A Critical Appraisal of the Most Recent Investigations on Ora-Pro-Nobis (Pereskia sp.): Economical, Botanical, Phytochemical, Nutritional, and Ethnopharmacological Aspects
Abstract
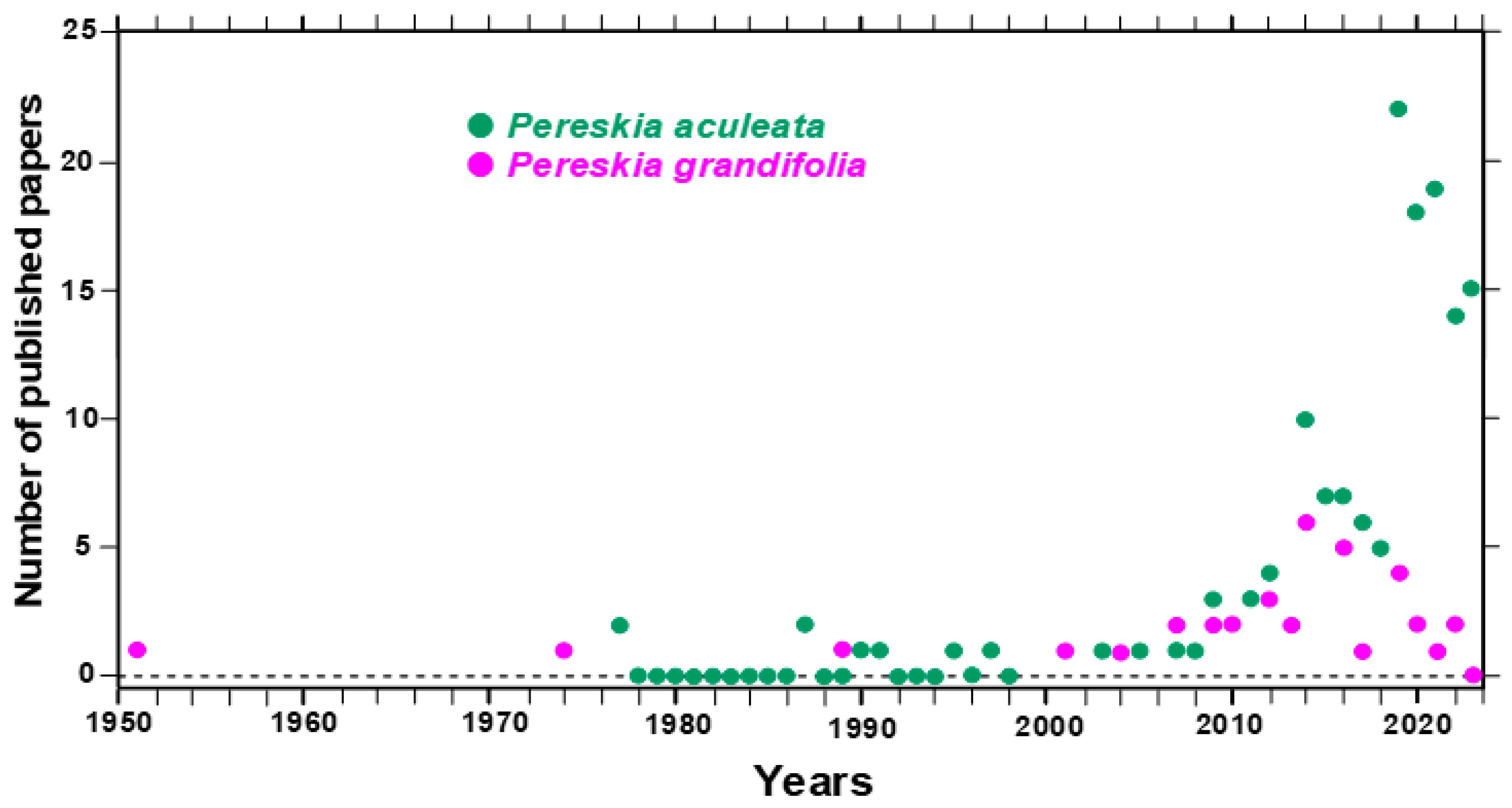
:1. Introduction
2. Search Methodology
3. Morphology, Chemical Characteristics, and Cultivation
| Ora-Pro-Nobis Species and Samples | Moisture (%) | Total Proteins (%) | Lipids (%) | Ash (%) | Carbohydrates (%) | Total Dietary Fiber (%) | Reference |
|---|---|---|---|---|---|---|---|
| P. aculeata fresh leaves | 89.5 ± 0.2 | 28.4 ± 0.4 | 4.1 ± 0.3 | 16.1 ± 0.1 | nd. | 39.1 | [20] |
| P. aculeata (dry basis) leaves * | 8.3 | 23 | 4 | 16.6 | 43.7 | 30.2 | [35] |
| P. aculeata (dry basis) leaves | 12.46 ± 0.47 | 28.99 ± 0.59 | 5.07 ± 0.15 | 14.81 ± 0.18 | 29.53 ± 1.28 | 21.60 ± 0.82 | [26] |
| P. grandifolia (dry basis) leaves | 10.94 ± 0.78 | 32.02 ± 0.46 | 6.72 ± 0.30 | 18.8 ±0.92 | 29.86 ± 1.32 | 18.82 ± 0.92 | [26] |
| P. grandifolia fresh leaves | 86.50 ± 0.13 | 14.64 ± 0.87 | 2.97 ± 0.86 | nd. | 24.47 ± 0.35 | nd. | [36] |
| P. aculeata fruit | 87.37 ± 0.26 | --- | 0.23 ± 0.05 | 0.93 ± 0.01 | 11.47 ± 0.22 | nd. | [37] |
| Ora-Pro-Nobis Species and Samples | Mineral Content (mg/100 g) | Vitamin Content (mg/100 g) | Reference |
|---|---|---|---|
| P. aculeata (fresh leaves) | Calcium= 3420 Magnesium = 1900 Potassium = 1632 Phosphorus = 156 Manganese = 4.2 Boron = 5.5 Copper = 1.4 | β-carotene = 4.2 ± 0.2 Vitamin A = 2333 IU/100 g Vitamin C = 185.8 ± 14.0 Folic acid = 19.3 | [20] |
| P. aculeata (dry basis, leaves) * | Iron = 19.8 Zinc = 5.7 Calcium = 2679.3 Magnesium = 1065.3 Phosphorous 447.4 Potassium = 3266 Copper = 0.7 Boron = 4.1 Manganese = 23.6 Sulphur = 640.8 | Total carotenoids = 186 µg/g | [35] |
| P. aculeata (dry basis, leaves) | n.a. | Thiamine (B1) = 1.18 ± 0.37 Nicotinamide (B3) = 2.75 ± 0.39 Pantothenic acid (B5) = 0.47 ± 0.43 Biotin (B7) = 35.12 ± 7.26 Ascorbic acid = 0.83 ± 0.14 | [39] |
4. Folk Medicinal Claims
5. Development of Food Products Added with Ora-Pro-Nobis Flour
| Food Product | Product Characterization | Reference |
|---|---|---|
| Yogurt | Approximate composition, pH, Lactobacillus acidophilus and Bifidobacterium BB-12 counting, and sensory analysis (acceptance test) | [54] |
| Milk-based beverage | Approximate composition, total phenolics, antioxidant activity, clinical trial (analysis of fecal microbiota, gastrointestinal symptoms, and anthropometric parameters) | [22] |
| Talharim pasta | Sensory analysis (acceptance and preference tests) and proximate composition | [55] |
| Bread | Approximate composition and sensory analysis (acceptance test) | [56] |
| Bread | Sensory acceptance test and purchase intention | |
| Cake | Protein content and sensory evaluation (acceptance test) | [57] |
| Bread and pie | Sensory acceptance test | [58] |
| Bread | Sensory acceptance test | [59] |
| Veggie pie | Sensory acceptance test | [60] |
| Cake | Approximate composition | [61] |
| Hot dog | Approximate composition, color, pH, texture profile analysis, and sensory acceptance tests | [62] |
| Extruded corn snack | Approximate composition and sensory analysis (acceptance test) | [52] |
| Vegan chickpea burger | Approximate composition, color, and sensory analysis (acceptance test) | [63] |
| Cereal bar | Protein content and sensory evaluation (acceptance test) | [53] |
| Paçoca (peanut candy) | Sensory acceptance test | [51] |
| Rice dumplings (leaves in natura) | Approximate composition and sensory analysis (acceptance test) | [64] |
6. Extraction of the Bioactives of P. aculeata and P. grandifolia
| Pereskia sp. Plant Part | Extraction Technique | Extraction Solvent | Bioactive/Functional Characterization | Reference |
|---|---|---|---|---|
| P. aculeata leaves | Hot extraction, pressing, and precipitation | Water | Mucilage: emulsifier | [21] |
| P. aculeata green fruit | Pressing and precipitation | None | Mucilage: emulsifier | [71] |
| P. aculeata fruits (unripe, intermediate, and ripe) | Homogenization with water | Water | Fruit pulp: antioxidant activity (DPPH, ABTS, ORAC, and total phenolic compounds), total carotenoids, total chlorophylls | [72] |
| P. aculeata leaves | Simple stirring | Water, ethanol, and acetone | Bioactive extract: in vitro antioxidant and antihemolytic activities | [73] |
| P. aculeata leaves | Sequential solvent extraction and hydro-distillation | Petroleum ether followed by chloroform and methanolWater | Bioactive extracts and essential oil: antimicrobial (Staphylococcus aureus, Bacillus cereus, and Bacillus cereus, Escherichia coli, Pseudomonas aeru-ginosa, Penicillium citrinum, P. ex-pansum and Aspergillus versicolor), antioxidant, cytotoxicity on neuro-blastoma SH-SY5Y cancer cell line and influence on adenylate cyclase (ADCY) expression | [9,45] |
| P. aculeata leaves | Simple stirring | Ethanol:water (70:30) | Bioactive extract: antioxidant (OxHLIA, TBARS, DPPH, and ABTS) and hepatotoxicity | [6] |
| P. aculeata leaves | Homogenization and hot extraction or pressing | Water | Mucilage: emulsifier | [74] |
| P. aculeata leaves | Hot extraction, pressing, and precipitation | Water | Mucilage: emulsifier applied to Petit Suisse cheeses | [75] |
| P. aculeata leaves | Turbo-extraction | Ethanol:water (95:5) | Bioactive extract: cytotoxicity, and in vitro wound healing property, fibroblast cell culture | [67,76] |
| P. aculeata leaves | Soxhlet pre-extraction to remove lipids followed by hot water extraction | Ethanol, followed by water | Mucilage: emulsifier | [77] |
| P. aculeata leaves | Simple stirring | Water | Bioactive extract: microencapsulation and Caco-2 cell line viability | [68] |
| P. aculeata leaves | Maceration followed by fractionation in solvents by polarity order | Methanol (maceration), hexane, dichloromethane, ethyl acetate, and butanol (fractions) | Bioactive extract: cytotoxicity against human promyelocytic leukemia (HL60) and human breast adeno-carcinoma (MCF-7) cells | [8] |
| P. aculeata leaves | Supercritical fluid extraction, pressurized liquid extraction, and Soxhlet | CO2, ethanol, water, hexane | Bioactive extract: acetylcholinesterase (AChE) and lipoxygenase (LOX) inhibitory activities | [36] |
| P. aculeata leaves | Simple stirring | Water | Mucilage: pigment clarification with activated carbon | [78] |
| P. aculeata leaves | Maceration and fractionation | Methanol (maceration), hexane (fraction) | Bioactive extract: topical anti-inflammatory activity on acute and chronic inflammation | [10] |
| P. aculeata leaves | Hot homogenization | Water | Mucilage: emulsifier | [79] |
| P. aculeata leaves | Simple stirring | Water | Mucilage: fat replacer in processed mortadella meat | [80] |
| P. aculeata leaves | Hot homogenization | Water | Mucilage: material for biodegradable films | [81] |
| P. aculeata leaves | Hot homogenization | Water (extraction) and ethanol (fiber remotion) | Mucilage: emulsifier | [82] |
| P. aculeata leaves | Hot homogenization | Water | Mucilage: emulsifier and encapsulating agent for α-tocopherol | [83] |
| P. aculeata leaves | Simple stirring | Water and ethanol | Bioactive extract: antibacterial activity (Enterococcus faecalis, Streptococcus mutans, and Lactobacillus casei) | [84] |
| P. grandifolia leaves | Microwave-assisted extraction and Soxhlet | Water and ethanol | Bioactive extract: FRAP, ABTS, DPPH, and total phenolic content | [85] |
| P. grandifolia leaves | Simple stirring | Methanol | Bioactive extract: acute oral toxicity in mice | [86] |
| P. grandifolia leaves | Maceration and fractionation | Methanol (maceration), hexane, ethyl acetate, and water (fractions) | Bioactive extract: β-Carotene bleaching, FRAP, DPPH, total phenolic content | [87] |
| P. grandifolia leaves | Simple stirring | Methanol | Bioactive extract: cytotoxicity against nasopharyngeal epidermoid carcinoma (KB), cervical carcinoma (CasKi), colon carcinoma (HCT 116), hormone-dependent breast carcinoma (MCF7), lung carcinoma (A549), and the non-cancer human fibroblast (MRC-5) cell lines | [46] |
| P. grandifolia leaves | Infusion and maceration | Water (infusion) and ethanol (maceration) | Bioactive extract: acute and prolonged diuretic activity in Wistar rats | [88] |
| P. grandifolia leaves | Simple stirring, reflux, and maceration | Ethanol and water | Bioactive extract: antioxidant activity (DPPH, FRAP) and antibacterial activity (Staphylococcus aureus and Pseudomonas aeruginosa) | [69] |
| P. grandifolia leaves | Maceration | Ethanol and water, hexane, dichloromethane, chloroform, ethyl acetate, and methanol | Bioactive extract: antioxidant activity (DPPH, FRAP) and antibacterial activity (S. aureus, Escherichia coli, Bacillus subtilis, and Pseudomonas aeruginosa) | [89] |
7. Mucilage Characteristics and Composition
8. Small Molecular Weight Bioactives of P. aculeata e P. grandifolia: Extraction Methods and Properties
9. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- De Pinto, N.C.C.; Scio, E. The biological activities and chemical composition of Pereskia Species (Cactaceae): A review. Plant Foods Hum. Nutr. 2014, 69, 189–195. [Google Scholar] [CrossRef]
- Francelin, M.F.; Vieira, T.F.; Amanda, J.; Garcia, A.; Carvalho, R.; Correa, G.; Roberto, A.; Monteiro, G.; Bracht, A.; Peralta, R.M. Phytochemical, nutritional and pharmacological properties of unconventional native fruits and vegetables from Brazil. In Phytochemicals in Vegetables: A Valuable Source of Bioactive Compounds; Petropoulos, S.A., Ferreira, C.F.R., Barros, L., Eds.; Bentham Science Publishers: Sharjah, United Arab Emirates, 2018; pp. 442–470. [Google Scholar]
- Da Agostini-Costa, T.S.; Wondraceck, D.C.; da Rocha, W.S.; da Silva, D.B. Carotenoids profile and total polyphenols in fruits of Pereskia aculeata Miller. Rev. Bras. Frutic. 2012, 34, 234–238. [Google Scholar] [CrossRef]
- Nogueira Silva, N.F.; Silva, S.H.; Baron, D.; Oliveira Neves, I.C.; Casanova, F. Pereskia aculeata Miller as a novel food source: A review. Foods 2023, 12, 2092. [Google Scholar] [CrossRef]
- Porto, F.G.S.; Campos, A.D.; Carreño, N.L.V.; Garcia, I.T.S. Pereskia aculeata leaves: Properties and potentialities for the development of new products. Nat. Prod. Res. 2022, 36, 4821–4832. [Google Scholar] [CrossRef]
- Garcia, J.A.A.; Corrêa, R.C.G.; Barros, L.; Pereira, C.; Abreu, R.M.V.; Alves, M.J.; Calhelha, R.C.; Bracht, A.; Peralta, R.M.; Ferreira, I.C.F.R. Phytochemical profile and biological activities of “Ora-pro-nobis” leaves (Pereskia aculeata Miller), an underexploited superfood from the Brazilian Atlantic Forest. Food Chem. 2019, 294, 302–308. [Google Scholar] [CrossRef]
- De Almeida, M.E.F.; Corrêa, A.D. Utilização de cactáceas do gênero Pereskia na alimentação humana em um município de Minas Gerais. Cienc. Rural 2012, 42, 751–756. Available online: http://repositorio.ufla.br/jspui/handle/1/12510 (accessed on 15 May 2023). [CrossRef]
- De Pinto, N.C.C.; dos Santos, R.C.; Machado, D.C.; Florêncio, J.R.; de Fagundes, E.M.S.; Antinarelli, L.M.R.; Coimbra, E.S.; Ribeiro, A.; Scio, E. Cytotoxic and antioxidant activity of Pereskia aculeata Miller. Pharmacologyonline 2012, 3, 63–69. [Google Scholar]
- Souza, L.F.; Caputo, L.; Bergman, I.; de Barros, I.; Fratianni, F.; Nazzaro, F.; de Feo, V. Pereskia aculeata Mill. (Cactaceae) leaves: Chemical composition and biological activities. Int. J. Mol. Sci. 2016, 17, 1478. [Google Scholar] [CrossRef]
- Pinto, N.D.C.C.; Machado, D.C.; da Silva, J.M.; Conegundes, J.L.M.; Gualberto, A.C.M.; Gameiro, J.; Moreira Chedier, L.; Castañon, M.C.M.N.; Scio, E. Pereskia aculeata Miller leaves present in vivo topical anti-inflammatory activity in models of acute and chronic dermatitis. J. Ethnopharmacol. 2015, 173, 330–337. [Google Scholar] [CrossRef]
- Pinto, N.D.C.C.; Duque, A.P.D.N.; Pacheco, N.R.; Mendes, R.D.F.; Motta, E.V.D.S.; Bellozi, P.M.Q.; Ribeiro, A.; Salvador, M.J.; Scio, E. Pereskia aculeata: A plant food with antinociceptive activity. Pharm. Biol. 2015, 53, 1780–1785. [Google Scholar] [CrossRef]
- Souza, L.F. Aspectos Fitotécnicos, Bromatológicos e Componentes Bioativos de Pereskia aculeata, Pereskia grandifolia e Anredera cordifolia. Ph.D. Thesis, Universidade Federal Do Rio Grande Do Sul, Porto Alegre, RS, Brazil, 2014. [Google Scholar]
- Sousa, R.M.F.; Lira, C.S.; Rodrigues, A.O.; Morais, S.A.L.; Queiroz, P.C.R.A.A.; Chang, R.; Aquino, F.J.T.; Muñoz, R.A.A.; de Oliveira, A. Antioxidant activity of ora-pro-nobis (Pereskia aculeata Mill.) leaves extracts using spectrophotometric and voltammetric assays in vitro. Biosci. J. 2014, 30, 448–457. [Google Scholar]
- Souza, L.F.; de Barros, I.B.I.; Mancini, E.; de Martino, L.; Scandolera, E.; de Feo, V. Chemical Composition and Biological Activities of the Essential Oils from Two Pereskia Species Grown in Brazil. Nat. Prod. Commun. 2014, 9, 1805–1808. [Google Scholar] [CrossRef]
- Carnevalli, D.B.; Ramos, C.N.; Mardigan, L.P.; Meurer, E.C.; Cardozo-Filho, L.; Corrêa, R.C.G.; Gonçalves, J.E. Ora-pro-nobis-chemical characterization and sourcing of crude extract through different extraction methods: A review. Res. Soc. Dev. 2022, 11, e55211629315. [Google Scholar] [CrossRef]
- Da Agostini-Costa, T.S. Bioactive compounds and health benefits of Pereskioideae and Cactoideae: A review. Food Chem. 2020, 327, 126961. [Google Scholar] [CrossRef]
- Legrand, R.; Merville, R.; Desruelles, J.; Robelet, A.F. Cardiovascular effects of a cactus, Pereskia grandifolia Effets cardio-vasculaires d’une Cactacèe “Pereskia grandifolia”. Thérapie 1951, 6, 103–107. [Google Scholar]
- Dayrell, M.S.; Vieira, E.C. Leaf protein concentrate of the cactacea Pereskia aculeata Mill. I. Extraction and composition. Nutr. Rep. Int. 1977, 15, 529–537. [Google Scholar]
- Dayrell, M.S.; Vieira, E.C. Leaf protein concentrate of the cactacea Pereskia aculeata Mill. II. Nutritive value. Nutr. Rep. Int. 1977, 15, 539–545. [Google Scholar]
- Takeiti, C.Y.; Antonio, G.C.; Motta, E.M.P.; Collares-Queiroz, F.P.; Park, K.J. Nutritive evaluation of a non-conventional leafy vegetable (Pereskia aculeata Miller). Int. J. Food Sci. Nutr. 2009, 60 (Suppl. 1), 148–160. [Google Scholar] [CrossRef]
- Lima Junior, F.A.; Conceição, M.C.; Vilela de Resende, J.; Junqueira, L.A.; Pereira, C.G.; Torres Prado, M.E. Response surface methodology for optimization of the mucilage extraction process from Pereskia aculeata Miller. Food Hydrocoll. 2013, 33, 38–47. [Google Scholar] [CrossRef]
- Vieira, C.R.; Grancieri, M.; Martino, H.S.D.; César, D.E.; Barra, R.R.S. A beverage containing ora-pro-nobis flour improves intestinal health, weight, and body composition: A double-blind randomized prospective study. Nutrition 2020, 78, 110869. [Google Scholar] [CrossRef]
- Edwards, E.J.; Nyffeler, R.; Donoghue, M.J. Basal cactus phylogeny: Implications of Pereskia (Cactaceae) paraphyly for the transition to the cactus life form. Am. J. Bot. 2005, 92, 1177–1188. [Google Scholar] [CrossRef]
- Maciel, V.B.V.; Yoshida, C.M.P.; Goycoolea, F.M. Agronomic cultivation, chemical composition, functional activities and applications of Pereskia species-A mini review. Curr. Med. Chem. 2019, 26, 4573–4584. [Google Scholar] [CrossRef]
- Boke, N.H. Structure and development of the flower and fruit of Pereskia diaz-romeroana. Am. J. Bot. 1968, 55, 1254–1260. [Google Scholar] [CrossRef]
- De Almeida, M.E.F.; Junqueira, A.M.B.; Simão, A.A.; Corrêa, A.D. Caracterização química das hortaliças não-convencionais conhecidas como ora-pro-nobis/Chemical characterization of the non-conventional vegetable known as ora-pro-nobis. Biosci. J. 2014, 30, 431–439. [Google Scholar]
- Duarte, M.R.; Hayashi, S.S. Estudo anatômico de folha e caule de Pereskia aculeata Mill. (Cactaceae). Rev. Bras. Farmacogn. 2005, 15, 103–109. [Google Scholar] [CrossRef]
- Da Rosa, S.M.; Souza, L.A. Morphology and anatomy of the fruit (hypanthium, pericarp and seed) development of Pereskia aculeata Miller (Cactaceae). Acta. Sci. Biol. Sci. 2003, 25, 415–428. [Google Scholar]
- Brasil. Ministério da Agricultura, Pecuária e Abastecimento. Secretaria de Desenvolvimento Agropecuário e Cooperativismo; Manual de Hortaliças Não Convencionais: Brasília, Brasil, 2010; 92p. [Google Scholar]
- Marsaro Júnior, A.L.; Souza Filho, M.F.; Adaime, R.; Strinkis, P.C. First report of natural infestation of Pereskia aculeata Mill. (cactaceae) by Ceratitis capitata (Wedemann) (diptera: Tephiritidae) in Brazil. Rev. Agric. 2011, 86, 151–154. [Google Scholar]
- Madeira, N.R.; Silva, P.C.; Botrel, N.; Mendonça, J.L.; Silveira, G.S.R.; Pedrosa, M.W. Manual de Produção de Hortaliças Tradicionais; Embrapa: Brasília, Brasil, 2013; p. 159. [Google Scholar]
- Queensland Government. Weeds of Australia, Biosecurity Queensland Edition; Queensland Government: Brisbane, Australia, 2018; Available online: https://keyserver.lucidcentral.org/weeds/data/media/Html/search.html (accessed on 15 October 2022).
- Useful Tropical Plants. Useful Tropical Plants Database. 2020. Available online: http://tropical.theferns.info/ (accessed on 20 October 2022).
- Cronquist, A. An Integrated System of Classification of Flowering Plants; Columbia University Press: New York, NY, USA, 1981. [Google Scholar]
- Egea, M.B.; Pierce, G. Bioactive compounds of Barbados gooseberry (Pereskia aculeata Mill.). In Bioactive Compounds in Underutilized Vegetables and Legumes; Murthy, H.N., Paek, K.Y., Eds.; Springer International Publishing: Berlin, Germany, 2021; pp. 1–14. [Google Scholar] [CrossRef]
- Torres, T.M.S.; Álvarez-Rivera, G.; Mazzutti, S.; Sánchez-Martínez, J.D.; Cifuentes, A.; Ibáñez, E.; Ferreira, S.R.S. Neuroprotective potential of extracts from leaves of ora-pro-nobis (Pereskia aculeata) recovered by clean compressed fluids. J. Supercrit. Fluids 2022, 179, 105390. [Google Scholar] [CrossRef]
- Queiroz, C.R.A.d.A.; Melo, C.M.T.; de Andrade, R.R.; Pavani, L.C.; de Morais, S.A.L. Composição centesimal de frutos de ora-pro-nóbis. In 34a Reunião Anual da Sociedade Brasileira de Química; Brazilian Chemical Society: Florianópolis, Brazil, 2011; p. 1. [Google Scholar]
- Botrel, N.; Godoy, R.L.d.O.; Madeira, N.R.; Amaro, G.B.; Melo, R.A.d.C. Estudo Comparativo da Composição Proteica e do Perfil de Aminoácidos em Cinco Clones de Ora-pro-Nóbis; Embrapa: Brasilia, Brazil, 2019; ISSN 1677-2229. [Google Scholar]
- Neves, B.V.; Mesquita, L.M.d.S.; Murador, D.C.; Cheberle, A.I.d.P.; Braga, A.R.C.; Mercadante, A.Z.; de Rosso, V.V. Improvement of bioactive compound levels, antioxidant activity, and bioaccessibility of carotenoids from Pereskia aculeata after different cooking techniques. ACS Food Sci. Technol. 2021, 1, 1285–1293. [Google Scholar] [CrossRef]
- Moran, V.C.; Zimmermann, H.G. Biological control of cactus weeds of minor importance in South Africa. Agric. Ecosyst. Environ. 1991, 37, 37–55. [Google Scholar] [CrossRef]
- Paterson, I.D.; Coetzee, J.A.; Hill, M.P.; Downie, D.D. A pre-release assessment of the relationship between the invasive alien plant, Pereskia aculeata Miller (Cactaceae), and native plant biodiversity in South Africa. Biol. Control 2011, 57, 59–65. [Google Scholar] [CrossRef]
- Muskett, P.C.; Paterson, I.D.; Coetzee, J.A. Ground-truthing climate-matching predictions in a post-release evaluation. Biol. Control 2020, 144, 104217. [Google Scholar] [CrossRef]
- Venter, N.; Cowie, B.W.; Paterson, I.D.; Witkowski, E.T.F.; Byrne, M.J. The interactive effects of CO2 and water on the growth and physiology of the invasive alien vine Pereskia aculeata (Cactaceae): Implications for its future invasion and management. Environ. Exp. Bot. 2022, 194, 104737. [Google Scholar] [CrossRef]
- Dos Queiroz, C.R.A.A.; dos Moraes, C.M.S.; de Andrade, R.R.; Pavani, L.C. Crescimento inicial e composição química de Pereskia aculeata Miller cultivada em diferentes luminosidades. Rev. Agrogeoambiental 2015, 7, 93–104. [Google Scholar] [CrossRef]
- Souza, L.F.; Gasparetto, B.F.; Lopes, R.R.; Barros, I.B.I. Temperature requirements for seed germination of Pereskia aculeata and Pereskia grandifolia. J. Therm. Biol. 2016, 57, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Nurestri, A.M.S.; Sim, K.S.; Norhanom, A.W. Phytochemical and cytotoxic investigations of Pereskia grandifolia Haw. (Cactaceae) leaves. J. Biol. Sci. 2009, 9, 488–493. [Google Scholar] [CrossRef]
- Massocatto, A.M.; Silva, N.F.d.S.; Kazama, C.C.; Pires, M.D.B.; Takemura, O.S.; Jacomassi, E.; Ruiz, A.L.T.G.; Laverde Junior, A. Biological activity survey of Pereskia aculeata Mill. and Pereskia grandifolia Haw. (Cactaceae). Pharm. Sci. 2021, 28, 156–165. [Google Scholar] [CrossRef]
- Silva, D.O.; Seifert, M.; Nora, F.R.; Bobrowski, V.L.; Freitag, R.A.; Kucera, H.R.; Nora, L.; Gaikwad, N.W. Acute toxicity and cytotoxicity of Pereskia aculeata, a highly nutritious cactaceae plant. J. Med. Food 2017, 20, 403–409. [Google Scholar] [CrossRef]
- Barbalho, S.M.; Guiguer, É.L.; Marinelli, P.S.; do Santos Bueno, P.C.; Pescinini-Salzedas, L.M.; dos Santos, M.C.B.; Oshiiwa, M.; Mendes, C.G.; de Menezes, M.L.; Nicolau, C.C.T.; et al. Pereskia aculeata Miller flour: Metabolic effects and composition. J. Med. Food 2016, 19, 890–894. [Google Scholar] [CrossRef]
- Mazon, S.; Menin, D.; Cella, B.M.; Lise, C.C.; Vargas, T.d.O.; Daltoé, M.L.M. Exploring consumers’ knowledge and perceptions of unconventional food plants: Case study of addition of Pereskia aculeata Miller to ice cream. Food Sci. Technol. 2020, 40, 215–221. [Google Scholar] [CrossRef]
- Patriota, G.A.R. Elaboração e Avaliação Sensorial de Paçoca Adicionada de Ora–pro–Nóbis (Pereskia Aculeata Miller) Trabalho de Conclusão de Curso; Universidade Federal de Campina Grande: Cuité, Brazil, 2020. [Google Scholar]
- Francelin, M.F.; Machado, L.M.; Silva, D.d.M.B.; Alves, E.d.S.; Peralta, R.M.; Costa, S.C.; Monteiro, A.R.G. Desenvolvimento e caracterização de snack de milho extrusado com adição de farinha de ora-pro-nóbis. Res. Soc. Dev. 2021, 10, e2910312850. [Google Scholar] [CrossRef]
- Faiom, A.; Savoldi, A.L.L.; Mattiello, E.R. Elaboração de barra de cereal a partir de farinha de ora-pro-nóbis e resíduo agroindustrial de abacaxi. Tecnol. Compet. Ind. 2021, 14, 139–154. [Google Scholar] [CrossRef]
- Pocai, A.V.; Laurindo, J.B.; Jiménez, M.S.E.; Richards, N.S.P. dos S. Produção de bebida fermentada enriquecida com ora-pro-nóbis (Pereskia aculeata). In Produtos Lácteos: Desenvolvimento & Tecnologia; Richards, N.S.P.S., Ed.; Mérida Publishers: Canoas, Brazil, 2020; pp. 43–52. [Google Scholar] [CrossRef]
- Rocha, D.R.C.; Pereira Juúnio, G.A.; Vieira, G.; Pantoja, L.; Santos, A.S.; Pinto, N.A.V.D. Macarrão adicionado de ora-pro-nóbis (Pereskia aculeata Miller) desidratado. Aliment. Nutr. Araraquara 2008, 19, 459–465. [Google Scholar]
- Martinevski, C.S.; de Oliveira, V.R.; de Rios, A.O.; Flores, S.H.; Venzke, J.G. Utilização de bertalha (Anredera cordifolia (ten.) Steenis) e ora-pro-nobis (Pereskia aculeata Mill.) na elaboração de pães. Aliment. Nutr. 2013, 24, 1–7. [Google Scholar]
- De Paula, M.C.; de Oliveira, R.B.; Felipe, D.F.; Magrine, I.C.O.; Sartor, C.F.P. Processamento de bolo com a planta Pereskia aculeata Mill. (ora-pro-nóbis). Rev. Bras. Prod. Agroind. 2016, 18, 167–174. [Google Scholar] [CrossRef]
- Izzo, S.; Domene, S.M.Á. Aceitabilidade de preparações culinárias com ora-pro-nóbis por escolares atendidos pelo Programa Nacional de Alimentação Escolar. Demetra 2021, 16, e53372. [Google Scholar] [CrossRef]
- Rover, C.H.; Sasaki, J.L.S.; dos Isepon, J.S.; Teixeira Filho, M.C.M.; Garcia, C.M.d.P.; Rosa, M.E. Aceitabilidade de pães processados com ora-pro-nobis. Agron. Crop J. 2013, 22, 35–44. [Google Scholar]
- Baroni, J.O.; Fernanda Volpini-Rapina, L.; Costa-Singh, T. Sensory evaluation of vegetable pie with non-conventional added vegetable ora-pro-nóbis (Pereskia aculeata). Nutrição 2017, 16, 5. [Google Scholar]
- Rosa, L.; Queiroz, C.R.A.D.A.; Melo, C.M.T. Fresh leaves of ora-pro-nobis in cakes prepared from commercial pre-mixture. Biosci. J. 2020, 36, 376–382. [Google Scholar] [CrossRef]
- Sobrinho, S.S.; Costa, L.L.; Gonçalves, C.A.A.; Campagnol, P.C.B. Emulsified cooked sausages enriched with flour from ora-pro-nobis leaves (Pereskia aculeata Miller). Int. Food Res. J. 2015, 22, 318–323. [Google Scholar]
- Moro, G.L.; Moro, G.L.M. Desenvolvimento e Caracterização de Hambúrguer Vegano de Grão de Bico (Cicer arietinum L.) com Adição de Ora-pro-Nóbis (Pereskia aculeata); Trabalho de Conclusão de Curso; Universidade Federal da Grande Dourados: Dourados-Mato Grosso do Sul, Brazil, 2019. [Google Scholar]
- De Silveira, A.Á.; dos Queiroz, C.R.A.A.; Melo, C.M.T.; Bonnas, D.S. Bolinho de arroz com ora-pro-nobis: Análise química e Sensorial. In Ciência e Tecnologia dos Alimentos; Andrade, D.F., Ed.; Editora Poisson: Belo Horizonte, Brazil, 2021; Volume 11, pp. 39–45. [Google Scholar]
- Saldaña, E.; Behrens, J.H.; Serrano, J.S.; Ribeiro, F.; de Almeida, M.A.; Contreras-Castillo, C.J. Microstructure, texture profile and descriptive analysis of texture for traditional and light mortadella. Food Struct. 2015, 6, 13–20. [Google Scholar] [CrossRef]
- Silveira, M.G.; Picinin, C.T.R.; Cirillo, M.Â.; Freire, J.M.; Barcelos, M.D.F.P. Nutritional assay Pereskia spp.: Unconventional vegetable. An. Acad. Bras. Ciênc. 2020, 92 (Suppl. 1), e20180757. [Google Scholar] [CrossRef]
- Carvalho, E.G.; Soares, C.P.; Blau, L.; Menegon, R.F.; Joaquim, W.M. Wound healing properties and mucilage content of Pereskia aculeata from different substrates. Rev. Bras. Farmacogn. 2014, 24, 677–682. [Google Scholar] [CrossRef]
- Maciel, V.B.V.; Yoshida, C.M.P.; Boesch, C.; Goycoolea, F.M.; Carvalho, R.A. Iron-rich chitosan-pectin colloidal microparticles laden with ora-pro-nobis (Pereskia aculeata Miller) extract. Food Hydrocoll. 2020, 98, 105313. [Google Scholar] [CrossRef]
- De Vicente, N.F.P.; de Martins, H.H.A.; Campidelli, M.L.L.; da Silva, D.M.; Aazza, S.; de Souza, E.C.; Bertolucci, S.K.V.; Piccoli, R.H. Determination of the phenolic, antioxidant and antimicrobial potential of leaf extracts of Pereskia grandifolia Haw. Res. Soc. Dev. 2020, 9, e2979108483. [Google Scholar] [CrossRef]
- Neves, I.C.O.; Silva, S.H.; Oliveira, N.L.; Lago, A.M.T.; Ng, N.; Sultani, A.; Campelo, P.H.; Veríssimo, L.A.A.; de Resende, J.V.; Rogers, M.A. Effect of carrier oil on α-tocopherol encapsulation in ora-pro-nobis (Pereskia aculeata Miller) mucilage-whey protein isolate microparticles. Food Hydrocoll. 2020, 105, 105716. [Google Scholar] [CrossRef]
- Silva, S.H.; Neves, I.C.O.; Oliveira, N.L.; de Oliveira, A.C.F.; Lago, A.M.T.; de Oliveira Giarola, T.M.; de Resende, J.V. Extraction processes and characterization of the mucilage obtained from green fruits of Pereskia aculeata Miller. Ind. Crops Prod. 2019, 140, 111716. [Google Scholar] [CrossRef]
- Da Silva, A.P.G.; Spricigo, P.C.; de Freitas, T.P.; da Acioly, T.M.S.; de Alencar, S.M.; Jacomino, A.P. Ripe ora-pro-nobis (Pereskia aculeata miller) fruits express high contents of bioactive compounds and antioxidant capacity. Rev. Bras. Frutic. 2018, 40, e-749. [Google Scholar] [CrossRef]
- Cruz, T.M.; Santos, J.S.; do Carmo, M.A.V.; Hellström, J.; Pihlava, J.M.; Azevedo, L.; Granato, D.; Marques, M.B. Extraction optimization of bioactive compounds from ora-pro-nobis (Pereskia aculeata Miller) leaves and their in vitro antioxidant and antihemolytic activities. Food Chem. 2021, 361, 130078. [Google Scholar] [CrossRef]
- Conceição, M.C.; Junqueira, L.A.; Guedes Silva, K.C.; Prado, M.E.T.; de Resende, J.V. Thermal and microstructural stability of a powdered gum derived from Pereskia aculeata Miller leaves. Food Hydrocoll. 2014, 40, 104–114. [Google Scholar] [CrossRef]
- Silva, S.H.; Neves, I.C.O.; de Meira, A.C.F.O.; Alexandre, A.C.S.; Oliveira, N.L.; de Resende, J.V. Freeze-dried Petit Suisse cheese produced with ora-pro-nóbis (Pereskia aculeata Miller) biopolymer and carrageenan mix. LWT 2021, 149, 111764. [Google Scholar] [CrossRef]
- Ribeiro Neto, J.A.; Pimenta Tarôco, B.R.; Batista dos Santos, H.; Thomé, R.G.; Wolfram, E.; Maciel de A Ribeiro, R.I. Using the plants of Brazilian Cerrado for wound healing: From traditional use to scientific approach. J. Ethnopharmacol. 2020, 260, 112547. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.A.; de Freitas, R.A.; Sassaki, G.L.; Evangelista, P.H.L.; Sierakowski, M.R. Chemical structure and physical-chemical properties of mucilage from the leaves of Pereskia aculeata. Food Hydrocoll. 2017, 70, 20–28. [Google Scholar] [CrossRef]
- Silva, K.C.G.; Amaral, T.N.; Junqueira, L.A.; de Oliveira Leite, N.; de Resende, J.V. Adsorption of protein on activated carbon used in the filtration of mucilage derived from Pereskia aculeata Miller. S. Afr. J Chem. Eng. 2017, 23, 42–49. [Google Scholar] [CrossRef]
- Junqueira, L.A.; da Silva, V.; Amaral, T.N.; Torres Prado, M.E.; Resende, J.V. Effects of temperature and concentration on the rheological properties of mucilage extracted from Pereskia aculeata Miller. J. Food Meas. Charact. 2019, 13, 2549–2562. [Google Scholar] [CrossRef]
- Lise, C.C.; Marques, C.; da Cunha, M.A.A.; Mitterer-Daltoé, M.L. Alternative protein from Pereskia aculeata Miller leaf mucilage: Technological potential as an emulsifier and fat replacement in processed mortadella meat. Eur. Food Res. Technol. 2021, 247, 851–863. [Google Scholar] [CrossRef]
- Oliveira, N.L.; Rodrigues, A.A.; Oliveira Neves, I.C.; Teixeira Lago, A.M.; Borges, S.V.; de Resende, J.V. Development and characterization of biodegradable films based on Pereskia aculeata Miller mucilage. Ind. Crops Prod. 2019, 130, 499–510. [Google Scholar] [CrossRef]
- Lago, A.M.T.; Neves, I.C.O.; Oliveira, N.L.; Botrel, D.A.; Minim, L.A.; de Resende, J.V. Ultrasound-assisted oil-in-water nanoemulsion produced from Pereskia aculeata Miller mucilage. Ultrason. Sonochem. 2018, 50, 339–353. [Google Scholar] [CrossRef]
- Neves, I.C.O.; Rodrigues, A.A.; Valentim, T.T.; de Meira, A.C.F.O.; Silva, S.H.; Veríssimo, L.A.A.; de Resende, J.V. Amino acid-based hydrophobic affinity cryogel for protein purification from ora-pro-nobis (Pereskia aculeata Miller) leaves. J. Chromatogr. B 2020, 1161, 122435. [Google Scholar] [CrossRef]
- Colacite, J.; Batista, A.P.; Reis, S.L.M.; Assumpção, J. Evaluation of the antimicrobial activity of different extracts of Ora-Pro-Nobis leaves. Braz. J. Dev. 2022, 8, 33207–33216. [Google Scholar] [CrossRef]
- Torres, T.M.S.; Mazzutti, S.; Castiani, M.A.; Saddique, I.; Vitali, L.; Ferreira, S.R.S. Phenolic compounds recovered from ora-pro-nobis leaves by microwave assisted extraction. Biocatal. Agric. Biotechnol. 2022, 39, 102238. [Google Scholar] [CrossRef]
- Nurestri, A.M.S.; Sinniah, S.K.; Kim, K.H.; Norhanom, A.W.; Sim, K.S. Acute oral toxicity of Pereskia bleo and Pereskia grandifolia in mice. Pharmacogn. Mag. 2010, 6, 67. [Google Scholar] [CrossRef] [PubMed]
- Nurestri, A.M.S.; Norhanom, A.W.; Sim, K.S. Phenolic content and antioxidant activity of Pereskia grandifolia Haw. (Cactaceae) extracts. Pharmacogn. Mag. 2010, 6, 248. [Google Scholar] [CrossRef] [PubMed]
- Kazama, C.C.; Uchida, D.T.; Canzi, K.N.; de Souza, P.; Crestani, S.; Gasparotto Junior, A.; Laverde-Junior, A. Involvement of arginine-vasopressin in the diuretic and hypotensive effects of Pereskia grandifolia Haw. (Cactaceae). J. Ethnopharmacol. 2012, 144, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Turra, A.F.; Marçal, F.J.B.; Baretta, I.P.; Takemura, O.S.; Laverde, A., Jr. Antioxidant properties and antimicrobial susceptibility evaluation of Pereskia grandifolia Haworth (Cactaceae). Arq. Ciências Saúde UNIPAR 2007, 11, 9–14. [Google Scholar]
- Dybka-Stępień, K.; Otlewska, A.; Góźdź, P.; Piotrowska, M.; Piotrowska, M. The renaissance of plant mucilage in health promotion and industrial applications: A review. Nutrients 2021, 13, 3354. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.W.; Lai, L.S. Characterization of the mucilage extracted from the edible fronds of bird’s nest fern (Asplenium australasicum) with enzymatic modifications. Food Hydrocoll. 2016, 53, 84–92. [Google Scholar] [CrossRef]
- Tosif, M.M.; Najda, A.; Bains, A.; Kaushik, R.; Dhull, S.B.; Chawla, P.; Walasek-Janusz, M. A Comprehensive review on plant-derived mucilage: Characterization, functional properties, applications, and its utilization for nanocarrier fabrication. Polymers 2021, 13, 1066. [Google Scholar] [CrossRef]
- Sierakowski, M.R. Aspectos Estruturais da Mucilagem de Pereskia aculeata, Mill (ora-pro-nobis). Ph.D. Thesis, Federal University of Paraná, Curitiba, Brazil, 1988. Available online: https://acervodigital.ufpr.br/handle/1884/30018 (accessed on 15 May 2023).
- Sierakowski, M.-R.; Gorin, P.A.J.; Reicher, F.; Corrêa, J.B.C. Some structural features of a heteropolysaccharide from the leaves of the cactus Pereskia aculeata. Phytochemistry 1987, 26, 1709–1713. [Google Scholar] [CrossRef]
- Sierakowski, M.-R.; Gorin, P.A.J.; Reicher, F.; Corrêa, J.B.C. Location of O-acetyl groups in the heteropolysaccharide of the cactus Pereskia aculeata. Carbohydr. Res. 1990, 201, 277–284. [Google Scholar] [CrossRef]
- Amaral, T.N.; Junqueira, L.A.; Prado, M.E.T.; Cirillo, M.A.; de Abreu, L.R.; Costa, F.F.; de Resende, J.V. Blends of Pereskia aculeata Miller mucilage, guar gum, and gum Arabic added to fermented milk beverages. Food Hydrocoll. 2018, 79, 331–342. [Google Scholar] [CrossRef]
- Junqueira, L.A.; Amaral, T.N.; Félix, P.C.; Botrel, D.A.; Prado, M.E.T.; de Resende, J.V. Effects of change in pH and addition of sucrose and NaCl on the emulsifying properties of mucilage obtained from Pereskia aculeata Miller. Food Bioproc. Technol. 2019, 12, 486–498. [Google Scholar] [CrossRef]
- Oliveira, N.L.; Oliveira, A.C.S.; Silva, S.H.; Rodrigues, A.A.; Borges, S.V.; de Oliveira, J.E.; de Resende, J.V. Development and characterization of starch-based films added ora-pro-nobis mucilage and study of biodegradation and photodegradation. J. Appl. Polym. Sci. 2022, 139, e52108. [Google Scholar] [CrossRef]
- Torres, T.M.S.; Guedes, J.A.C.; de Brito, E.S.; Mazzutti, S.; Ferreira, S.R.S. High-pressure biorefining of ora-pro-nobis (Pereskia aculeata). J. Supercrit. Fluids 2022, 181, 105514. [Google Scholar] [CrossRef]
- Coral, M.C. O Efeito do Processamento Térmico Sobre os Compostos Bioativos e a Atividade Antioxidante em Pereskia grandifolia Hawer e Talinum paniculatum (jacq.) Gaertn. Master’s Thesis, Universidade Estadual Paulista Julio Mesquita Filho, Botucatu, SP, Brazil, 2019. [Google Scholar]
- Hoff, R.; Daguer, H.; Deolindo, C.T.P.; de Melo, A.P.Z.; Durigon, J. Phenolic compounds profile and main nutrients parameters of two underestimated non-conventional edible plants: Pereskia aculeata Mill. (ora-pro-nóbis) and Vitex megapotamica (Spreng.) Moldenke (tarumã) fruits. Food Res. Int. 2022, 162, 112042. [Google Scholar] [CrossRef]
- Geran, R.I.; Greenberg, N.H.; Macdonald, M.M.; Schumacher, A.M.; Abbott, B.J. Protocols for screening chemical agents and natural products against animal tumors and other biological systems. Cancer Chemother. Rep. 1972, 3, 17–19. [Google Scholar]
- Wang, H.; Chan, G.C.-K.; Athos, J.; Storm, D.R. Synaptic concentration of type-I adenylyl cyclase in cerebellar neurons. J. Neurochem. 2002, 83, 946–954. [Google Scholar] [CrossRef]
- Yang, M.; Ding, Q.; Zhang, M.; Moon, C.; Wang, H. Forebrain overexpression of type 1 adenylyl cyclase promotes molecular stability and behavioral resilience to physical stress. Neurobiol. Stress 2020, 13, 100237. [Google Scholar] [CrossRef]
- Brand, C.S.; Hocker, H.J.; Gorfe, A.A.; Cavasotto, C.N.; Dessauer, C.W. Isoform selectivity of adenylyl cyclase inhibitors: Characterization of known and novel compounds. J. Pharmacol. Exp. Ther. 2013, 347, 265–275. [Google Scholar] [CrossRef]
- Tanyeli, A.; Ekinci, F.A.; Eraslan, E.; Güler, M.C.; Nacar, T. Antioxidant and anti-inflammatory effectiveness of caftaric acid on gastric ulcer induced by indomethacin in rats. Gen. Physiol. Biophys. 2019, 38, 175–181. [Google Scholar] [CrossRef]
- De Sato, R.; Cili, L.P.L.; de Oliveira, B.E.; Maciel, V.B.V.; Venturini, A.C.; Yoshida, C.M.P. Nutritional improvement of pasta with Pereskia aculeata Miller: An unconventional edible vegetable. Food Sci. Technol. 2019, 39, 28–34. [Google Scholar] [CrossRef]
- Omidian, K.; Rafiei, H.; Bandy, B. Increased mitochondrial content and function by resveratrol and select flavonoids protects against benzo[a]pyrene-induced bioenergetic dysfunction and ROS generation in a cell model of neoplastic transformation. Free Rad. Biol. Med. 2020, 152, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Gullón, B.; Lú-Chau, T.A.; Moreira, M.T.; Lema, J.M.; Eibes, G. Rutin: A review on extraction, identification and purification methods, biological activities and approaches to enhance its bioavailability. Trends Food Sci. Technol. 2017, 67, 220–235. [Google Scholar] [CrossRef]
- O’Mahony, L.; Akdis, M.; Akdis, C.A. Regulation of the immune response and inflammation by histamine and histamine receptors. J. Allergy Clin. Immunol. 2011, 128, 1153–1162. [Google Scholar] [CrossRef]
- Blanco, A.D.R.; Nascimento, B.D.; Cavalcante, E.R.; Gamito, S.A. Formulation of Vegan Facial Cream with Ora Pro-Nobis Essential Oil (Pereskia aculeata) and Aloe Vera. Monograph, São Paulo, Brazil. 2022. Available online: http://ric.cps.sp.gov.br/handle/123456789/11583 (accessed on 15 May 2023).
- Pimenta, P.C.; Belo, T.C.A.; Vanzele, P.A.R.; Nasser, T.F.; Santos, H.C.A.S.; Bani, G.M.A. Castro. Evaluation of the antimicrobial capacity of Pereskia aculeata essential oil: Interaction with microorganisms found in the coat of health professionals. Braz. J. Dev. 2020, 6, 40046–40058. [Google Scholar] [CrossRef]





| Ora-Pro-Nobis Species | Extraction Solvent | Extraction Technique | Major Compounds Identified | Reference |
|---|---|---|---|---|
| P. aculeata leaves | Ethanol:water (60:40) | Simple stirring | Phenolic compounds: caftaric acid; chicoric acid; caffeoyl-hexaric acid; coumaroyl-hexaric acid; quercetin-pentoside; kaempherol-rhamnoside-hexoside | [73] |
| P. aculeata leaves | Aqueous and ethanolic extracts | Subcritical water extraction and pressurized liquid extraction | Phenolic compounds: caftaric acid isomers; quercetin-O-pentoside-O-rutinoside; quercetin-3-O-rutinoside; isorhamnetin-O-pentoside-O-rutinoside; quercetin-glucoside; kaempferol-3-O-rutinoside; isorhamnetin-3-O-rutinoside; isorhamnetin-3-O-glucoside | [85,99] |
| P. aculeata leaves | Ethanol:water (70:30) | Simple stirring | Phenolic compounds: caftaric acid; trans caftaric acid; caffeic acid derivative quercetin-O-pentoside-O-rutinoside; quercetin-O-pentoside-O-hexoside; quercetin-3-O-rutinoside; isorhamnetin-O-pentoside-O-rutinoside; isorhamnetin-O-pentoside-O-hexoside; kaempferol-3-O-rutinoside; isorhamnetin-3-O-rutinoside | [6] |
| P. grandifolia leaves | Extracted with methanol and fractionated with hexane | Decane; 4,5-dimethyl-2,6-octadiene; undecane; nerolidol; 1-methoxy-p-tolyl-propan-2-ol; neophytadiene; methyl palmitate; palmitic acid; linolenic acid methyl ester; phytol; methyl octadecanoate; tricosane; cholesterol; campesterol; stigmasterol; sitosterol; taraxasterol taraxerol; | [11] | |
| P. grandifolia leaves | Ethanol, water, and hexane | Microwave-assisted extraction and Soxhlet extraction | Phenolic compounds: 4-aminobenzoic acid; 4-hydroxymethylbenzoic acid; caffeic acid; chlorogenic acid; cinnamic acid; ellagic acid; ferulic acid; gallic acid; mandelic acid; p-anisic acid; p-coumaric acid; protocatechuic acid; salicylic acid; sinapic acid; syringic acid; vanillic acid; epicatechin; kaempferol; myricetin; quercetin; rutin; syringaldehyde; vanillin, umbelliferone | [36] |
| P. aculeata leaves | Acetone | Maceration | Carotenoids: 9-cis-neoxanthin; 13-cis-violaxanthin; all-trans-neochrome; 13-cis-lutein; 15-cis-lutein; all-trans-lutein; all-trans-zeaxanthin; all-trans-β-cryptoxanthin; 13-cis-β-carotene; all-trans-α-carotene; di-cis-β-carotene; 9-cis-β-carotene; all-trans-β-carotene | [39] |
| P. grandifolia leaves | Water | Cooking in boiling water, water vapor and microwave | Phenolic compounds: caffeic acid; chlorogenic acid; catechin; rutin; p-coumaric acid; t-ferulic acid; luteolincarotenoids: lutein, α-carotene, β-carotene | [100] |
| P. aculeataFruit | 2,4-DHBA, 2,5-DHBA, caffeic acid, chlorogenic acid, ferulic acid, catechin, hesperidin, isoquercitrin, p-coumaric acid, quercetin, and rutin. | [101] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teixeira, V.M.C.; Oliveira, A.d.; Backes, E.; Souza, C.G.M.d.; Castoldi, R.; Sá-Nakanishi, A.B.d.; Bracht, L.; Comar, J.F.; Corrêa, R.C.G.; Leimann, F.V.; et al. A Critical Appraisal of the Most Recent Investigations on Ora-Pro-Nobis (Pereskia sp.): Economical, Botanical, Phytochemical, Nutritional, and Ethnopharmacological Aspects. Plants 2023, 12, 3874. https://doi.org/10.3390/plants12223874
Teixeira VMC, Oliveira Ad, Backes E, Souza CGMd, Castoldi R, Sá-Nakanishi ABd, Bracht L, Comar JF, Corrêa RCG, Leimann FV, et al. A Critical Appraisal of the Most Recent Investigations on Ora-Pro-Nobis (Pereskia sp.): Economical, Botanical, Phytochemical, Nutritional, and Ethnopharmacological Aspects. Plants. 2023; 12(22):3874. https://doi.org/10.3390/plants12223874
Chicago/Turabian StyleTeixeira, Valéria Maria Costa, Anielle de Oliveira, Emanueli Backes, Cristina Giatti Marques de Souza, Rafael Castoldi, Anacharis Babeto de Sá-Nakanishi, Lívia Bracht, Jurandir Fernando Comar, Rúbia Carvalho Gomes Corrêa, Fernanda Vitória Leimann, and et al. 2023. "A Critical Appraisal of the Most Recent Investigations on Ora-Pro-Nobis (Pereskia sp.): Economical, Botanical, Phytochemical, Nutritional, and Ethnopharmacological Aspects" Plants 12, no. 22: 3874. https://doi.org/10.3390/plants12223874
APA StyleTeixeira, V. M. C., Oliveira, A. d., Backes, E., Souza, C. G. M. d., Castoldi, R., Sá-Nakanishi, A. B. d., Bracht, L., Comar, J. F., Corrêa, R. C. G., Leimann, F. V., Bracht, A., & Peralta, R. M. (2023). A Critical Appraisal of the Most Recent Investigations on Ora-Pro-Nobis (Pereskia sp.): Economical, Botanical, Phytochemical, Nutritional, and Ethnopharmacological Aspects. Plants, 12(22), 3874. https://doi.org/10.3390/plants12223874










