Beneficial Microorganisms as Bioprotectants against Foliar Diseases of Cereals: A Review
Abstract
:1. Introduction
2. The Main Foliar Diseases Affecting Cereals
2.1. Fungal Leaf Diseases of Wheat
2.2. Fungal Leaf Diseases of Rice
2.3. Fungal Leaf Diseases of Maize

3. Current Control Strategies for Cereal Leaf Pathogens
3.1. Chemical Control
3.2. Management of Foliar Diseases Using Antagonists
3.2.1. Biocontrol with Bacillus spp. as BCAs
3.2.2. Biocontrol with Pseudomonas spp. as BCAs
3.2.3. Other Antagonistic Bacteria as BCAs
3.2.4. Biocontrol with Trichoderma spp. as BCAs
| Antagonist Strains | Origin | Target Organism Pathogen | Targeted Crop | Results | Antifungal Metabolites/Mode of Action | References |
|---|---|---|---|---|---|---|
| B. subtilis BBG13, BBG125, and Bs2504 | ProBioGEM, Centre Wallon de Biologie Industrielle | Zymoseptoria tritici | Wheat | In vitro and in vivo studies: Mycosubtiline formulations inhibit STB growth, with demi-maximal inhibitory doses of 1.4 mg L−1 for M and (M + S) and 4.5 mgL−1 for (M + S + F), respectively. | Mycosubtiline, surfactin, fengycin bacillomycin D | [15] |
| B. megaterium 6A Paneibacillus xylanexedens 7A | Yellow rust-resistant wheat | Puccinia striiformis tritici | Wheat | In semi-field: Decreased severity by 46.07% and 44.47% for the FLBC effect in curative, while 65.16% and 61.11% in protective effect, respectively. | Antioxidant enzymes: SOD, POD, PPO, and PAL; PR proteins | [145] |
| B. subtilis XZ16-1 | _ | Blumeria graminis | Wheat | Preventive and therapeutic efficacy against powdery mildew was 83.72% and 81.18, respectively. | Solubilize phosphate, fix nitrogen, hydrolases, lipopeptides, siderophores, IAA | [150] |
| B. subtilis QST713 | Serenade® ASO, Bayer CropScience | Puccinia striiformis, Blumeria graminis spp. | Wheat | In the field: Decreased the severity of stripe rust, offering up to 60% at BBCH development stages 65–69, and powdery mildew with moderate control between 20% and 65%. | Mycoparasitism/Metabolites | [45,139] |
| B. subtilis E1R-j | Wheat roots | Puccinia striiformis f. sp. tritici (Pst) | Wheat | In a condition-controlled greenhouse: Protective mode reduces the severity of disease, and the control efficacy ranged between 54.0% and 87.7%. | Mycoparasitism/Metabolites | [138] |
| B. subtilis TE3 | Native Wheat | Bipolaris sorokiniana | Wheat | In vivo biological control: Reduced the number of lesions/cm2 to 3.06 ± 0.6 and 3.74 ± 0.70 as well as the visual damage to 3–5 and 4–6, respectively. | Chitinase, glucanase; siderophores, indoles, and biosurfactants | [149] |
| B. velezensis (S1, S6) | Wheat ears | Zymoseptoria tritici | wheat | Regarding culture filtrates, the minimum inhibitory dilution and the semi-maximum inhibitory dilution were, 15% and 7.4% for strain S6 and 3.7% and 1.4% for strain S1, respectively. | Bacillomycine D | [136] |
| B. megaterium MKB135, P. fluorescens MKB21 and MKB91 | Barley leaves and grain, oat chaff, and wheat rhizospheres | Zymoseptoria tritici | wheat | STB development was postponed (by up to 80%). | Mycoparasitism/Metabolites | [175] |
| B. amyloliquefaciens S499 | Rhizosphere | Zymoseptoria tritici | Wheat | Surfactin provided wheat with a 70% defense against Z. tritici in greenhouse tests. | Surfactin, SA and JA signaling pathways | [148] |
| B. velezensis BZR 517 and BZR 336 g | Rhizosphere of winter wheat | Pyrenophora tritcii repentis | Wheat | In vitro: induced degenerative alterations in mycelium and decreased its development by 72.4–94.3%. In a three-year field study, BZR 517 and BZR 336 g increased yield by 5.0–7.6%. | Mycoparasitism/Metabolites | [57] |
| Pseudomonas putida BK8661 | Wheat leaves | Zymoseptoria tritici and Puccinia recondita f. sp. tritici | Wheat | On wheat leaves, Septoria tritici and Puccinia recondita f. sp. tritici are inhibited from growing in vitro. | Siderophores, antibiotics, HCN | [128,159] |
| P. fluorescens PFM2 | Wheat phyllosphere | Zymoseptoria tritici | Wheat | In vitro: After 3, 7, and 14 days, the zone of inhibition increased from 0 to 9 cm, 1 to 6, and 1 to 9 cm, respectively. | 2,4-diacetylphloroglucinol | [160] |
| Pseudomonas aeruginosa LEC 1 | Soil | Zymoseptoria tritici | Wheat | Inhibit Septoria tritici by 88% and Puccinia recondita by 98% when applied to wheat seedlings 3 h before inoculation with the pathogens. | 1-hyroxyphénazine (phOH), catalase | [161] |
| T. harzianum sensu lato TSM39 | Soil | Bipolaris sorokoniata | Wheat | Cellular elements of B. sorokiniana stimulate the chitinolytic system of strain TSM39. | Mycoparasitism | [172] |
| Streptomyces tauricus XF | Rhizospheric soil of peony | Puccinia striiformis | wheat | The control effects of FL and AC reached 68.25%, and 65.48%, respectively, in the greenhouse. Using XF fermentation broth, yellow rust disease indices were considerably decreased by 53.83%. in the field. | ROS, (PAL), β-1,3-endoglucanases, chitinases, endochitinase, and peroxidase | [164] |
| Cladosporium cladosporioides R23Bo | Puccinia striiformis | Puccinia striiformis | Wheat | Reduce the urediospore germination rate. The color of uredinia went from yellow to taupe. | Hyperparasitism | [165] |
| Epicoccum nigrum HE20 | Healthy wheat | Puccinia striiformis | Wheat | In the greenhouse: Reduction of severity by 87.5%. | POD, PPO, and CAT, butyric acid, hexanoic acid, α-linolenic acid, lactic acid, pentadecanoic acid, and 10,12-tricosadiynoic acid Defensive genes (JERF3, GLU, and PR1) | [176] |
| B. subtilis BJ-1 | Contaminated Magnaporthe oryzae culture plate | Magnaporthe oryzae | Rice | Detached leaves were inhibited by 108 CFUmL−1 (BC) or 5% (Fl) of BJ-1. | Surfactin, fengycin, subtilin, and bacilysin. ISR | [153] |
| Bacillus safensis B21 | Osmanthus fragrans Lour. Fruits | Magnaortae oryzae | Rice | Inhibition of hyphal growth. | Iturin A2, A6 | [150,177] |
| B. tequilensis JN-369 | Rice | Magnaortae oryzae | Rice | The efficacy of biocontrol in protective tests and therapeutic tests on detached rice leaves was up to 74.08% and 62.96%, respectively. | Plant growth and resistance induction | [154,177] |
| Bacillus cereus YN917 | Rice leaf | Magnaporthe oryzae | Rice | The efficacity before and after inoculation was 68.15% 65.61%, respectively, under detached leaf and greenhouse conditions. | IAA, siderophores, protease, ACC deaminase, cellulase, amylase, β-1,3-glucanase, and phosphate solubilization | [155] |
| B. subtilis B47 | Tomato | Bipolaris maydis | Maize | In the field, the control efficacy increased to 64.2% when iturin A2 concentration was raised to 500 mg kg−1. | Iturin A2 | [97] |
| Trichoderma harzianum SH2303 T. atroviride SG3403 | Soil | Cochliobolus heterostrophus | Maize | In-field and greenhouse conditions: synergistic application difenoconazole-propiconazole (DP). +SH2303 showed 60% of control. | SAR, PAL, CAT, SOD SA pathway (PR1) | [96,173] |
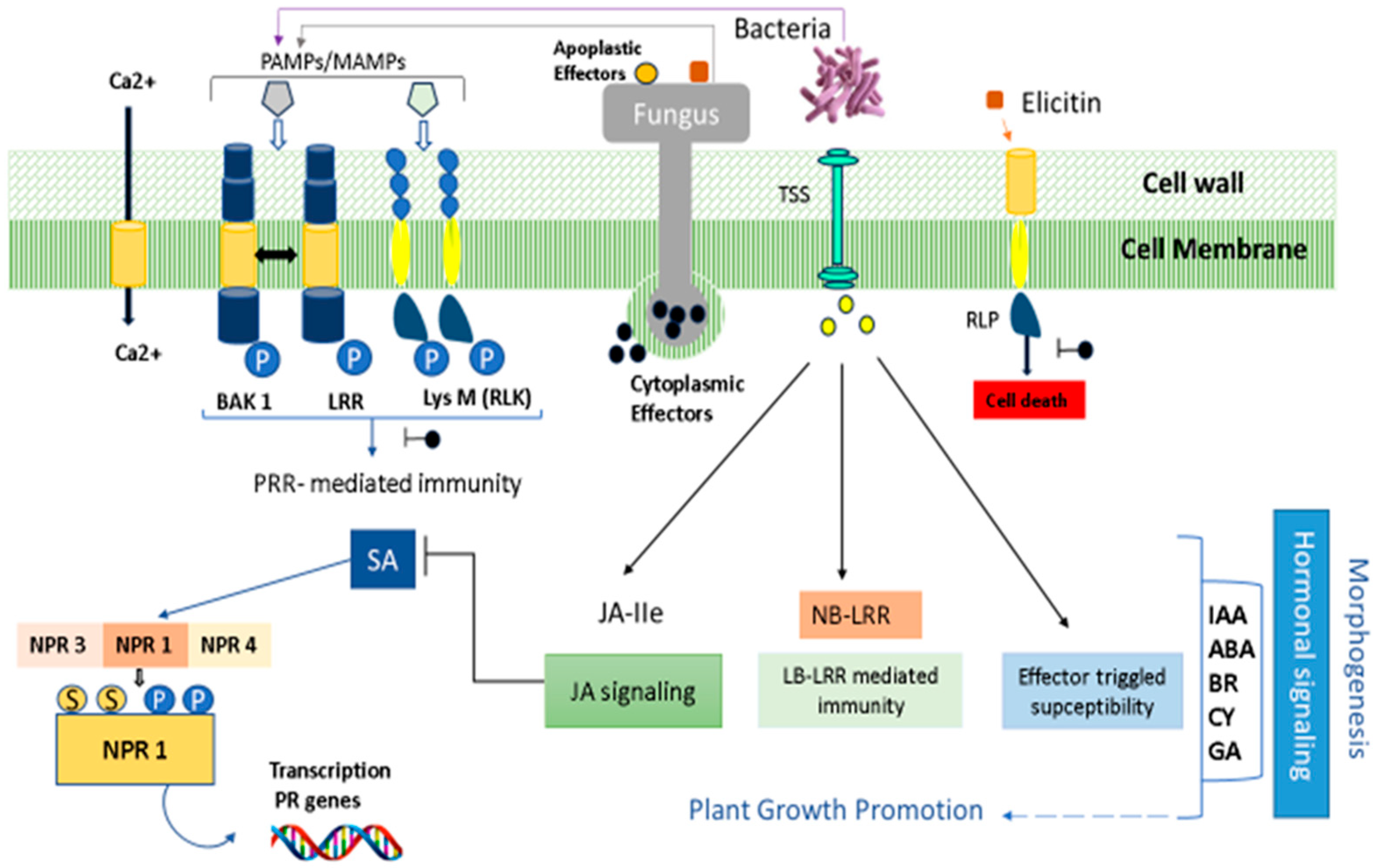
4. Induction of Cereals Defense Mechanisms
5. Advancements in BCAs: Discussion on Screening, Application, and Future Perspectives
5.1. Challenges of Conventional Practices
5.2. BCAs as Sustainable Alternatives
5.3. Field Application and Challenges
5.4. Biotechnological Insights and Environmental Impact
5.5. Systemic Resistance and Molecular Insights
5.6. Commercial Landscape and Future Perspectives
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sandhu, N.; Sethi, M.; Kumar, A.; Dang, D.; Singh, J.; Chhuneja, P. Biochemical and Genetic Approaches Improving Nitrogen Use Efficiency in Cereal Crops: A Review. Front. Plant Sci. 2021, 12, 657629. [Google Scholar] [CrossRef]
- Biel, W.; Kazimierska, K.; Bashutska, U. Nutritional Value of Wheat, Triticale, Barley and Oat Grains. Acta Sci. Pol. Zootech. 2020, 19, 19–28. [Google Scholar] [CrossRef]
- Jauhar, P.P. Modern Biotechnology as an Integral Supplement to Conventional Plant Breeding: The Prospects and Challenges. Crop Sci. 2006, 46, 1841–1859. [Google Scholar] [CrossRef]
- Blanco, A.; Cenci, A.; Simeone, R.; Gadaleta, A. The Cytogenetics and Molecular Characteristics of a Translocated Chromosome 1AS. 1AL-1DL with a Glu-D1 Locus in Durum Wheat. Cell. Mol. Biol. Lett. 2002, 7, 559–568. [Google Scholar] [PubMed]
- Lin, F.; Li, X.; Jia, N.; Feng, F.; Huang, H.; Huang, J.; Fan, S.; Ciais, P.; Song, X.P. The Impact of Russia-Ukraine Conflict on Global Food Security. Glob. Food Sec. 2023, 36, 100661. [Google Scholar] [CrossRef]
- Appels, R.; Eversole, K.; Feuillet, C.; Keller, B.; Rogers, J.; Stein, N.; Pozniak, C.J.; Choulet, F.; Distelfeld, A.; Poland, J.; et al. Shifting the Limits in Wheat Research and Breeding Using a Fully Annotated Reference Genome. Science 2018, 361, 6403. [Google Scholar] [CrossRef]
- Fei, W.; Liu, Y. Biotrophic Fungal Pathogens: A Critical Overview. Appl. Biochem. Biotechnol. 2023, 195, 1–16. [Google Scholar] [CrossRef]
- Barnes, G.; Saunders, D.G.O.; Williamson, T. Banishing Barberry: The History of Berberis Vulgaris Prevalence and Wheat Stem Rust Incidence across Britain. Plant Pathol. 2020, 69, 1193–1202. [Google Scholar] [CrossRef]
- Hafeez, A.N.; Arora, S.; Ghosh, S.; Gilbert, D.; Bowden, R.L.; Wulff, B.B.H. Creation and Judicious Application of a Wheat Resistance Gene Atlas. Mol. Plant 2021, 14, 1053–1070. [Google Scholar] [CrossRef]
- Dracatos, P.M. Resistance That Stacks up: Engineering Rust and Mildew Disease Control in the Cereal Crops Wheat and Barley. Plant Biotechnol. J. 2023, 21, 1938–1951. [Google Scholar] [CrossRef]
- Simón, M.R.; Börner, A.; Struik, P.C. Editorial: Fungal Wheat Diseases: Etiology, Breeding, and Integrated Ma agement. Front. Plant Sci. 2021, 12, 671060. [Google Scholar] [CrossRef]
- Tucker, M.A.; Moffat, C.S.; Ellwood, S.R.; Tan, K.C.; Jayasena, K.; Oliver, R.P. Development of Genetic SSR Markers in Blumeria graminis f. sp. Hordei and Application to Isolates from Australia. Plant Pathol. 2015, 64, 337–343. [Google Scholar] [CrossRef]
- Figueroa, M.; Hammond-Kosack, K.E.; Solomon, P.S. A Review of Wheat Diseases—A Field Perspective. Mol. Plant Pathol. 2018, 19, 1523–1536. [Google Scholar] [CrossRef]
- Lahlali, R.; Ezrari, S.; Radouane, N.; Kenfaoui, J.; Esmaeel, Q.; El Hamss, H.; Belabess, Z.; Barka, E.A. Biological Control of Plant Pathogens: A Global Perspective. Microorganisms 2022, 10, 596. [Google Scholar] [CrossRef] [PubMed]
- Mejri, S.; Siah, A.; Coutte, F.; Magnin-Robert, M.; Randoux, B.; Tisserant, B.; Krier, F.; Jacques, P.; Reignault, P.; Halama, P. Biocontrol of the Wheat Pathogen Zymoseptoria tritici Using Cyclic Lipopeptides from Bacillus subtilis. Environ. Sci. Pollut. Res. 2018, 25, 29822–29833. [Google Scholar] [CrossRef] [PubMed]
- Marrone, P.G. Status and Potential of Bioprotection Products for Crop Protection. In Recent Highlights in the Discovery and Optimization of Crop Protection Products; Elsevier: Amsterdam, The Netherlands, 2021; pp. 25–38. ISBN 9780128210352. [Google Scholar]
- Robert, C.; Bancal, M.O.; Ney, B.; Lannou, C. Wheat Leaf Photosynthesis Loss Due to Leaf Rust, with Respect to Lesion Development and Leaf Nitrogen Status. New Phytol. 2005, 165, 227–241. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.K.M.; Hovmøll, M.S. Aerial Dispersal of Pathogens on the Global and Continental Scales and Its Impact on Plant Disease. Science 2002, 297, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Wan, A.M.; Chen, X.M.; He, Z.H. Wheat Stripe Rust in China. Aust. J. Agric. Res. 2007, 58, 605–619. [Google Scholar] [CrossRef]
- Zhao, X.L.; Zheng, T.C.; Xia, X.C.; He, Z.H.; Liu, D.Q.; Yang, W.X.; Yin, G.H.; Li, Z.F. Molecular Mapping of Leaf Rust Resistance Gene LrZH84 in Chinese Wheat Line Zhou 8425B. Theor. Appl. Genet. 2008, 117, 1069–1075. [Google Scholar] [CrossRef]
- Singh, S.S.; Sharma, J.B.; Baranwal, A.; Ahamed, M.L.; Singh, J.B. Identification of Fast Leaf Ruster Local Wheat for Use in Genetic Analysis. Ann. Plant Prot. Sci. 2004, 12, 448–450. [Google Scholar]
- Wellings, C.R. Global Status of Stripe Rust: A Review of Historical and Current Threats. Euphytica 2011, 179, 129–141. [Google Scholar] [CrossRef]
- Chen, X.M. Epidemiology and Control of Stripe Rust [Puccinia striiformis f. sp. tritici] on Wheat. Can. J. Plant Pathol. 2005, 27, 314–337. [Google Scholar] [CrossRef]
- Oliver, R. Diseases Affecting Wheat and Barley: Powdery Mildew Javier Sánchez-Martín, Salim Bourras and Beat Keller, University of Zürich. In Integrated Disease Management of Wheat and Barley; Burleigh Dodds Science Publishing: Cambridge, UK, 2018; pp. 89–114. [Google Scholar]
- Singh, R.P.; Hodson, D.P.; Jin, Y.; Lagudah, E.S.; Ayliffe, M.A.; Bhavani, S.; Rouse, M.N.; Pretorius, Z.A.; Szabo, L.J.; Huerta-Espino, J.; et al. Emergence and Spread of New Races of Wheat Stem Rust Fungus: Continued Threat to Food Security and Prospects of Genetic Control. Phytopathology 2015, 105, 872–884. [Google Scholar] [CrossRef]
- Thach, T.; Ali, S.; Justesen, A.F.; Rodriguez-Algaba, J.; Hovmøller, M.S. Recovery and Virulence Phenotyping of the Historic “Stubbs Collection” of the Yellow Rust Fungus Puccinia Striiformis from Wheat. Ann. Appl. Biol. 2015, 167, 314–326. [Google Scholar] [CrossRef]
- Wellings, C.R.; Boyd, L.A.; Chen, X.M. Resistance to Stripe Rust in Wheat: Pathogen Biology Driving Resistance Breeding. In Disease Resistance in Wheat; CABI: Wallingford, UK, 2012; pp. 63–83. [Google Scholar] [CrossRef]
- Jin, Y.; Szabo, L.J.; Carson, M. Century-Old Mystery of Puccinia striiformis Life History Solved with the Identification of Berberis as an Alternate Host. Phytopathology 2010, 100, 432–435. [Google Scholar] [CrossRef]
- Moldenhauer, J.; Moerschbacher, B.M.-P.; Van Der Westhuizen, A.J. Histological Investigation of Stripe Rust (Puccinia striiformis f.sp. tritici) Development in Resistant and Susceptible Wheat Cultivars. Plant Pathol. 2006, 55, 469–474. [Google Scholar] [CrossRef]
- Ma, Q.; Shang, H.S. Ultrastructure of Stripe Rust (Puccinia striiformis f. sp. tritici) Interacting with Slow-Rusting, Highly Resistant, and Susceptible Wheat Cultivars. J. Plant Pathol. 2009, 91, 597–606. [Google Scholar]
- Voegele, R.T.; Hahn, M.; Mendgen, K. The Uredinales: Cytology, Biochemistry, and Molecular Biology. In The Mycota; Springer: Berlin/Heidelberg, Germany, 2009; pp. 69–98. [Google Scholar] [CrossRef]
- Hovmøller, M.S.; Sørensen, C.K.; Walter, S.; Justesen, A.F. Diversity of Puccinia striiformis on Cereals and Grasses. Annu. Rev. Phytopathol. 2011, 49, 197–217. [Google Scholar] [CrossRef]
- Sørensen, C.K.; Justesen, A.F.; Hovmøller, M.S. 3-D Imaging of Temporal and Spatial Development of Puccinia striiformis Haustoria in Wheat. Mycologia 2012, 104, 1381–1389. [Google Scholar] [CrossRef]
- Bolton, M.D.; Kolmer, J.A.; Garvin, D.F. Wheat Leaf Rust Caused by Puccinia triticina. Mol. Plant Pathol. 2008, 9, 563–575. [Google Scholar] [CrossRef]
- Roelfs, A.P.; Singh, R.P.; Saari, E.E. Rust Diseases of Wheat: Concepts and Methods of Disease Management; International Maize and Wheat Improvement Center (CIMMYT): México-Veracruz, Mexico, 1992; ISBN 968612747X. [Google Scholar]
- Baka, Z.A.; Larous, L.; Lösel, D.M. Distribution of ATPase Activity at the Host-Pathogen Interfaces of Rust Infections. Physiol. Mol. Plant Pathol. 1995, 47, 67–82. [Google Scholar] [CrossRef]
- Jørgensen, L.N.; Hovmøller, M.S.; Hansen, J.G.; Lassen, P.; Clark, B.; Bayles, R.; Rodemann, B.; Flath, K.; Jahn, M.; Goral, T.; et al. IPM Strategies and Their Dilemmas Including an Introduction to www.eurowheat.org. J. Integr. Agric. 2014, 13, 265–281. [Google Scholar] [CrossRef]
- Chakraborty, S.; Newton, A.C. Climate Change, Plant Diseases and Food Security: An Overview. Plant Pathol. 2011, 60, 2–14. [Google Scholar] [CrossRef]
- El Jarroudi, M.; Giraud, F.; Delfosse, P.; Hoffmann, L.; Maraite, H.; Tychon, B. Time Spray Strategies for Septoria Leaf Blotch Disease Progress on Winter Wheat: The Use of Forecasting Model. Phytopathology 2010, 100, 32. [Google Scholar]
- Lovell, D.J.; Hunter, T.; Powers, S.J.; Parker, S.R.; Van Den Bosch, F. Effect of Temperature on Latent Period of Septoria Leaf Blotch on Winter Wheat under Outdoor Conditions. Plant Pathol. 2004, 53, 170–181. [Google Scholar] [CrossRef]
- Cowger, C.; Hoffer, M.E.; Mundt, C.C. Specific Adaptation by Mycosphaerella graminicola to a Resistant Wheat Cultivar. Plant Pathol. 2000, 49, 445–451. [Google Scholar] [CrossRef]
- Cheval, P.; Siah, A.; Bomble, M.; Popper, A.D.; Reignault, P.; Halama, P. Evolution of QoI Resistance of the Wheat Pathogen Zymoseptoria tritici in Northern France. Crop Prot. 2017, 92, 131–133. [Google Scholar] [CrossRef]
- Siah, A.; Reignault, P.; Halama, P. Genetic diversity of Mycosphaerella graminicola isolates from a single field. Commun. Agric. Appl. Biol. Sci. 2013, 78, 437–442. [Google Scholar] [PubMed]
- El Chartouni, L.; Tisserant, B.; Siah, A.; Duyme, F.; Leducq, J.B.; Deweer, C.; Fichter-Roisin, C.; Sanssene, J.; Durand, R.; Halama, P.; et al. Genetic Diversity and Population Structure in French Populations of Mycosphaerella graminicola. Mycologia 2011, 103, 764–774. [Google Scholar] [CrossRef] [PubMed]
- Matzen, N.; Heick, T.M.; Jørgensen, L.N. Control of Powdery Mildew (Blumeria graminis spp.) in Cereals by Serenade®ASO (Bacillus amyloliquefaciens (Former subtilis) Strain QST 713). Biol. Control 2019, 139, 104067. [Google Scholar] [CrossRef]
- Both, M.; Csukai, M.; Stumpf, M.P.H.; Spanu, P.D. Gene Expression Profiles of Blumeria graminis Indicate Dynamic Changes to Primary Metabolism during Development of an Obligate Biotrophic Pathogen. Plant Cell 2005, 17, 2107–2122. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Riederer, M.; Hildebrandt, U. Very-Long-Chain Aldehydes Induce Appressorium Formation in Ascospores of the Wheat Powdery Mildew Fungus Blumeria graminis. Fungal Biol. 2017, 121, 716–728. [Google Scholar] [CrossRef] [PubMed]
- Jankovics, T.; Komáromi, J.; Fábián, A.; Jäger, K.; Vida, G.; Kiss, L. New Insights into the Life Cycle of the Wheat Powdery Mildew: Direct Observation of Ascosporic Infection in Blumeria graminis f. sp. tritici. Phytopathology 2015, 105, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Duan, X.; Cai, P.; Li, Y.F.; Qiu, Z. Deciphering the Genome of Simplicillium aogashimaense to Understand Its Mechanisms against the Wheat Powdery Mildew Fungus Blumeria graminis f. sp. tritici. Phytopathol. Res. 2022, 4, 16. [Google Scholar] [CrossRef]
- Singh, R.P.; Singh, P.K.; Rutkoski, J.; Hodson, D.P.; He, X.; Jørgensen, L.N.; Hovmøller, M.S.; Huerta-Espino, J. Disease Impact on Wheat Yield Potential and Prospects of Genetic Control. Annu. Rev. Phytopathol. 2016, 54, 303–322. [Google Scholar] [CrossRef] [PubMed]
- Fraaije, B.A.; Butters, J.A.; Coelho, J.M.; Jones, D.R.; Hollomon, D.W. Following the Dynamics of Strobilurin Resistance in Blumeria graminis f.sp. tritici Using Quantitative Allele-Specific Real-Time PCR Measurements with the Fluorescent Dye SYBR Green I. Plant Pathol. 2002, 51, 45–54. [Google Scholar] [CrossRef]
- Moreno, M.; Stenglein, S.A.; Perelló, A.E. Pyrenophora tritici-repentis, causal agent of tan spot: A review of intraspecific genetic diversity. In The Molecular Basis of Plant Genetic Diversity; IntechOpen: London, UK, 2012. [Google Scholar]
- Bertagnolli, V.V.; Ferreira, J.R.; Liu, Z.; Rosa, A.C.; Deuner, C.C. Phenotypical and Genotypical Characterization of Pyrenophora tritici-Repentis Races in Brazil. Eur. J. Plant Pathol. 2019, 154, 995–1007. [Google Scholar] [CrossRef]
- del Pozo, A.; Méndez-Espinoza, A.M.; Castillo, D. Triticale. In Neglected and Underutilized Crops; Academic Press: Cambridge, MA, USA, 2023; pp. 325–362. [Google Scholar] [CrossRef]
- Chaitanya, A.K.; Jamedar, H.V.R.; Shanmugam, A.; Kaniganti, S.; Devi, Y.L.; Kumar, P.G.; Mekala, R.; Pitha, C.C.; Wani, S.H. Advances in QTL Mapping for Biotic Stress Tolerance in Wheat. In QTL Mapping in Crop Improvement; Academic Press: Cambridge, MA, USA, 2023; pp. 119–148. [Google Scholar] [CrossRef]
- Ciuffetti, L.M.; Manning, V.A.; Pandelova, I.; Faris, J.D.; Friesen, T.L.; Strelkov, S.E.; Weber, G.L.; Goodwin, S.B.; Wolpert, T.J.; Figueroa, M. Pyrenophora tritici-repentis: A Plant Pathogenic Fungus with Global Impact. In Genomics of Plant-Associated Fungi: Monocot Pathogens; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–39. [Google Scholar] [CrossRef]
- Asaturova, A.; Zhevnova, N.; Tomashevich, N.; Pavlova, M.; Kremneva, O.; Volkova, G.; Sidorov, N. Efficacy of New Local Bacterial Agents against Pyrenophora tritici-Repentis in Kuban Region, Russia. Agronomy 2022, 12, 373. [Google Scholar] [CrossRef]
- Francl, L.J. Local and Mesodistance Dispersal of Pyrenophora tritici-Repentis Conidia. Can. J. Plant Pathol. 1997, 19, 247–255. [Google Scholar] [CrossRef]
- Lamari, L.; Strelkov, S.E. The Wheat/Pyrenophora tritici-Repentis Interaction: Progress towards an Understanding of Tan Spot Disease. Can. J. Plant Pathol. 2010, 32, 4–10. [Google Scholar] [CrossRef]
- Kokhmetova, A.M.; Kovalenko, N.M.; Kumarbaeva, M.T. Pyrenophora tritici-Repentis Population Structure in the Republic of Kazakhstan and Identification of Wheat Germplasm Resistant to Tan Spot. Vavilovskii Zhurnal Genet. Selektsii 2020, 24, 722–729. [Google Scholar] [CrossRef] [PubMed]
- See, P.T.; Chen, K.; Marathamuthu, K.A.; Wood, B.; Schultz, N.; Shankar, M.; Moffat, C.S. Virulence Assessment of Australian Pyrenophora tritici-Repentis Isolates. Plant Pathol. 2022, 71, 556–565. [Google Scholar] [CrossRef]
- Corsi, B.; Percival-Alwyn, L.; Downie, R.C.; Venturini, L.; Iagallo, E.M.; Campos Mantello, C.; McCormick-Barnes, C.; See, P.T.; Oliver, R.P.; Moffat, C.S.; et al. Genetic Analysis of Wheat Sensitivity to the ToxB Fungal Effector from Pyrenophora tritici-Repentis, the Causal Agent of Tan Spot. Theor. Appl. Genet. 2020, 133, 935–950. [Google Scholar] [CrossRef]
- Gourlie, R.; McDonald, M.; Hafez, M.; Ortega-Polo, R.; Low, K.E.; Abbott, D.W.; Strelkov, S.E.; Daayf, F.; Aboukhaddour, R. The Pangenome of the Wheat Pathogen Pyrenophora tritici-Repentis Reveals Novel Transposons Associated with Necrotrophic Effectors ToxA and ToxB. BMC Biol. 2022, 20, 239. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Kariyawasam, G.; Liu, S.; Leng, Y.; Zhong, S.; Ali, S.; Moolhuijzen, P.; Moffat, C.S.; Rasmussen, J.B.; Friesen, T.L.; et al. A Conserved Hypothetical Gene Is Required but Not Sufficient for Ptr ToxC Production in Pyrenophora tritici-Repentis. Mol. Plant. Microbe. Interact. 2022, 35, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Devi, H.M.; Mahapatra, S.; Das, S. Assessment of Yield Loss of Wheat Caused by Spot Blotch Using Regression Model. Indian Phytopathol. 2018, 71, 291–294. [Google Scholar] [CrossRef]
- Gultyaeva, E.I.; Kovalenko, N.M.; Shamanin, V.P.; Tyunin, V.A.; Shreyder, E.R.; Shaydayuk, E.L.; Morgunov, A.I. Population Structure of Leaf Pathogens of Common Spring Wheat in the West Asian Regions of Russia and North Kazakhstan in 2017. Вавилoвский журнал генетики и селекции 2018, 22, 363–369. [Google Scholar] [CrossRef]
- Gupta, P.K.; Vasistha, N.K.; Aggarwal, R.; Joshi, A.K. Biology of B. sorokiniana (Syn. Cochliobolus sativus) in Genomics Era. J. Plant Biochem. Biotechnol. 2018, 27, 123–138. [Google Scholar] [CrossRef]
- Gupta, P.K.; Chand, R.; Vasistha, N.K.; Pandey, S.P.; Kumar, U.; Mishra, V.K.; Joshi, A.K. Spot Blotch Disease of Wheat: The Current Status of Research on Genetics and Breeding. Plant Pathol. 2018, 67, 508–531. [Google Scholar] [CrossRef]
- Tembo, B.; Sibiya, J.; Tongoona, P. Genetic Variability among Wheat (Triticum aestivum L.) Germplasm for Resistance to Spot Blotch Disease. J. Agric. Rural Dev. Trop. Subtrop. 2018, 119, 85–93. [Google Scholar]
- Aggarwal, R.; Sharma, S.; Singh, K.; Gurjar, M.S.; Saharan, M.S.; Gupta, S.; Bashyal, B.M.; Gaikwad, K. First Draft Genome Sequence of Wheat Spot Blotch Pathogen Bipolaris sorokiniana BS_112 from India, Obtained Using Hybrid Assembly. Microbiol. Resour. Announc. 2019, 8, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Al-sadi, A.M. Bipolaris sorokiniana -Induced Black Point, Common Root Rot, and Spot Blotch Diseases of Wheat: A Review. Front. Cell. Infect. Microbiol. 2021, 11, 584899. [Google Scholar] [CrossRef]
- Sharma, P.; Gupta, O.P.; Gupta, V.; Singh, G.; Singh, G.P. Differential Expression Profiling of MicroRNAs and Their Target Genes during Wheat-Bipolaris sorokiniana Pathosystem. Physiol. Mol. Biol. Plants 2021, 27, 2567–2577. [Google Scholar] [CrossRef] [PubMed]
- Shoemaker, S.; Spot, C.; Disease, B. Phylogeographic Diversity Analysis of Bipolaris sorokiniana. Genes 2022, 13, 2206. [Google Scholar]
- Al-Sadi, A.M. Variation in Resistance to Spot Blotch and the Aggressiveness of Bipolaris sorokiniana on Barley and Wheat Cultivars. J. Plant Pathol. 2016, 98, 97–103. [Google Scholar] [CrossRef]
- Raguchander, T.; Kulkarni, S.; Hegde, R.K. Studies on Leaf Blight of Triticale Caused by Bipolaris sorokiniana Sacc. Shoem. Anamorph of Cochliobolus sativus Ito and Kurib. Dreschler Ex Dastur. Plant Pathol. News 1990, 6, 43–44. [Google Scholar]
- Langner, T.; Białas, A.; Kamoun, S. The Blast Fungus Decoded: Genomes in Flux. MBio 2018, 9, 10-1128. [Google Scholar] [CrossRef]
- Devanna, B.N.; Jain, P.; Solanke, A.U.; Das, A.; Thakur, S.; Singh, P.K.; Kumari, M.; Dubey, H.; Jaswal, R.; Pawar, D.; et al. Understanding the Dynamics of Blast Resistance in Rice-Magnaporthe oryzae Interactions. J. Fungi 2022, 8, 584. [Google Scholar] [CrossRef]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The Global Burden of Pathogens and Pests on Major Food Crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef]
- Eseola, A.B.; Ryder, L.S.; Osés-Ruiz, M.; Findlay, K.; Yan, X.; Cruz-Mireles, N.; Molinari, C.; Garduño-Rosales, M.; Talbot, N.J. Investigating the Cell and Developmental Biology of Plant Infection by the Rice Blast Fungus Magnaporthe oryzae. Fungal Genet. Biol. 2021, 154, 103562. [Google Scholar] [CrossRef]
- Barnwal, M.K.; Kotasthane, A.; Magculia, N.; Mukherjee, P.K.; Savary, S.; Sharma, A.K.; Singh, H.B.; Singh, U.S.; Sparks, A.H.; Variar, M.; et al. A Review on Crop Losses, Epidemiology and Disease Management of Rice Brown Spot to Identify Research Priorities and Knowledge Gaps. Eur. J. Plant Pathol. 2013, 136, 443–457. [Google Scholar] [CrossRef]
- Ahmadpour, A.; Castell-Miller, C.; Javan-Nikkhah, M.; Naghavi, M.R.; Dehkaei, F.P.; Leng, Y.; Puri, K.D.; Zhong, S. Population Structure, Genetic Diversity, and Sexual State of the Rice Brown Spot Pathogen Bipolaris Oryzae from Three Asian Countries. Plant Pathol. 2018, 67, 181–192. [Google Scholar] [CrossRef]
- Abrol, S.; Singh, S.; Mehta, A.; Ahanger, S.A.; Basu, U.; Vaid, A.; Singh, R.; Singh, A.; Singh, V. Distribution Pattern and Prevalence of Brown Spot of Rice (Bipolaris oryzae) in Jammu Region. Pharma Innov. J. 2022, 11, 732–736. [Google Scholar]
- Leonard, K.J.; Suggs, E.G. Setosphaeria Prolata, The Ascigerous State of Exserohilum Prolatum. Mycologia 1974, 66, 281–297. [Google Scholar] [CrossRef]
- Kotze, R.G.; van der Merwe, C.F.; Crampton, B.G.; Kritzinger, Q. A Histological Assessment of the Infection Strategy of Exserohilum turcicum in Maize. Plant Pathol. 2019, 68, 504–512. [Google Scholar] [CrossRef]
- Chung, C.L.; Longfellow, J.M.; Walsh, E.K.; Kerdieh, Z.; Van Esbroeck, G.; Balint-Kurti, P.; Nelson, R.J. Resistance Loci Affecting Distinct Stages of Fungal Pathogenesis: Use of Introgression Lines for QTL Mapping and Characterization in the Maize—Setosphaeria turcica Pathosystem. BMC Plant Biol. 2010, 10, 103. [Google Scholar] [CrossRef] [PubMed]
- Weems, J.D.; Bradley, C.A. Exserohilum turcicum Race Population Distribution in the North Central United States. Plant Dis. 2018, 102, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Wise, K. Diseases of Corn: Northern Corn Leaf Blight. Purdue Ext. 2011, 6, 1–3. [Google Scholar]
- Zhu, X.; Kebede, A.; Woldemariam, T.; Wu, J.; Jindal, K.K.; Reid, L.M. Resistance Breeding to Northern Corn Leaf Blight with Dominant Genes, Polygene, and Their Combinations—Effects to Yield Traits. Agronomy 2023, 13, 1269. [Google Scholar] [CrossRef]
- Pataky, J.K.; Raid, R.N.; Du Toit, L.J.; Schueneman, T.J. Disease Severity and Yield of Sweet Corn Hybrids with Resistance to Northern Leaf Blight. Plant Dis. 1998, 82, 57–63. [Google Scholar] [CrossRef]
- Langenhoven, B.; Murray, S.L.; Crampton, B.G. Quantitative Detection of Exserohilum turcicum in Northern Leaf Blight Diseased Sorghum and Maize Leaves. Australas. Plant Pathol. 2020, 49, 609–617. [Google Scholar] [CrossRef]
- Hooda, K.S.; Bagaria, P.K.; Khokhar, M.; Kaur, H.; Rakshit, S. Mass Screening Techniques for Resistance to Maize Diseases; ICAR-ICAR Indian Institute of Maize Research: Ludhiana, India, 2018; Volume 1004, 14p.
- Elsharkawy, M.M.; Omara, R.I.; Mostafa, Y.S.; Alamri, S.A.; Hashem, M.; Alrumman, S.A.; Ahmad, A.A. Mechanism of Wheat Leaf Rust Control Using Chitosan Nanoparticles and Salicylic Acid. J. Fungi 2022, 8, 304. [Google Scholar] [CrossRef]
- Wang, M.; Wang, S.; Ma, J.; Yu, C.; Gao, J.; Chen, J. Detection of Cochliobolus heterostrophus Races in South China. J. Phytopathol. 2017, 165, 681–691. [Google Scholar] [CrossRef]
- Turgeon, B.G.; Baker, S.E. Genetic and Genomic Dissection of the Cochliobolus heterostrophus Tox1 Locus Controlling Biosynthesis of the Polyketide Virulence Factor T-Toxin. Adv. Genet. 2007, 57, 219–261. [Google Scholar] [CrossRef] [PubMed]
- Vasmatkar, P.; Kaur, K.; Pannu, P.P.S. Field Based Assessment of Yield-Related Traits and Flowering Response in Zea mays towards Southern Corn Leaf Blight. Indian Phytopathol. 2021, 74, 969–979. [Google Scholar] [CrossRef]
- Kutawa, A.B.; Ahmad, K.; Ali, A.; Hussein, M.Z.; Wahab, M.A.A.; Sijam, K. State of the Art on Southern Corn Leaf Blight Disease Incited by Cochliobolus heterostrophus: Detection, Pathogenic Variability and Novel Control Measures. Bulg. J. Agric. Sci. 2021, 27, 147–155. [Google Scholar]
- Ye, Y.F.; Li, Q.Q.; Fu, G.; Yuan, G.Q.; Miao, J.H.; Lin, W. Identification of Antifungal Substance (Iturin A2) Produced by Bacillus subtilis B47 and Its Effect on Southern Corn Leaf Blight. J. Integr. Agric. 2012, 11, 90–99. [Google Scholar] [CrossRef]
- Lucas, G.B.; Campbell, C.L.; Lucas, L.T. Diseases Caused by Airborne Fungi. In Introduction to Plant Diseases; Springer: Boston, MA, USA, 1992; pp. 192–242. [Google Scholar] [CrossRef]
- Naz, I. Optimization of Cultural Conditions for Cochliobolus heterostrophus Isolates from Infected Maize Plants from Different Agricultural Zones of Pakistan. Br. Microbiol. Res. J. 2012, 2, 233–242. [Google Scholar] [CrossRef]
- Cheng, Z.; Lv, X.; Duan, C.; Zhu, H.; Wang, J.; Xu, Z.; Yin, H.; Zhou, X.; Li, M.; Hao, Z.; et al. Pathogenicity Variation in Two Genomes of Cercospora Species Causing Gray Leaf Spot in Maize. Mol. Plant-Microbe Interact. 2023, 36, 14–25. [Google Scholar] [CrossRef]
- Ranum, P.; Peña-Rosas, J.P.; Garcia-Casal, M.N. Global Maize Production, Utilization, and Consumption. Ann. N. Y. Acad. Sci. 2014, 1312, 105–112. [Google Scholar] [CrossRef]
- Sun, H.; Zhai, L.; Teng, F.; Li, Z.; Zhang, Z. QRgls1.06, a Major QTL Conferring Resistance to Gray Leaf Spot Disease in Maize. Crop J. 2021, 9, 342–350. [Google Scholar] [CrossRef]
- Rehman, F.U.; Adnan, M.; Kalsoom, M.; Naz, N.; Husnain, M.G.; Ilahi, H.; Ilyas, M.A.; Yousaf, G.; Tahir, R.; Ahmad, U. Seed-Borne Fungal Diseases of Maize (Zea mays L.): A Review. Agrinula J. Agroteknologi Perkeb. 2021, 4, 43–60. [Google Scholar] [CrossRef]
- Smith, D.R.; White, D.G. Diseases of Corn. In Corn and Corn Improvement, 3rd ed.; Wiley: New York, NY, USA, 2015; pp. 687–766. [Google Scholar] [CrossRef]
- Yu, Y.; Shi, J.; Li, X.; Liu, J.; Geng, Q.; Shi, H.; Ke, Y.; Sun, Q. Transcriptome Analysis Reveals the Molecular Mechanisms of the Defense Response to Gray Leaf Spot Disease in Maize. BMC Genom. 2018, 19, 742. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Tong, L.; Xu, M.; Zhong, T. Correction to: Genetic Dissection of Maize Disease Resistance and Its Applications in Molecular Breeding (Molecular Breeding, (2021), 41, 5, (32), 10.1007/S11032-021-01219-Y). Mol. Breed. 2021, 41, 58. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, L.; Li, Z.; Zhang, Y.; Kang, M.S.; Wang, Y.; Fan, X. High-Density Mapping for Gray Leaf Spot Resistance Using Two Related Tropical Maize Recombinant Inbred Line Populations. Mol. Biol. Rep. 2021, 48, 3379–3392. [Google Scholar] [CrossRef]
- Carmona, M.; Sautua, F.; Pérez-Hérnandez, O.; Reis, E.M. Role of Fungicide Applications on the Integrated Management of Wheat Stripe Rust. Front. Plant Sci. 2020, 11, 733. [Google Scholar] [CrossRef]
- Holmes, G. Wheat Leaf Rust (Puccinia recondita). Available online: https://www.ipmimages.org/browse/detail.cfm?imgnum=1573112 (accessed on 2 November 2023).
- Desm, R. ex Zymoseptoria tritici—Wikipedia. Available online: https://en.wikipedia.org/wiki/Zymoseptoria_tritici (accessed on 2 November 2023).
- Cattlin, N. Tan Spot Lesion on Wheat Leaves—Stock Image—C012/4700—Science Photo Library. Available online: https://www.sciencephoto.com/media/448342/view/tan-spot-lesion-on-wheat-leaves (accessed on 2 November 2023).
- Alkan, M.; Bayraktar, H.; İmren, M.; Özdemir, F.; Lahlali, R.; Mokrini, F.; Paulitz, T.; Dababat, A.A.; Özer, G. Monitoring of Host Suitability and Defense-Related Genes in Wheat to Bipolaris sorokiniana. J. Fungi 2022, 8, 149. [Google Scholar] [CrossRef]
- Rice Blast Fungus Discovery Will Drive Crop Innovation. Available online: https://www.oeaw.ac.at/de/gmi/detail/news/rice-blast-fungus-discovery-will-drive-crop-innovation (accessed on 2 November 2023).
- Ashfaq, B. Brown Spot—IRRI Rice Knowledge Bank. Available online: http://www.knowledgebank.irri.org/training/fact-sheets/pest-management/diseases/item/brown-spot (accessed on 2 November 2023).
- Muller, D. Crop Protection Network. Available online: https://cropprotectionnetwork.org/encyclopedia/northern-corn-leaf-blight-of-corn (accessed on 2 November 2023).
- CIMMYT Maydis Leaf Blight on Maize|Maize Leaf Showing Lesions Cau…|Flickr. Available online: https://www.flickr.com/photos/cimmyt/4886128233 (accessed on 2 November 2023).
- Isaacs, J. Northern Corn Leaf Blight, Grey Leaf Spot Top Ontario Corn Diseases—Top Crop ManagerTop Crop Manager. Available online: https://www.topcropmanager.com/northern-corn-leaf-blight-grey-leaf-spot-top-ontario-corn-diseases-19935/ (accessed on 2 November 2023).
- Jørgensen, L.N.; Nielsen, B.J. Control of Yellow Rust (Puccinia striiformis) on Winter Wheat by Ergosterol Inhibitors at Full and Reduced Dosages. Crop Prot. 1994, 13, 323–330. [Google Scholar] [CrossRef]
- Peng, F.; Si, M.; Zizhu, Y.; Fu, Y.; Yang, Y.; Yu, Y.; Bi, C. Rapid Quantification of Fungicide Effectiveness on Inhibiting Wheat Stripe Rust Pathogen (Puccinia striiformis f. sp. tritici). Plant Dis. 2020, 104, 2434–2439. [Google Scholar] [CrossRef]
- Chen, W.; Wellings, C.; Chen, X.; Kang, Z.; Liu, T. Wheat Stripe (Yellow) Rust Caused by Puccinia striiformis f. sp. tritici. Mol. Plant Pathol. 2014, 15, 433–446. [Google Scholar] [CrossRef]
- Chen, X.M. Challenges and Solutions for Stripe Rust Control in the United States. Aust. J. Agric. Res. 2007, 58, 648–655. [Google Scholar] [CrossRef]
- Silva, T.S.; da Fonseca, L.F.; Yamada, J.K.; de Carvalho Pontes, N. Flutriafol and Azoxystrobin: An Efficient Combination to Control Fungal Leaf Diseases in Corn Crops. Crop Prot. 2021, 140, 105394. [Google Scholar] [CrossRef]
- Xin, W.; Mao, Y.; Lu, F.; Li, T.; Wang, J.; Duan, Y.; Zhou, M. In Vitro Fungicidal Activity and in Planta Control Efficacy of Coumoxystrobin against Magnaporthe oryzae. Pestic. Biochem. Physiol. 2020, 162, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Oliver, R.P. A Reassessment of the Risk of Rust Fungi Developing Resistance to Fungicides. Pest Manag. Sci. 2014, 70, 1641–1645. [Google Scholar] [CrossRef] [PubMed]
- Zhan, G.; Ji, F.; Zhao, J.; Liu, Y.; Zhou, A.; Xia, M.; Zhang, J.; Huang, L.; Guo, J.; Kang, Z. Sensitivity and Resistance Risk Assessment of Puccinia striiformis f. sp. tritici to Triadimefon in China. Plant Dis. 2022, 106, 1690–1699. [Google Scholar] [CrossRef] [PubMed]
- Ravensberg, W.J. Commercialisation of Microbes: Present Situation and Future Prospects. In Principles of Plant-Microbe Interactions: Microbes for Sustainable Agriculture; Springer International Publishing: Cham, Switzerland, 2015; pp. 309–317. [Google Scholar] [CrossRef]
- Singh, D.P.; Gupta, V.K.; Prabha, R. Microbial Interventions in Agriculture and Environment: Volume 1: Research Trends, Priorities and Prospects; Springer: Singapore, 2019; ISBN 9789811383915. [Google Scholar]
- Dutilloy, E.; Oni, F.E.; Esmaeel, Q.; Clément, C.; Barka, E.A. Plant Beneficial Bacteria as Bioprotectants against Wheat and Barley Diseases. J. Fungi 2022, 8, 632. [Google Scholar] [CrossRef] [PubMed]
- Fedele, G.; Bove, F.; González-Domínguez, E.; Rossi, V. A Generic Model Accounting for the Interactions among Pathogens, Host Plants, Biocontrol Agents, and the Environment, with Parametrization for Botrytis cinerea on Grapevines. Agronomy 2019, 10, 222. [Google Scholar] [CrossRef]
- Bardin, M.; Ajouz, S.; Comby, M.; Lopez-Ferber, M.; Graillot, B.; Siegwart, M.; Nicot, P.C. Is the Efficacy of Biological Control against Plant Diseases Likely to Be More Durable than That of Chemical Pesticides? Front. Plant Sci. 2015, 6, 566. [Google Scholar] [CrossRef]
- Kempler, C.; Muehlchen, A.M.; Forge, T.A. Screening for resistance to phytophthora root rot in raspberries: Identifying new sources of resistance. Acta Hortic. 2012, 59–64. [Google Scholar] [CrossRef]
- Szczech, M.; Shoda, M. The Effect of Mode of Application of Bacillus subtilis RB14-C on Its Efficacy as a Biocontrol Agent against Rhizoctonia Solani. J. Phytopathol. 2006, 154, 370–377. [Google Scholar] [CrossRef]
- Pérez-Montaño, F.; Alías-Villegas, C.; Bellogín, R.A.; Del Cerro, P.; Espuny, M.R.; Jiménez-Guerrero, I.; López-Baena, F.J.; Ollero, F.J.; Cubo, T. Plant Growth Promotion in Cereal and Leguminous Agricultural Important Plants: From Microorganism Capacities to Crop Production. Microbiol. Res. 2014, 169, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Ongena, M.; Jacques, P. Bacillus Lipopeptides: Versatile Weapons for Plant Disease Biocontrol. Trends Microbiol. 2008, 16, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Raaijmakers, J.M.; de Bruijn, I.; Nybroe, O.; Ongena, M. Natural Functions of Lipopeptides from Bacillus and Pseudomonas: More than Surfactants and Antibiotics. FEMS Microbiol. Rev. 2010, 34, 1037–1062. [Google Scholar] [CrossRef]
- Platel, R.; Sawicki, M.; Esmaeel, Q.; Randoux, B.; Trapet, P.; El Guilli, M.; Chtaina, N.; Arnauld, S.; Bricout, A.; Rochex, A.; et al. Isolation and Identification of Lipopeptide-Producing Bacillus velezensis Strains from Wheat Phyllosphere with Antifungal Activity against the Wheat Pathogen Zymoseptoria tritici. Agronomy 2022, 12, 95. [Google Scholar] [CrossRef]
- Qiao, H.; Huang, L.; Kang, Z. Endophytic bacteria isolated from wheat and their antifungal activities to soil-borne disease pathogens. J. Appl. Ecol. 2006, 17, 690–694. [Google Scholar]
- Li, H.; Zhao, J.; Feng, H.; Huang, L.; Kang, Z. Biological Control of Wheat Stripe Rust by an Endophytic Bacillus subtilis Strain E1R-j in Greenhouse and Field Trials. Crop Prot. 2013, 43, 201–206. [Google Scholar] [CrossRef]
- Reiss, A.; Jørgensen, L.N. Biological Control of Yellow Rust of Wheat (Puccinia striiformis) with Serenade®ASO (Bacillus subtilis Strain QST713). Crop Prot. 2017, 93, 1–8. [Google Scholar] [CrossRef]
- Rytter, J.L. Biological Control of Geranium Rust by Bacillus subtilis. Phytopathology 1989, 79, 367. [Google Scholar] [CrossRef]
- Ongena, M.; Jourdan, E.; Adam, A.; Paquot, M.; Brans, A.; Joris, B.; Arpigny, J.L.; Thonart, P. Surfactin and Fengycin Lipopeptides of Bacillus subtilis as Elicitors of Induced Systemic Resistance in Plants. Environ. Microbiol. 2007, 9, 1084–1090. [Google Scholar] [CrossRef]
- Choudhary, D.K.; Johri, B.N. Interactions of Bacillus spp. and Plants – With Special Reference to Induced Systemic Resistance (ISR). Microbiol. Res. 2009, 164, 493–513. [Google Scholar] [CrossRef]
- Fischer, S.; Príncipe, A.; Alvarez, F.; Cordero, P.; Castro, M.; Godino, A.; Jofré, E.; Mori, G. Fighting Plant Diseases Through the Application of Bacillus and Pseudomonas Strains. In Symbiotic Endophytes; Springer: Berlin/Heidelberg, Germany, 2013; pp. 165–193. [Google Scholar] [CrossRef]
- Romero, D.; De Vicente, A.; Olmos, J.L.; Dávila, J.C.; Pérez-García, A. Effect of Lipopeptides of Antagonistic Strains of Bacillus subtilis on the Morphology and Ultrastructure of the Cucurbit Fungal Pathogen Podosphaera Fusca. J. Appl. Microbiol. 2007, 103, 969–976. [Google Scholar] [CrossRef]
- Kiani, T.; Mehboob, F.; Hyder, M.Z.; Zainy, Z.; Xu, L.; Huang, L.; Farrakh, S. Control of Stripe Rust of Wheat Using Indigenous Endophytic Bacteria at Seedling and Adult Plant Stage. Sci. Rep. 2021, 11, 14473. [Google Scholar] [CrossRef]
- Pérez-García, A.; Romero, D.; de Vicente, A. Plant Protection and Growth Stimulation by Microorganisms: Biotechnological Applications of Bacilli in Agriculture. Curr. Opin. Biotechnol. 2011, 22, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Mejri, S.; Ghinet, A.; Magnin-Robert, M.; Randoux, B.; Abuhaie, C.M.; Tisserant, B.; Gautret, P.; Rigo, B.; Halama, P.; Reignault, P.; et al. New Plant Immunity Elicitors from a Sugar Beet Byproduct Protect Wheat against Zymoseptoria tritici. Sci. Rep. 2023, 13, 90. [Google Scholar] [CrossRef] [PubMed]
- Le Mire, G.; Siah, A.; Brisset, M.N.; Gaucher, M.; Deleu, M.; Jijakli, M.H. Surfactin Protects Wheat against Zymoseptoria tritici and Activates Both Salicylic Acid- and Jasmonic Acid-Dependent Defense Responses. Agriculture 2018, 8, 11. [Google Scholar] [CrossRef]
- Villa-Rodríguez, E.; Parra-Cota, F.; Castro-Longoria, E.; López-Cervantes, J.; de los Santos-Villalobos, S. Bacillus subtilis TE3: A Promising Biological Control Agent against Bipolaris sorokiniana, the Causal Agent of Spot Blotch in Wheat (Triticum turgidum L. subsp. durum). Biol. Control 2019, 132, 135–143. [Google Scholar] [CrossRef]
- Yi, Y.J.; Yin, Y.N.; Yang, Y.A.; Liang, Y.Q.; Shan, Y.T.; Zhang, C.F.; Zhang, Y.R.; Liang, Z.P. Antagonistic Activity and Mechanism of Bacillus subtilis XZ16-1 Suppression of Wheat Powdery Mildew and Growth Promotion of Wheat. Phytopathology 2022, 112, 2476–2485. [Google Scholar] [CrossRef] [PubMed]
- Rong, S.; Xu, H.; Li, L.; Chen, R.; Gao, X.; Xu, Z. Antifungal Activity of Endophytic Bacillus Safensis B21 and Its Potential Application as a Biopesticide to Control Rice Blast. Pestic. Biochem. Physiol. 2020, 162, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Shan, H.; Zhao, M.; Chen, D.; Cheng, J.; Li, J.; Feng, Z.; Ma, Z.; An, D. Biocontrol of Rice Blast by the Phenaminomethylacetic Acid Producer of Bacillus methylotrophicus Strain BC79. Crop Prot. 2013, 44, 29–37. [Google Scholar] [CrossRef]
- He, Y.; Zhu, M.; Huang, J.; Hsiang, T.; Zheng, L. Biocontrol Potential of a Bacillus subtilis Strain BJ-1 against the Rice Blast Fungus Magnaporthe oryzae. Can. J. Plant Pathol. 2019, 41, 47–59. [Google Scholar] [CrossRef]
- Zhou, H.; Zhu, H.; Ren, Z.; Li, X.; Zhong, J.; Liu, E. Efficacy of Bacillus Tequilensis Strain JN-369 to Biocontrol of Rice Blast and Enhance Rice Growth. Biol. Control 2021, 160, 104652. [Google Scholar] [CrossRef]
- Zhou, H.; Ren, Z.H.; Zu, X.; Yu, X.Y.; Zhu, H.J.; Li, X.J.; Zhong, J.; Liu, E.M. Efficacy of Plant Growth-Promoting Bacteria Bacillus Cereus YN917 for Biocontrol of Rice Blast. Front. Microbiol. 2021, 12, 684888. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhao, L.; Dong, Y.; Chen, W.; Li, C.; Gao, X.; Chen, R.; Li, L.; Xu, Z. The Antagonistic Mechanism of Bacillus Velezensis ZW10 against Rice Blast Disease: Evaluation of ZW10 as a Potential Biopesticide. PLoS ONE 2021, 16, e0256807. [Google Scholar] [CrossRef]
- Arseneault, T.; Filion, M. Phenazine-Producing Pseudomonas spp. as Biocontrol Agents of Plant Pathogens. In Microbial Inoculants in Sustainable Agricultural Productivity: Vol. 2: Functional Applications; Springer: Berlin/Heidelberg, Germany, 2016; pp. 53–68. [Google Scholar] [CrossRef]
- Mishra, J.; Arora, N.K. Secondary Metabolites of Fluorescent Pseudomonads in Biocontrol of Phytopathogens for Sustainable Agriculture. Appl. Soil Ecol. 2018, 125, 35–45. [Google Scholar] [CrossRef]
- Flaishman, M.A.; Eyal, Z.; Zilberstein, A.; Voisard, C.; Haas, D. Suppression of Septoria tritici Blotch and Leaf Rust of Wheat by Recombinant Cyanide-Producing Strains of Peudomonas putida. Mol. Plant-Microbe Interact. 1996, 9, 642–645. [Google Scholar] [CrossRef]
- Levy, E.; Gough, F.J.; Berlin, K.D.; Guiana, P.W.; Smith, J.T. Inhibition of Septoria Tritici and Other Phytopathogenic Fungi and Bacteria by Pseudomonas Fluorescens and Its Antibiotics. Plant Pathol. 1992, 41, 335–341. [Google Scholar] [CrossRef]
- Levy, E.; Eyal, Z.; Carmely, S.; Kashman, Y.; Chet, I. Suppression of Septoria tritici and Puccinia recondita of Wheat by an Antibiotic-Producing Fluorescent Pseudomonad. Plant Pathol. 1989, 38, 564–570. [Google Scholar] [CrossRef]
- Levy, E.; Eyal, Z.; Chet, I.; Hochman, A. Resistance Mechanisms of Septoria tritici to Antifungal Products of Pseudomonas. Physiol. Mol. Plant Pathol. 1992, 40, 163–171. [Google Scholar] [CrossRef]
- Han, X.; Wang, J.; Liu, L.; Shen, F.; Meng, Q.; Li, X.; Li, Y.; Liu, D. Correction for Han et al., “Identification and Predictions Regarding the Biosynthesis Pathway of Polyene Macrolides Produced by Streptomyces roseoflavus Men-Myco-93-63”. Appl. Environ. Microbiol. 2021, 87, e0080221. [Google Scholar] [CrossRef]
- Jia, R.; Xiao, K.; Yu, L.; Chen, J.; Hu, L.; Wang, Y. A Potential Biocontrol Agent Streptomyces Tauricus XF for Managing Wheat Stripe Rust. Phytopathol. Res. 2023, 5, 14. [Google Scholar] [CrossRef]
- Zhang, H.; He, M.; Fan, X.; Dai, L.; Zhang, S.; Hu, Z.; Wang, N. Isolation, Identification and Hyperparasitism of a Novel Cladosporium cladosporioides Isolate Hyperparasitic to Puccinia striiformis f. sp. tritici, the Wheat Stripe Rust Pathogen. Biology 2022, 11, 892. [Google Scholar] [CrossRef]
- Dennis, C.; Webster, J. Antagonistic Properties of Species-Groups of Trichoderma. Trans. Br. Mycol. Soc. 1971, 57, 41-IN4. [Google Scholar] [CrossRef]
- Harman, G.E. Myths and Dogmas of Biocontrol: Changes in Perceptions Derived from Research on Trichoderma Harzianum T-22. Plant Dis. 2000, 84, 377–393. [Google Scholar] [CrossRef]
- Perelló, A.; Mónaco, C.; Simón, M.; Sisterna, M.; Bello, G.D. Biocontrol Efficacy of Trichoderma Isolates for Tan Spot of Wheat in Argentina. Crop Prot. 2003, 22, 1099–1106. [Google Scholar] [CrossRef]
- Howell, C.R. Mechanisms Employed by Trichoderma Species in the Biological Control of Plant Diseases: The History and Evolution of Current Concepts. Plant Dis. 2003, 87, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Brunner, K.; Zeilinger, S.; Ciliento, R.; Woo, S.L.; Lorito, M.; Kubicek, C.P.; Mach, R.L. Improvement of the Fungal Biocontrol Agent Trichoderma Atroviride to Enhance Both Antagonism and Induction of Plant Systemic Disease Resistance. Appl. Environ. Microbiol. 2005, 71, 3959–3965. [Google Scholar] [CrossRef]
- Lynch, K.M.; Zannini, E.; Guo, J.; Axel, C.; Arendt, E.K.; Kildea, S.; Coffey, A. Control of Zymoseptoria tritici Cause of Septoria tritici Blotch of Wheat Using Antifungal Lactobacillus Strains. J. Appl. Microbiol. 2016, 121, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Villa-Rodriguez, E.; Lugo-Enríquez, C.; Ferguson, S.; Parra-Cota, F.I.; Cira-Chávez, L.A.; de los Santos-Villalobos, S. Trichoderma harzianum Sensu Lato TSM39: A Wheat Microbiome Fungus That Mitigates Spot Blotch Disease of Wheat (Triticum turgidum L. subsp. durum) Caused by Bipolaris sorokiniana. Biol. Control 2022, 175, 105055. [Google Scholar] [CrossRef]
- Wang, S.; Ma, J.; Wang, M.; Wang, X.; Li, Y.; Chen, J. Combined Application of Trichoderma harzianum SH2303 and Difenoconazole-Propiconazolein Controlling Southern Corn Leaf Blight Disease Caused by Cochliobolus heterostrophus in Maize. J. Integr. Agric. 2019, 18, 2063–2071. [Google Scholar] [CrossRef]
- Limdolthamand, S.; Songkumarn, P.; Suwannarat, S.; Jantasorn, A.; Dethoup, T. Biocontrol Efficacy of Endophytic Trichoderma spp. in Fresh and Dry Powder Formulations in Controlling Northern Corn Leaf Blight in Sweet Corn. Biol. Control 2023, 181, 105217. [Google Scholar] [CrossRef]
- Kildea, S.; Ransbotyn, V.; Khan, M.R.; Fagan, B.; Leonard, G.; Mullins, E.; Doohan, F.M. Bacillus megaterium Shows Potential for the Biocontrol of Septoria tritici Blotch of Wheat. Biol. Control 2008, 47, 37–45. [Google Scholar] [CrossRef]
- El-Sharkawy, H.H.A.; Rashad, Y.M.; Elazab, N.T. Biocontrol Potential of the Endophytic Epicoccum nigrum HE20 against Stripe Rust of Wheat. Pestic. Biochem. Physiol. 2023, 194, 105517. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, M.; Mahmud, N.U.; Ullah, C.; Rahman, M.; Islam, T. Biological and Biorational Management of Blast Diseases in Cereals Caused by Magnaporthe oryzae. Crit. Rev. Biotechnol. 2021, 41, 994–1022. [Google Scholar] [CrossRef] [PubMed]
- Hardoim, P.R.; de Carvalho, T.L.G.; Ballesteros, H.G.F.; Bellieny-Rabelo, D.; Rojas, C.A.; Venancio, T.M.; Ferreira, P.C.G.; Hemerly, A.S. Genome-Wide Transcriptome Profiling Provides Insights into the Responses of Maize (Zea mays L.) to Diazotrophic Bacteria. Plant Soil 2020, 451, 121–143. [Google Scholar] [CrossRef]
- Choudhary, D.K.; Prakash, A.; Johri, B.N. Induced Systemic Resistance (ISR) in Plants: Mechanism of Action. Indian J. Microbiol. 2007, 47, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, D.K.; Sharma, K.P.; Gaur, R.K. Biotechnological Perspectives of Microbes in Agro-Ecosystems. Biotechnol. Lett. 2011, 33, 1905–1910. [Google Scholar] [CrossRef]
- Kamel, M.; Borah, R.; Bora, H.; Jaiswal, A.K.; Singh, R.K.; Kumar, P. Systemic Acquired Resistance (SAR) and Induced Systemic Resistance (ISR): Role and Mechanism of Action Against Phytopathogens. In Fungal Biotechnology and Bioengineering; Springer: Cham, Switzerland, 2020; pp. 457–470. [Google Scholar]
- Kumari, P.; Singh, A.; Kharwar, R.N. Phytostimulation and ISR Responses of Fungi. In Fungi Bio-Prospects in Sustainable Agriculture, Envronment and Nano-Technology; Academic Press: Cambridge, MA, USA, 2021; pp. 459–473. [Google Scholar] [CrossRef]
- Kaur, H.; Kohli, S.K.; Bhardwaj, R. Nitric Oxide. In Nitric Oxide in Plants: A Molecule with Dual Roles; Wiley: New York, NY, USA, 2022; pp. 248–264. [Google Scholar] [CrossRef]
- Mustafa, G.; Khong, N.G.; Tisserant, B.; Randoux, B.; Fontaine, J.; Magnin-Robert, M.; Reignault, P.; Sahraoui, A.L.H. Defence Mechanisms Associated with Mycorrhiza-Induced Resistance in Wheat against Powdery Milde. Funct. Plant Biol. 2017, 44, 443–454. [Google Scholar] [CrossRef]
- Xie, D.; Cai, X.; Yang, C.; Xie, L.; Qin, G.; Zhang, M.; Huang, Y.; Gong, G.; Chang, X.; Chen, H. Studies on the Control Effect of Bacillus subtilis on Wheat Powdery Mildew. Pest Manag. Sci. 2021, 77, 4375–4382. [Google Scholar] [CrossRef]
- Bennett, J.S.; Isakeit, T.; Borrego, E.J.; Odvody, G.; Murray, S.; Kolomiets, M.V. Identification of Naturally Occurring Atoxigenic Strains of Fusarium verticillioides and Their Potential as Biocontrol Agents of Mycotoxins and Ear Rot Pathogens of Maize. Crop Prot. 2023, 167, 106197. [Google Scholar] [CrossRef]
- Savita; Sharma, A. Fungi as Biological Control Agents. In Biofertilizers for Sustainable Agriculture and Environment; Springer: Cham, Switzerland, 2019; pp. 395–411. [Google Scholar] [CrossRef]
- Bale, J.S.; Van Lenteren, J.C.; Bigler, F. Biological Control and Sustainable Food Production. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 761–776. [Google Scholar] [CrossRef]
- Colnot, T.; Dekant, W. Approaches for Grouping of Pesticides into Cumulative Assessment Groups for Risk Assessment of Pesticide Residues in Food. Regul. Toxicol. Pharmacol. 2017, 83, 89–99. [Google Scholar] [CrossRef]
- Anderson, S.E.; Meade, B.J. Potential Health Effects Associated with Dermal Exposure to Occupational Chemicals. Environ. Health Insights 2014, 8, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Usta, C. Microorganisms in Biological Pest Control—A Review (Bacterial Toxin Application and Effect of Environmental Factors). In Current Progress in Biological Research; IntechOpen: London, UK, 2013. [Google Scholar] [CrossRef]
- Siegwart, M.; Graillot, B.; Lopez, C.B.; Besse, S.; Bardin, M.; Nicot, P.C.; Lopez-Ferber, M. Resistance to Bio-Insecticides or How to Enhance Their Sustainability: A Review. Front. Plant Sci. 2015, 6, 381. [Google Scholar] [CrossRef] [PubMed]
- Hernandez Nopsa, J.F.; Thomas-Sharma, S.; Garrett, K.A. Climate Change and Plant Disease. Encycl. Agric. Food Syst. 2014, 2, 232–243. [Google Scholar] [CrossRef]
- Wade, M.R.; Zalucki, M.P.; Wratten, S.D.; Robinson, K.A. Conservation Biological Control of Arthropods Using Artificial Food Sprays: Current Status and Future Challenges. Biol. Control 2008, 45, 185–199. [Google Scholar] [CrossRef]
- Dutta, P.; Das, B.C. Management of Collar Rot of Tomato by Trichoderma spp. and Chemicals. Indian Phytopath 2002, 55, 235–237. [Google Scholar]
- Basumatary, M.; Dutta, B.K.; Singha, D.M.; Das, N. Some in Vitro Observations on the Biological Control of Sclerotium rolfsii, a Serious Pathogen of Various Agricultural Crop Plants. IOSR J. Agric. Vet. Sci. Ver. II 2015, 8, 2319–2372. [Google Scholar] [CrossRef]
- Parikh, K.; Jha, A. Biocontrol Features in an Indigenous Bacterial Strain Isolated from Agricultural Soil of Gujarat, India. J. Soil Sci. Plant Nutr. 2012, 12, 245–252. [Google Scholar] [CrossRef]
- Bhuiyan, S.A.; Stringer, J.K.; Croft, B.J.; Olayemi, M.E. Resistance of Sugarcane Varieties to Smut (Sporisorium Scitamineum), Development over Crop Classes, and Impact on Yield. Crop Pasture Sci. 2022, 73, 1180–1187. [Google Scholar] [CrossRef]
- Duveiller, E.; Singh, R.P.; Nicol, J.M. The Challenges of Maintaining Wheat Productivity: Pests, Diseases, and Potential Epidemics. Euphytica 2007, 157, 417–430. [Google Scholar] [CrossRef]
- Droby, S.; Wisniewski, M.; Teixidó, N.; Spadaro, D.; Jijakli, M.H. The Science, Development, and Commercialization of Postharvest Biocontrol Products. Postharvest Biol. Technol. 2016, 122, 22–29. [Google Scholar] [CrossRef]
- Yao, H.J.; Tian, S.P. Effects of a Biocontrol Agent and Methyl Jasmonate on Postharvest Diseases of Peach Fruit and the Possible Mechanisms Involved. J. Appl. Microbiol. 2005, 98, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Rane, N.R.; Tapase, S.; Kanojia, A.; Watharkar, A.; Salama, E.S.; Jang, M.; Kumar Yadav, K.; Amin, M.A.; Cabral-Pinto, M.M.S.; Jadhav, J.P.; et al. Molecular Insights into Plant–Microbe Interactions for Sustainable Remediation of Contaminated Environment. Bioresour. Technol. 2022, 344, 126246. [Google Scholar] [CrossRef] [PubMed]
- Spadaro, D.; Droby, S. Development of Biocontrol Products for Postharvest Diseases of Fruit: The Importance of Elucidating the Mechanisms of Action of Yeast Antagonists. Trends Food Sci. Technol. 2016, 47, 39–49. [Google Scholar] [CrossRef]
- Antle, J.M.; Ray, S. Sustainable Agricultural Development; Springer: Dordrecht, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Bejarano, A.; Puopolo, G. Bioformulation of Microbial Biocontrol Agents for a Sustainable Agriculture. Prog. Biol. Control 2020, 21, 275–293. [Google Scholar] [CrossRef]
- Abd-Elsalam, K.A.; Mohamed, H.I. Cereal Diseases: Nanobiotechnological Approaches for Diagnosis and Management; Springer: Dordrecht, The Netherlands, 2022. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dehbi, I.; Achemrk, O.; Ezzouggari, R.; El Jarroudi, M.; Mokrini, F.; Legrifi, I.; Belabess, Z.; Laasli, S.-E.; Mazouz, H.; Lahlali, R. Beneficial Microorganisms as Bioprotectants against Foliar Diseases of Cereals: A Review. Plants 2023, 12, 4162. https://doi.org/10.3390/plants12244162
Dehbi I, Achemrk O, Ezzouggari R, El Jarroudi M, Mokrini F, Legrifi I, Belabess Z, Laasli S-E, Mazouz H, Lahlali R. Beneficial Microorganisms as Bioprotectants against Foliar Diseases of Cereals: A Review. Plants. 2023; 12(24):4162. https://doi.org/10.3390/plants12244162
Chicago/Turabian StyleDehbi, Ilham, Oussama Achemrk, Rachid Ezzouggari, Moussa El Jarroudi, Fouad Mokrini, Ikram Legrifi, Zineb Belabess, Salah-Eddine Laasli, Hamid Mazouz, and Rachid Lahlali. 2023. "Beneficial Microorganisms as Bioprotectants against Foliar Diseases of Cereals: A Review" Plants 12, no. 24: 4162. https://doi.org/10.3390/plants12244162
APA StyleDehbi, I., Achemrk, O., Ezzouggari, R., El Jarroudi, M., Mokrini, F., Legrifi, I., Belabess, Z., Laasli, S.-E., Mazouz, H., & Lahlali, R. (2023). Beneficial Microorganisms as Bioprotectants against Foliar Diseases of Cereals: A Review. Plants, 12(24), 4162. https://doi.org/10.3390/plants12244162










