Do Traits Travel? Multiple-Herbicide-Resistant A. tuberculatus, an Alien Weed Species in Israel
Abstract
:1. Introduction
2. Results
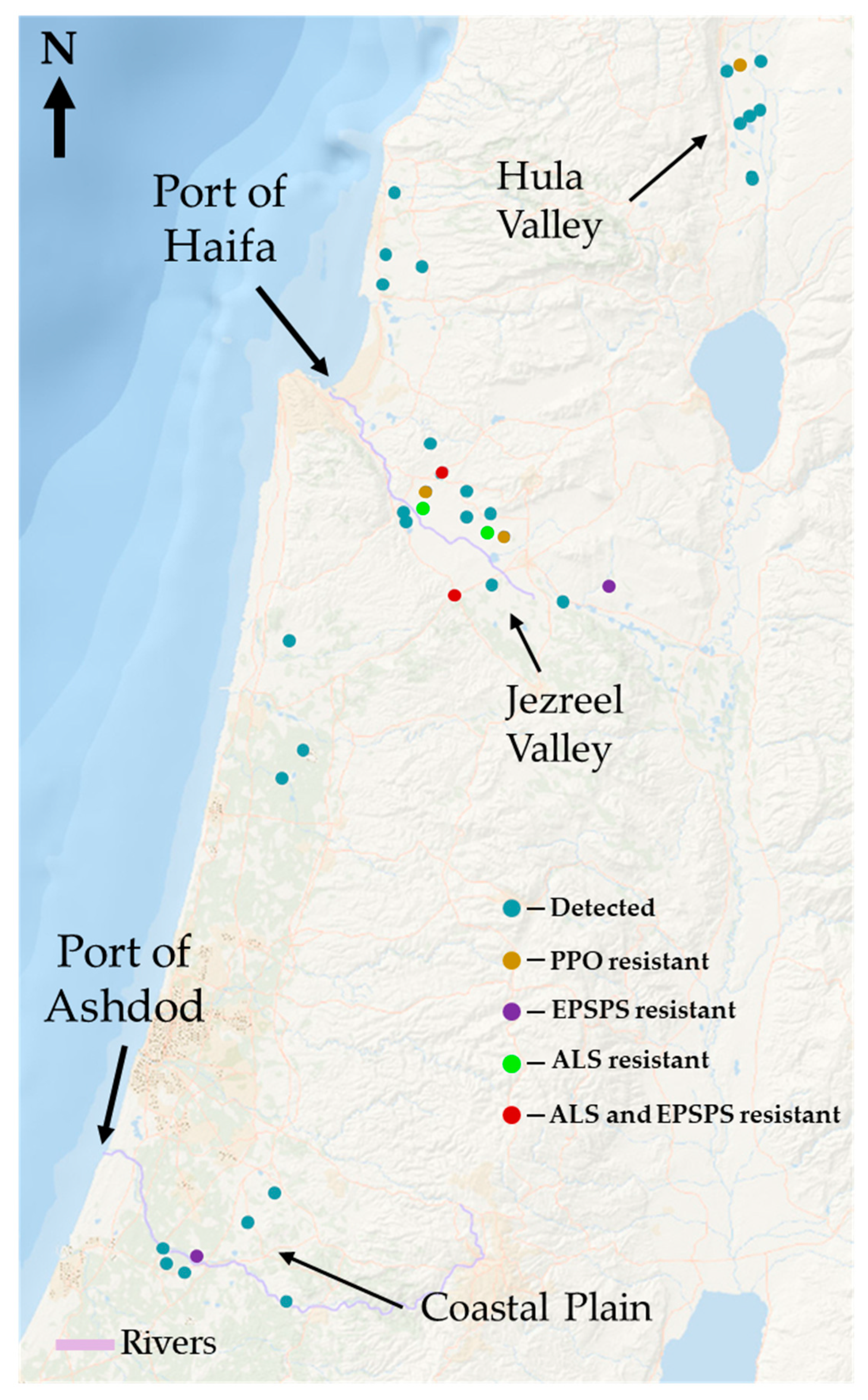
2.1. Distribution of A. tuberculatus: Survey Results
2.2. Herbicide Screening
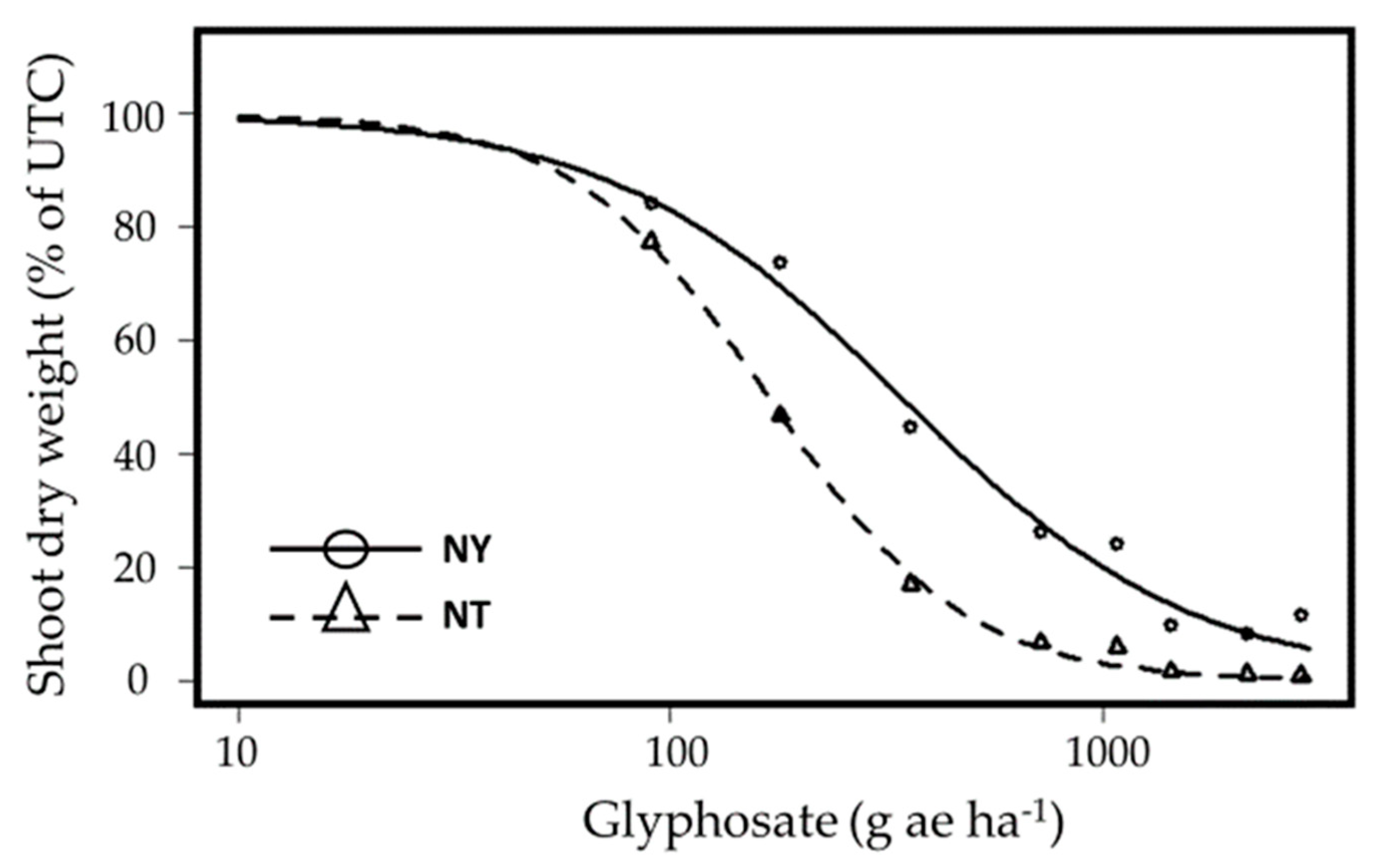
2.3. Glyphosate Resistance
2.4. ALS Resistance
2.5. PPO Resistance
2.6. Multiple Herbicide Resistance
3. Discussion
3.1. Distribution of A. tuberculatus
3.2. Glyphosate Resistance
3.3. ALS Resistance
3.4. PPO Resistance
3.5. Multiple Herbicide Resistance
4. Materials and Methods
4.1. Survey and Plant Material
4.2. Herbicide-Response Screening
4.3. Dose–Response Studies
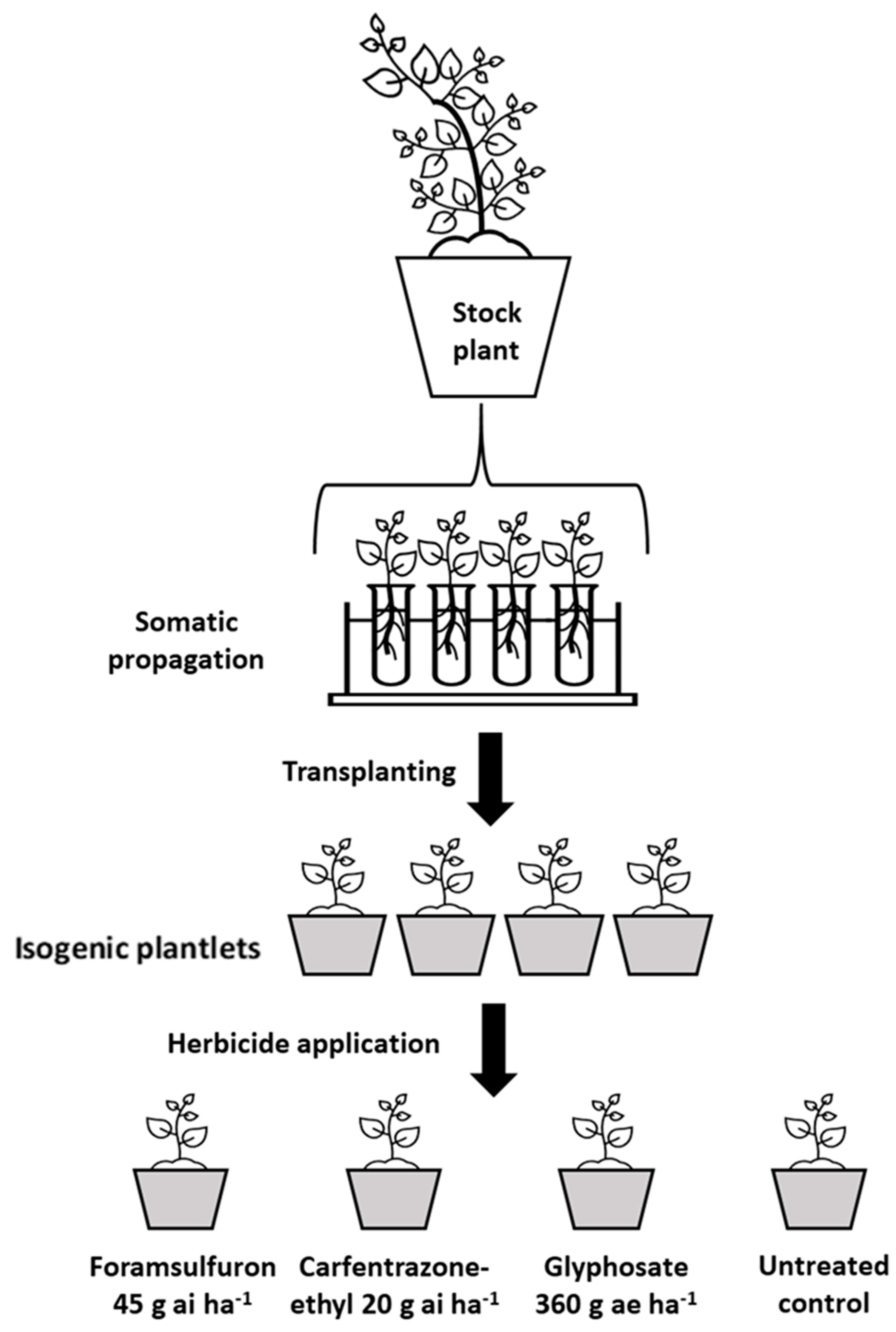
4.4. Clonal Cutting: Multiple Herbicide Resistance Assay
4.5. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tranel, P.J. Herbicide resistance in Amaranthus tuberculatus. Pest Manag. Sci. 2021, 77, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Assad, R.; Reshi, Z.A.; Jan, S.; Rashid, I. Biology of amaranths. Bot. Rev. 2017, 83, 382–436. [Google Scholar] [CrossRef]
- Costea, M.; Weaver, S.E.; Tardif, F.J. The biology of invasive alien plants in Canada. 3. Amaranthus tuberculatus (Mo var. rudis (Sauer) Costea & Tardif). Can. J. Plant Sci. 2005, 85, 507–522. [Google Scholar]
- Wu, C.; Owen, M.D. When is the best time to emerge—II: Seed mass, maturation, and after ripening of common waterhemp (Amaranthus tuberculatus) natural cohorts. Weed Sci. 2015, 63, 846–854. [Google Scholar] [CrossRef]
- Van Wychen, L. Survey of the Most Common and Troublesome Weeds in Broadleaf Crops, Fruits & Vegetables in the United States and Canada. Weed Science Society of America National Weed Survey Dataset. 2022. Available online: http://wssa.net/wp-content/uploads/2022 Weed-Survey Broadleaf crops.xlsx (accessed on 12 July 2023).
- Heap, I. The International Herbicide-Resistant Weed Database. 13 March 2023. Available online: www.weedscience.org (accessed on 14 July 2023).
- Yadid, I.; Peleg, Z.; Rubin, B. Amaranthus palmeri and Amaranthus tuberculatus (rudis)—Troublesome weeds in irrigated crops. In Proceedings of the 18th European Weed Research Society Symposium, Ljubljana, Slovenia, 17–21 June 2018. [Google Scholar]
- Milani, A.; Scarabel, L.; Sattin, M. A family affair: Resistance mechanism and alternative control of three Amaranthus species resistant to acetolactate synthase inhibitors in Italy. Pest Manag. Sci. 2019, 76, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Milani, A.; Lutz, U.; Galla, G.; Scarabel, L.; Weigel, D.; Sattin, M. Population structure and evolution of resistance to acetolactate synthase (ALS)-inhibitors in Amaranthus tuberculatus in Italy. Pest Manag. Sci. 2021, 77, 2971–2980. [Google Scholar] [CrossRef]
- The National Natural History Collections—Herbarium. June 2023. Available online: https://en-nnhc.huji.ac.il/herbarium (accessed on 23 July 2023).
- De Rouw, A.; Ribolzi, O.; Douillet, M.; Tjantahosong, H.; Soulileuth, B. Weed seed dispersal via runoff water and eroded soil. Agric. Ecosyst. Environ. 2018, 265, 488–502–502. [Google Scholar] [CrossRef]
- Lorentz, L.; Gaines, T.A.; Nissen, S.J.; Westra, P.; Strek, H.J.; Dehne, H.W.; Juan Pedro Ruiz, S.; Beffa, R. Characterization of glyphosate resistance in Amaranthus tuberculatus populations. J. Agric. Food Chem. 2014, 62, 8134–8142.–8142. [Google Scholar] [CrossRef]
- Tranel, P.J.; Riggins, C.W.; Bell, M.S.; Hager, A.G. Herbicide resistances in Amaranthus tuberculatus: A call for new options. J. Agric. Food Chem. 2011, 59, 5808–5812. [Google Scholar] [CrossRef]
- Van Horn, C.R.; Moretti, M.L.; Robertson, R.R.; Segobye, K.; Weller, S.C.; Young, B.G.; Johnson, W.G.; Schulz, B.; Green, A.C.; Jeffery, T.; et al. Glyphosate resistance in Ambrosia trifida: Part 1. Novel rapid cell death response to glyphosate. Pest Manag. Sci. 2018, 5, 1071–1078. [Google Scholar]
- United States Department of Agriculture. PSD Online—Statistics by Country. 2023. Available online: https://apps.fas.usda.gov/psdonline/circulars/production.pdf (accessed on 20 September 2023).
- EPPO. Amaranthus Tuberculatus; (Moq) J.D. Sauer: Isny im Allgäu, Germany, 2020; EPPO Bull; Volume 50, pp. 543–548. [Google Scholar]
- Lu, S.; Luo, X.; Wang, H.; Gentili, R.; Citterio, S.; Yang, J.; Jin, J.; Li, J.; Yang, J. China-US grain trade shapes the spatial genetic pattern of common ragweed in East China cities. Commun. Biol. 2023, 6, 1072. [Google Scholar] [CrossRef] [PubMed]
- Nemtzov, S.C. Management of wildlife-human conflicts in Israel: A wide variety of vertebrate pest problems in a difficult and compact environment. In Proceedings of the 20th Vertebrate Pest Conference, Reno NV, USA, 4–7 March 2002; Timm, R.M., Schmidt, R.H., Eds.; University of California: Davis, CA, USA, 2002; pp. 348–353. [Google Scholar]
- Barnea, I.; Kaplan, D.; Amar, D. Monitoring of the Hula project, Final Report 2008-2018. Project 4501334109 Submitted to the National Water Authority and KKL-JNF. 2020. Available online: https://www.gov.il/BlobFolder/reports/hula-project1/he/hula_HulaMontoringReport_2008-2018.pdf (accessed on 13 July 2023).
- Rubin, B. Herbicide-resistant weeds—The inevitable phenomenon: Mechanisms, distribution, and significance. Z. Fur Pflanzenkrankh. Und Plfantzenschuts 1996, 15, 17–32. [Google Scholar]
- Matzrafi, M.; Rubin, B. Multiple Herbicide Resistance in Rigid Ryegrass (Lolium rigidum) in Israel. In Proceedings of the 6th International Weed Science Congress, Hangzhou, China, 17–22 June 2012; Volume 67. [Google Scholar]
- Kleinman, Z.; Ben-Ami, G.; Rubin, B. From sensitivity to resistance—Factors affecting the response of Conyza spp. to glyphosate. Pest Manag. Sci. 2016, 72, 1681–1688. [Google Scholar] [CrossRef] [PubMed]
- Kleinman, Z.; Rubin, B. Non-target-site glyphosate resistance in Conyza bonariensis is based on modified subcellular distribution of the herbicide. Pest Manag. Sci. 2017, 73, 246–253. [Google Scholar] [CrossRef]
- Gaines, T.A.; Ward, S.M.; Bukun, B.; Preston, C.; Leach, J.E.; Westra, P. Interspecific hybridization transfers a previously unknown glyphosate resistance mechanism in Amaranthus species. Evol. Appl. 2012, 5, 29–38.–38. [Google Scholar] [CrossRef]
- Gaines, T.A.; Duke, S.O.; Morran, S.; Rigon, C.A.; Tranel, P.J.; Küpper, A.; Dayan, F.E. Mechanisms of evolved herbicide resistance. J. Biol. Chem. 2020, 295, 10307–10330. [Google Scholar] [CrossRef] [PubMed]
- Barker, A.L.; Pawlak, J.; Duke, S.O.; Beffa, R.; Tranel, P.J.; Wuerffel, J.; Young, B.; Porri, A.; Liebl, R.; Aponte, R.; et al. Discovery, mode of action, resistance mechanisms, and plan of action for sustainable use of Group 14 herbicides. Weed Sci. 2023, 71, 173–188. [Google Scholar] [CrossRef]
- Shoup, D.E.; Al-Khatib, K.; Peterson, D.E. Common waterhemp (Amaranthus rudis) resistance to protoporphyrinogen oxidase-inhibiting herbicides. Weed Sci. 2003, 51, 145–150. [Google Scholar] [CrossRef]
- Obenland, O.A.; Ma, R.; O’Brien, S.R.; Lygin, A.V.; Riechers, D.E. Carfentrazone-ethyl resistance in an Amaranthus tuberculatus population is not mediated by amino acid alterations in the PPO2 protein. PLoS ONE 2019, 14, e0215431. [Google Scholar] [CrossRef]
- Thinglum, K.A.; Riggins, C.W.; Davis, A.S.; Bradley, K.W.; Al-Khatib, K.; Tranel, P.J. Wide distribution of the waterhemp (Amaranthus tuberculatus) ΔG210 PPX2 mutation, which confers resistance to PPO-inhibiting herbicides. Weed Sci. 2011, 59, 22–27. [Google Scholar] [CrossRef]
- Patzoldt, W.L.; Hager, A.G.; McCormick, J.S.; Tranel, P.J. A codon deletion confers resistance to herbicides inhibiting protoporphyrinogen oxidase. Proc. Nat. Acad. Sci. USA 2006, 103, 12329–12334. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, 2023, Vienna, Austria. Available online: https://www.R-project.org/ (accessed on 2 July 2023).
- Ritz, C.; Baty, F.; Streibig, J.; Gerhard, D. Dose-response analysis using R. PLoS ONE 2015, 10, e0146021. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H.; Chang, W.; Wickham, M.H. Package ‘ggplot2’: Create Elegant Data Visualisations Using the Grammar of Graphics. Version 2016, 2, 1–189. [Google Scholar]

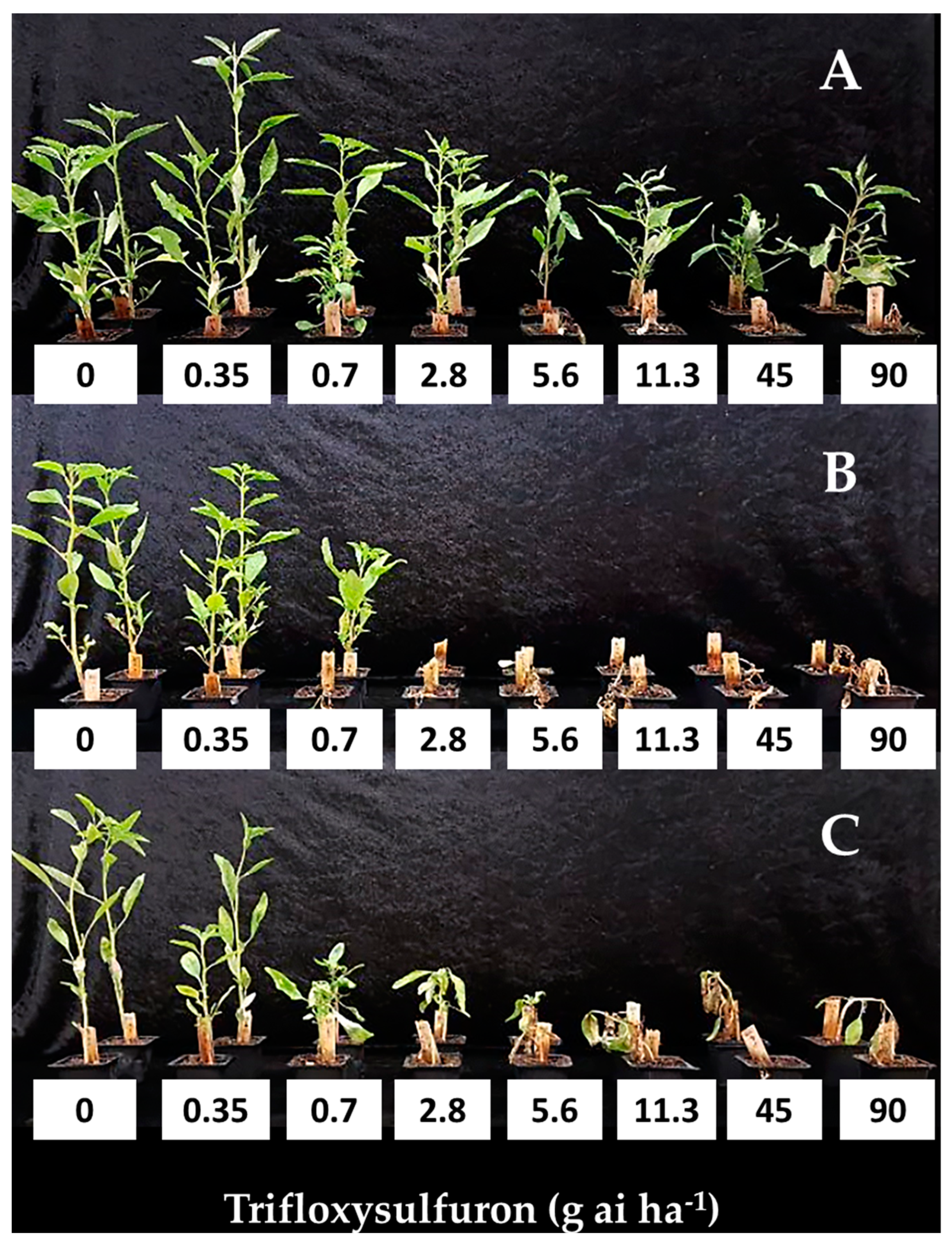
 ) AT-Tel Nof, (
) AT-Tel Nof, ( ) ZM-Tzora, (ALS-intermediate), (
) ZM-Tzora, (ALS-intermediate), ( ) NY-Newe Ya’ar and (
) NY-Newe Ya’ar and ( ) GA-Ginegar (ALS-resistant). Trifloxysulfuron (0, 0.35, 0.7, 2.8, 5.6, 11.3, 45 and 90 g ai ha−1) was applied at the four-to-six-leaf stage. The data represent the mean shoot dry weights (% of control) of plants harvested at 21 DAA. Plants were grown in a net-house under ambient summer conditions. UTC-untreated control.
) GA-Ginegar (ALS-resistant). Trifloxysulfuron (0, 0.35, 0.7, 2.8, 5.6, 11.3, 45 and 90 g ai ha−1) was applied at the four-to-six-leaf stage. The data represent the mean shoot dry weights (% of control) of plants harvested at 21 DAA. Plants were grown in a net-house under ambient summer conditions. UTC-untreated control.
 ) AT-Tel Nof, (
) AT-Tel Nof, ( ) ZM-Tzora, (ALS-intermediate), (
) ZM-Tzora, (ALS-intermediate), ( ) NY-Newe Ya’ar and (
) NY-Newe Ya’ar and ( ) GA-Ginegar (ALS-resistant). Trifloxysulfuron (0, 0.35, 0.7, 2.8, 5.6, 11.3, 45 and 90 g ai ha−1) was applied at the four-to-six-leaf stage. The data represent the mean shoot dry weights (% of control) of plants harvested at 21 DAA. Plants were grown in a net-house under ambient summer conditions. UTC-untreated control.
) GA-Ginegar (ALS-resistant). Trifloxysulfuron (0, 0.35, 0.7, 2.8, 5.6, 11.3, 45 and 90 g ai ha−1) was applied at the four-to-six-leaf stage. The data represent the mean shoot dry weights (% of control) of plants harvested at 21 DAA. Plants were grown in a net-house under ambient summer conditions. UTC-untreated control.
 ) ZM-Tzora; (
) ZM-Tzora; ( ) GA-Ginegar; carfentrazone-ethyl-resistant (
) GA-Ginegar; carfentrazone-ethyl-resistant ( ) KY-Kfar Yehoshua and (
) KY-Kfar Yehoshua and ( ) HG-Havat Gadash. Carfentrazone-ethyl (0, 5, 10, 20, 40, 60, 80, and 120 g ai ha−1) was applied post-emergence to seedlings at the four-to-six-leaf stage, which were grown in a net-house under ambient summer conditions. The experiment was repeated twice, and the data represent the mean shoot dry weights (% of control) of plants harvested at 21 DAA. UTC-untreated control.
) HG-Havat Gadash. Carfentrazone-ethyl (0, 5, 10, 20, 40, 60, 80, and 120 g ai ha−1) was applied post-emergence to seedlings at the four-to-six-leaf stage, which were grown in a net-house under ambient summer conditions. The experiment was repeated twice, and the data represent the mean shoot dry weights (% of control) of plants harvested at 21 DAA. UTC-untreated control.
 ) ZM-Tzora; (
) ZM-Tzora; ( ) GA-Ginegar; carfentrazone-ethyl-resistant (
) GA-Ginegar; carfentrazone-ethyl-resistant ( ) KY-Kfar Yehoshua and (
) KY-Kfar Yehoshua and ( ) HG-Havat Gadash. Carfentrazone-ethyl (0, 5, 10, 20, 40, 60, 80, and 120 g ai ha−1) was applied post-emergence to seedlings at the four-to-six-leaf stage, which were grown in a net-house under ambient summer conditions. The experiment was repeated twice, and the data represent the mean shoot dry weights (% of control) of plants harvested at 21 DAA. UTC-untreated control.
) HG-Havat Gadash. Carfentrazone-ethyl (0, 5, 10, 20, 40, 60, 80, and 120 g ai ha−1) was applied post-emergence to seedlings at the four-to-six-leaf stage, which were grown in a net-house under ambient summer conditions. The experiment was repeated twice, and the data represent the mean shoot dry weights (% of control) of plants harvested at 21 DAA. UTC-untreated control.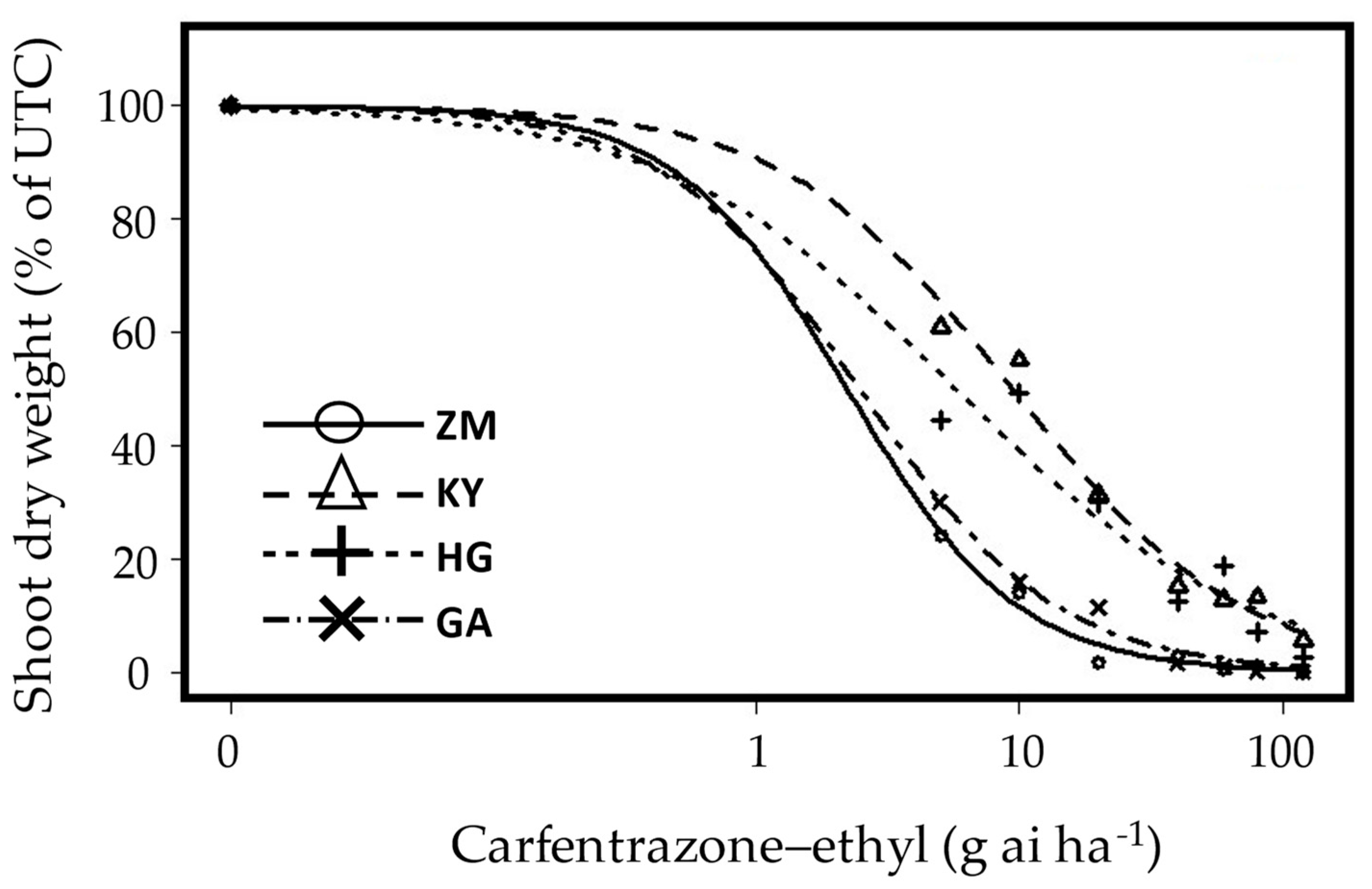



| Population | Protoporphyrinogen oxidase (PPO) inhibitors | |||||||||
| Carfentrazone-ethyl | Oxyfluorfen | Oxadiazon | Sulfentrazone | Pyraflufen-ethyl | ||||||
| Shoot DW | Survival | Shoot DW | Survival | Shoot DW | Survival | Shoot DW | Survival | Shoot DW | Survival | |
| % of untreated control | ||||||||||
| Tzora (S) | 0.0 | 0.0 | 0.0 | 0.0 | 9.3 | 50.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Havat Gadash | 22.4 * | 66.6 | 14.1 | 100.0 | 25.1 | 100.0 | 2.1 | 16.6 | 9.9 * | 50.0 |
| Newe Ya’ar | 2.3 | 16.6 | 6.4 | 33.3 | 6.2 | 50.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Megiddo | 59.7 * | 100.0 | 28.4 * | 66.6 | 42.3 * | 83.3 | 0.4 | 16.6 | 3.1 | 16.6 |
| Kfar Yehoshua | 19.4 * | 66.6 | 0.8 | 16.6 | 8.0 | 50.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Ginegar | 3.2 | 33.3 | 12.9 | 83.3 | 18.8 | 100.0 | 0.0 | 0.0 | 2.5 | 16.6 |
| Nahal Timnah | 0.0 | 0.0 | 0.0 | 0.0 | 8.2 | 66.6 | 0.0 | 0.0 | 0.0 | 0.0 |
| Population | PSII inhibitor | Acetolactate synthase (ALS) inhibitors | EPSPS inhibitor | |||||||
| Atrazine | Trifloxysulfuron | Pyrithiobac-sodium | Glyphosate | |||||||
| Shoot DW | Survival | Shoot DW | Survival | Shoot DW | Survival | Shoot DW | Survival | |||
| % of untreated control | ||||||||||
| Hulata | 0.2 | 0.0 | NA | NA | NA | NA | NA | NA | ||
| Havat Gadash (S) | 6.0 | 40.0 | 0.1 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | ||
| Tzora | 0.2 | 80.0 * | 5.8 | 20.0 | 0.2 | 0.0 | 2.7 | 20.0 | ||
| Newe Ya’ar | 19.1 | 80.0 * | 48.1 * | 80.0 * | 72.4 * | 80.0 * | 27.3 * | 100.0 * | ||
| Megiddo | 26.3 | 100.0 | 108.5 * | 100.0 * | 64.6 * | 100.0 * | 29.3 * | 80.0 * | ||
| Kfar Yehoshua | 36.1 | 80.0 * | 65.2 * | 80.0 * | 60.7 * | 60.0 * | 46.7 * | 100.0 * | ||
| Ginegar | 6.0 | 40.0 | 110.7 * | 100.0 * | 88.0 * | 100.0 * | 9.4 | 60.0 | ||
| Herbicide (MoA) | Population | Response | ED50 (g ai ha−1) | RI |
|---|---|---|---|---|
| Glyphosate (EPSPS) | Nahal Timnah | S | 168.2 ± 11.8 | -- |
| Newe Ya’ar | R | 341.5 ± 28.6 | 2.0 | |
| Trifloxysulfuron (ALS) | Tel-Nof | S | 0.4 ± 0.17 | -- |
| Tzora | I | 2.9 ± 1.0 | 6.8 | |
| Newe-Ya’ar | R | 11.1 ± 6.3 | 25.8 | |
| Ginegar | R | 56.8 ± 24.4 | 132.3 | |
| Carfentrazone-ethyl (PPO) | Tzora | S | 2.2 ± 1.4 | -- |
| R | 9.5 ± 1.8 | 4.3 | ||
| Havat Gadash | R | 5.8 ± 1.7 | 2.6 |
| Mode of Action * | Active Ingredient | Dose (g ai/ae ha−1) |
|---|---|---|
| ALS | Trifloxysulfuron | 11.3 |
| Pyrithiobac–sodium | 51.7 | |
| PPO | Oxyfluorfen | 480 |
| Carfentrazone–ethyl | 20 | |
| Sulfentrazone | 336 | |
| Pyraflufen | 7.2 | |
| Oxadiazon | 500 | |
| Aclonifen | 600 | |
| Flumioxazin | 40 | |
| Fomesafen | 200 | |
| EPSPS | Glyphosate | 1080 |
| HPPD | Tembotrione | 99 |
| PSII | Atrazine | 500 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roth, I.S.; Singer, A.; Yadid, I.; Sibony, M.; Peleg, Z.; Rubin, B. Do Traits Travel? Multiple-Herbicide-Resistant A. tuberculatus, an Alien Weed Species in Israel. Plants 2023, 12, 4002. https://doi.org/10.3390/plants12234002
Roth IS, Singer A, Yadid I, Sibony M, Peleg Z, Rubin B. Do Traits Travel? Multiple-Herbicide-Resistant A. tuberculatus, an Alien Weed Species in Israel. Plants. 2023; 12(23):4002. https://doi.org/10.3390/plants12234002
Chicago/Turabian StyleRoth, Idan S., Aviv Singer, Inon Yadid, Moshe Sibony, Zvi Peleg, and Baruch Rubin. 2023. "Do Traits Travel? Multiple-Herbicide-Resistant A. tuberculatus, an Alien Weed Species in Israel" Plants 12, no. 23: 4002. https://doi.org/10.3390/plants12234002
APA StyleRoth, I. S., Singer, A., Yadid, I., Sibony, M., Peleg, Z., & Rubin, B. (2023). Do Traits Travel? Multiple-Herbicide-Resistant A. tuberculatus, an Alien Weed Species in Israel. Plants, 12(23), 4002. https://doi.org/10.3390/plants12234002








