Effects of Vegetation Restoration on Soil Nitrogen Fractions and Enzyme Activities in Arable Land on Purple Soil Slopes
Abstract
:1. Introduction
2. Results
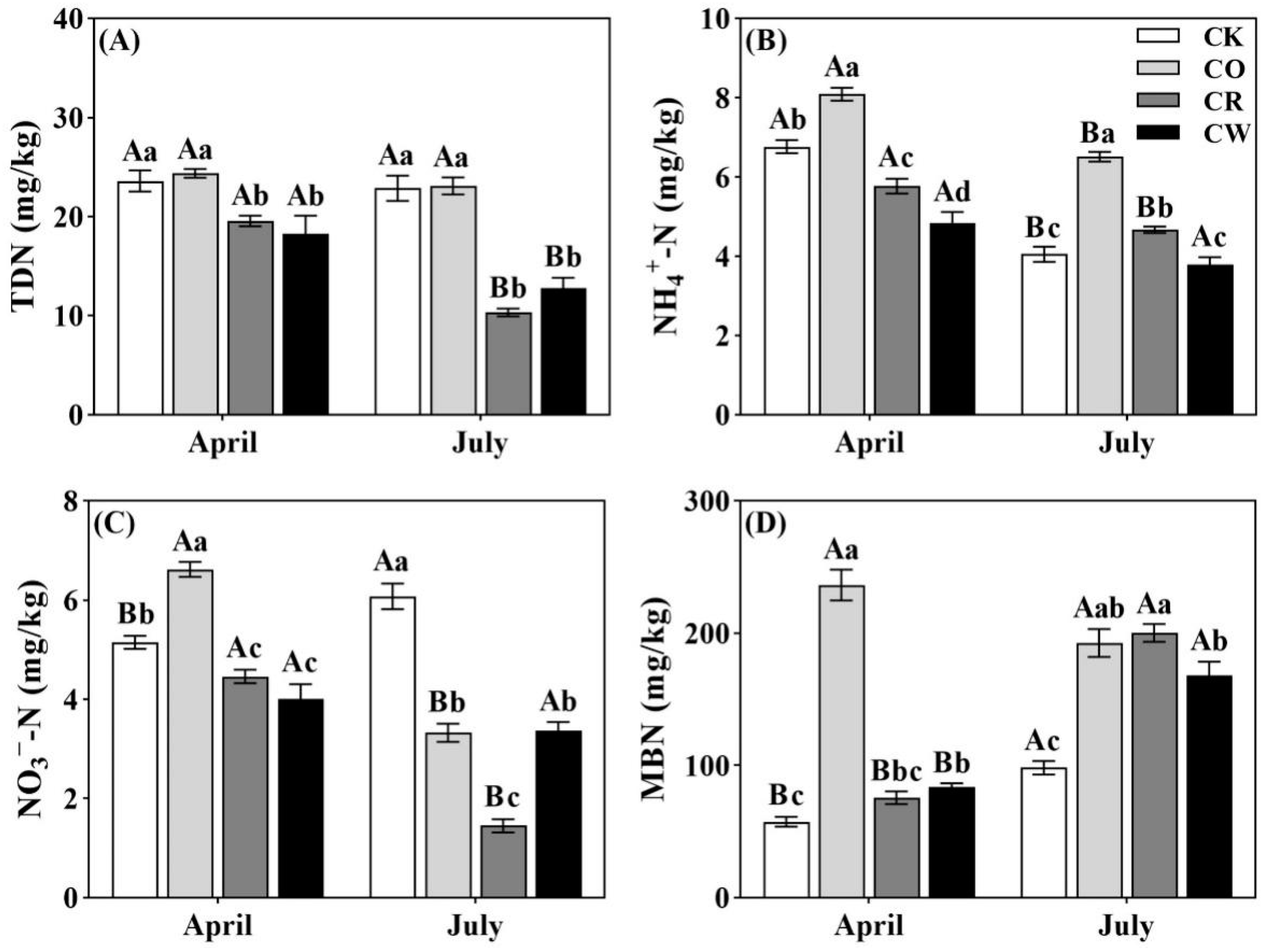
2.1. Changes in Soil N Fractions in Different Vegetation Types
2.2. Changes in Soil N Cycle Enzyme Activities in Different Vegetation Types
2.3. Correlation Analysis
3. Materials and Methods
3.1. Experimental Site
3.2. Materials and Experimental Design
3.3. Soil Sampling and Analysis
3.4. Statistical Analysis
4. Discussion
4.1. Effect of Vegetation-Restoration Measures on the Content of Soil N Fractions
4.2. Effects of Vegetation-Restoration Measures on the Activities of Soil N Cycle Enzyme Activities
4.3. Correlation of Soil N Cycle Enzyme Activities with Soil Environmental Factors
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lozano, Y.M.; Hortal, S.; Armas, C.; Pugnaire, F.I. Interactions among soil, plants, and microorganisms drive secondary succession in a dry environment. Soil Biol. Biochem. 2014, 78, 298–306. [Google Scholar] [CrossRef]
- Ma, R.T.; Hu, F.N.; Xu, C.Y.; Liu, J.F.; Zhao, S.W. Response of soil aggregate stability and splash erosion to different breakdown mechanisms along natural vegetation restoration. Catena 2022, 208, 105–775. [Google Scholar] [CrossRef]
- Feng, J.; Turner, B.L.; Wei, K.; Tian, J.H.; Chen, Z.H.; Lü, X.T.; Wang, C.; Chen, L.J. Divergent composition and turnover of soil organic nitrogen along a climate gradient in arid and semiarid grasslands. Geoderma 2018, 327, 36–44. [Google Scholar] [CrossRef]
- Xu, L.; He, N.P.; Yu, G.R. Nitrogen storage in China’s terrestrial ecosystems. Sci. Total Environ. 2020, 709, 136–201. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.B.; Ni, H.Q.; Su, W.H. Effects of management measures on organic carbon, nitrogen and chemical structure of different soil fractions in Phyllostachys edulis plantations. J. Appl. Ecol. 2020, 31, 25–34. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, D.; Wu, J. Soil nitrogen–hydrolyzing enzyme activity and stoichiometry following a subtropical land use change. Land Degrad. Dev. 2021, 32, 4277–4287. [Google Scholar] [CrossRef]
- Zhang, C.Q.; Zhang, B.; Yang, Y.G. Research on Soil Nutrients of Forests Nearby Xitiaoxi River in the Upper Reaches of Taihu Lake Basin. J. Soil Water Conserv. 2011, 25, 53–58. [Google Scholar] [CrossRef]
- Omidvar, N.; Xu, Z.; Nguyen, T. A global meta–analysis shows soil nitrogen pool increases after revegetation of riparian zones. J. Soils Sediments. 2021, 21, 665–677. [Google Scholar] [CrossRef]
- Cuia, Y.; Fanga, L.; Guoc, X.; Wanga, X.; Zhangd, Y. Ecoenzymatic stoichiometry and microbial nutrient limitation in rhizosphere soil in the arid area of the northern Loess Plateau, China. Soil Biol. Biochem. 2018, 116, 11–21. [Google Scholar] [CrossRef]
- Weintraub, S.R.; Brooks, P.D.; Bowen, G.J. Interactive effects of vegetation type and topographic position on nitrogen availability and loss in a temperate montane ecosystem. Ecosystems 2017, 20, 1073–1088. [Google Scholar] [CrossRef]
- Jat, H.S.; Choudhary, M.; Datta, A.; Yadav, A.K.; Meena, M.D.; Devi, R.; Gathala, M.K.; Jat, M.L.; McDonald, A.; Sharma, P.C. Temporal changes in soil microbial properties and nutrient dynamics under climate smart agriculture practices. Soil Tillage Res. 2020, 199, 104595. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fu, X.; Ghimire, R.; Sainju, U.M.; Jia, Y.; Zhao, F. Responses of soil bacterial community and enzyme activity to organic matter components under long-term fertilization on the loess plateau of China. Appl Soil. Ecol. 2021, 166, 103992. [Google Scholar] [CrossRef]
- Holík, L.; Hlisnikovský, L.; Honzík, R.; Trögl, J.; Burdová, H.; Popelka, J. Soil microbial communities and enzyme activities after long-term application of inorganic and organic fertilizers at different depths of the soil profile. Sustainability 2019, 11, 3251. [Google Scholar] [CrossRef]
- Hu, P.L.; Zhao, Y.; Xiao, D.; Xu, Z.H.; Zhang, W.; Xiao, J.; Wang, K.L. Dynamics of soil nitrogen availability following vege–tation restoration along a climatic gradient of a subtropical karst region in China. Soils Sediments 2021, 21, 2167–2178. [Google Scholar] [CrossRef]
- Guan, H.L.; Fan, J.W.; Lu, X.K. Soil specific enzyme stoichiometry reflects nitrogen limitation of microorganisms under different types of vegetation restoration in the karst areas. Appl Soil. Ecol. 2021, 169, 104–253. [Google Scholar] [CrossRef]
- Yang, Y.; Liang, C.; Wang, Y.Q.; Cheng, H.; An, S.S.; Chang, S.X. Soil extracellular enzyme stoichiometry reflects the shift from P– to N–limitation of microorganisms with grassland restoration. Soil Biol. Biochem. 2020, 149, 107–928. [Google Scholar] [CrossRef]
- Li, X.J.; Yang, H.T.; Shi, W.L.; Li, Y.F.; Guo, Q. Afforestation with xerophytic shrubs accelerates soil net nitrogen nitrification and mineralization in the Tengger Desert, Northern China. Catena 2018, 169, 11–20. [Google Scholar] [CrossRef]
- Pastore, G.; Tobin, B.; Nieuwenhuis, M. Quantifying carbon and nitrogen losses by respiration and leaching from decomposing woody debris in reforested coniferous stands in Ireland. Agric. For. Meteorol. 2019, 265, 195–207. [Google Scholar] [CrossRef]
- Ding, X.Q.; Chang, Y.; Hou, H.B.; Peng, P.Q.; Xiang, W.H. Quantification of the sources of soluble organic N (SON) from new litter or indigenous soil in a typical subtropical forest. Land Degrad. Dev. 2021, 32, 2528–2539. [Google Scholar] [CrossRef]
- Yang, N.; Zou, D.S.; Yang, M.Y. Variations of soil microbial community diversity in purple soils at different re-vegetation stages on sloping–land in Hengyang, Hunan Province. Sci. Silvae Sin. 2016, 52, 146–156. [Google Scholar] [CrossRef]
- Yang, N.; Zou, D.S.; Fu, M.Y. Properties of soil aggregates in purple soils during re–vegetation on sloping land in relation to soil characteristics. Chin. J. Ecol. 2016, 35, 2361–2368. [Google Scholar] [CrossRef]
- Zou, X.; Zhao, X.; Zhao, H. Spatial heterogeneity of soil properties and vegetation–soil relationships following vegetation restoration of mobile dunes in Horqin Sandy Land, Northern China. Plant Soil. 2009, 318, 153–167. [Google Scholar] [CrossRef]
- Yang, N.; Zou, D.S.; Yang, M.Y. Dynamic changes of soil microbial biomass and soil nutrients along re–vegetation on sloping–land with purple soils in Hengyang of Hunan Province, South-central China. Sci. Silvae Sin. 2014, 50, 144–150. [Google Scholar] [CrossRef]
- Yang, N.; Zou, D.S.; Li, J.G. The vegetation restoration mode construction in sloping–land with purple soils in Hengyang basin. Pratac. Sci. 2010, 27, 10–16. [Google Scholar]
- Angelova, V.; Akova, V.; Ivanov, K. Comparative study of the methods for the determination of organic carbon and organic matter in soils, compost and sludge. Bulg. Chem. Commun. 2019, 51, 342–347. [Google Scholar] [CrossRef]
- Dolobeshkin, E.; Gumbarov, A.; Bandurin, M. Monitoring of arable land fertility based on agrochemical analysis and dynamics of changes in soil organic matter reserves. Proc. IOP Conf. Ser. Earth Environ. Sci. 2021, 666, 052064. [Google Scholar] [CrossRef]
- Guo, J.; Feng, H.; Roberge, G.; Feng, L.; Pan, C.; McNie, P.; Yu, Y. The negative effect of Chinese fir (Cunninghamia lanceolata) monoculture plantations on soil physicochemical properties, microbial biomass, fungal communities, and enzymatic activities. For. Ecol. Manag. 2022, 519, 120–297. [Google Scholar] [CrossRef]
- Wang, C.Q.; Xue, L.; Jiao, R.Z. Soil organic carbon fractions, C–cycling associated hydrolytic enzymes, and microbial carbon metabolism vary with stand age in Cunninghamia lanceolate (Lamb.) Hook plantations. For. Ecol. Manag. 2021, 482, 118–887. [Google Scholar] [CrossRef]
- Sheng, M.Y.; Liu, Y.; Xiong, K.N. Response of soil physical–chemical properties to rocky desertification succession in south China karst. Acta Ecol. Sin. 2018, 33, 6303–6313. [Google Scholar] [CrossRef]
- Mishra, U.; Hugelius, G.; Shelef, E.; Yang, Y.; Strauss, J.; Lupachev, A.; Harden, J.; Jastrow, J.; Ping, C.; Riley, W. Spatial heterogeneity and environmental predictors of permafrost region soil organic carbon stocks. Sci. Adv. 2021, 7, eaaz5236. [Google Scholar] [CrossRef]
- Li, H.; Cai, J.J.; Liu, M.; Sui, X.; Hu, Y.; Feng, F. Microbial community structure and the relationship with soil carbon and nitrogen in an original Korean pine forest of Changbai Mountain, China. BMC Microbiol. 2019, 19, 218. [Google Scholar] [CrossRef] [PubMed]
- Jian, J.; Du, X.; Reiter, M.S.; Stewart, R.D. A meta-analysis of global cropland soil carbon changes due to cover cropping. Soil Biol. Biochem. 2020, 143, 107735. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, J.; Mary, B. Soil pH has contras–ting effects on gross and net nitrogen mineralizations in adjacent forest and grassland soils in central Alberta, Canada. Soil Biol. Biochem. 2013, 57, 848–857. [Google Scholar] [CrossRef]
- Cai, Z.; Yan, X.; Gu, B. Applying C:N ratio to assess the rationality of estimates of carbon sequestration in terrestrial ecosystems and nitrogen budgets. Carbon Res. 2022, 1, 2. [Google Scholar] [CrossRef]
- Ashraf, M.N.; Hu, C.; Wu, L.; Duan, Y.; Zhang, W.; Aziz, T.; Cai, A.; Abrar, M.M.; Xu, M. Soil and microbial biomass stoichiometry regulate soil organic carbon and nitrogen mineralization in rice-wheat rotation subjected to long-term fertilization. J. Soils Sediments 2020, 20, 3103–3113. [Google Scholar] [CrossRef]
- Watanabe, S.; Shibata, M.; Kosugi, Y.; Marryanna, L.; Fukushima, K.; Hartono, A.; Funakawa, S. Investigating drivers of active nitrification in organic horizons of tropical forest soils. Soil Ecol. Lett. 2023, 5, 220167. [Google Scholar] [CrossRef]
- Cui, Y.; Fang, L.; Guo, X.; Han, F.; Ju, W.; Ye, L.; Wang, X.; Tan, W.; Zhang, X. Natural grassland as the optimal pattern of vegetation restoration in arid and semi-arid regions: Evidence from nutrient limitation of soil microbes. Sci. Total Environ. 2019, 648, 388–397. [Google Scholar] [CrossRef]
- Kooch, Y.; Moghimian, N.; Wirth, S.; Noghre, N. Effects of grazing management on leaf litter decomposition and soil microbial activities in northern Iranian rangeland. Geoderma 2020, 361, 114100. [Google Scholar] [CrossRef]
- Seabloom, E.W.; Borer, E.T.; Tilman, D. Grassland ecosystem recovery after soil disturbance depends on nutrient supply rate. Ecol. Lett. 2020, 23, 1756–1765. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Liang, C.; Ai, Z.; Wu, Y.; Xu, H.; Xue, S.; Liu, G. Glomalin-related soil protein affects soil aggregation and recovery of soil nutrient following natural revegetation on the Loess Plateau. Geoderma 2020, 357, 113921. [Google Scholar] [CrossRef]
- Li, J.; Nie, M.; Powell, J.R.; Bissett, A.; Pendall, E. Soil physico-chemical properties are critical for predicting carbon storage and nutrient availability across Australia. Environ. Res. Lett. 2020, 15, 094088. [Google Scholar] [CrossRef]
- Taikui, L.; Xiangning, Z.; Changlin, K.; Jinling, L.; Zhanling, G.; Xiaosheng, L. Effects of different agronomic measures on runoff, water and phosphorous losses of tea garden located in sloping cropland in Danjiangkou reservoir area. Ecol. Environ. 2021, 30, 2324–2330. [Google Scholar]
- Li, J.; Liu, Y.; Hai, X.; Shangguan, Z.; Deng, L. Dynamics of soil microbial C: N: P stoichiometry and its driving mechanisms following natural vegetation restoration after farmland abandonment. Sci. Total Environ. 2019, 693, 133613. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Dai, Q.; Yi, X.; Wang, Y.; Peng, X.; Yan, Y. Effects of soil and rock microhabitats on soil organic carbon stability in a karst peak-cluster depression region of Southwestern China. Geoderma Regional 2023, 32, e00623. [Google Scholar] [CrossRef]
- Fan, Z.; Lu, S.; Liu, S.; Guo, H.; Wang, T.; Zhou, J.; Peng, X. Changes in plant rhizosphere microbial communities under different vegetation restoration patterns in karst and non-karst ecosystems. Sci. Rep. 2019, 9, 8761. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wu, C.; Chen, D.; Liu, H.; Sun, X.; Zhang, S. Changes in soil carbon and nutrients and related extracellular enzymes in successive rotations of Japanese larch plantations. Catena 2021, 204, 105386. [Google Scholar] [CrossRef]
- Liu, X.; Guo, K.L.; Huang, L.; Ji, Z.Y.; Jiang, H.M.; Li, H.; Zhang, J.F. Responses of absolute and specific enzyme activity to consecutive application of composted sewage sludge in a Fluventic Ustochrept. PLoS ONE 2017, 12, 177–796. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.H.; Chen, Z.H.; Jiang, N.; Jiang, H.; Chen, L.J. Effects of long–term no–tillage with different residue application rates on soil nitrogen cycling. Soil Tillage Res. 2021, 212, 105044. [Google Scholar] [CrossRef]
- Zaman, T.; Iqbal, A.; Shaukat, A.; Nazir, R.; Pervez, A.; Bilal, M.; Faridullah, M.R.; Ali, S.; Alkahtani, S.; Abdel–Daim, M.M. Assessing the N cycling ecosystem function–processes and the involved functional guilds upon plant litter amendment in lower Himalaya. J. Environ. Stud. 2021, 30, 917–926. [Google Scholar] [CrossRef]
- Qiang, L.; Jibo, S.; Guangdi, L.; Juan, H.; Ruonan, M. Extracellular enzyme stoichiometry and microbial resource limitation following various grassland reestablishment in abandoned cropland. Sci. Total. Environ. 2023, 870, 161–746. [Google Scholar] [CrossRef]
- Singh, J.; Kumar, S. Seasonal changes of soil carbon fractions and enzyme activities in response to winter cover crops under long-term rotation and tillage systems. Eur. J. Soil Sci. 2021, 72, 886–899. [Google Scholar] [CrossRef]
- Ma, W.; Li, G.; Wu, J.; Xu, G.; Wu, J. Response of soil labile organic carbon fractions and carbon-cycle enzyme activities to vegetation degradation in a wet meadow on the Qinghai–Tibet Plateau. Geoderma 2020, 377, 114565. [Google Scholar] [CrossRef]
- Jia, X.; Zhong, Y.; Liu, J.; Zhu, G.; Shangguan, Z.; Yan, W. Effects of nitrogen enrichment on soil microbial characteristics: From biomass to enzyme activities. Geoderma 2020, 366, 114256. [Google Scholar] [CrossRef]
- Pokharel, P.; Ma, Z.; Chang, S.X. Biochar increases soil microbial biomass with changes in extra-and intracellular enzyme activities: A global meta-analysis. Biochar 2020, 2, 65–79. [Google Scholar] [CrossRef]
- Luo, M.X.; Hu, Z.D.; Liu, X.L.; Li, Y.F.; Hu, J.; Ou, D.H.; Wu, D.Y. Characteristics of soil microbial biomass carbon, nitrogen and enzyme activities in Picea asperata plantations with different ages in subalpine of western Sichuan, China. Acta Ecol. Sin. 2021, 41, 5632–5642. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, X.; Xu, Y.; Jin, M.; Ye, X.; Gao, H.; Chu, W.; Mao, J.; Thompson, M.L. Soil labile organic carbon fractions and soil enzyme activities after 10 years of continuous fertilization and wheat residue incorporation. Sci. Rep. 2020, 10, 11318. [Google Scholar] [CrossRef]
- Wang, H.Y.; Wu, J.Q.; Li, G.; Yan, L.J. Changes in soil carbon fractions and enzyme activities under different vegetation types of the northern Loess Plateau. BMC Ecol. Evol. 2020, 10, 12211–12223. [Google Scholar] [CrossRef]
- Fan, Z.Z.; Lu, S.Y.; Liu, S.; Li, Z.R.; Hong, J.X.; Zhou, J.X.; Peng, X.W. The effects of vegetation restoration strategies and seasons on soil enzyme activities in the karst landscapes of Yunnan, southwest China. J. For. Res. 2019, 31, 1949–1957. [Google Scholar] [CrossRef]



| Period | Symbol of Plot | pH | Total Soil Organic Carbon Content (g·kg−1) | Total Nitrogen Content (g·kg−1) | Total Phosphorus Content (g·kg−1) |
|---|---|---|---|---|---|
| Lush period (April) | CK | 4.48 ± 0.020 Aa | 3.20 ± 0.128 Aab | 0.32 ± 0.008 Ab | 0.046 ± 0.001 Bb |
| CO | 4.36 ± 0.038 Ab | 2.82 ± 0.134 Bb | 0.29 ± 0.006 Ac | 0.052 ± 0.003 Ba | |
| CR | 4.41 ± 0.008 Aab | 3.36 ± 0.053 Aab | 0.27 ± 0.009 Ac | 0.035 ± 0.001 Bc | |
| CW | 4.45 ± 0.004 Aa | 3,63 ± 0.124 Aa | 0.40 ± 0.011 Aa | 0.046 ± 0.002 Bab | |
| Wilting period (July) | CK | 4.20 ± 0.027 Bb | 2.56 ± 0.081 Bb | 0.26 ± 0.008 Bab | 0.076 ± 0.003 Ab |
| CO | 4.26 ± 0.013 Bb | 3.26 ± 0.100 Aa | 0.29 ± 0.014 Aa | 0.087 ± 0.005 Aa | |
| CR | 4.45 ± 0.019 Aa | 3.41 ± 0.170 Aa | 0.25 ± 0.007 Ab | 0.081 ± 0.003 Aab | |
| CW | 4.42 ± 0.026 Aa | 2.44 ± 0.030 Bb | 0.26 ± 0.007 Bb | 0.053 ± 0.002 Ac |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Zhang, Y.; Yao, Y.; Dang, P.; Farooq, T.H.; Wu, X.; Wang, J.; Yan, W. Effects of Vegetation Restoration on Soil Nitrogen Fractions and Enzyme Activities in Arable Land on Purple Soil Slopes. Plants 2023, 12, 4188. https://doi.org/10.3390/plants12244188
Li B, Zhang Y, Yao Y, Dang P, Farooq TH, Wu X, Wang J, Yan W. Effects of Vegetation Restoration on Soil Nitrogen Fractions and Enzyme Activities in Arable Land on Purple Soil Slopes. Plants. 2023; 12(24):4188. https://doi.org/10.3390/plants12244188
Chicago/Turabian StyleLi, Bowen, Yi Zhang, Yuxin Yao, Peng Dang, Taimoor Hassan Farooq, Xiaohong Wu, Jun Wang, and Wende Yan. 2023. "Effects of Vegetation Restoration on Soil Nitrogen Fractions and Enzyme Activities in Arable Land on Purple Soil Slopes" Plants 12, no. 24: 4188. https://doi.org/10.3390/plants12244188
APA StyleLi, B., Zhang, Y., Yao, Y., Dang, P., Farooq, T. H., Wu, X., Wang, J., & Yan, W. (2023). Effects of Vegetation Restoration on Soil Nitrogen Fractions and Enzyme Activities in Arable Land on Purple Soil Slopes. Plants, 12(24), 4188. https://doi.org/10.3390/plants12244188







