5605. [Google Scholar] [CrossRef] [PubMed]
- Iminjan, M.; Amat, N.; Li, X.H.; Upur, H.; Ahmat, D.; He, B. Investigation into the toxicity of traditional uyghur medicine quercus infectoria galls water extract. PLoS ONE 2014, 9, e90756. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).