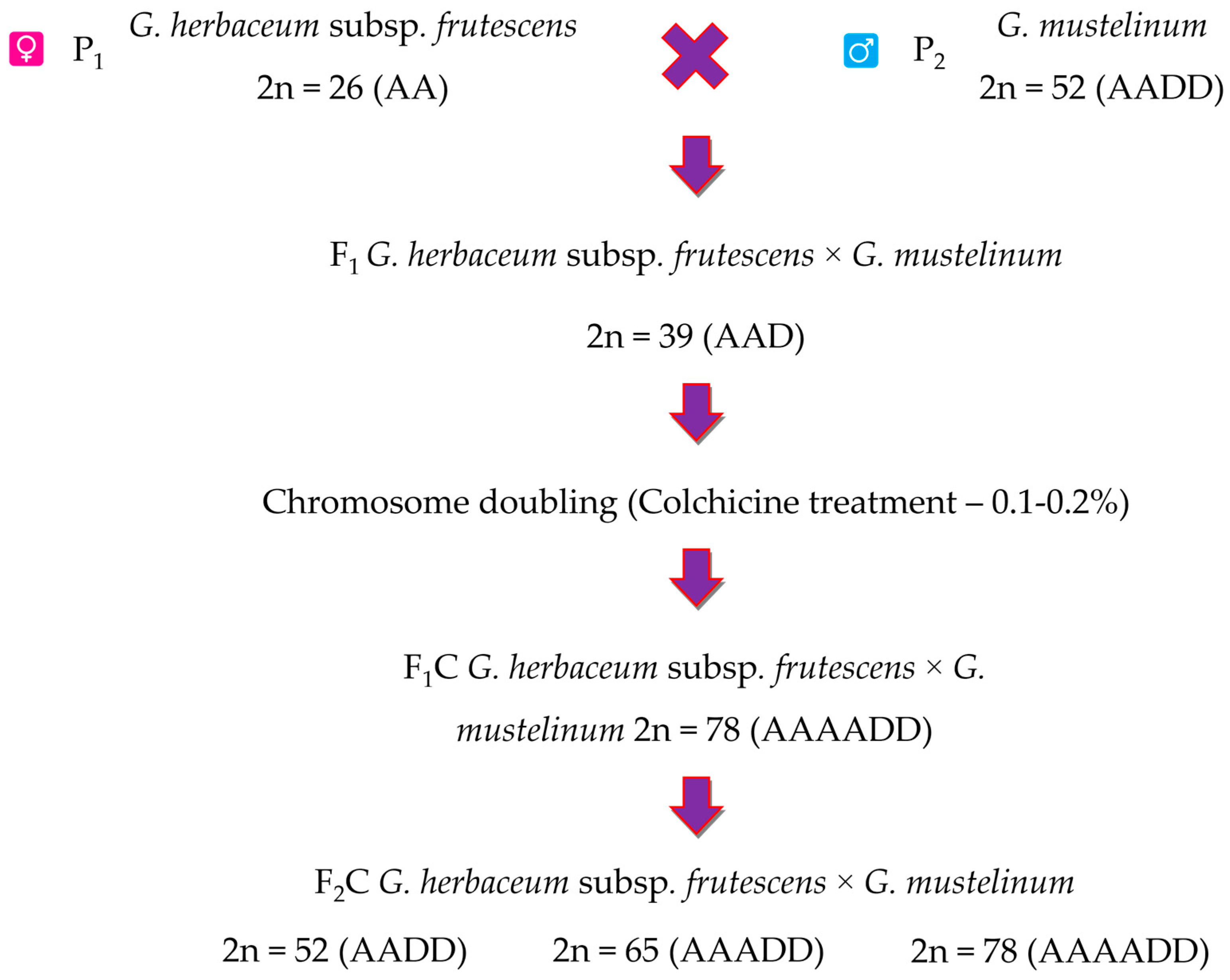
2.2. Inheritance of Morphological and Economically Valuable Traits in Interspecific Polyploid Hybrids F1C and F2C
2.2.1. The Number of Bolls per Plant and the Fertility of Seeds
It is known that parameters such as the number of bolls in a plant, fiber yield, weight of 1000 seeds, weight of cotton in one boll and the number of fully born seeds in one boll play an important role in determining the level of productivity. Since these indicators are inextricably linked with each other and have a correlative effect on the development of the character. In addition, qualitative and quantitative indicators of seeds born in one bag are used as a basis for determining the phylogenetic relationships of species based on classical methods. Therefore, the initial sources used in the rese—arch and the yield indicators of the polyploid hybrids obtained on their basis were analyzed. In terms of the number of bolls per plant among the subspecies of
G. herbaceum, the highest rate (36.0 pieces) was recorded in the form of subsp.
pseudoarboreum f.
harga. In the analyzed bolls, the rate of complete seed (or, fertility of seeds) was 89.2 ± 1.9%. Also, the number of bolls per plant showed positive indicators on subspecies of subsp.
africanum—27.0 pieces, and the yield of complete seeds in the boll was 94.5 ± 1.8%, in the variety A-833 (subsp.
euherbaceum)—28.0 pieces, and the rate of complete seeds was 86.9 ± 1.9%. In
G. mustelinum—a Brazilian endemic species, the average number of bolls per plant was—16.0, and the rate of complete seed was—86.0 ± 2.1% (
Table 2).
In F
1C subsp.
frutescens ×
G. mustelinum polyploid hybrids, the number of bolls per plant was—21.0, and the “fertility rate of seeds per bolls” was very low (19.4 ± 1.9%) (
Table 3). An average of 14.3 seeds were set in one boll, of which 2.8 whole seeds and 11.5 empty seeds were set. In F
1C subsp.
pseudoarboreum f.
harga ×
G. mustelinum hybrids, the number of bolls per plant was—1.0, the number of complete seeds was 2.0, and the number of empty seeds was 7.0. F
1C subsp.
africanum ×
G. mustelinum hybrid plants were sterile, and no yield components were observed.
In hybrid plants of subsp. frutescens × G. mustelinum F2C generation, there was a variation of 18.0–26.0 units in terms of “number of bolls per plant”. Relatively low values (between 30.6 ± 1.2% and 49.2 ± 2.1%) were found in the “seeds fertility” indicator, also. According to the results of the analysis, in polyploid hybrids of the F1C and F2C generations, there was a slight decrease in the number of pods per plant compared to the parental samples, as well as cases of sterility in the subsp. africanum × G. mustelinum combination. A sharp decrease was noted in terms of the rate of complete seed fertility, which is the main factor of productivity. This indicates that the parental lines are phylogenetically distant and isolated species.
2.2.2. Vegetative Growth Duration
According to the results of the research, wild (subsp. africanum) and ruderal (subsp. pseudoarboreum f. harga) forms of G. herbaceum of which, the vegetative growth duration was 137.4 and 127.2 days, therefore they would require breeding for short day conditions. The vegetative growth duration in the cultivated-tropical subsp. frutescens, ruderal subsp. pseudoarboreum subspecies and cultivated subsp. euherbaceum A-833 variety is 117.2, 119.6, and 117.4 days respectively, in field conditions since they do not require breeding for short days. The wild tetraploid species G. mustelinum also requires breeding for short days since the duration of its vegetative growth duration was 151.8 days.
F
1C hybrid plants obtained by cross-species with representatives of the diploid
G. herbaceum species and tetraploid
G. mustelinum species was between 128.6–145.2 days. Including F
1C subsp.
frutescens ×
G. mustelinum the vegetative duration of polyploid hybrid plants was 128.6 days, the coefficient of variation was 2.3%. The coefficient of dominance for the sign is equal to
hp = 0.34, and the state of partial dominance of the form with a positive indicator (subsp.
frutescens) was noted. The vegetative growth duration of F
1C subsp.
pseudoarboreum f.
harga ×
G. mustelinum polyploid hybrid combination plants was 139.6 days on average, the variation coefficient was 1.5%, and the dominance coefficient was
hp = −0.01. In this combination, the character was inherited with a partial dominance of the negative indicator form (
G. mustelinum). The average vegetative growth duration of polyploid hybrid plants was 139.6 days, the variation coefficient was 1.5%, and the dominance coefficient was
hp = −0.01. In this combination, the character was inherited in the partial dominance of the
G. mustelinum species with a negative indicator. The polyploid hybrid plants of F
1C subsp.
africanum ×
G. mustelinum combination had an average vegetative growth duration of 145.2 days, and the coefficient of variation was 2.2% (
Table 4). The coefficient of dominance for the studied character is equal to
hp = −0.08, and in this combination, the character was inherited in the case of partial dominance of the negative indicator form (
G. mustelinum).
From the above-mentioned F1C polyploid hybrids, only F2C hybrids of subsp. frutescens × G. mustelinum combination were obtained. Also, in this generation, the degree of variability of the indicators of the period from the germination to the opening of the first bud was studied in 50% of the plants. A total of 134 plants were obtained in the F2C generation of the subsp. frutescens × G. mustelinum combination, of which 53 strongly required breeding for short days and thus affected yield components under field conditions. In the remaining 81 F2C hybrids, the growth duration showed a wide range of variability between 116.6–152.2 days, which were divided into eight classes. Among the studied hybrid combinations, the growth duration was 115–119 day and faster recombinant forms around 120–124 were isolated.
2.2.3. Fiber Length and Strength
Cotton has been cultivated for centuries mainly for its fiber and has undergone natural and artificial selection. The wild diploid and tetraploid species are the natural resources with high genetic diversity for improving the fiber quality traits of elite cultivars. Therefore, fiber length is considered one of the major indicators of valuable economic traits in the breeding process.
According to the results of fiber quality tests the fiber length was in the range of 17.2–25.2 mm in
G. herbaceum subspecies (
Table 5). In this case, a relatively low result was observed in subsp.
frutescens (17.2 ± 0.2 mm) and a relatively high result was observed in cultivated variety subsp.
euherbaceum A-833 (25.2 ± 0.3 mm). As well as 25.1 ± 0.4 mm was in ruderal subsp.
pseudoarboreum, 20.1 ± 0.2 mm was in subsp.
pseudoarboreum f.
harga and 24.7 ± 0.3 mm was in wild-type subsp.
africanum. In the case of tetraploid
G. mustelinum, the fiber length was 25.6 ± 0.2 mm.
The fiber strength varied in all of the cotton samples including the highest values of 35.5 cN/tex and 32.6 cN/tex, respectively, in subsp.
frutescens and subsp.
africanum. The fiber strength in A-833 variety of subsp.
euherbaceum was 27.2 cN/tex, in subsp.
pseudoarbareum f.
harga was 24.1 cN/tex, and in
G. mustelinum was 17.3 cN/tex (
Table 5).
Due to two F1C allohexaploid combinations were dead after the germination and the other two being male sterile, therefore all analyses were conducted only with G. herbaceum subsp. frutescens × G. mustelinum F1C allohexaploids since they were fertile. The fiber length was 28.7 ± 0.9 mm, and a positive heterosis (hp = 1.74) was observed.
Variability was observed in the plants of F2C subsp. frutescens × G. mustelinum hybrid combinations. The analyzed results were divided into eight classes. Accordingly, two transgressive plants with long fiber of 35.1–37.0 mm, and one transgressive plant with extra-long fiber of 39.1–41.0 mm were identified. Since the fiber of the Brazilian endemic species G. mustelinum differs from that of the allotetraploid species G. hirsutum in terms of strength, maturity and length, a genetically rich source for improving fiber qualities of cultivars.
The identification of major QTL loci for fiber quality and application of a set of beneficial alleles from
G. mustelinum can contribute significantly to the long-term improvement of cultivated cotton germplasm. To introduce important alleles from
G. mustelinum species into the genome of
G. hirsutum species for fiber quality improvement, interspecific populations consisting of plants obtained by crossing
G. mustelinum species with
G. hirsutum species were created [
43,
44,
45]. In the studied families, it was determined that the genes responsible for fiber strength and fineness in alleles of
G. mustelinum are dominantly inherited. Based on the previous work with
G. barbadense,
G. tomentosum, and
G. darwinii, which included introgression of
G. mustelinum alleles [
43,
44,
45]. These hybrid genotypes identified in our study would serve as a valuable source for the development of long-fiber cultivars and future breeding programs.
2.3. Cytogenetic and Genomic Studies of Interspecific Hybrids
Chromosome conjugation, spore and pollen viability analysis at metaphase I (MI) stage of meiosis is one of the effective methods for determining the ploidy level of intergenomic hybrids (allopolyploids, autopolyploids). Cytogenetic analyzes of hybrids resulting from the crosses between diploid and tetraploid cotton species were conducted in the study, and interesting data were obtained regarding their genomic structure and their partial homology.
In the three hexaploid plants studied, disturbances in the process of the first division of meiosis (MI) were detected. F
1C subsp.
frutescens ×
G. mustelinum 38.22 ± 0.25 bivalents, 0.45 ± 0.29 univalents (the number of univalents was up to six) and 0.36 ± 0.14 quadrivalents. F
1C subsp.
pseudoarboreum f.
harga ×
G. mustelinum and F
1C subsp.
africanum ×
G. mustelinum combinations had a higher number of univalents than the above combination (up to 2–10) (
Table 6,
Figure 3). The analysis showed that due to structural differences in chromosomes and the impossibility of normal chromosome conjugation in hybrids, there was a desynaptic effect due to early and asynchronous divergence in individual bivalents (
Figure 3 a,b).
In general, the number of univalents found in maternal pollen cells was recorded from two to ten. It was known that there is weak desynapsis (meeting of several univalents along with bivalents in the female pollen cell), medium desynapsis (meeting of many univalents along with bivalents in the female pollen cell), and complete desynapsis (meeting of mainly univalents and sometimes several bivalents in the female pollen cell) [
46]. Such univalents were formed as a result of early divergence of chromosomes. In the cotton samples of our study, this level—from two to ten univalent encounters—corresponded to the medium desynapsis.
As a result of research, the MI phase of meiosis was observed in some plants of the F
2C subsp.
frutescens ×
G. mustelinum hexaploid hybrid (
Figure 4). The cotton plant samples with different levels of ploidy (24.73
II; 38.18
II; 38.00
II) were identified in second generation (F
2C). Disruptions in the mentioned chromosomal conjugation led to partial sterility of anthers in hexaploid hybrids and abnormal spore formation at the sporulation stage. It should also be noted that the MI phase of meiosis was carried out normally (26.00
II) in two hybrid plants (
Table 6).
Thus, the cause of partial infertility observed in allopolyploids was likely due to the presence of disturbances in the meiosis phase (disruption of the synchronous distribution of chromosomes in the anaphase or desynapsis phenomenon), instability of the number of chromosomes. One of the unique features of hybrids derived from the crosses between cotton species far from each other was with chromosomal aberrations. In such cases, plants of with chromosomal aberrations are due to the absence of homologous chromosomes of different species or the presence of their only partial homologue. Hybrids with homeologous genomes are viable, but sterile, i.e., sterile, due to genome duplication [
47]. According to the conclusions of Sanamyan [
48], hybrid combinations obtained from the diploid species (
G. thurberi ×
G. raimondii,
G. arboreum ×
G. thurberi, and
G. herbaceum ×
G. thurberi) are productive in generations, on the contrary, intergenomic hybrids are very a large percentage of plants without flowers to appear.
There were difficulties in spore analysis to determine the required stage due to the very low number of spikelets and pollen grains in polyploid hybrids. As a result, ten additional samples had to be analyzed. According to the analysis of the conducted tetrads, 90.3–96.8% meiotic stage in three hybrid forms (F
1C subsp.
frutescens ×
G. mustelinum, F
1C subsp.
pseudoarboreum f.
harga ×
G. mustelinum, F
1C subsp.
africanum ×
G. mustelinum) index was defined. It was noted that certain disturbances of the meiotic index in the form of micronuclear tetrads and polyads were observed in all forms. In F
1C subsp.
frutescens ×
G. mustelinum polyploid hybrid, the meiotic index was 90.36%, the rate of micronuclear tetrads was 3.27% and that of polyads was 6.37% (
Table 7).
Micronuclear tetrads were not found in F1C subsp. pseudoarboreum f. harga × G. mustelinum polyploid hybrid unlike other plants. F2C subsp. frutescens × G. mustelinum polyploid hybrids T 38-12, T 30-6, T 30-7, T 30-8, T 38-3 forms meiotic index 96.05–98.54%, polyads 1.42–3.12%, micronuclear tetrads were not observed. In T 1-8 and T 51-13 forms, meiotic index was 96.15–97.45%, polyads 2.11–3.07%, micronuclear tetrads 0.69–0.89%.
In tetrads with identified micronuclei, up to 1–8 micronuclei (
Figure 5c), and from polyads up to pentad, hexad, heptad, octad (
Figure 5d) aneuploid spores were noted.
It was known that pollen grains formed as result of meiosis are not equal in terms of genetic characters and functional capabilities. When the chromosomal complex of pollen grains was insufficient (i.e., when the frequency of meeting homologous chromosomes was low, the pollen grains were underdeveloped, and the viable pollen decreased significantly). When a complete chromosome complex was obtained, that is, when the homologous row of chromosomes was restored, the pollen grains became pink and fertile again. As a result of cytogenetic analysis, pollen graininess in hybrid plants F
1C subsp.
pseudoarboreum f.
harga ×
G. mustelinum 63.89% in the combination of, F
1C subsp.
frutescens ×
G. mustelinum combination had a low yield—32.67%, F
1C subsp.
africanum ×
G. mustelinum combination, pollen sterility was determined (
Table 8,
Figure 6).
Since most of the hybrid plants showed short day and late tolerance in F
2C generation of the combination subsp.
frutescens × G. mustelinum, 24 out of 134 plants were studied for pollen graininess (
Table 9). Until late autumn, reproductive organs were not formed in many hybrids, abnormal forms were noted without pollen development in hybrids that started flowering. There were a few cases where dust grains were not formed.
The pollen fertility in the studied hybrids had different indicators. The pollen fertility of 14 hybrids was low (1.49–40.43%). Among them T 33-6, T 30-8, T49-2, T 38-7 plants were very low (1.49–10.23%), T 33-2, T 37-4, plants up to 50% or more, T 38-3, T 30-6, T 30-7, T 38-12, more than 60% (
Table 8,
Figure 7) indicators of dustiness were found in plants.
2.4. Molecular and Phylogenetic Analysis of Allopolyploid Forms of Cotton
The PCR analysis was conducted using 72 simple sequence repeat (SSR) markers (
Table S1) associated with economically important traits to determine the genetic polymorphisms between parental genotypes as well as to determine some genomic changes in allohexaploid hybrids (
Figure 8). As a result of PCR analysis, 57 DNA markers were determined as polymorphic markers (
Table S2), while 14 DNA markers were monomorphic, and one DNA marker was not amplified.
Based on the results of PCR analysis, the genomic changes were determined in the genomic regions of 18 (31.6%) out of 57 polymorphic DNA markers or in 40 (26.7%) PCR amplicons (alleles) of 150 alleles in allohexaploid hybrids. In particular, 31 (26.0%), 29 (24.3%), and 27 (22.6%) alleles had a change in three F1C combinations (G. herbaceum subsp. frutescens × G. mustelinum), F1C (G. herbaceum subsp. pseudoarboreum f. harga × G. mustelinum) and F1C (G. herbaceum subsp. africanum × G. mustelinum) hybrids, of 119, 120 and 116 alleles, respectively. In these 3 allohexaploid hybrids, alleles 23, 21, and 20 were missing although they were present in parental genotypes, respectively. On the other hand, non-parental alleles 8, 8, and 7 appeared in allohexaploid hybrids, respectively.
A phylogenetic tree construction indicated that the cotton accessions were clustered into two main groups (
Figure 9). The first group included three
Gossypium herbaceum subspecies such as subsp.
frutescens, subsp.
pseudoarboreum f.
harga and subsp.
africanum.
G. herbaceum subsp.
pseudoarboreum f.
harga and subsp.
africanum are genetically more similar to each other than subsp.
frutescens.
G. mustelinum and three interspecific allohexaploid hybrids F
1C (
G. herbaceum subsp.
frutescens ×
G. mustelinum), F
1C (
G. herbaceum subsp.
pseudoarboreum f.
harga ×
G. mustelinum) and F
1C (
G. herbaceum subsp.
africanum ×
G. mustelinum) were presented in the second group of the phylogenetic tree.






 —lack alleles,
—lack alleles,  —additional alleles. 1—G. mustelinum; 2—G. herbaceum subsp. frutescens; 3—F1C (G. herbaceum subsp. frutescens × G. mustelinum); 4—G. herbaceum subsp. pseudoarboreum f. harga; 5—F1C (G. herbaceum subsp. pseudoarboreum f. harga × G. mustelinum); 6—G. herbaceum subsp. africanum; 7—F1C (G. herbaceum subsp. africanum × G. mustelinum).
—additional alleles. 1—G. mustelinum; 2—G. herbaceum subsp. frutescens; 3—F1C (G. herbaceum subsp. frutescens × G. mustelinum); 4—G. herbaceum subsp. pseudoarboreum f. harga; 5—F1C (G. herbaceum subsp. pseudoarboreum f. harga × G. mustelinum); 6—G. herbaceum subsp. africanum; 7—F1C (G. herbaceum subsp. africanum × G. mustelinum).
 —lack alleles,
—lack alleles,  —additional alleles. 1—G. mustelinum; 2—G. herbaceum subsp. frutescens; 3—F1C (G. herbaceum subsp. frutescens × G. mustelinum); 4—G. herbaceum subsp. pseudoarboreum f. harga; 5—F1C (G. herbaceum subsp. pseudoarboreum f. harga × G. mustelinum); 6—G. herbaceum subsp. africanum; 7—F1C (G. herbaceum subsp. africanum × G. mustelinum).
—additional alleles. 1—G. mustelinum; 2—G. herbaceum subsp. frutescens; 3—F1C (G. herbaceum subsp. frutescens × G. mustelinum); 4—G. herbaceum subsp. pseudoarboreum f. harga; 5—F1C (G. herbaceum subsp. pseudoarboreum f. harga × G. mustelinum); 6—G. herbaceum subsp. africanum; 7—F1C (G. herbaceum subsp. africanum × G. mustelinum).










