In Vitro and In Vivo Anti-Inflammatory Potential of Cannabichromene Isolated from Hemp
Abstract
:1. Introduction
2. Results and Discussion
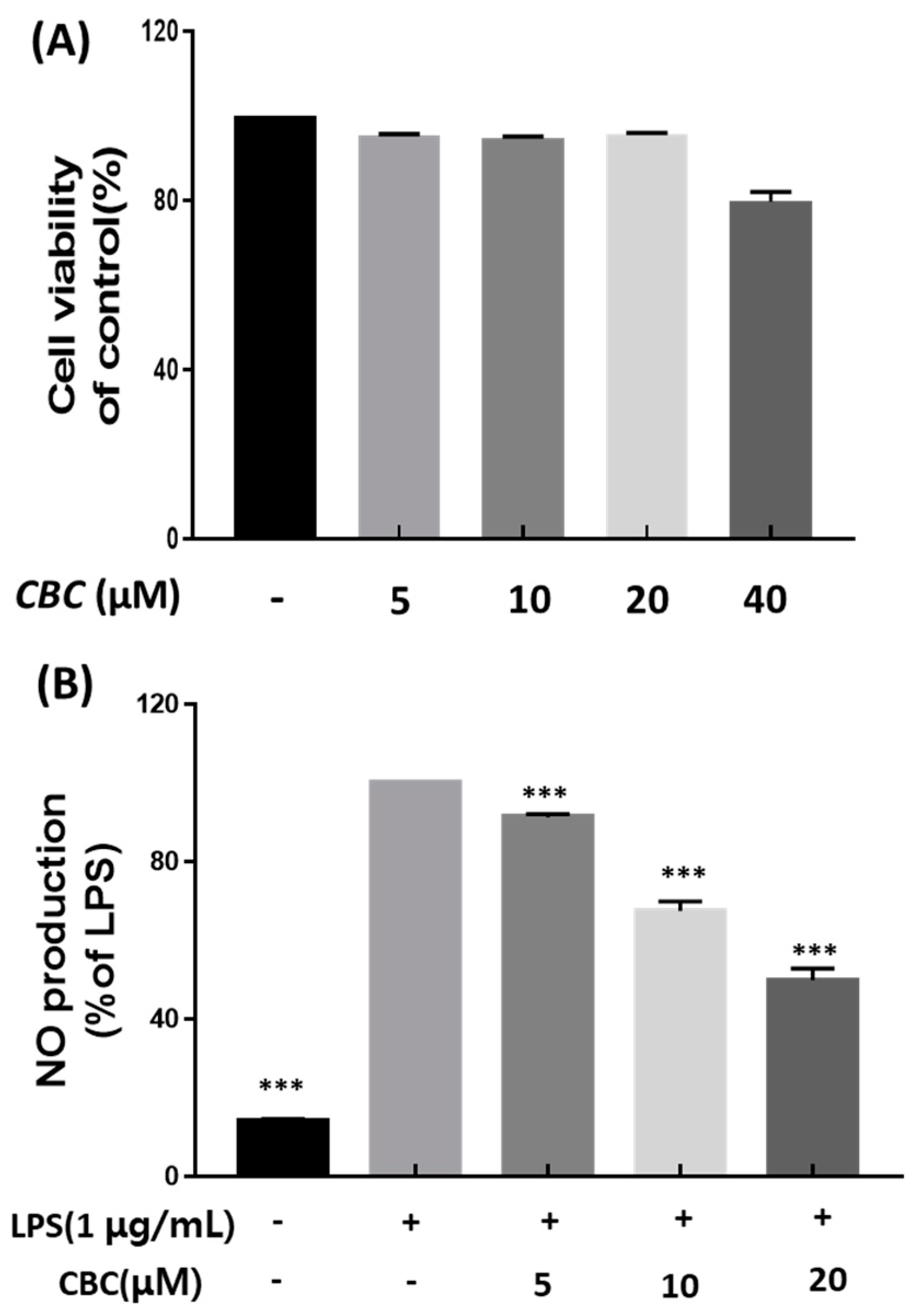
2.1. Cell Viability and Suppression of NO Production in RAW 264.7 Cells in response to Cannabichromene
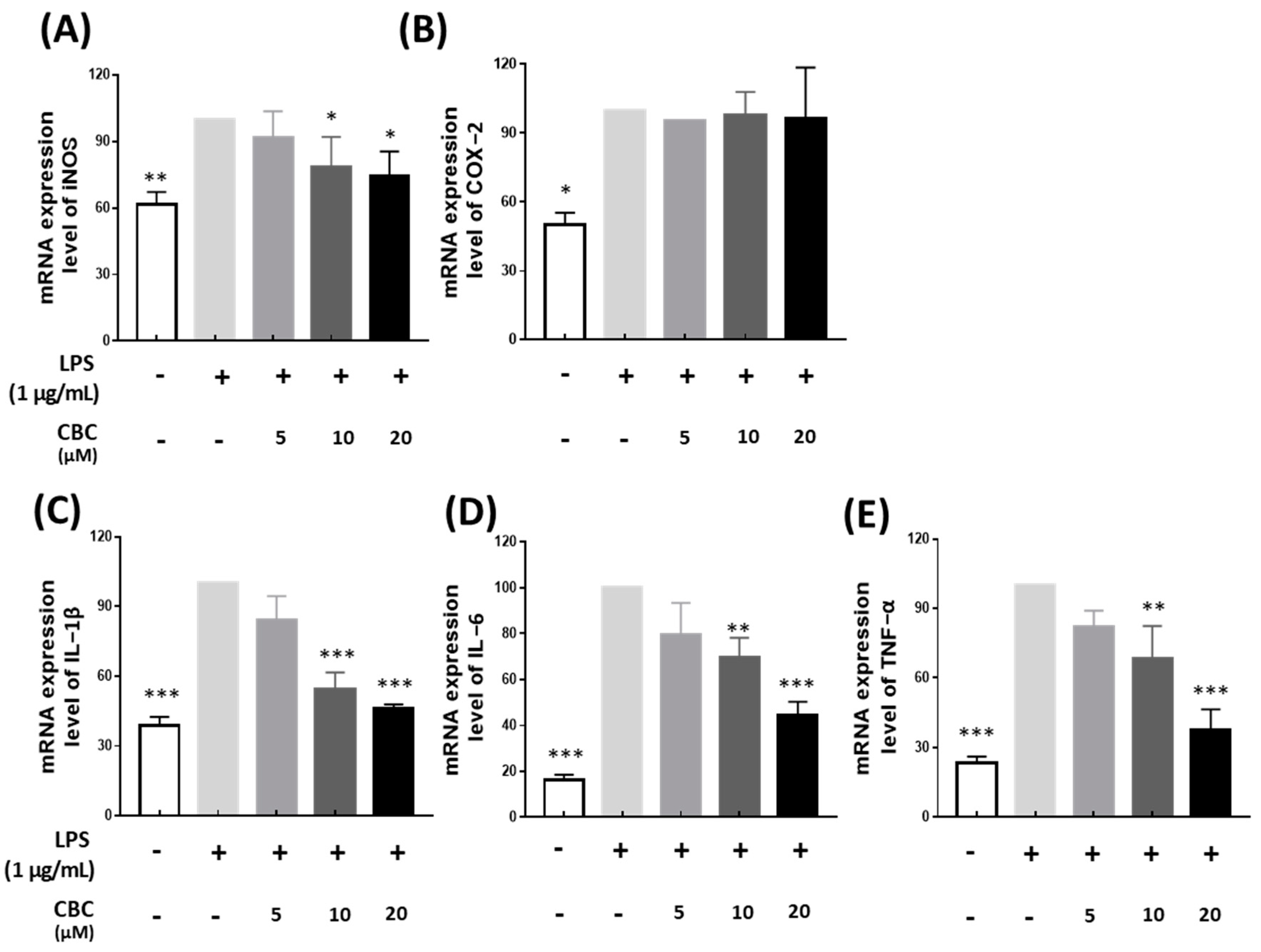
2.2. Effects of Cannabichromene on mRNA Expression of Inflammation-Related Genes
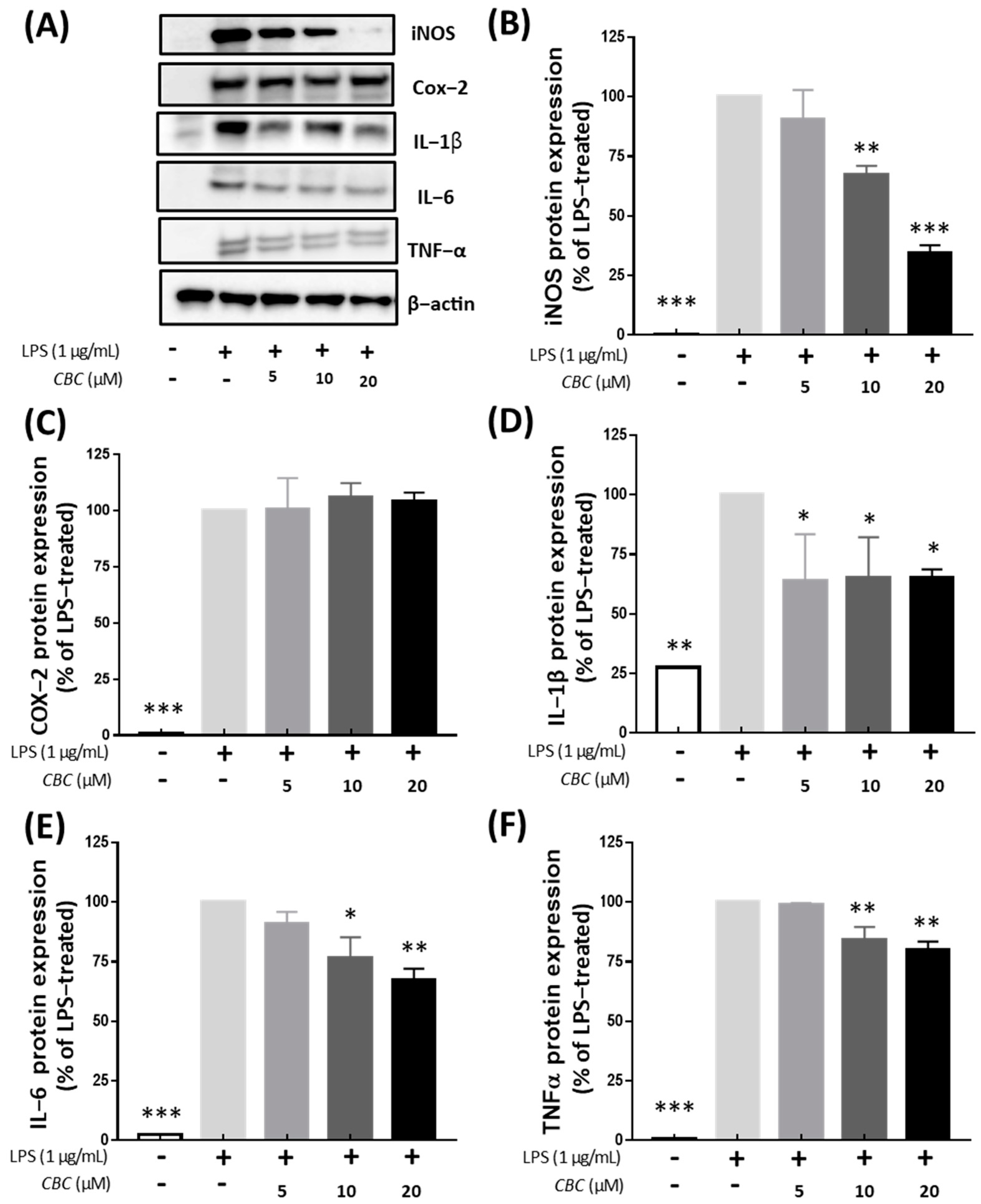
2.3. Investigation of Protein Expression of iNOS, COX-2 and Inflammatory Cytokines According to Cannabichromene Treatment in Lipopolysaccharide-Treated RAW 246.7 Cells
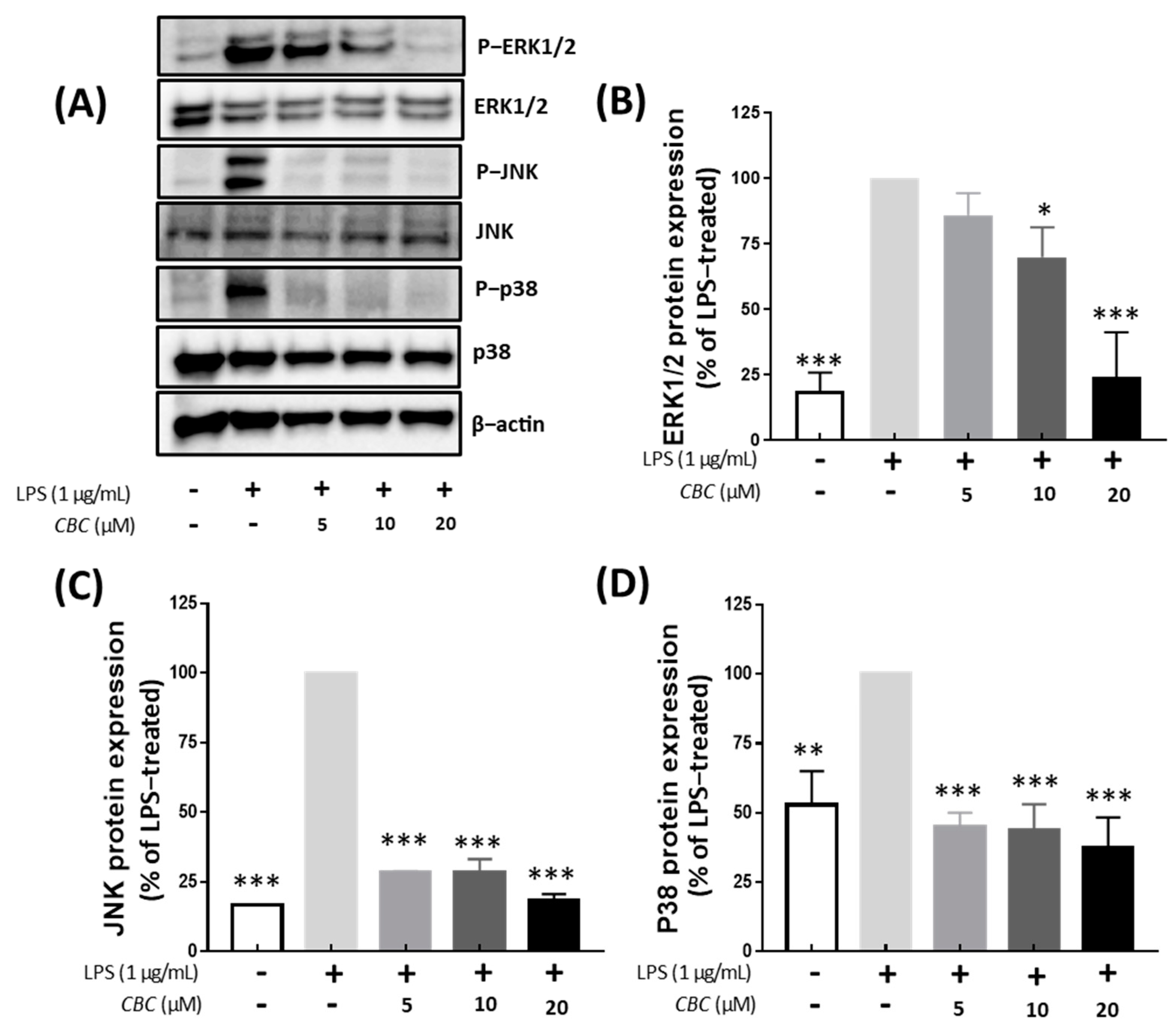
2.4. Inflammation Regulation of Cannabichromene in the MAPK Pathway
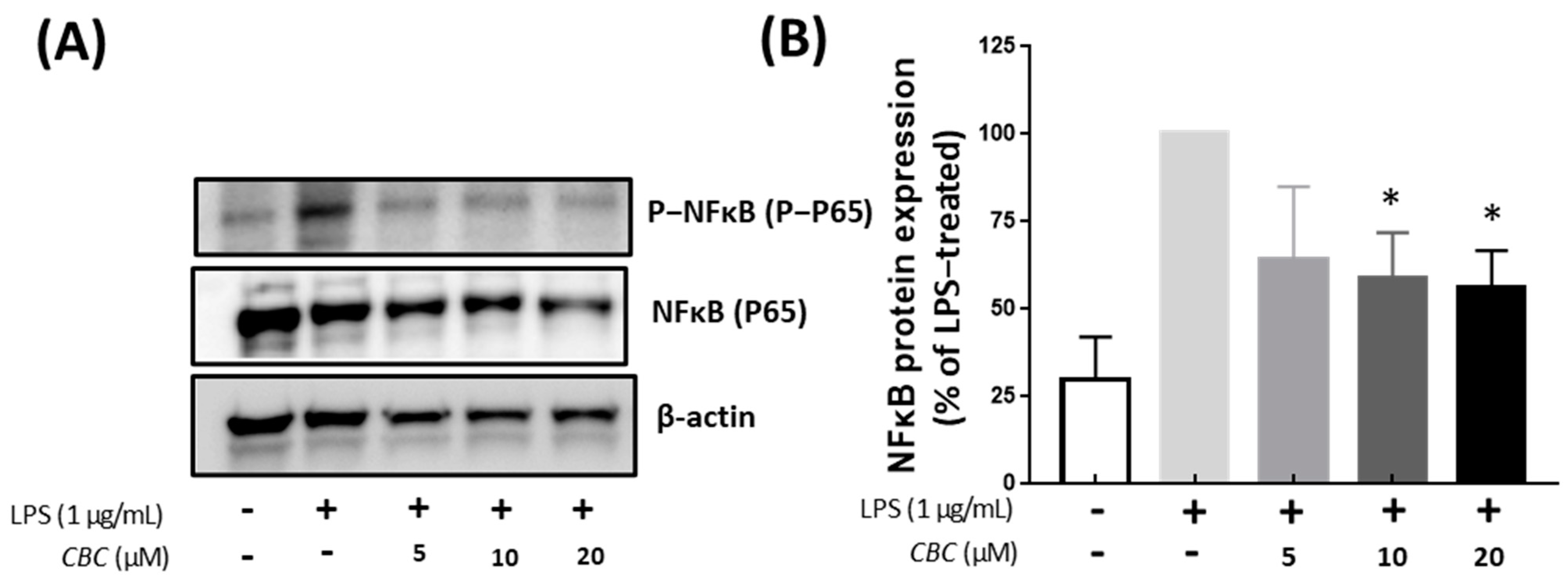
2.5. Cannabichromene Inhibition of NF-κB Phosphorylation
2.6. Inhibitory Effects of Cannabichromene on λ-Carrageenan-Induced Mouse Model
3. Materials and Methods
3.1. Plant Materials
3.2. Supercritical Fluid Extraction Procedure for Cannabichromene Extract
3.3. Cannabichromene Purification
3.4. Cell Culture
3.5. Cell Viability and Production of Nitric Oxide
3.6. RT-qPCR
3.7. Cell Lysate Preparation, Isolation of Nuclear Extracts, and Subsequent Western Blotting Analysis
3.8. Mouse Experiment
3.9. Data Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Anderson, L.L.; Ametovski, A.; Lin Luo, J.; Everett-Morgan, D.; McGregor, I.S.; Banister, S.D.; Arnold, J.C. Cannabichromene, related phytocannabinoids, and 5-fluoro-cannabichromene have anticonvulsant properties in a mouse model of Dravet Syndrome. ACS Chem. Neurosci. 2021, 12, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yu, T.; Jang, H.J.; Byeon, S.E.; Song, S.Y.; Lee, B.H.; Rhee, M.H.; Kim, T.W.; Lee, J.; Hong, S.; et al. In vitro and in vivo anti-inflammatory activities of Polygonum hydropiper methanol extract. J. Ethnopharmacol. 2012, 139, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, E.R.; Toliver-Kinsky, T. Mechanisms of the inflammatory response. Best Pract. Res. Clin. Anaesthesiol. 2004, 18, 385–405. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef]
- Kim, E.O.; Min, K.J.; Kwon, T.K.; Um, B.H.; Moreau, R.A.; Choi, S.W. Anti-inflammatory activity of hydroxycinnamic acid derivatives isolated from corn bran in lipopolysaccharide-stimulated Raw 264.7 macrophages. Food Chem. Toxicol. 2012, 50, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Lee, Y.J.; Jang, H.J.; Kim, A.R.; Hong, S.; Kim, T.W.; Kim, M.Y.; Lee, J.; Lee, Y.G.; Cho, J.Y. Anti-inflammatory activity of Sorbus commixta water extract and its molecular inhibitory mechanism. J. Ethnopharmacol. 2011, 134, 493–500. [Google Scholar] [CrossRef]
- Reddy, D.B.; Reddanna, P. Chebulagic acid (CA) attenuates LPS-induced inflammation by suppressing NF-κB and MAPK activation in RAW 264.7 macrophages. Biochem. Biophys. Res. Commun. 2009, 381, 112–117. [Google Scholar] [CrossRef]
- Finch, C.E. Developmental origins of aging in brain and blood vessels: An overview. Neurobiol. Aging 2005, 26, 281–291. [Google Scholar] [CrossRef]
- Caruso, C.; Lio, D.; Cavallone, L.; Franceschi, C. Aging, longevity, inflammation, and cancer. Ann. N. Y. Acad. Sci. 2004, 1028, 1–13. [Google Scholar] [CrossRef]
- Cahill, M.R.; Perry, I.J. Inflammation, aspirin, and the risk of cardiovascular disease. N. Engl. J. Med. 1997, 337, 422–423. [Google Scholar]
- Zarev, Y.; Marinov, L.; Momekova, D.; Ionkova, I. Exploring phytochemical composition and in vivo anti-inflammatory potential of grape seed oil from an alternative source after traditional fermentation processes: Implications for phytotherapy. Plants 2023, 12, 2795. [Google Scholar] [CrossRef] [PubMed]
- Grigore, A.; Vulturescu, V.; Neagu, G.; Ungureanu, P.; Panteli, M.; Rasit, I. Antioxidant–anti-inflammatory evaluation of a polyherbal formula. Pharmaceuticals 2022, 15, 114. [Google Scholar] [CrossRef] [PubMed]
- Ashton, J.C. Cannabinoids for the treatment of inflammation. Curr. Opin. Investig. Drugs 2007, 8, 373–384. [Google Scholar]
- Marsh, D.T.; Sugiyama, A.; Imai, Y.; Kato, R.; Smid, S.D. The structurally diverse phytocannabinoids cannabichromene, cannabigerol and cannabinol significantly inhibit amyloid β-evoked neurotoxicity and changes in cell morphology in PC12 cells. Basic Clin. Pharmacol. Toxicol. 2023. online ahead of print. [Google Scholar] [CrossRef]
- Pollastro, F.; Caprioglio, D.; Del Prete, D. Cannabichromene. Nat. Prod. Commun. 2018, 13, 1934578X1801300922. [Google Scholar] [CrossRef]
- Huntsman, R.J.; Tang-Wai, R.; Alcorn, J.; Vuong, S.; Acton, B.; Corley, S.; Laprairie, R.; Lyon, A.W.; Meier, S.; Mousseau, D.D.; et al. Dosage related efficacy and tolerability of cannabidiol in children with treatment-resistant epileptic encephalopathy: Preliminary results of the CARE-E study. Front. Neurol. 2019, 10, 716. [Google Scholar] [CrossRef] [PubMed]
- DeLong, G.T.; Wolf, C.E.; Poklis, A.; Lichtman, A.H. Pharmacological evaluation of the natural constituent of Cannabis sativa, cannabichromene and its modulation by Δ9-tetrahydrocannabinol. Drug Alcohol Depend. 2010, 112, 126–133. [Google Scholar] [CrossRef]
- Turner, C.E.; Elsohly, M.A. Biological activity of cannabichromene, its homologs and isomers. J. Clin. Pharmacol. 1981, 21 (Supp S1), 283S–291S. [Google Scholar] [CrossRef]
- Tortolani, D.; Di Meo, C.; Standoli, S.; Ciaramellano, F.; Kadhim, S.; Hsu, E.; Rapino, C.; Maccarrone, M. Rare phytocannabinoids exert anti-inflammatory effects on human keratinocytes via the endocannabinoid system and MAPK signaling pathway. Int. J. Mol. Sci. 2023, 24, 2721. [Google Scholar] [CrossRef]
- Glivar, T.; Eržen, J.; Kreft, S.; Zagožen, M.; Čerenak, A.; Čeh, B.; Benković, E.T. Cannabinoid content in industrial hemp (Cannabis sativa L.) varieties grown in Slovenia. Ind. Crops Prod. 2022, 145, 112082. [Google Scholar] [CrossRef]
- Lim, J.D. Cannabis sativa Isolate KNU-18-1 (Cultivar: Pink Pepper), Whole Genome Sequencing Project, GenBank. 2022. Available online: https://www.ncbi.nlm.nih.gov/datasets/genome/GCA_029168945.1/ (accessed on 13 March 2023).
- Lim, J.D. This Research Was Supported by the Ministry of Science and ICT (MSIT, Korea), (Project No.: 2021-DD-UP-0379), figshare. Dataset. 2022. Available online: https://figshare.com/articles/dataset/This_research_was_supported_by_the_Ministry_of_Science_and_ICT_MSIT_Korea_Project_No_2021-DD-UP-0379_/21391449/1 (accessed on 13 March 2023).
- Calcaterra, A.; Cianfoni, G.; Tortora, C.; Manetto, S.; Grassi, G.; Botta, B.; Gasparrini, F.; Mazzoccanti, G.; Appendino, G. Natural cannabichromene (CBC) shows distinct scalemicity grades and enantiomeric dominance in Cannabis sativa strains. J. Nat. Prod. 2023, 86, 909–914. [Google Scholar] [CrossRef]
- Mackie, K. Cannabinoid receptors: Where they are and what they do. J. Neuroendocrinol. 2008, 20, 10–14. [Google Scholar] [CrossRef]
- Onaivi, E.S.; Ishiguro, H.; GONG, J.P.; Patel, S.; Perchuk, A.; Meozzi, P.A.; Myers, L.; Mora, Z.; Tagliaferro, P.; Gardner, E.; et al. Discovery of the presence and functional expression of cannabinoid CB2 receptors in brain. Ann. N. Y. Acad. Sci. 2006, 1074, 514–536. [Google Scholar] [CrossRef]
- Peyravian, N.; Deo, S.; Daunert, S.; Jimenez, J.J. Cannabidiol as a novel therapeutic for immune modulation. ImmunoTargets Ther. 2020, 9, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Udoh, M.; Santiago, M.; Devenish, S.; McGregor, I.S.; Connor, M. Cannabichromene is a cannabinoid CB2 receptor agonist. Br. J. Pharmacol. 2019, 176, 4537–4547. [Google Scholar] [CrossRef] [PubMed]
- Walsh, K.B.; Holmes, A.E. Pharmacology of minor cannabinoids at the cannabinoid CB1 receptor: Isomer-and ligand-dependent antagonism by tetrahydrocannabivarin. Receptors 2022, 1, 3–12. [Google Scholar] [CrossRef]
- Peng, J.; Fan, M.; An, C.; Ni, F.; Huang, W.; Luo, J. A narrative review of molecular mechanism and therapeutic effect of cannabidiol (CBD). Basic Clin. Pharmacol. Toxicol. 2022, 130, 439–456. [Google Scholar] [CrossRef] [PubMed]
- Khorasani, M.M.; Hassanshahi, G.; Brodzikowska, A.; Khorramdelazad, H. Role (s) of cytokines in pulpitis: Latest evidence and therapeutic approaches. Cytokine 2020, 126, 154896. [Google Scholar] [CrossRef]
- Lechner, M.; Lirk, P.; Rieder, J. Inducible nitric oxide synthase (iNOS) in tumor biology: The two sides of the same coin. Semin. Cancer Biol. 2005, 15, 277–289. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. Interleukin (IL-6) immunotherapy. Cold Spring Harb. Perspect. Biol. 2018, 10, a028456. [Google Scholar] [CrossRef]
- Gliozzi, M.; Scicchitano, M.; Bosco, F.; Musolino, V.; Carresi, C.; Scarano, F.; Maiuolo, J.; Nucera, S.; Maretta, A.; Paone, S.; et al. Modulation of nitric oxide synthases by oxidized LDLs: Role in vascular inflammation and atherosclerosis development. Int. J. Mol. Sci. 2019, 20, 3294. [Google Scholar] [CrossRef]
- Bresalier, R.S.; Sandler, R.S.; Quan, H.; Bolognese, J.A.; Oxenius, B.; Horgan, K.; Lines, C.; Riddell, R.; Morton, D.; Lanas, A.; et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N. Engl. J. Med. 2005, 352, 1092–1102. [Google Scholar] [CrossRef] [PubMed]
- Greenhough, A.; Smartt, H.J.; Moore, A.E.; Roberts, H.R.; Williams, A.C.; Paraskeva, C.; Kaidi, A. The COX-2/PGE 2 pathway: Key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis 2009, 30, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Tak, P.P.; Firestein, G.S. NF-κB: A key role in inflammatory diseases. J. Clin. Investig. 2011, 107, 7–11. [Google Scholar] [CrossRef]
- Kim, J.C.; Lee, Y.H.; Yu, M.K.; Lee, N.H.; Park, J.D.; Bhattarai, G.; Yi, H.K. Anti-inflammatory mechanism of PPARγ on LPS-induced pulp cells: Role of the ROS removal activity. Arch. Oral Biol. 2012, 57, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Lee, N.H.; Bhattarai, G.; Kim, G.E.; Lee, I.K.; Yun, B.S.; Hwang, P.H.; Yi, H.K. Anti-inflammatory effect of pachymic acid promotes odontoblastic differentiation via HO-1 in dental pulp cells. Oral Dis. 2013, 19, 193–199. [Google Scholar] [CrossRef]
- Morales, P.; Hurst, D.P.; Reggio, P.H. Molecular targets of the phytocannabinoids: A complex picture. Prog. Chem. Org. Nat. Prod. 2017, 103, 103–131. [Google Scholar]
- Turner, S.E.; Williams, C.M.; Iversen, L.; Whalley, B.J. Molecular pharmacology of phytocannabinoids. Prog. Chem. Org. Nat. Prod. 2017, 103, 61–101. [Google Scholar]
- Powles, T.; Poele, R.T.; Shamash, J.; Chaplin, T.; Propper, D.; Joel, S.; Oliver, T.; Liu, W.M. Cannabis-induced cytotoxicity in leukemic cell lines: The role of the cannabinoid receptors and the MAPK pathway. Blood 2005, 105, 1214–1221. [Google Scholar] [CrossRef]
- Tedesco, L.; Valerio, A.; Dossena, M.; Cardile, A.; Ragni, M.; Pagano, C.; Pagotto, U.; Carruba, M.O.; Vettor, R.; Nisoli, E. Cannabinoid receptor stimulation impairs mitochondrial biogenesis in mouse white adipose tissue, muscle, and liver: The role of eNOS, p38 MAPK, and AMPK pathways. Diabetes 2010, 59, 2826–2836. [Google Scholar] [CrossRef]
- Turu, G.; Hunyady, L. Signal transduction of the CB1 cannabinoid receptor. J. Mol. Endocrinol. 2010, 44, 75–85. [Google Scholar] [CrossRef]
- Do, Y.; McKallip, R.J.; Nagarkatti, M.; Nagarkatti, P.S. Activation through cannabinoid receptors 1 and 2 on dendritic cells triggers NF-κB-dependent apoptosis: Novel role for endogenous and exogenous cannabinoids in immunoregulation. J. Immunol. 2004, 173, 2373–2382. [Google Scholar] [CrossRef] [PubMed]
- Holland, S.; Coste, O.; Zhang, D.D.; Pierre, S.C.; Geisslinger, G.; Scholich, K. The ubiquitin ligase MYCBP2 regulates transient receptor potential vanilloid receptor 1 (TRPV1) internalization through inhibition of p38 MAPK signaling. J. Biol. Chem. 2011, 286, 3671–3680. [Google Scholar] [CrossRef]
- Huang, K.F.; Ma, K.H.; Liu, P.S.; Chen, B.W.; Chueh, S.H. Baicalein increases keratin 1 and 10 expression in hacat keratinocytes via trpv 4 receptor activation. Exp. Dermatol. 2016, 25, 623–629. [Google Scholar] [CrossRef]
- Xie, C.; Kang, J.; Li, Z.; Schauss, A.G.; Badger, T.M.; Nagarajan, S.; Wu, T.; Wu, X. The açaí flavonoid velutin is a potent anti-inflammatory agent: Blockade of LPS-mediated TNF-α and IL-6 production through inhibiting NF-κB activation and MAPK pathway. J. Nutr. Biochem. 2012, 23, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Zhou, K.; Wang, S.; Li, P.; Chen, S.; Lin, G.; Zhao, Y.; Wang, T. Involvement of MAPK/NF-κB signaling in the activation of the cholinergic anti-inflammatory pathway in experimental colitis by chronic vagus nerve stimulation. PLoS ONE 2013, 8, e69424. [Google Scholar] [CrossRef] [PubMed]
- Myers, M.J.; Deaver, C.M.; Lewandowski, A.J. Molecular mechanism of action responsible for carrageenan-induced inflammatory response. Mol. Immunol. 2019, 109, 38–42. [Google Scholar] [CrossRef]
- Halici, Z.; Dengiz, G.O.; Odabasoglu, F.; Suleyman, H.; Cadirci, E.; Halici, M. Amiodarone has anti-inflammatory and anti-oxidative properties: An experimental study in rats with carrageenan-induced paw edema. Eur. J. Pharmacol. 2007, 566, 215–221. [Google Scholar] [CrossRef]
- Burayk, S.; Oh-Hashi, K.; Kandeel, M. Drug discovery of new anti-inflammatory compounds by targeting cyclooxygenases. Pharmaceuticals 2022, 15, 282. [Google Scholar] [CrossRef]
- Kasama, T.; Miwa, Y.; Isozaki, T.; Odai, T.; Adachi, M.; Kunkel, S.L. Neutrophil-derived cytokines: Potential therapeutic targets in inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 273–279. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, M.; Kim, J.-H.; Han, J.-H.; Ryu, B.-R.; Lim, Y.-S.; Lim, J.-D.; Park, S.-H.; Kim, C.-H.; Lee, S.-U.; Kwon, T.-H. In Vitro and In Vivo Anti-Inflammatory Potential of Cannabichromene Isolated from Hemp. Plants 2023, 12, 3966. https://doi.org/10.3390/plants12233966
Hong M, Kim J-H, Han J-H, Ryu B-R, Lim Y-S, Lim J-D, Park S-H, Kim C-H, Lee S-U, Kwon T-H. In Vitro and In Vivo Anti-Inflammatory Potential of Cannabichromene Isolated from Hemp. Plants. 2023; 12(23):3966. https://doi.org/10.3390/plants12233966
Chicago/Turabian StyleHong, Min, Jong-Hui Kim, Joon-Hee Han, Byeong-Ryeol Ryu, Young-Seok Lim, Jung-Dae Lim, Sang-Hyuck Park, Chang-Hyeug Kim, Soo-Ung Lee, and Tae-Hyung Kwon. 2023. "In Vitro and In Vivo Anti-Inflammatory Potential of Cannabichromene Isolated from Hemp" Plants 12, no. 23: 3966. https://doi.org/10.3390/plants12233966
APA StyleHong, M., Kim, J.-H., Han, J.-H., Ryu, B.-R., Lim, Y.-S., Lim, J.-D., Park, S.-H., Kim, C.-H., Lee, S.-U., & Kwon, T.-H. (2023). In Vitro and In Vivo Anti-Inflammatory Potential of Cannabichromene Isolated from Hemp. Plants, 12(23), 3966. https://doi.org/10.3390/plants12233966





