Genome-Wide Identification and Analysis of the WNK Kinase Gene Family in Upland Cotton
Abstract
1. Introduction
2. Results
2.1. The G. hirsutum Genome Contains 26 WNK Genes
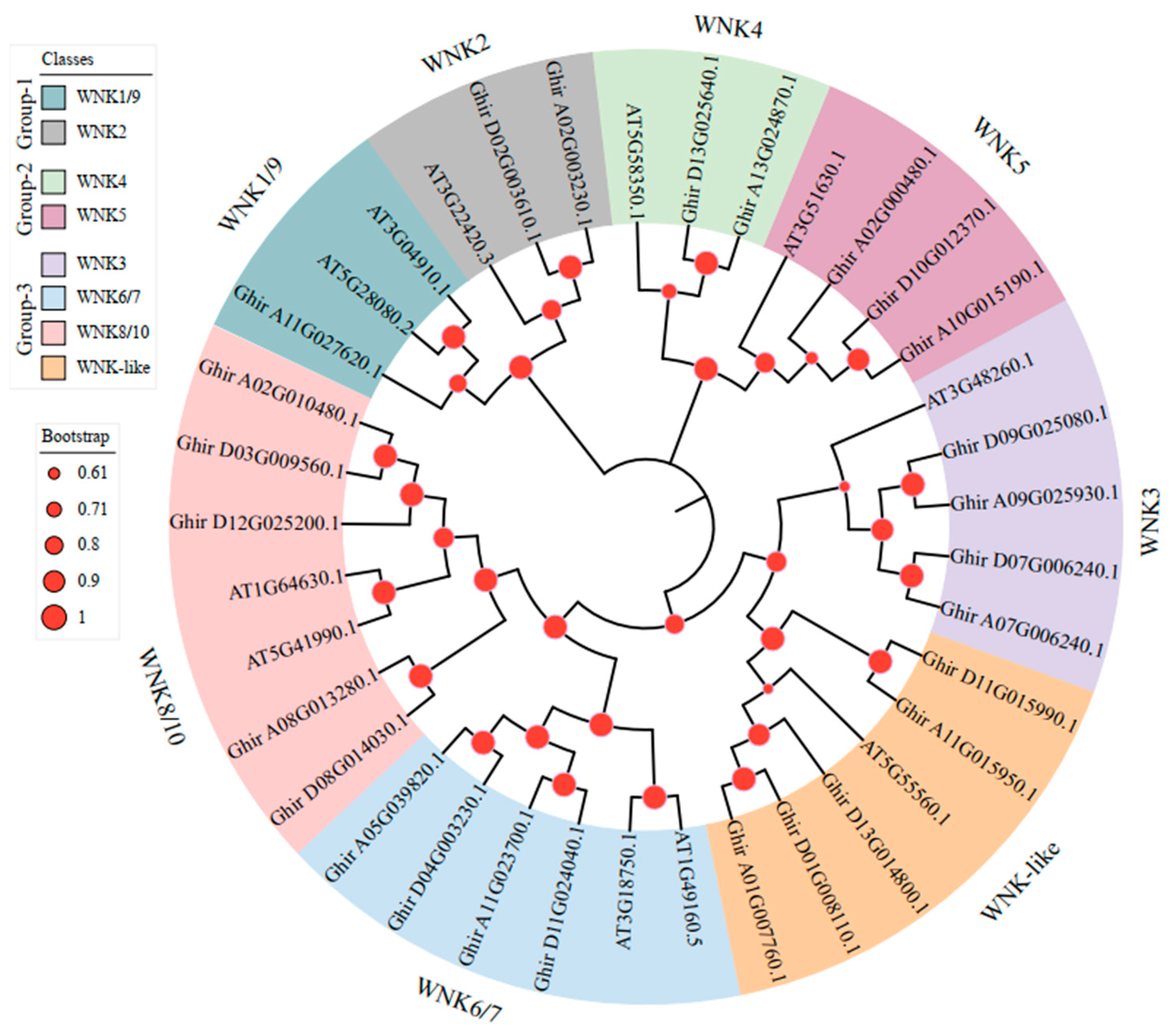
2.2. Phylogenetic Analysis of GhWNKs
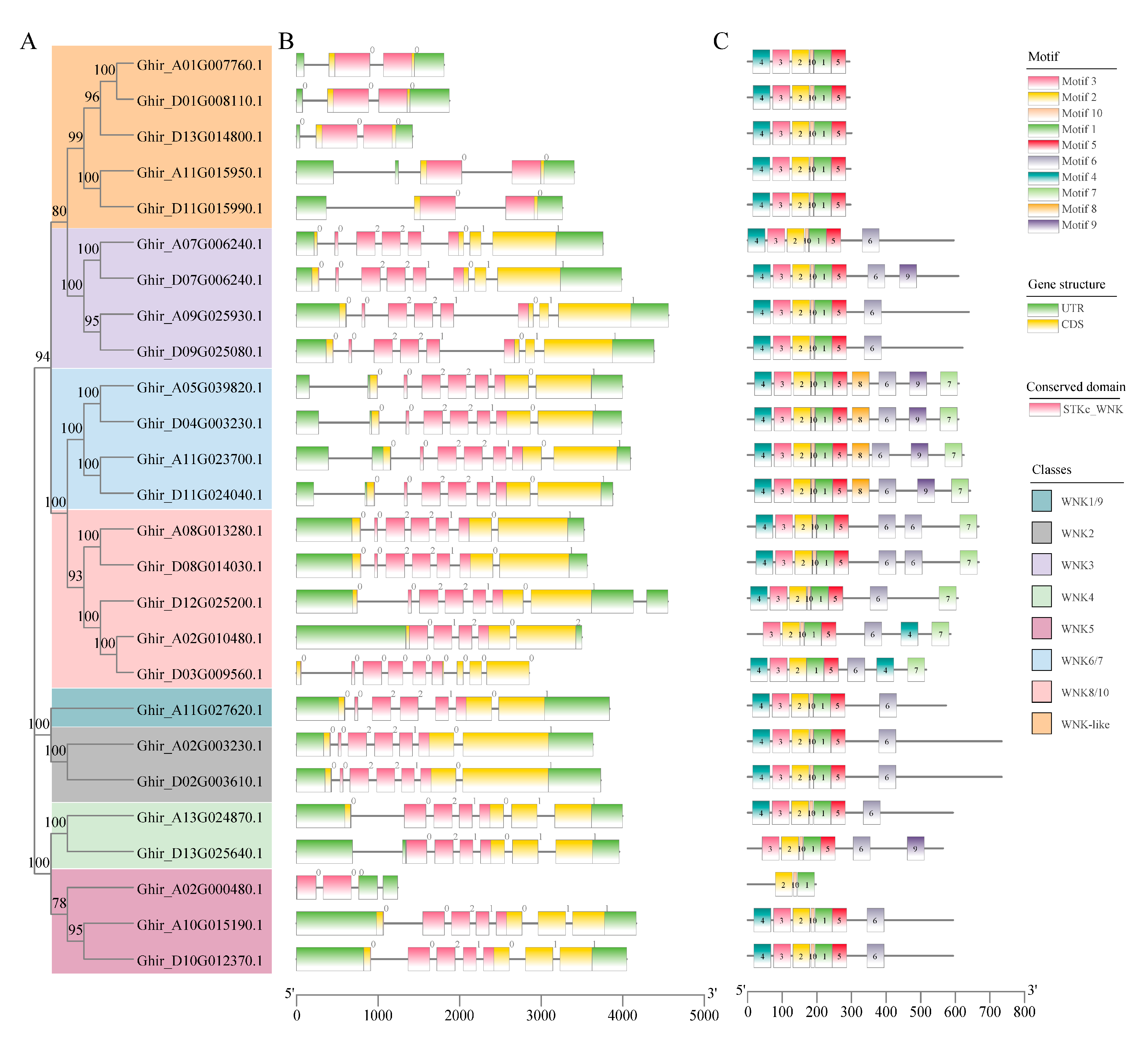
2.3. Structural and Conserved Motifs Characteristics of GhWNKs
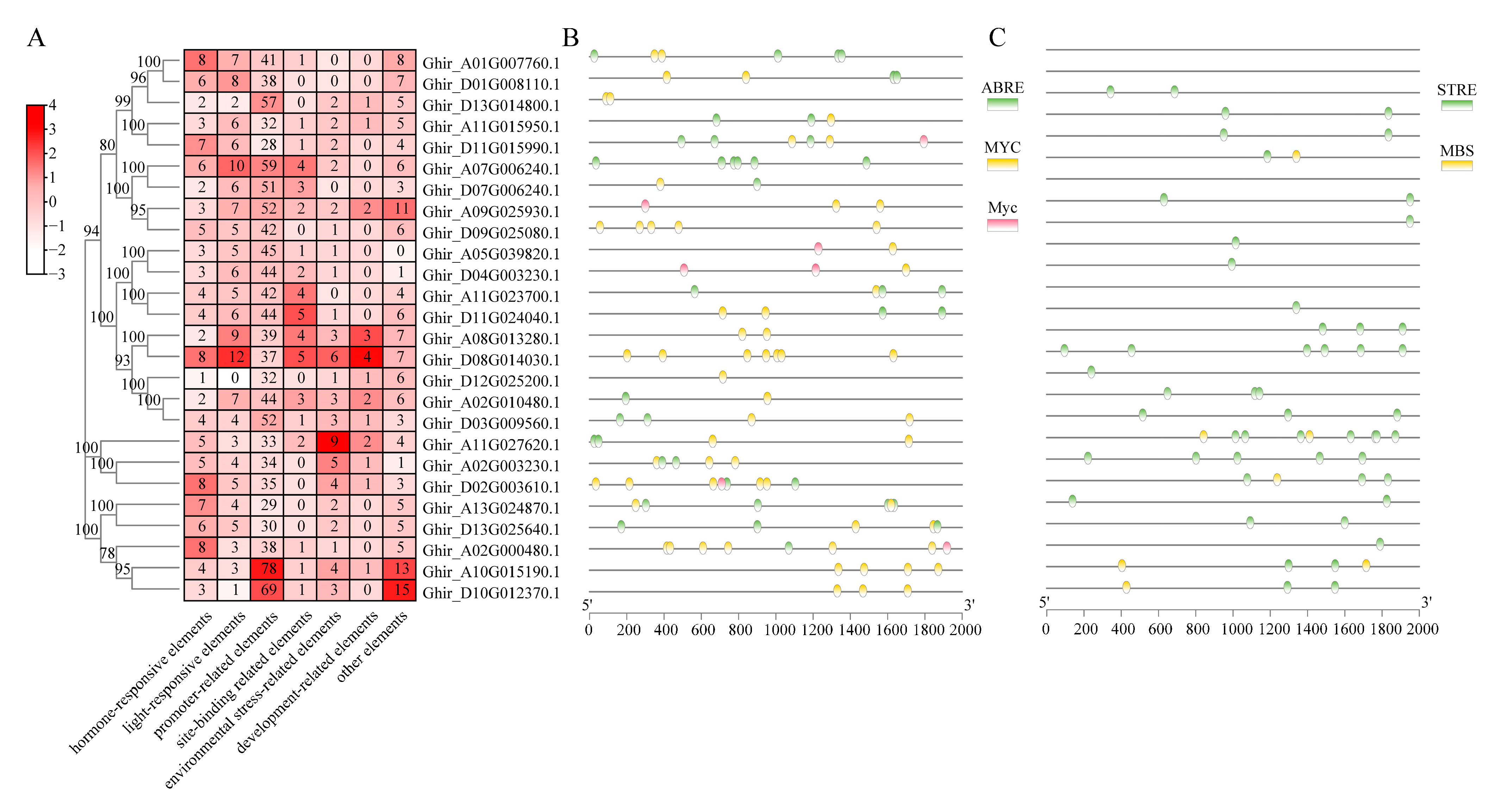
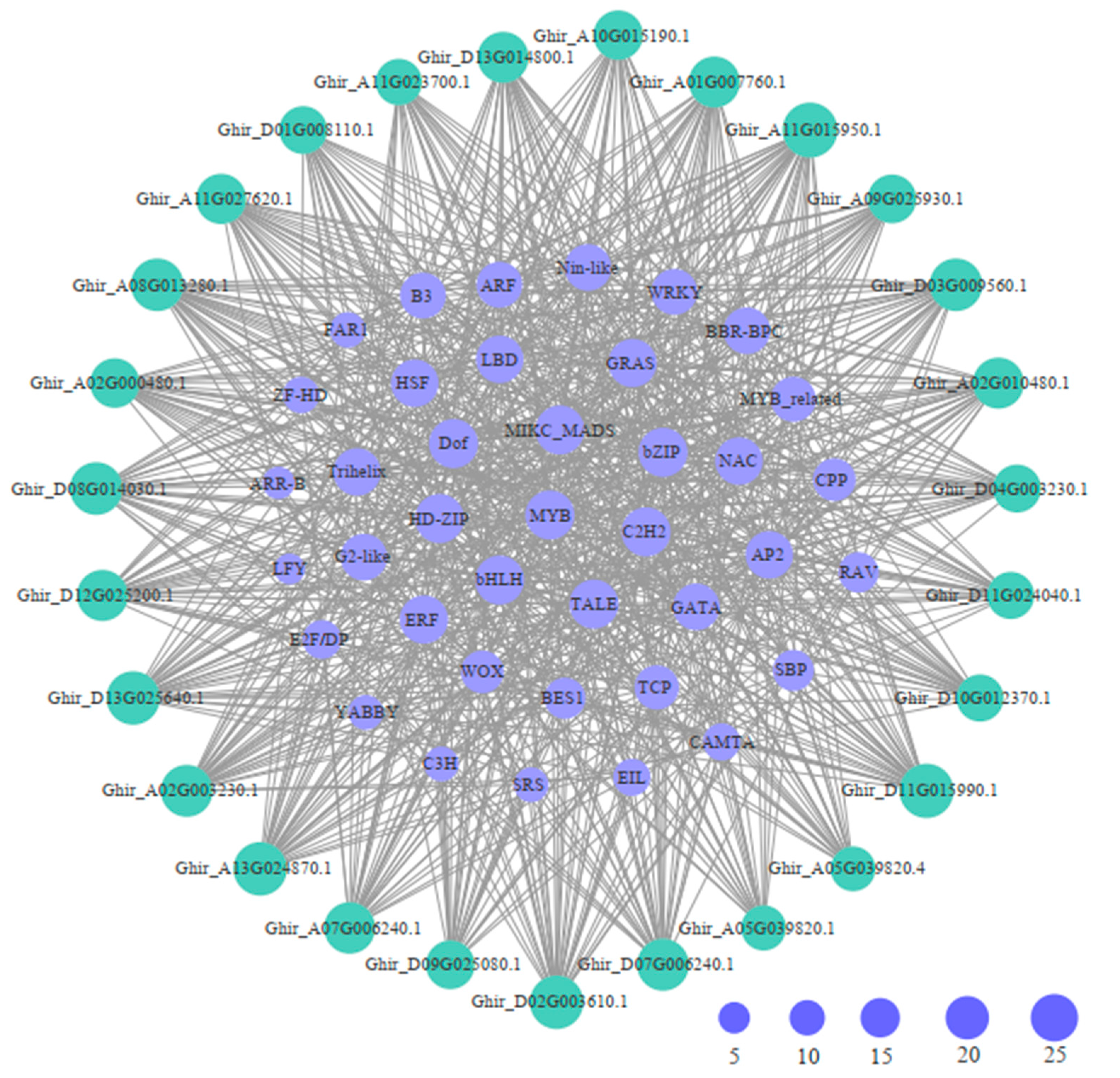
2.4. Cis-Acting Elements and Transcription Factors Were Detected in GhWNKs
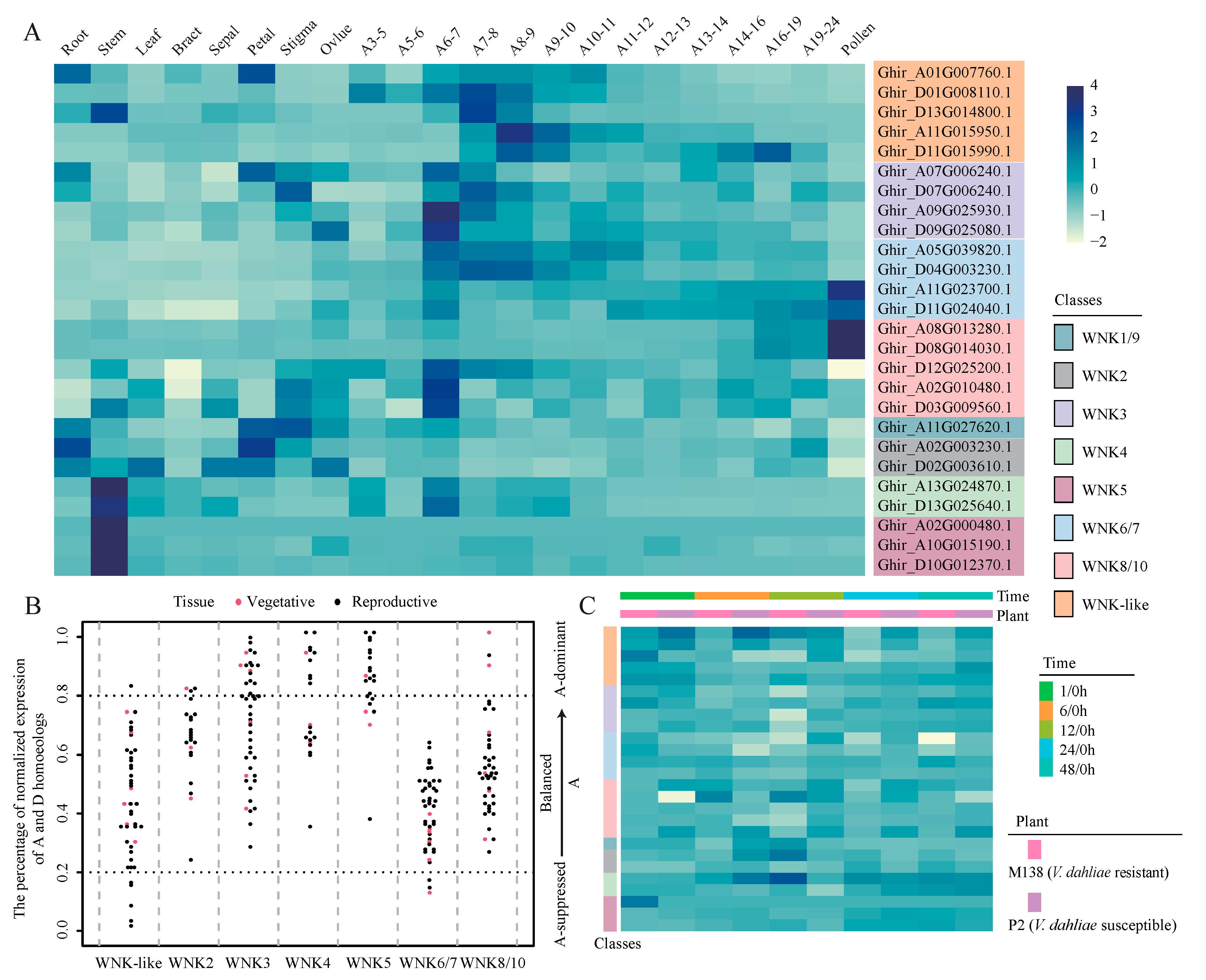
2.5. Tissue-Specific Expression of GhWNKs
2.6. Expression of GhWNKs under Biotic and Abiotic Stress
3. Discussion
3.1. The Conserved Motif and Sequence Characterization of GhWNKs
3.2. Functions of GhWNK Genes in Abiotic and Biotic Stresses
3.3. The Bias Expression Patterns of GhWNKs Homologous Gene Pairs
4. Materials and Methods
4.1. Identification of the WNK Kinase Gene Family in Upland Cotton
4.2. Phylogenetic Analysis and Molecular Evolution Analyses
4.3. Analysis of Conserved Motifs, Gene Structure, Functional Domains, 3D Structure, and uORFs
4.4. Promoter cis-Acting Elements and TFs Prediction
4.5. Gene Expression Analysis Based on RNA-Seq and Digital Gene Expression Data
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shekarabi, M.; Zhang, J.W.; Khanna, A.R.; Ellison, D.H.; Delpire, E.; Kahle, K.T. WNK Kinase Signaling in Ion Homeostasis and Human Disease. Cell Metab. 2017, 25, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Saddhe, A.A.; Karle, S.B.; Aftab, T.; Kumar, K. With no lysine kinases. the key regulatory networks and phytohormone cross talk in plant growth, development and stress response. Plant Cell Rep. 2021, 40, 2097–2109. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, K.; Liao, H.; Zhuang, C.; Ma, H.; Yan, X. The plant WNK gene family and regulation of flowering time in Arabidopsis. Plant Biol. 2008, 10, 548–562. [Google Scholar] [CrossRef] [PubMed]
- Manuka, R.; Saddhe, A.A.; Kumar, K. Genome-wide identification and expression analysis of WNK kinase gene family in rice. Comput. Biol. Chem. 2015, 59 Pt A, 56–66. [Google Scholar] [CrossRef]
- Li, L.; Zhang, C.; Huang, J.; Liu, Q.; Wei, H.; Wang, H.; Liu, G.; Gu, L.; Yu, S. Genomic analyses reveal the genetic basis of early maturity and identification of loci and candidate genes in upland cotton (Gossypium hirsutum L.). Plant Biotechnol. J. 2021, 19, 109–123. [Google Scholar] [CrossRef]
- Li, X.; Wu, Y.; Chi, H.; Wei, H.; Wang, H.; Yu, S. Genomewide Identification and Characterization of the Genes Involved in the Flowering of Cotton. Int. J. Mol. Sci. 2022, 23, 7940. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Rao, K.P.; Biswas, D.K.; Sinha, A.K. Rice WNK1 is regulated by abiotic stress and involved in internal circadian rhythm. Plant Signal. Behav. 2011, 6, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Matsushika, A.; Imamura, A.; Yamashino, T.; Mizuno, T. Aberrant Expression of the Light-Inducible and Circadian-Regulated APRR9 Gene Belonging to the Circadian-Associated APRR1/TOC1 Quintet Results in the Phenotype of Early Flowering in Arabidopsis thaliana. Plant Cell Physiol. 2002, 43, 833–843. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.; Suo, H.; Zheng, Y.; Liu, K.; Zhuang, C.; Kahle, K.T.; Ma, H.; Yan, X. The soybean root-specific protein kinase GmWNK1 regulates stress-responsive ABA signaling on the root system architecture. Plant J. 2010, 64, 230–242. [Google Scholar] [CrossRef]
- Singh, M.; Mas, P. A Functional Connection between the Circadian Clock and Hormonal Timing in Arabidopsis. Genes 2018, 9, 567. [Google Scholar] [CrossRef]
- Berens, M.L.; Wolinska, K.W.; Spaepen, S.; Ziegler, J.; Nobori, T.; Nair, A.; Krüler, V.; Winkelmüller, T.M.; Wang, Y.; Mine, A.; et al. Balancing trade-offs between biotic and abiotic stress responses through leaf age-dependent variation in stress hormone cross-talk. Proc. Natl. Acad. Sci. USA 2019, 116, 2364–2373. [Google Scholar] [CrossRef]
- Zhang, B.G.; Liu, K.D.; Zheng, Y.; Wang, Y.X.; Wang, J.X.; Liao, H. Disruption of AtWNK8 Enhances Tolerance of Arabidopsis to Salt and Osmotic Stresses via Modulating Proline Content and Activities of Catalase and Peroxidase. Int. J. Mol. Sci. 2013, 14, 7032–7047. [Google Scholar] [CrossRef] [PubMed]
- Waadt, R.; Jawurek, E.; Hashimoto, K.; Li, Y.; Scholz, M.; Krebs, M.; Czap, G.; Hong-Hermesdorf, A.; Hippler, M.; Grill, E.; et al. Modulation of ABA responses by the protein kinase WNK8. FEBS Lett. 2019, 593, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Wu, D.; Duan, G.; Wang, L.; He, R.; Li, X.; Tang, D.; Zhao, X.; Liu, X. AtWNK9 is regulated by ABA and dehydration and is involved in drought tolerance in Arabidopsis. Plant Physiol. Biochem. 2014, 77, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Urano, D.; Czarnecki, O.; Wang, X.; Jones, A.M.; Chen, J.-G. Arabidopsis Receptor of Activated C Kinase1 Phosphorylation by with no lysine8 kinase1 open. Plant Physiol. 2015, 167, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Lo, S.; Fatokun, C.; Boukar, O.; Gepts, P.; Close, T.J.; Munoz-Amatriain, M. Identification of QTL for perenniality floral scent in cowpea (Vigna unguiculata, L. Walp.). PLoS ONE 2020, 15, e0229167. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.C.; Du, Y.Y.; Li, Y.A.; Ren, D.T.; Song, C.P. Hydrogen Peroxide-Mediated Activation of MAP Kinase 6 Modulates Nitric Oxide Biosynthesis and Signal Transduction in Arabidopsis. Plant Cell 2010, 22, 2981–2998. [Google Scholar] [CrossRef] [PubMed]
- Hong-Hermesdorf, A.; Brux, A.; Gruber, A.; Gruber, G.; Schumacher, K. A WNK kinase binds and phosphorylates V-ATPase subunit C. FEBS Lett. 2006, 580, 932–939. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, J.; Fang, L.; Zhang, Z.; Ma, W.; Niu, Y.; Ju, L.; Deng, J.; Zhao, T.; Lian, J.; et al. Gossypium barbadense and Gossypium hirsutum genomes provide insights into the origin and evolution of allotetraploid cotton. Nat. Genet. 2019, 51, 739–748. [Google Scholar] [CrossRef]
- Chen, L.; Sun, H.; Wang, F.; Yue, D.; Shen, X.; Sun, W.; Zhang, X.; Yang, X. Genome-wide identification of MAPK cascade genes reveals the GhMAP3K14–GhMKK11–GhMPK31 pathway is involved in the drought response in cotton. Plant Mol. Biol. 2020, 103, 211–223. [Google Scholar] [CrossRef]
- Qiu, P.; Li, J.; Zhang, L.; Chen, K.; Shao, J.; Zheng, B.; Yuan, H.; Qi, J.; Yue, L.; Hu, Q.; et al. Polyethyleneimine-coated MXene quantum dots improve cotton tolerance to Verticillium dahliae by maintaining ROS homeostasis. Nat. Commun. 2023, 14, 7392. [Google Scholar] [CrossRef] [PubMed]
- An, Q.; Pan, Z.; Aini, N.; Han, P.; Wu, Y.; You, C.; Nie, X. Identification of candidate genes for aphid resistance in upland cotton by QTL mapping and expression analysis. Crop J. 2023, 11, 1600–1604. [Google Scholar] [CrossRef]
- Wang, J.; Liu, J.; Guo, Z. Natural uORF variation in plants. Trends Plant Sci. 2023. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Suo, H.; Zhuang, C.; Ma, H.; Yan, X. Overexpression of the soybean GmWNK1 altered the sensitivity to salt and osmotic stress in Arabidopsis. J. Plant Physiol. 2011, 168, 2260–2267. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Vasupalli, N.; Hou, D.; Stalin, A.; Wei, H.; Zhang, H.; Lin, X. Genome-wide identification and evolution of WNK kinases in Bambusoideae and transcriptional profiling during abiotic stress in Phyllostachys edulis. PeerJ 2022, 10, e12718. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.H.; Hao, P.P.; Shu, W.S.; Wang, G.M.; Xie, Z.H.; Gu, C.; Zhang, S.L. Phylogenetic and Expression Analyses of With-No-Lysine Kinase Genes Reveal Novel Gene Family Diversity in Fruit Trees. Hortic. Plant J. 2019, 5, 47–58. [Google Scholar] [CrossRef]
- Nakamichi, N.; Murakami-Kojima, M.; Sato, E.; Kishi, Y.; Yamashino, T.; Mizuno, T. Compilation and characterization of a novel WNK family of protein kinases in Arabiodpsis thaliana with reference to circadian rhythms. Biosci. Biotechnol. Biochem. 2002, 66, 2429–2436. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef]
- Ngou, B.P.M.; Ding, P.; Jones, J.D.G. Thirty years of resistance. Zig-zag through the plant immune system. Plant Cell 2022, 34, 1447–1478. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, B.; Sun, Z.; Liu, Z.; Cui, Y.; Ke, H.; Wang, Z.; Wu, L.; Zhang, G.; Wang, G.; et al. A large-scale genomic association analysis identifies a fragment in Dt11 chromosome conferring cotton Verticillium wilt resistance. Plant Biotechnol. J. 2021, 19, 2126–2138. [Google Scholar] [CrossRef]
- Li, W.; Mi, X.; Jin, X.; Zhang, D.; Zhu, G.; Shang, X.; Zhang, D.; Guo, W. Thiamine functions as a key activator for modulating plant health and broad-spectrum tolerance in cotton. Plant J. 2022, 111, 374–390. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.H.; Wu, Y.; Luo, L.; Ma, Y.; Li, Y.; Ma, H.; Luo, A.; Zhang, R.; Zhu, L.; Lin, Y.; et al. Proteomic analysis reveals that the heat shock proteins 70-17 and BiP5 enhance cotton male fertility under high-temperature stress by reducing the accumulation of ROS in anthers. Ind. Crops Prod. 2022, 188, 115693. [Google Scholar] [CrossRef]
- Hu, W.; Liu, Y.; Loka, D.A.; Zahoor, R.; Wang, S.; Zhou, Z. Drought limits pollen tube growth rate by altering carbohydrate metabolism in cotton (Gossypium hirsutum) pistils. Plant Sci. Int. J. Exp. Plant Biol. 2019, 286, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhu, L.; Xu, L.; Yuan, D.; Min, L.; Zhang, X. Cotton cytochrome P450 CYP82D regulates systemic cell death by modulating the octadecanoid pathway. Nat. Commun. 2014, 5, 5372. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Sun, Y.; Li, X.; Li, Y. Cysteine-Rich Receptor-Like Kinase5 (CRK5) and CRK22 regulate the response to Verticillium dahliae toxins. Plant Physiol. 2022, 190, 714–731. [Google Scholar] [CrossRef]
- Hu, Q.; Min, L.; Yang, X.; Jin, S.; Zhang, L.; Li, Y.; Ma, Y.; Qi, X.; Li, D.; Liu, H.; et al. Laccase GhLac1 Modulates Broad-Spectrum Biotic Stress Tolerance via Manipulating Phenylpropanoid Pathway and Jasmonic Acid Synthesis. Plant Physiol. 2018, 176, 1808–1823. [Google Scholar] [CrossRef]
- Gao, W.; Long, L.; Zhu, L.-F.; Xu, L.; Gao, W.-H.; Sun, L.-Q.; Liu, L.-L.; Zhang, X.-L. Proteomic and Virus-induced Gene Silencing (VIGS) analyses reveal that gossypol, brassinosteroids, and jasmonic acid contribute to the resistance of cotton to Verticillium dahliae. Mol. Cell. Proteom. 2013, 12, 3690–3703. [Google Scholar] [CrossRef]
- Ramirez-Gonzalez, R.H.; Borrill, P.; Lang, D.; Harrington, S.A.; Brinton, J.; Venturini, L.; Davey, M.; Jacobs, J.; van Ex, F.; Pasha, A.; et al. The transcriptional landscape of polyploid wheat. Science 2018, 361, eaar6089. [Google Scholar] [CrossRef]
- Akagi, T.; Jung, K.; Masuda, K.; Shimizu, K.K. Polyploidy before and after domestication of crop species. Curr. Opin. Plant Biol. 2022, 69, 102255. [Google Scholar] [CrossRef]
- Wang, M.; Tu, L.; Lin, M.; Lin, Z.; Wang, P.; Yang, Q.; Ye, Z.; Shen, C.; Li, J.; Zhang, L.; et al. Asymmetric subgenome selection and cis-regulatory divergence during cotton domestication. Nat. Genet. 2017, 49, 579–587. [Google Scholar] [CrossRef]
- Wang, M.; Tu, L.; Yuan, D.; Zhu, D.; Shen, C.; Li, J.; Liu, F.; Pei, L.; Wang, P.; Zhao, G.; et al. Reference genome sequences of two cultivated allotetraploid cottons, Gossypium hirsutum and Gossypium barbadense. Nat. Genet. 2019, 51, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, M.R.; Gasteiger, E.; Bairoch, A.; Sanchez, J.C.; Hochstrasser, D.F. Protein Identification and Analysis Tools in the ExPASy Server. Methods Mol. Biol. 1999, 112, 531–552. [Google Scholar]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools. An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Chou, K.-C.; Shen, H.-B. Plant-mPLoc. a top-down strategy to augment the power for predicting plant protein subcellular localization. PLoS ONE 2010, 5, e11335. [Google Scholar] [CrossRef] [PubMed]
- Larkin, M. Clustal W and Clustal X v. 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL). an online tool for phylogenetic tree display and annotation. Bioinformatics 2006, 23, 127–128. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z. PAML 4. phylogenetic analysis by maximum likelihood. Mol. Biol. Evol. 2007, 24, 1586–1591. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME Suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef]
- Marchler-Bauer, A.; Derbyshire, M.K.; Gonzales, N.R.; Lu, S.; Chitsaz, F.; Geer, L.Y.; Geer, R.C.; He, J.; Gwadz, M.; Hurwitz, D.I.; et al. CDD. NCBI’s conserved domain database. Nucleic Acids Res. 2014, 43, D222–D226. [Google Scholar] [CrossRef]
- Marchler-Bauer, A.; Bo, Y.; Han, L.; He, J.; Lanczycki, C.J.; Lu, S.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; et al. CDD/SPARCLE. functional classification of proteins via subfamily domain architectures. Nucleic Acids Res. 2016, 45, D200–D203. [Google Scholar] [CrossRef]
- Niu, R.; Zhou, Y.; Zhang, Y.; Mou, R.; Tang, Z.; Wang, Z.; Zhou, G.; Guo, S.; Yuan, M.; Xu, G. uORFlight. a vehicle toward uORF-mediated translational regulation mechanisms in eukaryotes. Database 2020, 2020, baaa007. [Google Scholar] [CrossRef] [PubMed]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Tian, F.; Yang, D.-C.; Meng, Y.-Q.; Kong, L.; Luo, J.; Gao, G. PlantTFDB 4.0. toward a central hub for transcription factors and regulatory interactions in plants. Nucleic Acids Res. 2016, 45, D1040–D1045. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P. Cytoscape. A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhou, L.; Li, Y.; Ma, H.; Li, Y.; Ma, Y.; Lv, R.; Yang, J.; Wang, W.; Alifu, A.; et al. Rapid Identification of Pollen- and Anther-Specific Genes in Response to High-Temperature Stress Based on Transcriptome Profiling Analysis in Cotton. Int. J. Mol. Sci. 2022, 23, 3378. [Google Scholar] [CrossRef]
- Nurimanguli, A.I.; Wu, Y.L.; Pan, Z.Y.; An, Q.S.; Shui, G.L.; Shao, P.X.; Yang, D.Y.; Lin, H.R.; Tang, B.H.; Xin, W.E.; et al. Cotton ethylene response factor GhERF91 involved in the defense against Verticillium dahliae. J. Integr. Agric. 2023, in press.
- Zhu, T.; Liang, C.; Meng, Z.; Sun, G.; Meng, Z.; Guo, S.; Zhang, R. CottonFGD: An integrated functional genomics database for cotton. BMC Plant Biol. 2017, 17, 101. [Google Scholar] [CrossRef]
| Gene ID | Gene Name | Gene Location | Strand | CDS | Protein Length (aa) | Amino Acids MW (kDa) | PI | Loctree3 | Instability Index | Aliphatic Index | Grand Average of Hydropathicity |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ghir_A01G007760.1 | GhWNK-like | Ghir_A01:12971108–12972916 | − | 882 | 294 | 33.51 | 5.28 | cytoplasm | 32.81 | 83.5 | −0.471 |
| Ghir_D01G008110.1 | GhWNK-like | Ghir_D01:11157645–11159519 | − | 885 | 295 | 33.70 | 5.28 | cytoplasm | 34.52 | 83.9 | −0.47 |
| Ghir_D13G014800.1 | GhWNK-like | Ghir_D13:48337800–48339222 | + | 1692 | 564 | 63.88 | 6.37 | cytoplasm | 47.56 | 81.06 | −0.483 |
| Ghir_A11G015950.1 | GhWNK-like | Ghir_A11:18534787–18538191 | + | 1719 | 573 | 65.83 | 4.96 | cytoplasm | 42.57 | 76.88 | −0.557 |
| Ghir_D11G015990.1 | GhWNK-like | Ghir_D11:15838773–15842033 | + | 1929 | 643 | 72.09 | 5.55 | cytoplasm | 46.74 | 82.94 | −0.402 |
| Ghir_A07G006240.1 | GhWNK3 | Ghir_A07:7499395–7503152 | + | 1785 | 595 | 66.98 | 5.49 | cytoplasm | 41.77 | 83.87 | −0.525 |
| Ghir_D07G006240.1 | GhWNK3 | Ghir_D07:6786435–6790420 | + | 1827 | 609 | 68.64 | 5.17 | cytoplasm | 41.34 | 78.1 | −0.628 |
| Ghir_A09G025930.1 | GhWNK3 | Ghir_A09:81714196–81718754 | − | 1917 | 639 | 72.13 | 5.29 | cytoplasm | 43.48 | 86.67 | −0.528 |
| Ghir_D09G025080.1 | GhWNK3 | Ghir_D09:52477764–52482145 | − | 1863 | 621 | 69.96 | 5.22 | cytoplasm | 44.82 | 85.89 | −0.548 |
| Ghir_A05G039820.1 | GhWNK6/7 | Ghir_A05:104554042–104558039 | − | 1830 | 610 | 68.01 | 5.25 | cytoplasm | 45.82 | 88.07 | −0.362 |
| Ghir_D04G003230.1 | GhWNK6/7 | Ghir_D04:4406163–4410147 | + | 1827 | 609 | 67.81 | 5.25 | cytoplasm | 44.7 | 87.9 | −0.376 |
| Ghir_A11G023700.1 | GhWNK6/7 | Ghir_A11:71644048–71648140 | + | 891 | 297 | 33.68 | 5.89 | cytoplasm | 39.07 | 87.27 | −0.328 |
| Ghir_D11G024040.1 | GhWNK6/7 | Ghir_D11:43781586–43785463 | + | 891 | 297 | 33.70 | 5.81 | cytoplasm | 37.84 | 88.59 | −0.304 |
| Ghir_A08G013280.1 | GhWNK8/10 | Ghir_A08:95894478–95898002 | − | 2001 | 667 | 75.44 | 4.92 | cytoplasm | 47.98 | 83.01 | −0.41 |
| Ghir_D08G014030.1 | GhWNK8/10 | Ghir_D08:48419558–48423120 | − | 2004 | 668 | 75.62 | 4.96 | cytoplasm | 45.85 | 83.19 | −0.418 |
| Ghir_D12G025200.1 | GhWNK8/10 | Ghir_D12:59173626–59178177 | + | 1821 | 607 | 68.55 | 5.01 | cytoplasm | 41.94 | 84.83 | −0.404 |
| Ghir_A02G010480.1 | GhWNK8/10 | Ghir_A02:44867018–44870515 | + | 1758 | 586 | 66.39 | 4.94 | cytoplasm | 45.49 | 84.16 | −0.371 |
| Ghir_D03G009560.1 | GhWNK8/10 | Ghir_D03:33375034–33377884 | + | 1548 | 516 | 58.96 | 4.9 | cytoplasm | 45.57 | 82.95 | −0.456 |
| Ghir_A11G027620.1 | GhWNK1/9 | Ghir_A11:108616362–108620199 | + | 1872 | 624 | 70.39 | 6 | cytoplasm | 50.46 | 83.61 | −0.402 |
| Ghir_A02G003230.1 | GhWNK2 | Ghir_A02:3714173–3717808 | + | 591 | 197 | 23.07 | 6.89 | secreted | 41.99 | 90.51 | −0.295 |
| Ghir_D02G003610.1 | GhWNK2 | Ghir_D02:4679685–4683413 | + | 2202 | 734 | 82.80 | 5.07 | cytoplasm | 45.16 | 81.4 | −0.497 |
| Ghir_A13G024870.1 | GhWNK4 | Ghir_A13:108318875–108322870 | + | 1779 | 593 | 67.41 | 6.2 | cytoplasm | 45.42 | 78.25 | −0.535 |
| Ghir_D13G025640.1 | GhWNK4 | Ghir_D13:63297294–63301250 | + | 900 | 300 | 34.30 | 5.29 | cytoplasm | 40.34 | 80.87 | −0.513 |
| Ghir_A02G000480.1 | GhWNK5 | Ghir_A02:293503–294742 | + | 2202 | 734 | 83.11 | 5.03 | cytoplasm | 45.31 | 81.01 | −0.515 |
| Ghir_A10G015190.1 | GhWNK5 | Ghir_A10:83087865–83092029 | − | 1779 | 593 | 67.69 | 5.31 | cytoplasm | 41.81 | 76.44 | −0.487 |
| Ghir_D10G012370.1 | GhWNK5 | Ghir_D10:19346669–19350715 | + | 1779 | 593 | 67.72 | 5.36 | cytoplasm | 42.28 | 76.93 | −0.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Zhang, C.; Pan, Z.; Lin, H.; Li, Z.; Hou, X.; Liu, J.; Nie, X.; Wu, Y. Genome-Wide Identification and Analysis of the WNK Kinase Gene Family in Upland Cotton. Plants 2023, 12, 4036. https://doi.org/10.3390/plants12234036
Zhang Q, Zhang C, Pan Z, Lin H, Li Z, Hou X, Liu J, Nie X, Wu Y. Genome-Wide Identification and Analysis of the WNK Kinase Gene Family in Upland Cotton. Plants. 2023; 12(23):4036. https://doi.org/10.3390/plants12234036
Chicago/Turabian StyleZhang, Qi, Caidie Zhang, Zhenyuan Pan, Hairong Lin, Zhibo Li, Xinhe Hou, Jinshan Liu, Xinhui Nie, and Yuanlong Wu. 2023. "Genome-Wide Identification and Analysis of the WNK Kinase Gene Family in Upland Cotton" Plants 12, no. 23: 4036. https://doi.org/10.3390/plants12234036
APA StyleZhang, Q., Zhang, C., Pan, Z., Lin, H., Li, Z., Hou, X., Liu, J., Nie, X., & Wu, Y. (2023). Genome-Wide Identification and Analysis of the WNK Kinase Gene Family in Upland Cotton. Plants, 12(23), 4036. https://doi.org/10.3390/plants12234036







