Evidence That Field Muskmelon (Cucumis melo L. var. agrestis Naud.) Fruits Are Solids of Revolution
Abstract
:1. Introduction
2. Materials and Methods
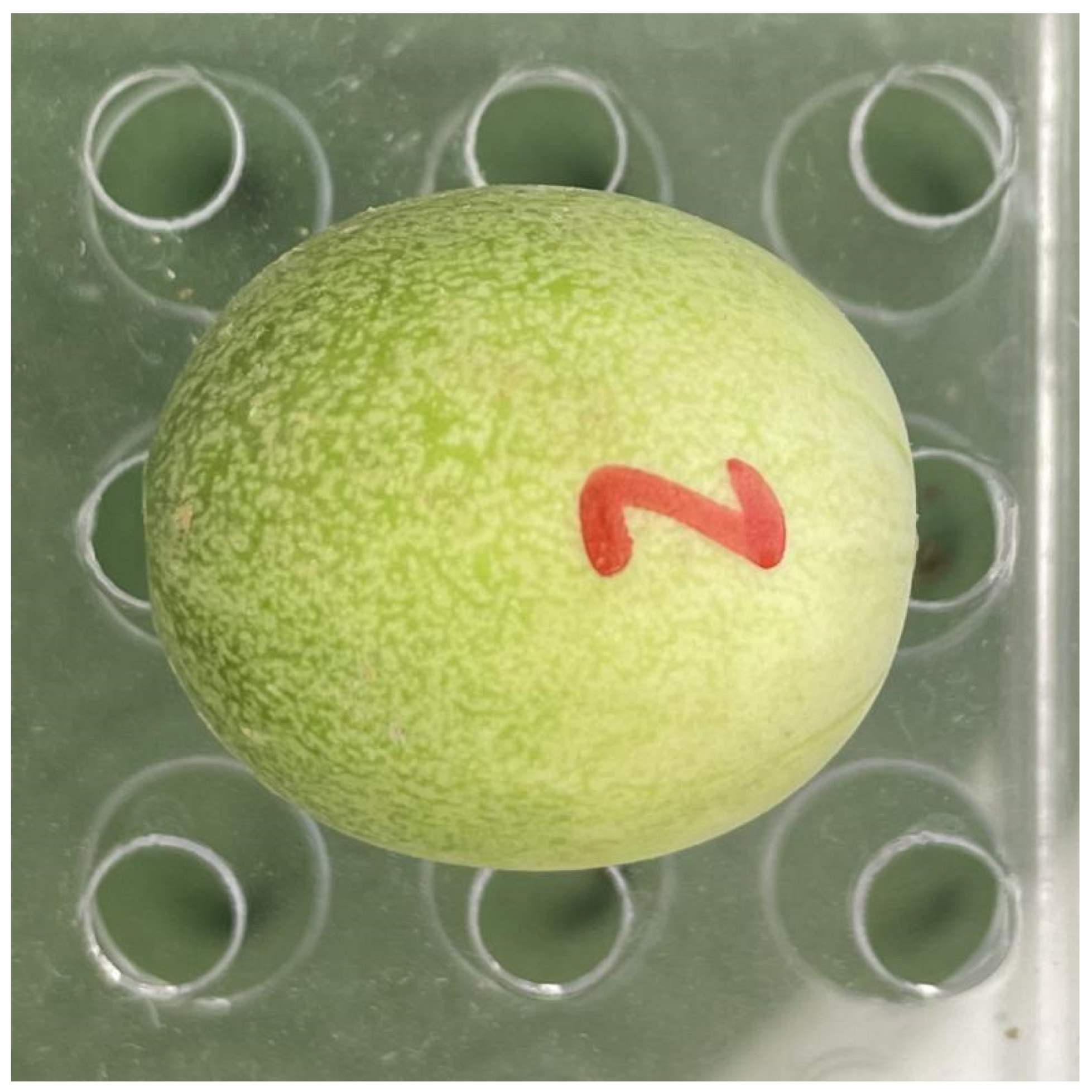
2.1. Fruit Sampling
2.2. Photographing and Data Acquisition
2.3. Modeling and Data Fitting
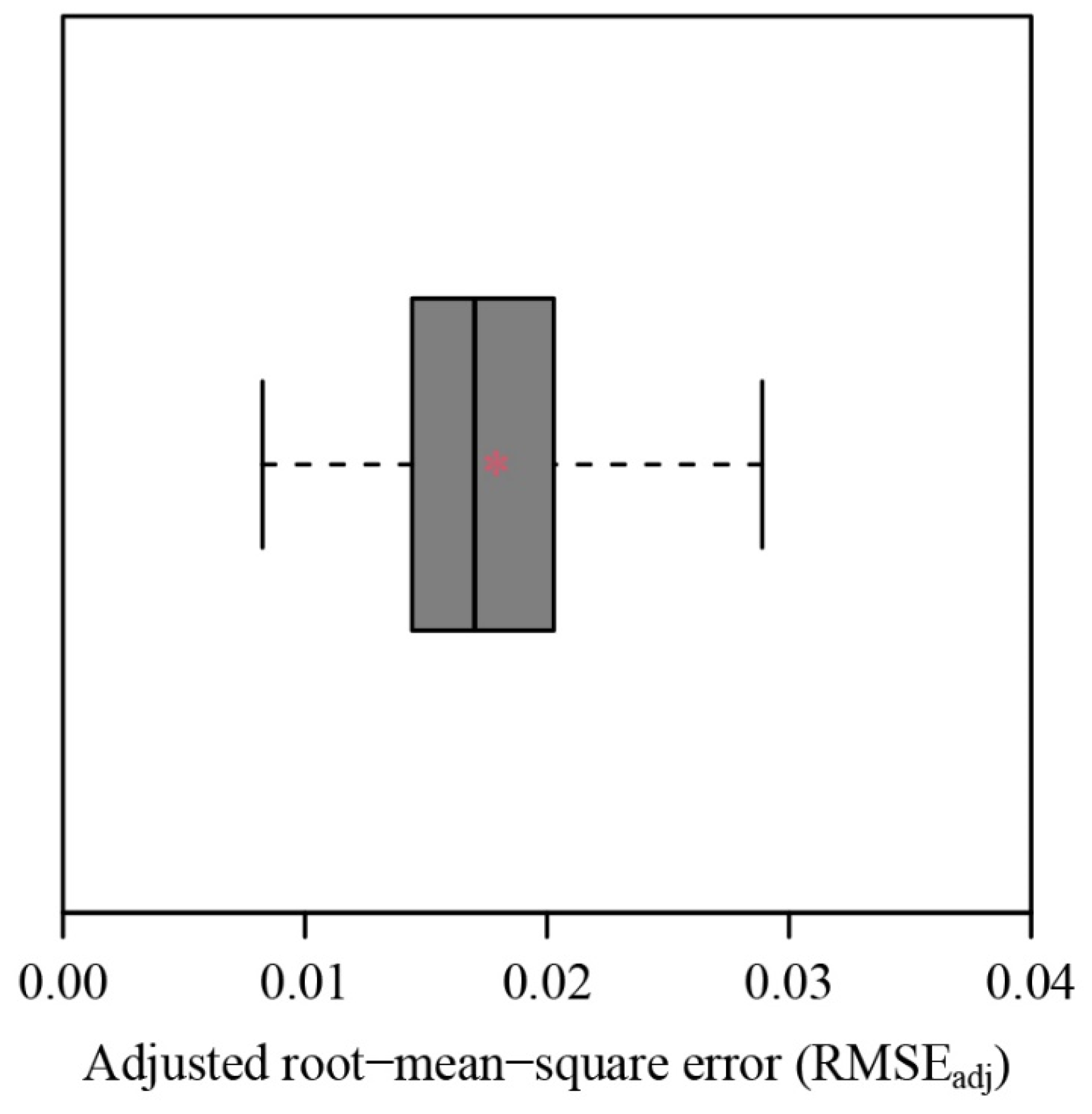
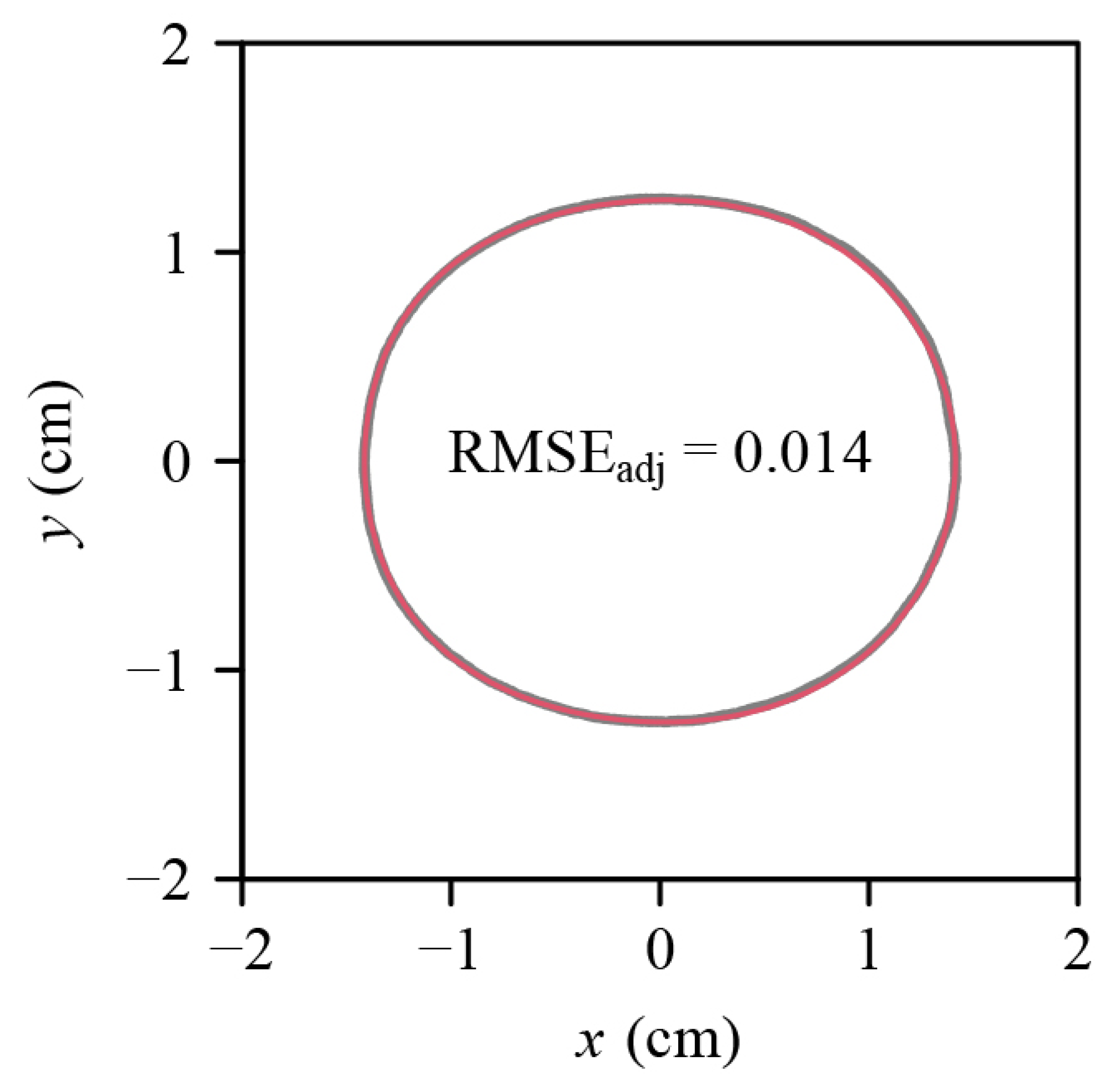
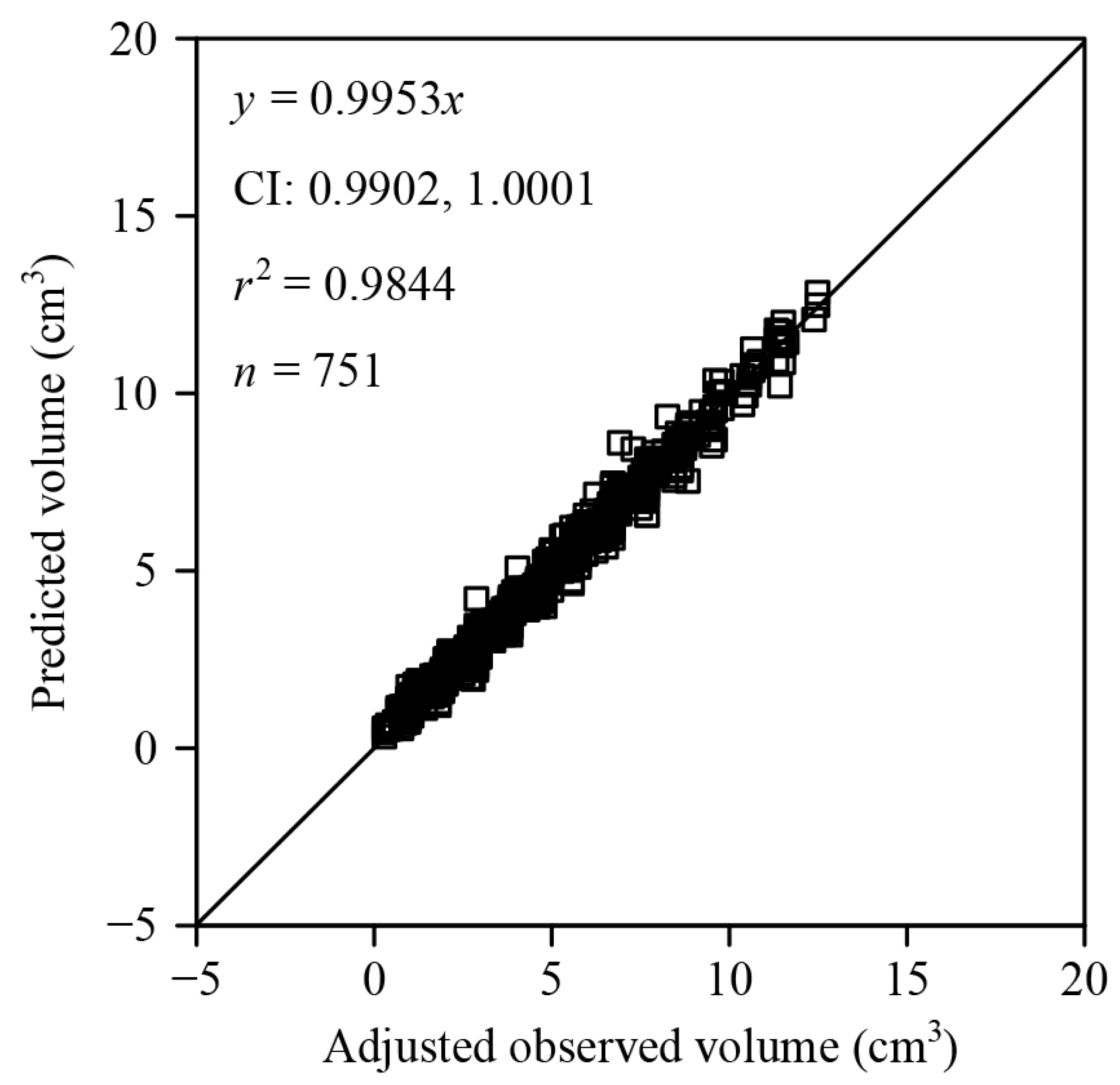
2.4. Testing the Solid of Revolution Hypothesis
2.5. Statistical Analysis
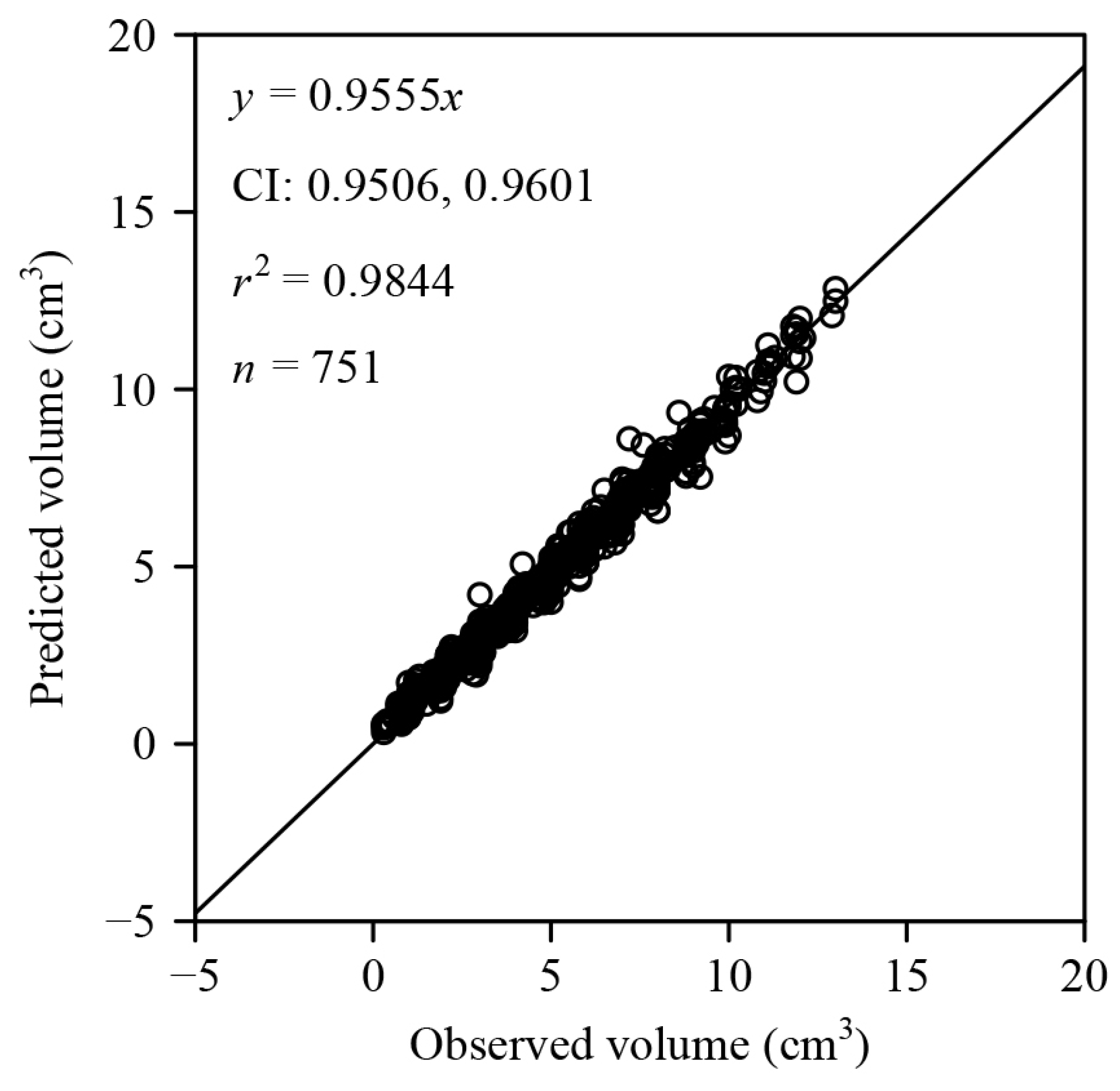
3. Results
4. Discussion
4.1. Validity of the Explicit Preston Equation for Describing Fruits
4.2. Are C. melo var. agrestis Fruits Solids of Revolution?
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferrándiz, C.; Pelaz, S.; Yanofsky, M.F. Control of carpel and fruit development in Arabidopsis. Annu. Rev. Biochem. 1999, 68, 321–354. [Google Scholar] [CrossRef] [PubMed]
- Linkies, A.; Graeber, K.; Knight, C.; Leubner-Metzger, G. The evolution of seeds. New Phytol. 2010, 186, 817–831. [Google Scholar] [CrossRef] [PubMed]
- van der Pijl, L. Principles of Dispersal in Higher Plants; Springer: Berlin, Germany, 1969. [Google Scholar]
- Shi, P.; Sandhu, H.S.; Reddy, G.V.P. Dispersal distance determines the exponent of the spatial Taylor’s power law. Ecol. Model. 2016, 335, 48–53. [Google Scholar] [CrossRef]
- Snow, D.W. Evolutionary aspects of fruit-eating by birds. Ibis 1971, 113, 194–202. [Google Scholar] [CrossRef]
- Stiles, E.W. The influence of pulp lipids on fruit preference by birds. In Frugivory and Seed Dispersal: Ecological and Evolutionary Aspects; Fleming, T.H., Estrada, A., Eds.; Springer: Dordrecht, The Netherlands, 1993; Advances in Vegetation Science; Volume 15, pp. 227–235. [Google Scholar]
- Levin, D.A.; Kerster, H.W. Gene flow in seed plants. Evol. Biol. 1974, 7, 139–220. [Google Scholar]
- Janzen, D.H. Herbivores and the number of tree species in tropical forests. Am. Nat. 1970, 104, 501–528. [Google Scholar] [CrossRef]
- Mazer, S.J.; Wheelwright, N.T. Fruit size and shape: Allometry at different taxonomic levels in bird-dispersed plants. Evol. Ecol. 1993, 7, 556–575. [Google Scholar] [CrossRef]
- Bach, C.E.; Kelly, D. Effects of forest edges, fruit display size, and fruit colour on bird seed dispersal in a New Zealand mistletoe, Alepis flavida. N. Z. J. Ecol. 2004, 28, 93–103. [Google Scholar]
- Hodgkison, R.; Ayasse, M.; Häberlein, C.; Schulz, S.; Zubaid, A.; Mustapha, W.A.W.; Kunz, T.H.; Kalko, E.K.V. Fruit bats and bat fruits: The evolution of fruit scent in relation to the foraging behaviour of bats in the New and Old World tropics. Funct. Ecol. 2013, 27, 1075–1084. [Google Scholar] [CrossRef]
- Shi, Y.; Li, B.; Su, G.; Zhang, M.; Grierson, D.; Chen, K. Transcriptional regulation of fleshy fruit texture. J. Integr. Plant Biol. 2022, 64, 1649–1672. [Google Scholar] [CrossRef]
- Wang, L.; Shi, P.; Chen, L.; Gielis, J.; Niklas, K.J. Evidence that Chinese white olive (Canarium album (Lour.) DC.) fruits are solids of revolution. Bot. Lett. 2023, in press. [Google Scholar] [CrossRef]
- Jordano, P. Avian fruit removal: Effects of fruit variation, crop size, and insect damage. Ecology 1987, 68, 1711–1723. [Google Scholar] [CrossRef] [PubMed]
- Herrera, C.M. The fruiting ecology of Osyris quadripartita: Individual variation and evolutionary potential. Ecology 1988, 69, 233–249. [Google Scholar] [CrossRef]
- Rey, P.J.; Gutiérrez, J.E.; Alcántara, J.; Valera, F. Fruit size in wild olives: Implications for avian seed dispersal. Funct. Ecol. 1997, 11, 611–618. [Google Scholar] [CrossRef]
- Levey, D.J. Seed size and fruit-handling techniques of avian frugivores. Am. Nat. 1987, 129, 471–485. [Google Scholar] [CrossRef]
- Snouffer, A.; Kraus, C.; van der Knaap, E. The shape of things to come: Ovate family proteins regulate plant organ shape. Curr. Opin. Plant Biol. 2020, 53, 98–105. [Google Scholar] [CrossRef]
- Clark, J.R. Grape breeding at the University of Arkansas: Approaching forty years of progress. Acta Horitic. 2003, 603, 357–360. [Google Scholar] [CrossRef]
- Olatunji, J.R.; Redding, G.P.; Rowe, C.L.; East, A.R. Reconstruction of kiwifruit fruit geometry using a CGAN trained on a synthetic dataset. Comput. Electron. Agric. 2020, 177, 105699. [Google Scholar] [CrossRef]
- Chomicki, G.; Schaefer, H.; Renner, S.S. Origin and domestication of Cucurbitaceae crops: Insights from phylogenies, genomics and archaeology. New Phytol. 2020, 226, 1240–1255. [Google Scholar] [CrossRef]
- Xu, H.; Su, W.; Lu, C.; Zhang, Z.; Li, H.; Xue, F.; Yang, M.; Wu, R. Differential sensitivity of field muskmelon (Cucumis melo L. var. agrestis Naud.) populations to nicosulfuron, imazapic, fomesafen and bentazon. Crop Prot. 2018, 106, 58–63. [Google Scholar] [CrossRef]
- Hui, L.; Xu, W.; Chen, W.; Zhou, Z.; Li, J.; Ma, T.; Feng, S.; Xu, Y.; Zhao, T.; Yang, H. Characterization of the complete chloroplast genome of Cucunmis melo L. var. Agrestis Naud. Mitochondrial DNA Part B 2020, 5, 2744–2745. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lu, Y.; Zhang, Y.; Fu, C.; Huang, D. Physicochemical and functional properties of red lentil protein isolates from three origins at different pH. Food Chem. 2021, 358, 129749. [Google Scholar] [CrossRef]
- Preston, F.W. The shapes of birds’ eggs. Auk 1953, 70, 160–182. [Google Scholar] [CrossRef]
- Troscianko, J. A simple tool for calculating egg shape, volume and surface area from digital images. Ibis 2014, 156, 874–878. [Google Scholar] [CrossRef]
- Biggins, J.D.; Thompson, J.E.; Birkhead, T.R. Accurately quantifying the shape of birds’ eggs. Ecol. Evol. 2018, 8, 9728–9738. [Google Scholar] [CrossRef] [PubMed]
- Biggins, J.D.; Montgomerie, R.; Thompson, J.E.; Birkhead, T.R. Preston’s universal formula for avian egg shape. Ornithology 2022, 139, ukac028. [Google Scholar] [CrossRef]
- Narushin, V.G.; Romanov, M.N.; Griffin, D.K. Egg and math: Introducing a universal formula for egg shape. Ann. N. Y. Acad. Sci. 2021, 1505, 169–177. [Google Scholar] [CrossRef]
- Shi, P.; Gielis, J.; Niklas, K.J. Comparison of a universal (but complex) model for avian egg shape with a simpler model. Ann. N. Y. Acad. Sci. 2022, 1514, 34–42. [Google Scholar] [CrossRef]
- Shi, P.; Chen, L.; Quinn, B.K.; Yu, K.; Miao, Q.; Guo, X.; Lian, M.; Gielis, J.; Niklas, K.J. A simple way to calculate the volume and surface area of avian eggs. Ann. N. Y. Acad. Sci. 2023, 1524, 118–131. [Google Scholar] [CrossRef]
- Shi, P.; Wang, L.; Quinn, B.K.; Gielis, J. A new program to estimate the parameters of Preston’s equation, a general formula for describing the egg shape of birds. Symmetry 2023, 15, 231. [Google Scholar] [CrossRef]
- Shi, P.; Ratkowsky, D.A.; Li, Y.; Zhang, L.; Lin, S.; Gielis, J. A general leaf area geometric formula exists for plants—Evidence from the simplified Gielis equation. Forests 2018, 9, 714. [Google Scholar] [CrossRef]
- Su, J.; Niklas, K.J.; Huang, W.; Yu, X.; Yang, Y.; Shi, P. Lamina shape does not correlate with lamina surface area: An analysis based on the simplified Gielis equation. Glob. Ecol. Conserv. 2019, 19, e00666. [Google Scholar] [CrossRef]
- Shi, P.; Gielis, J.; Quinn, B.K.; Niklas, K.J.; Ratkowsky, D.A.; Schrader, J.; Ruan, H.; Wang, L.; Niinemets, Ü. “Biogeom”: An R package for simulating and fitting natural shapes. Ann. N. Y. Acad. Sci. 2022, 1516, 123–134. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.r-project.org/ (accessed on 1 June 2022).
- Narushin, V.G.; Romanov, M.N.; Mishra, B.; Griffin, D.K. Mathematical progression of avian egg shape with associated area and volume determinations. Ann. N. Y. Acad. Sci. 2022, 1513, 65–78. [Google Scholar] [CrossRef]
- Nelder, J.A.; Mead, R. A simplex method for function minimization. Comput. J. 1965, 7, 308–313. [Google Scholar] [CrossRef]
- Niklas, K.J. Plant Allometry; University of Chicago Press: Chicago, CA, USA, 1994. [Google Scholar]
- Quinn, G.P.; Keough, M.J. Experimental Design and Data Analysis for Biologists; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Efron, B.; Tibshirani, R.J. An Introduction to the Bootstrap; Chapman and Hall/CRC: Boca Raton, FL, USA, 1993. [Google Scholar]
- Sandhu, H.S.; Shi, P.; Kuang, X.; Xue, F.; Ge, F. Applications of the bootstrap to insect physiology. Fla. Entomol. 2011, 94, 1036–1041. [Google Scholar] [CrossRef]
- Gielis, J. A general geometric transformation that unifies a wide range of natural and abstract shapes. Am. J. Bot. 2003, 90, 333–338. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, K.; Hui, C.; Yao, W.; Wang, J.; Wang, L.; Li, Q.; Shi, P. Evidence That Field Muskmelon (Cucumis melo L. var. agrestis Naud.) Fruits Are Solids of Revolution. Plants 2023, 12, 4186. https://doi.org/10.3390/plants12244186
He K, Hui C, Yao W, Wang J, Wang L, Li Q, Shi P. Evidence That Field Muskmelon (Cucumis melo L. var. agrestis Naud.) Fruits Are Solids of Revolution. Plants. 2023; 12(24):4186. https://doi.org/10.3390/plants12244186
Chicago/Turabian StyleHe, Ke, Cang Hui, Weihao Yao, Jinfeng Wang, Lin Wang, Qiying Li, and Peijian Shi. 2023. "Evidence That Field Muskmelon (Cucumis melo L. var. agrestis Naud.) Fruits Are Solids of Revolution" Plants 12, no. 24: 4186. https://doi.org/10.3390/plants12244186
APA StyleHe, K., Hui, C., Yao, W., Wang, J., Wang, L., Li, Q., & Shi, P. (2023). Evidence That Field Muskmelon (Cucumis melo L. var. agrestis Naud.) Fruits Are Solids of Revolution. Plants, 12(24), 4186. https://doi.org/10.3390/plants12244186







