Nitrogen Application Can Optimize Form of Selenium in Soil in Selenium-Rich Areas to Affect Selenium Absorption and Accumulation in Black Wheat
Abstract
:1. Introduction
2. Results
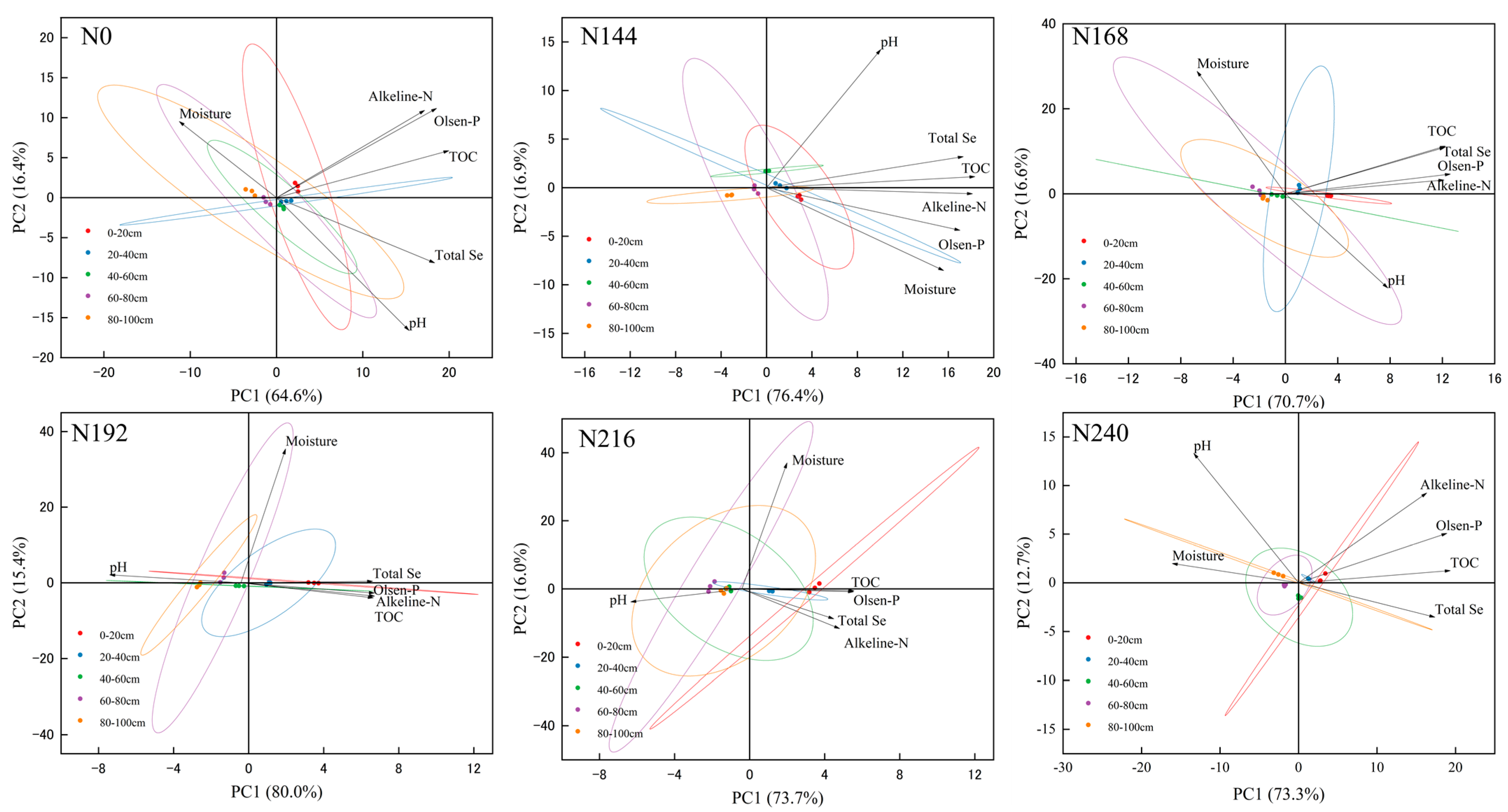
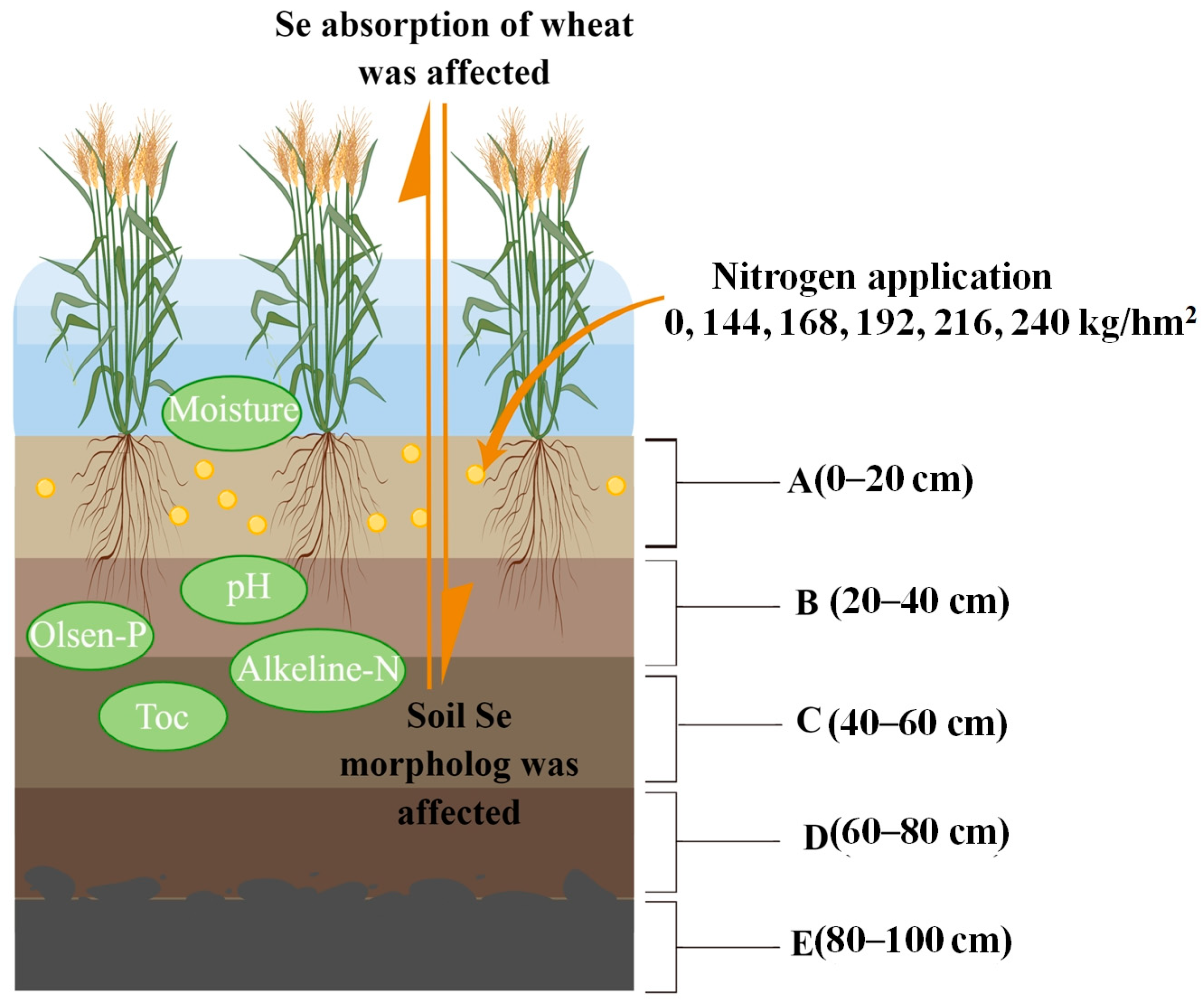
2.1. Changes in Environmental Characteristics in 0–100 cm Soil Layer of Selenium-Rich Soil under Different Nitrogen Application Rates
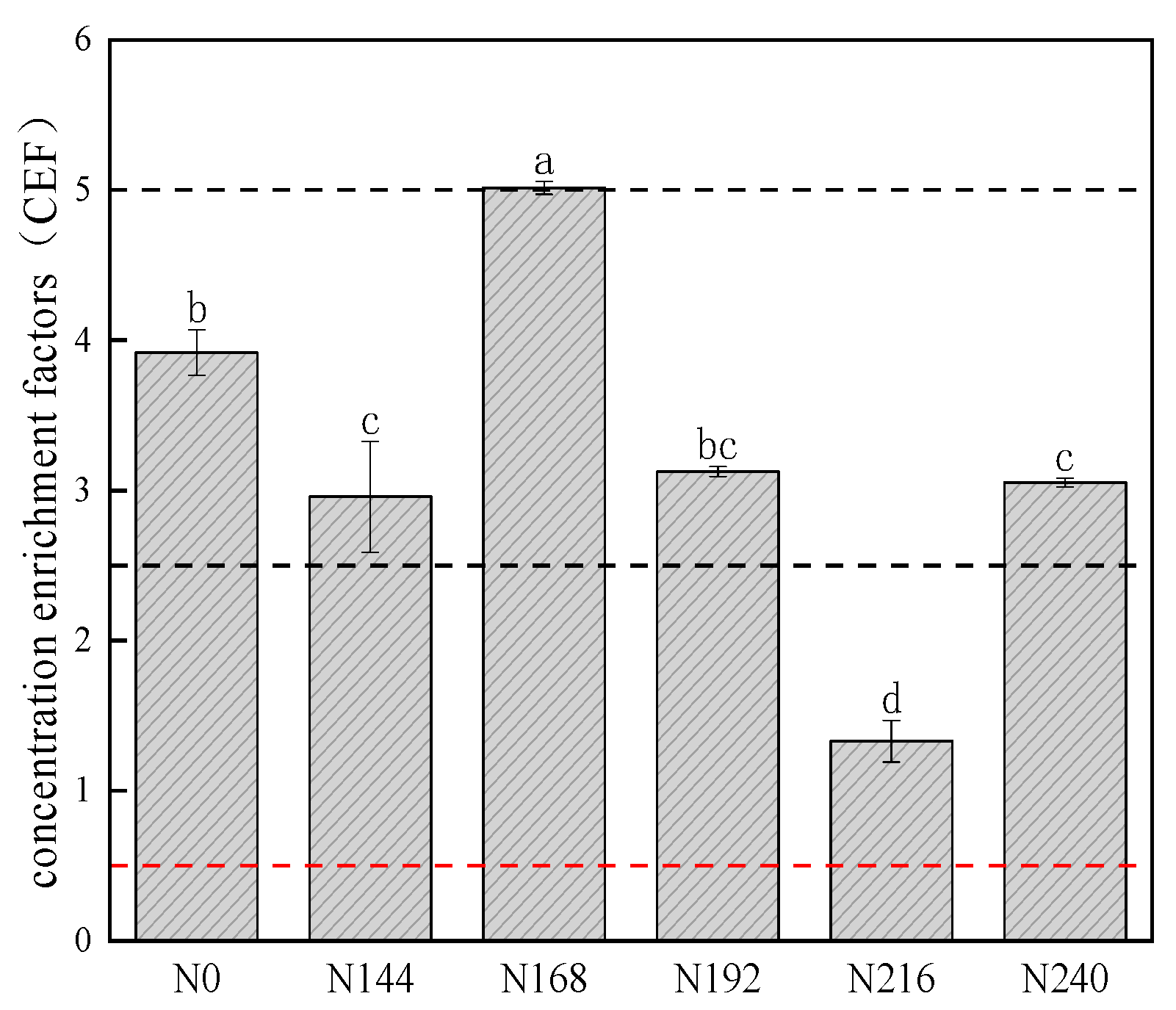
2.2. Differences in Selenium Concentration Enrichment Factor (CEF) Values in 0–100 cm Layer of Selenium-Rich Soil under Different Nitrogen Application Rates
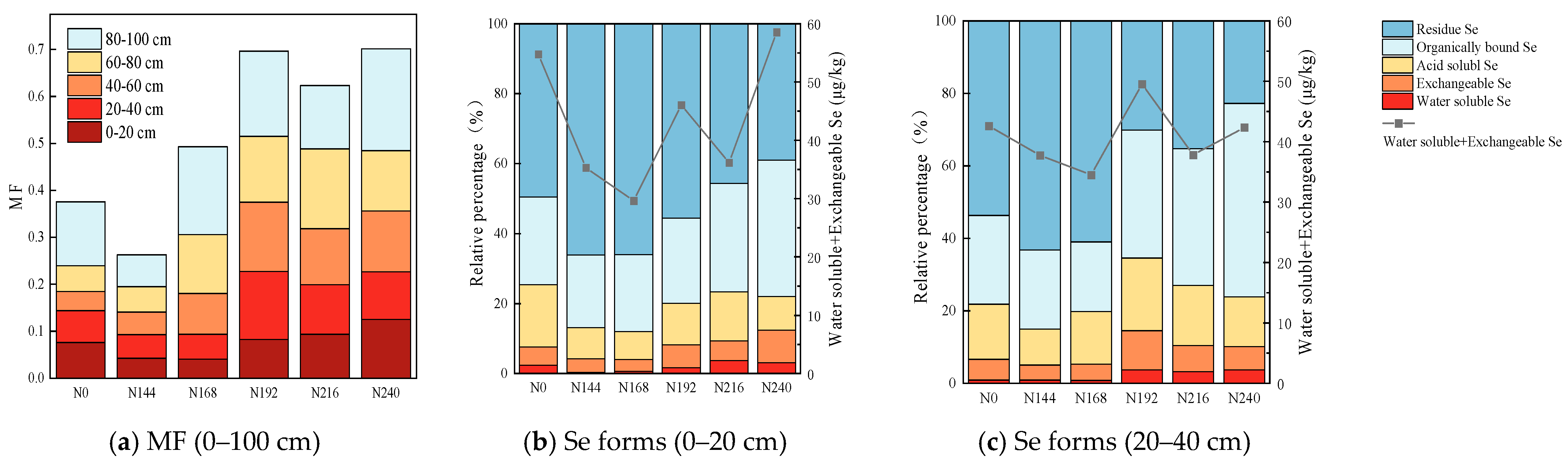
2.3. Differences in Soil Selenium Mobility (MF) and Forms of Selenium under Different Nitrogen Application Rates
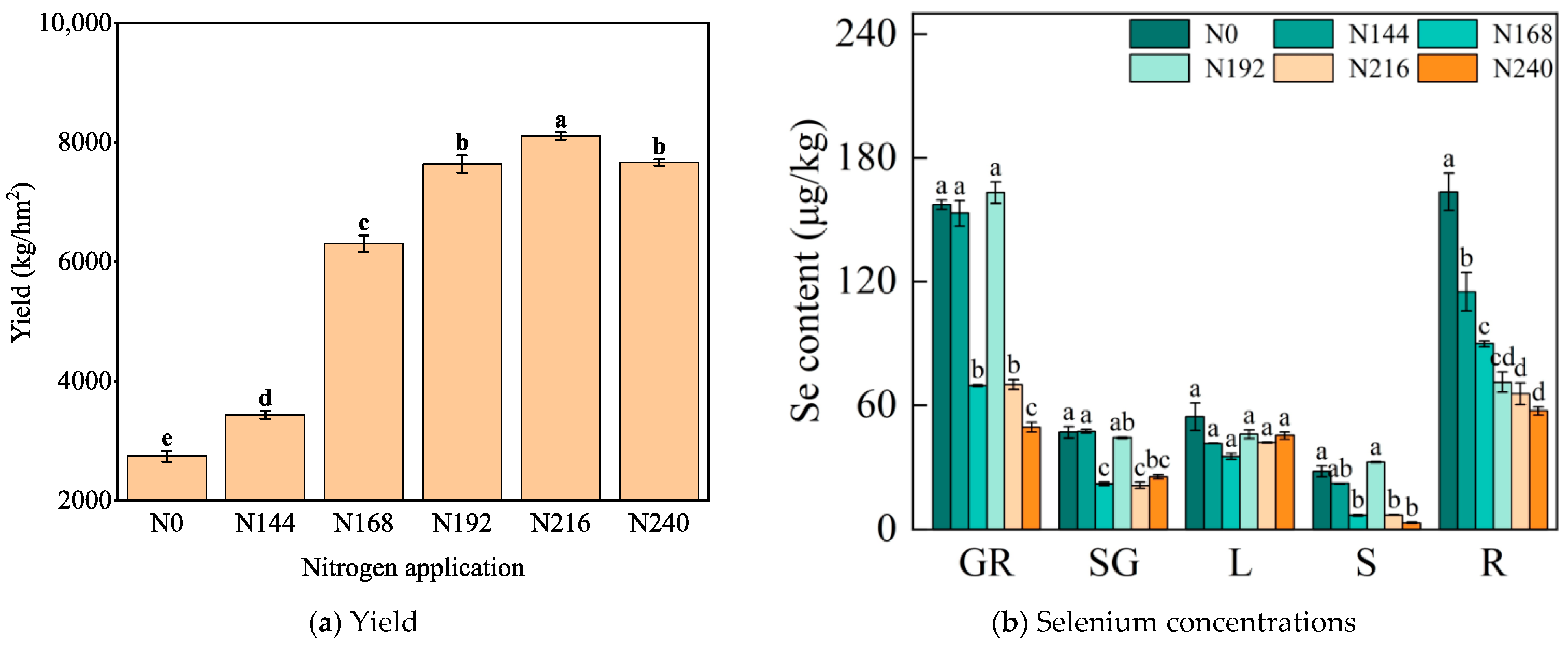
2.4. Changes in Selenium Absorption and Transport in Black Wheat Plants under Different Nitrogen Application Rates
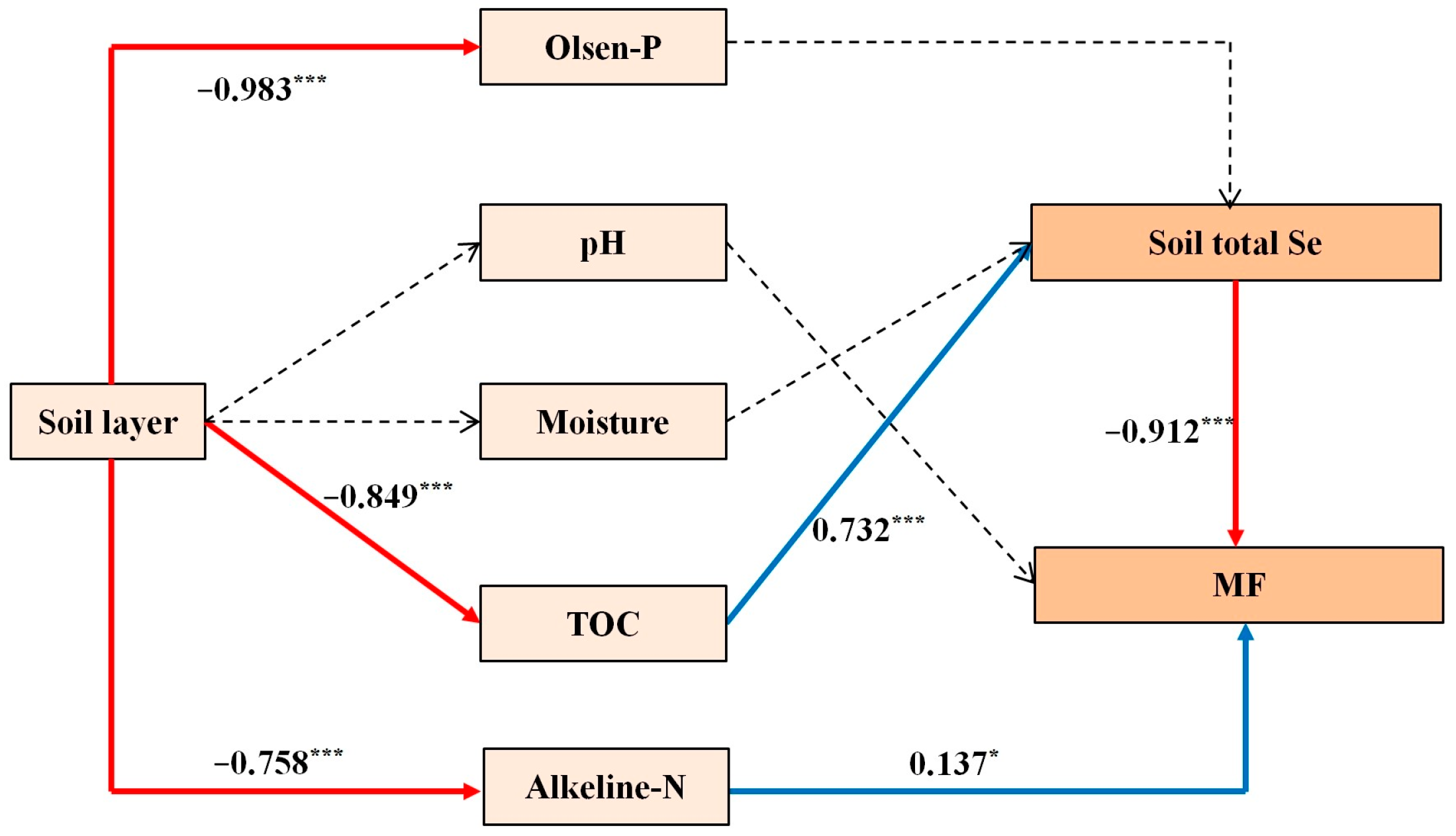
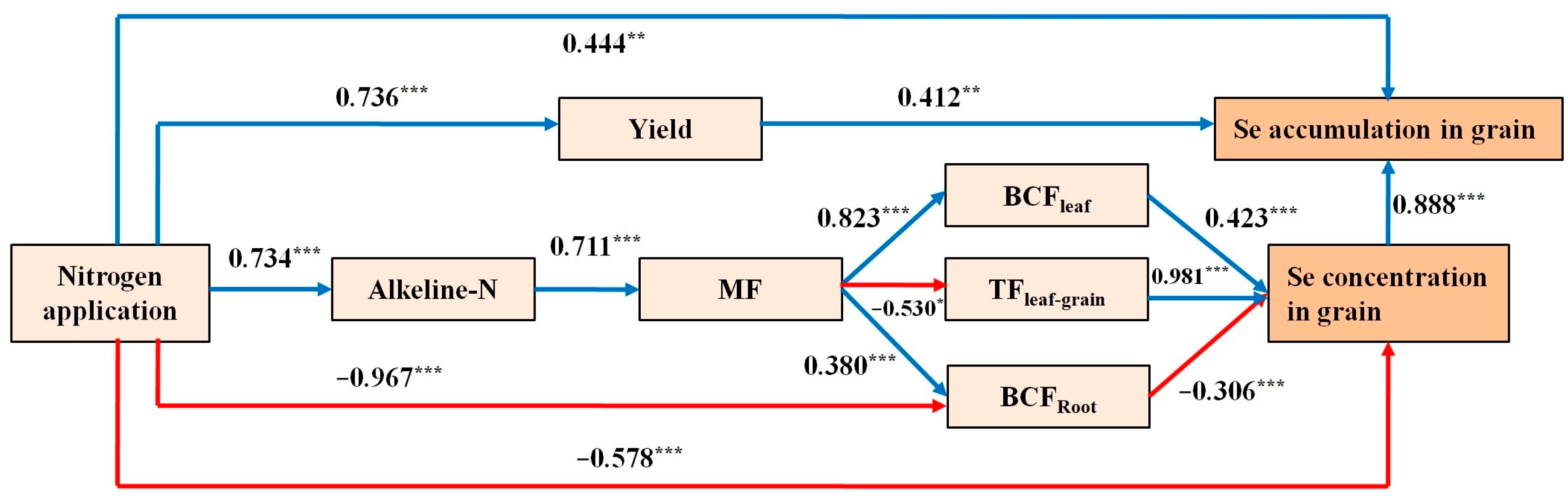
2.5. Comprehensive Path Analysis Based on Soil Selenium Contents, Nitrogen Application Rate, and Black Wheat Responses
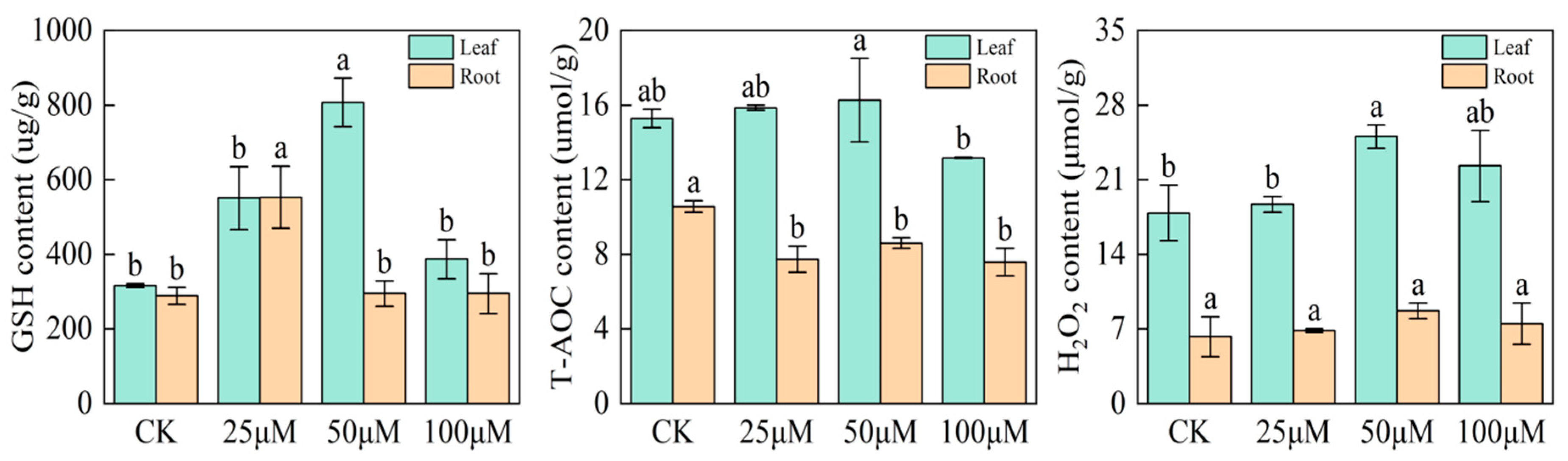
2.6. Effects of Sodium Selenite Solution on Glutathione (GSH), Total Antioxidant Capacity (T-AOC), and Hydrogen Peroxide (H2O2) Contents of Black Wheat Seedlings
3. Discussion
3.1. Effects of Environmental Factors on Soil Total Selenium Content and MF in Vertical Distribution of Soil
3.2. Effects of Nitrogen Application on Form of Soil Selenium
3.3. Effects of Nitrogen Application on Selenium Translocation and Distribution in Organs of Black Wheat
3.4. Effects of Nitrogen Application on Selenium Accumulation in Surface Soil and Grain
3.5. Effects of Nitrogen Application on Selenium Accumulation in Soil Plow Layer and Grain
4. Materials and Methods
4.1. Experimental Area
4.2. Experimental Method
4.3. Sampling and Chemical Analysis
4.4. Data Analysis
4.4.1. Plant Selenium Transport Factor (TF) and Bioconcentration Factor (BCF)
4.4.2. Soil Concentration Enrichment Factor (CEF)
4.4.3. Mobility Factor (MF)
4.4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, J.; Liu, R.F.; Zhang, C.Y.; Yang, J.; Lyu, L.H.; Shi, Z.Y.; Man, Y.B.; Wu, F.Y. Selenium uptake and accumulation in winter wheat as affected by level of phosphate application and arbuscular mycorrhizal fungi. J. Hazard. Mater. 2022, 433, 128762. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Zhu, L.F.; Chen, C.C.; Feng, H.Q.; Li, D.D.; Sun, S.Q.; Xing, Z.F.; Wan, X.Y.; Wang, X.H.; Li, F.; et al. Association of selenium levels with the prevention and control of Keshan disease: A cross-sectional study. J. Trace Elem. Med. Biol. 2021, 68, 126832. [Google Scholar] [CrossRef] [PubMed]
- Combs, G.F., Jr. Selenium in global food systems. Br. J. Nutr. 2001, 85, 517–547. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.A.; Zhu, W.; Wang, W.; Li, R.; Hou, S.; Wang, D.; Yang, L.S. Selenium in soil and endemic diseases in China. Sci. Total Environ. 2002, 284, 227–235. [Google Scholar] [CrossRef]
- Kipp, A.P.; Strohm, D.; Brigelius-Flohe, R.; Schomburg, L.; Bechthold, A.; Leschik-Bonnet, E.; Heseker, H.; German Nutrition Society (DGE). Revised reference values for selenium intake. J. Trace Elem. Med. Biol. 2015, 32, 195–199. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Z.Y.; Gu, M.H.; Li, H.; Shohag, M.J.I.; Shen, F.K.; Wang, X.L.; Wei, Y.Y. Combined use of arbuscular mycorrhizal fungus and selenium fertilizer shapes microbial community structure and enhances organic selenium accumulation in rice grain. Sci. Total Environ. 2020, 748, 141166. [Google Scholar] [CrossRef]
- Shardendu, U.; Salhani, N.; Boulyga, S.F.; Stengel, E. Phytoremediation of selenium by two helophyte species in subsurface flow constructed wetland. Chemosphere 2003, 50, 967–973. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Liu, H.; Yang, Y.E.; Wang, Z.H.; Li, F.C.; Li, K.Y.; Yang, N.; Wang, S.; Wang, H.; He, G.; Dai, J. Selenium content of wheat grain and its regulation in different wheat production regions of China. Sci. Agric. Sin. 2016, 49, 1715–1728. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Biofortifying crops with essential mineral elements. Trends Plant Sci. 2005, 10, 586–593. [Google Scholar] [CrossRef]
- Tian, S.Q.; Chen, Z.C.; Wei, Y.C. Measurement of colour-grained wheat nutrient compounds and the application of combination technology in dough. J. Cereal Sci. 2018, 83, 63–67. [Google Scholar] [CrossRef]
- Pasqualone, A.; Bianco, A.M.; Paradiso, V.M.; Summo, C.; Gambacorta, G.; Caponio, F.; Blanco, A. Production and characterization of functional biscuits obtained from purple wheat. Food Chem. 2015, 180, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Fallahi, E.; Colt, W.M.; Fallahi, B. Optimum ranges of leaf nitrogen for yield, fruit quality, and photosynthesis in ‘BC-2 Fuji’ apple. J. Am. Pomol. Soc. 2001, 55, 68–75. [Google Scholar]
- Soltanpour, P.N.; Olsen, S.R.; Goos, R.J. Effect of nitrogen fertilization of dryland wheat on grain selenium concentration. Soil Sci. Soc. Am. J. 1982, 46, 430–433. [Google Scholar] [CrossRef]
- Govasmark, E.; Grimmett, M.G. A method for determination of selenium in organic tissues using microwave digestion and liquid chromatography. J. AOAC Int. 2007, 90, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Govasmark, E.; Singh, B.R.; MacLeod, J.A.; Grimmett, M.G. Selenium concentration in spring wheat and leaching water as influenced by application times of selenium and nitrogen. J. Plant Nutr. 2008, 31, 193–203. [Google Scholar] [CrossRef]
- Wang, A.T.; Wang, Q.; Li, J.; Yuan, G.L.; Albanese, S.; Petrik, A. Geo-statistical and multivariate analyses of potentially toxic elements’ distribution in the soil of Hainan Island (China): A comparison between the topsoil and subsoil at a regional scale. J. Geochem. Explor. 2019, 197, 48–59. [Google Scholar] [CrossRef]
- Fan, J.X.; Zeng, Y.; Sun, J.X. The transformation and migration of selenium in soil under different Eh conditions. J. Soils Sediments 2018, 18, 2935–2947. [Google Scholar] [CrossRef]
- Elrashidi, M.A.; Adriano, D.C.; Workman, S.M.; Lindsay, W.L. Chemical equilibrium of selenium in soils: A theoretical development. Soil Sci. 1987, 144, 141–152. [Google Scholar] [CrossRef]
- Wang, D.; Zhou, F.; Yang, W.X.; Peng, Q.; Man, N.; Liang, D.L. Selenate redistribution during aging different Chinese soils and the dominant influential factors. Chemosphere 2017, 182, 284–292. [Google Scholar] [CrossRef]
- Eich-Greatorex, S.; Sogn, T.A.; Øgaard, A.F.; Aasen, I. Plant availability of inorganic and organic selenium fertiliser as influenced by soil organic matter content and pH. Nutr. Cycl. Agroecosyst. 2007, 79, 221–231. [Google Scholar] [CrossRef]
- Chang, C.; Yin, R.; Wang, X.; Shao, S.; Chen, C.; Zhang, H. Selenium translocation in the soil-rice system in the Enshi seleniferous area, Central China. Sci. Total Environ. 2019, 669, 83–90. [Google Scholar] [CrossRef]
- Tuttle, M.L.W.; Fahy, J.W.; Elliott, J.G.; Grauch, R.I.; Stilings, L.L. Contaminants from Cretaceous black shale: I. Natural weathering processes controlling contaminant cycling in Mancos Shale, southwestern United States, with emphasis on salinity and selenium. Appl. Geochem. 2014, 46, 57–71. [Google Scholar] [CrossRef]
- Gabos, M.B.; Alleoni, L.R.F.; Abreu, C.A. Background levels of selenium in some selected Brazilian tropical soils. J. Geochem. Explor. 2014, 145, 35–39. [Google Scholar] [CrossRef]
- Harris, J.; Schneberg, K.A.; Pilon-Smits, E.A.H. Sulfur–selenium–molybdenum interactions distinguish selenium hyperaccumulator Stanleya pinnata from non-hyperaccumulator Brassica juncea (Brassicaceae). Planta 2014, 239, 479–491. [Google Scholar] [CrossRef]
- Qu, L.L.; Xu, J.Y.; Dai, Z.H.; Elyamine, A.M.; Huang, W.X.; Han, D.; Dang, B.J.; Xu, Z.C.; Jia, W. Selenium in soil-plant system: Transport, detoxification and bioremediation. J. Hazard. Mater. 2023, 452, 131272. [Google Scholar] [CrossRef]
- Abrams, M.M.; Burau, R.G.; Zasoski, R.J. Organic selenium distribution in selected California soils. Soil Fence Soc. Am. J. 1990, 54, 979–982. [Google Scholar] [CrossRef]
- Lukas, S. Polar paths of diffusion across plant cuticles: New evidence for an old hypothesis. Ann. Bot. 2005, 95, 1069–1073. [Google Scholar] [CrossRef]
- Pu, Z.E.; Wei, G.H.; Liu, Z.H.; Chen, L.; Guo, H.; Li, Y.; Li, Y.; Dai, S.F.; Wang, J.R.; Li, W.; et al. Selenium and anthocyanins share the same transcription factors R2R3MYB and bHLH in wheat. Food Chem. 2021, 356, 129699. [Google Scholar] [CrossRef]
- Yin, N.; Mu, L.; Liang, Y.L.; Hao, W.L.; Yin, H.F.; Zhu, S.M.; An, X.J. Effects of foliar selenium fertilizer on fruit yield, quality and selenium content of three varieties of Vitis vinifera. J. Appl. Ecol. 2020, 31, 953–958. [Google Scholar] [CrossRef]
- Shalaby, T.A.; Abd-Alkarim, E.; El-Aidy, F.; Hamed, E.S.; Sharaf-Eldin, M.; Taha, N.; El-Ramady, H.; Bayoumi, Y.; Reis, A.R.D. Nano-selenium, silicon and H2O2 boost growth and productivity of cucumber under combined salinity and heat stress. Ecotoxicol. Environ. Saf. 2021, 212, 111962. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Raza, A.; Hawrylak-Nowak, B.; Matraszek Gawron, R.; Mahmud, J.A.; Fujita, M. Selenium in plants: Boon or bane? Environ. Exp. Bot. 2020, 178, 104170. [Google Scholar] [CrossRef]
- Fergusson, J.E. (Ed.) The Heavy Elements: Chemistry, Environmental Impact, and Health Effects; Pergamon: Oxford, UK, 1990. [Google Scholar]
- Lee, P.K.; Touray, J.C.; Baillif, P.; Ildefonse, J.P. Heavy metal contamination of settling particles in a retention pond along the A-71 motorway in Sologne, France. Sci. Total Environ. 1997, 201, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, R.A. Bed sediment-associated trace metals in an urban stream, Oahu, Hawaii. Environ. Geol. 2000, 39, 611–627. [Google Scholar] [CrossRef]
- Reimann, C.; Kashulina, G.; Caritat, D.P.; Niskavaara, H. Multi-element, multi-medium regional geochemistry in the European Arctic: Element concentration, variation and correlation. Appl. Geochem. 2001, 16, 759–780. [Google Scholar] [CrossRef]
- Yu, T.; Yang, Z.F.; Lv, Y.Y.; Hou, Q.Y.; Xia, X.Q.; Feng, H.Y.; Zhang, M.; Jin, L.X.; Kan, Z.Z. The origin and geochemical cycle of soil selenium in a Se-rich area of China. J. Geochem. Explor. 2014, 139, 97–108. [Google Scholar] [CrossRef]
- Hao, L.T.; Zhang, J.D.; Zhang, S.X.; Ma, S.M.; Li, B.; Long, G.; Fan, J.S.; Luo, K.L. Distribution characteristics and main influencing factors of selenium in surface soil of natural selenium-rich area: A case study in Langao County, China. Environ. Geochem. Health 2021, 43, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.S.; Cicchella, D.; Liu, T.; Yang, S.F.; Zhou, Y.H. Origin, distribution and enrichment of selenium in oasis farmland of Aksu, Xinjiang, China. J. Geochem. Explor. 2021, 223, 106723. [Google Scholar] [CrossRef]
- Kul’chitskii, N.A.; Naumov, A.V. Modern state of markets of selenium and selenium-based compounds. Russ. J. Non-Ferrous Metals 2015, 56, 409–416. [Google Scholar] [CrossRef]
- Stroud, J.L.; McGrath, S.P.; Zhao, F.J. Selenium speciation in soil extracts using LC-ICP-MS. Int. J. Environ. Anal. Chem. 2012, 92, 222–236. [Google Scholar] [CrossRef]
- Blazina, T.; Sun, Y.B.; Voegelin, A.; Lenz, M.; Berg, M.; Winkel, L.H.E. Terrestrial selenium distribution in China is potentially linked to monsoonal climate. Nat. Commun. 2014, 5, 4717. [Google Scholar] [CrossRef]
- GB/T 35876-2018; Inspection of Grain and Oils—Determination of Sodium, Mgnesium, Kalium, Calcium, Chromium, Manganese, Iron, Copper, Zinc, Arsenic, Selenium, Cadmium and Plumbum in Cereals and Derived Products—Iductively Cupled Pasma-Mss Sectrometric Method. China National Standardization Administration Committee: Beijing, China, 2018. Available online: https://openstd.samr.gov.cn/bzgk/gb/newGbInfo?hcno=D81757395C1739C3653784C91C372011 (accessed on 15 November 2023). (In Chinese)
- Martens, D.A.; Suarez, D.L. Selenium Speciation of Soil/Sediment Determined with Sequential Extractions and Hydride Generation Atomic Absorption Spectrophotometry. Environ. Sci. Technol. 1997, 31, 133–139. [Google Scholar] [CrossRef]
- Cui, W.T.; Fu, G.Q.; Wu, H.H.; Shen, W.B. Cadmium-induced heme oxygenase-1 gene expression is associated with the depletion of glutathione in the roots of Medicago sativa. Biometals 2011, 24, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, N.; Serafini, M.; Salvatore, S.; Del Rio, D.; Bianchi, M.; Brighenti, F. Total antioxidant capacity of spices, dried fruits, nuts, pulses, cereals and sweets consumed in Italy assessed by three different in vitro assays. Mol. Nutr. Food Res. 2006, 50, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Sima, Y.H.; Yao, J.M.; Hou, Y.S. Variations of hydrogen peroxide and catalase expression in Bombyx eggs during diapause initiation and termination. Arch. Insect Biochem. Physiol. 2011, 77, 72–80. [Google Scholar] [CrossRef]
- Wu, C.; Dun, Y.; Zhang, Z.J.; Li, M.L.; Wu, G.Q. Foliar application of selenium and zinc to alleviate wheat (Triticum aestivum L.) cadmium toxicity and uptake from cadmium-contaminated soil. Ecotoxicol. Environ. Saf. 2020, 190, 110091. [Google Scholar] [CrossRef]
- Gong, J.J.; Yang, J.Z.; Wu, H.; Gao, J.W.; Tang, S.X.; Ma, S.M. Spatial distribution and environmental impact factors of soil selenium in Hainan Island, China. Sci. Total Environ. 2022, 81, 151329. [Google Scholar] [CrossRef]
- China National Environmental Monitoring Centre. Background Values of Soil Elements in China. 1990. Available online: http://www.irgrid.ac.cn/handle/1471x/723390 (accessed on 15 November 2023).
- Ali, U.; Shaaban, M.; Bashir, S.; Fu, Q.L.; Zhu, J.; Islam, M.S.; Hu, H.Q. Effect of rice straw, biochar and calcite on maize plant and Ni bio-availability in acidic Ni contaminated soil. J. Environ. Manag. 2020, 259, 109674. [Google Scholar] [CrossRef]
- Liu, N.N.; Wang, M.; Zhou, F.; Zhai, H.; Qi, M.X.; Liu, Y.; Li, Y.A.; Zhang, N.C.; Ma, Y.Z.; Huang, J.; et al. Selenium bioavailability in soil-wheat system and its dominant influential factors: A field study in Shaanxi province, China. Sci. Total Environ. 2021, 770, 144664. [Google Scholar] [CrossRef]
| N Application Rate | Soil Layer | Olsen-P | pH | Moisture | TOC | Alkaline-N | Total Se |
|---|---|---|---|---|---|---|---|
| (kg hm−2) | (cm) | (mg kg−1) | (%) | (g kg−1) | (mg kg−1) | (μg kg−1) | |
| N0 | 0–20 | 56.82 | 7.53 | 10.11 | 18.18 | 67.67 | 723.06 |
| 20–40 | 42.27 | 7.57 | 9.67 | 14.78 | 46.67 | 647.77 | |
| 40–60 | 18.28 | 7.58 | 10.03 | 9.31 | 51.33 | 810.15 | |
| 60–80 | 3.26 | 7.54 | 10.88 | 5.11 | 46.67 | 491.36 | |
| 80–100 | 1.61 | 7.44 | 11.02 | 2.53 | 30.33 | 201.56 | |
| N144 | 0–20 | 66.28 | 7.54 | 9.90 | 18.42 | 77.00 | 825.95 |
| 20–40 | 34.12 | 7.56 | 8.78 | 15.93 | 72.33 | 754.04 | |
| 40–60 | 18.18 | 7.60 | 7.22 | 12.47 | 65.33 | 673.14 | |
| 60–80 | 2.99 | 7.52 | 7.94 | 11.22 | 53.67 | 642.60 | |
| 80–100 | 2.44 | 7.50 | 7.08 | 4.29 | 37.33 | 317.20 | |
| N168 | 0–20 | 67.23 | 7.57 | 9.26 | 10.53 | 81.67 | 734.12 |
| 20–40 | 40.37 | 7.55 | 10.15 | 9.36 | 46.67 | 651.35 | |
| 40–60 | 13.37 | 7.57 | 10.25 | 6.05 | 35.00 | 284.10 | |
| 60–80 | 4.08 | 7.53 | 10.26 | 4.00 | 30.33 | 216.20 | |
| 80–100 | 2.86 | 7.55 | 9.62 | 3.19 | 32.67 | 159.62 | |
| N192 | 0–20 | 59.91 | 7.02 | 10.32 | 12.44 | 86.33 | 558.47 |
| 20–40 | 28.00 | 7.12 | 10.07 | 9.94 | 65.33 | 341.56 | |
| 40–60 | 12.87 | 7.15 | 9.30 | 7.49 | 44.33 | 263.30 | |
| 60–80 | 2.50 | 7.25 | 10.82 | 4.48 | 32.67 | 256.23 | |
| 80–100 | 1.97 | 7.34 | 9.23 | 2.86 | 21.00 | 194.88 | |
| N216 | 0–20 | 60.06 | 7.27 | 10.75 | 13.39 | 93.33 | 386.62 |
| 20–40 | 36.94 | 7.36 | 9.71 | 9.35 | 65.33 | 362.26 | |
| 40–60 | 15.71 | 7.44 | 9.76 | 6.96 | 39.67 | 313.55 | |
| 60–80 | 1.09 | 7.44 | 10.14 | 5.14 | 44.33 | 216.24 | |
| 80–100 | 2.36 | 7.46 | 9.51 | 5.39 | 53.67 | 320.36 | |
| N240 | 0–20 | 59.88 | 7.34 | 9.06 | 18.96 | 102.67 | 470.41 |
| 20–40 | 44.05 | 7.38 | 9.81 | 13.42 | 84.00 | 418.48 | |
| 40–60 | 14.05 | 7.29 | 9.93 | 10.35 | 51.33 | 348.23 | |
| 60–80 | 2.03 | 7.44 | 11.08 | 8.34 | 28.00 | 347.45 | |
| 80–100 | 1.15 | 7.46 | 11.22 | 5.20 | 56.00 | 168.10 |
| Nitrogen Application Rate | TFroot-stem | TFstem-leaf | TFstem-spike glume | TFleaf-spike glume | TFspike glume-grain | TFstem-grain | TFleaf-grain | BCFroot | BCFstem | BCFleaf | BCFspike glume | BCFgrain |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N0 | 0.17 | 1.95 | 1.68 | 0.86 | 3.35 | 5.62 | 2.89 | 0.226 | 0.039 | 0.075 | 0.065 | 0.218 |
| N144 | 0.19 | 1.88 | 2.14 | 1.14 | 3.22 | 6.91 | 3.68 | 0.139 | 0.027 | 0.050 | 0.057 | 0.185 |
| N168 | 0.08 | 5.14 | 3.20 | 0.62 | 3.17 | 10.14 | 1.97 | 0.122 | 0.009 | 0.048 | 0.030 | 0.095 |
| N192 | 0.46 | 1.42 | 1.37 | 0.96 | 3.68 | 5.02 | 3.54 | 0.128 | 0.058 | 0.082 | 0.079 | 0.292 |
| N216 | 0.11 | 6.03 | 3.05 | 0.51 | 3.29 | 10.05 | 1.67 | 0.170 | 0.018 | 0.109 | 0.055 | 0.181 |
| N240 | 0.05 | 15.04 | 8.40 | 0.56 | 1.95 | 16.38 | 1.09 | 0.122 | 0.006 | 0.097 | 0.054 | 0.105 |
| Soil Layer | Co | Cr | Cu | Mn | Ni | Pb | V | Zn |
|---|---|---|---|---|---|---|---|---|
| Topsoil | 9.9 | 61.8 | 26.9 | 554 | 32 | 15.8 | 68.3 | 75.5 |
| Subsoil | 10.8 | 61.5 | 28.3 | 573 | 33.7 | 18.1 | 70.6 | 71.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, W.; Huo, R.; Lu, Y.; Fan, Z.; Yue, R.; Ren, A.; Li, L.; Ding, P.; Ren, Y.; Gao, Z.; et al. Nitrogen Application Can Optimize Form of Selenium in Soil in Selenium-Rich Areas to Affect Selenium Absorption and Accumulation in Black Wheat. Plants 2023, 12, 4160. https://doi.org/10.3390/plants12244160
Kong W, Huo R, Lu Y, Fan Z, Yue R, Ren A, Li L, Ding P, Ren Y, Gao Z, et al. Nitrogen Application Can Optimize Form of Selenium in Soil in Selenium-Rich Areas to Affect Selenium Absorption and Accumulation in Black Wheat. Plants. 2023; 12(24):4160. https://doi.org/10.3390/plants12244160
Chicago/Turabian StyleKong, Weilin, Ruiwen Huo, Yu Lu, Zhenjie Fan, Runqing Yue, Aixia Ren, Linghong Li, Pengcheng Ding, Yongkang Ren, Zhiqiang Gao, and et al. 2023. "Nitrogen Application Can Optimize Form of Selenium in Soil in Selenium-Rich Areas to Affect Selenium Absorption and Accumulation in Black Wheat" Plants 12, no. 24: 4160. https://doi.org/10.3390/plants12244160




