A Biostimulant Based on Silicon Chelates Enhances Growth and Modulates Physiological Responses of In-Vitro-Derived Strawberry Plants to In Vivo Conditions
Abstract
:1. Introduction
2. Results
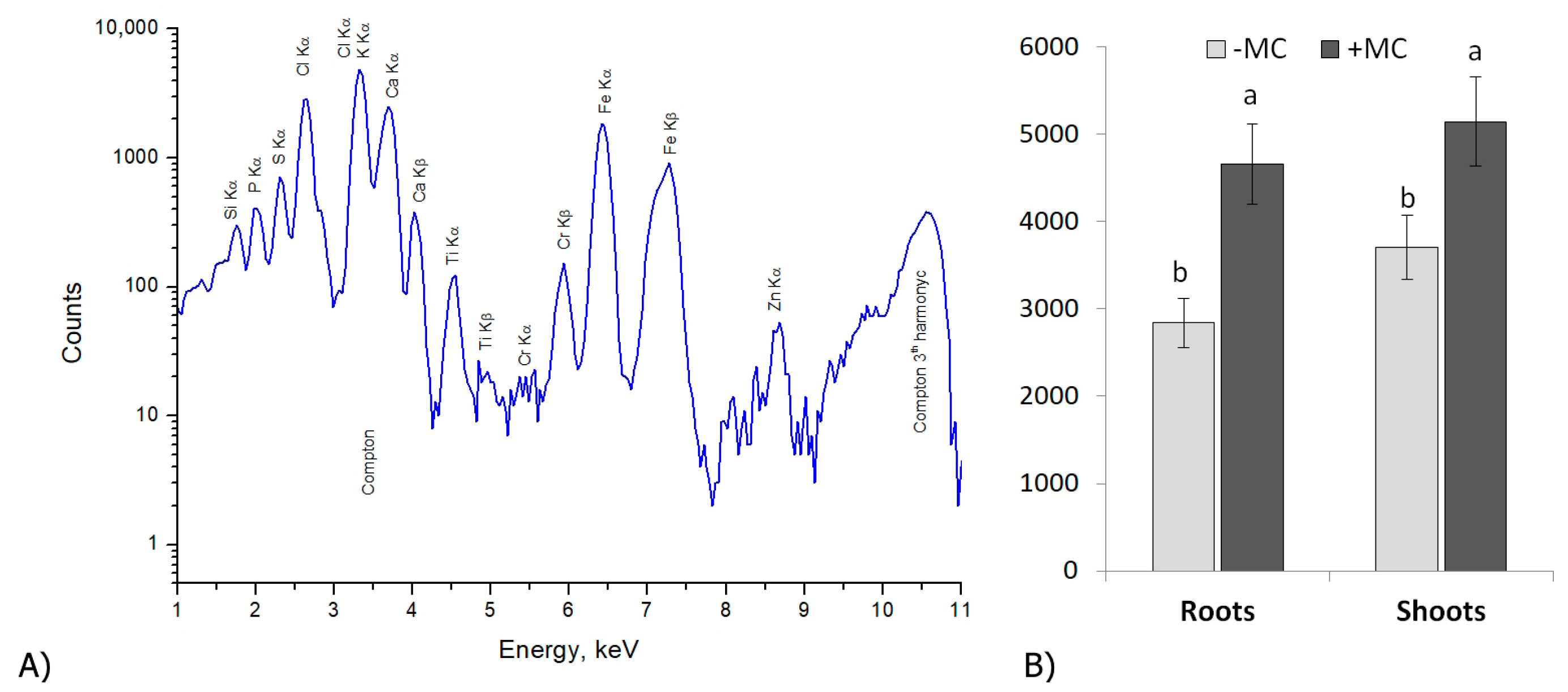
2.1. The Si Content of In-Vitro-Derived Strawberry Plants
2.2. Growth Status and Vegetative Productivity of In-Vitro-Derived Strawberry Plants
2.3. Physiological Characteristics of In-Vitro-Derived Strawberry Plants
2.4. Cuticle Thickness in In-Vitro-Derived Strawberry Plants
3. Discussion
4. Materials and Methods
4.1. Preparation and Characterization of Silicon Chelates
4.2. Experimental Design
4.3. Analysis of Growth Characteristics
4.4. Determination of the RWC
4.5. Determination of Pigment Concentrations
4.6. Analysis of Hydrogen Peroxide and Enzymatic Antioxidants
4.7. Phytohormone Quantification
4.8. High-Performance Liquid Chromatography (HPLC) Analysis of Phenolic Compounds
4.9. X-ray Fluorescence Analysis of Si
4.10. The Cuticle Thickness Measurement
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Durán-Lara, E.F.; Valderrama, A.; Marican, A. Natural organic compounds for application in organic farming. Agriculture 2020, 10, 41. [Google Scholar] [CrossRef]
- Garza-Alonso, C.A.; Olivares-Sáenz, E.; González-Morales, S.; Cabrera-De la Fuente, M.; Juárez-Maldonado, A.; González-Fuentes, J.A.; Tortella, G.; Valdés-Caballero, M.V.; Benavides-Mendoza, A. Strawberry biostimulation: From mechanisms of action to plant growth and fruit quality. Plants 2022, 11, 3463. [Google Scholar] [CrossRef] [PubMed]
- Rana, V.S.; Sharma, S.; Rana, N.; Sharma, U. Sustainable production through biostimulants under fruit orchards. CABI Agric. Biosci. 2022, 3, 38. [Google Scholar] [CrossRef]
- Savvas, D.; Ntatsi, G. Biostimulant activity of silicon in horticulture. Sci. Hortic. 2015, 196, 66–81. [Google Scholar] [CrossRef]
- Schaller, J.; Cramer, A.; Carminati, A.; Mohsen, Z. Biogenic amorphous silica as main driver for plant available water in soils. Sci. Rep. 2020, 10, 2424. [Google Scholar] [CrossRef]
- Ma, J.F. Role of silicon in enhancing the resistance of plants to biotic and abiotic stresses. Soil Sci. Plant Nutr. 2004, 50, 11–18. [Google Scholar] [CrossRef]
- Zargar, S.M.; Mahajan, R.; Bhat, J.A.; Nazir, M.; Deshmukh, R. Role of silicon in plant stress tolerance: Opportunities to achieve a sustainable cropping system. 3 Biotech 2019, 9, 73. [Google Scholar] [CrossRef]
- Verma, K.K.; Song, X.-P.; Tian, D.-D.; Guo, D.-J.; Chen, Z.-L.; Zhong, C.-S.; Nikpay, A.; Singh, M.; Rajput, V.D.; Singh, R.K.; et al. Influence of silicon on biocontrol strategies to manage biotic stress for crop protection, performance, and improvement. Plants 2021, 10, 2163. [Google Scholar] [CrossRef]
- Matichenkov, V.V.; Bocharnikova, E.A. Chapter 13. The relationship between silicon and soil physical and chemical properties. In Silicon in Agriculture; Datnoff, L.E., Snyder, G.H., Korndorfer, G.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2001; pp. 209–219. [Google Scholar] [CrossRef]
- Vatansever, R.; Ozyigit, I.I.; Filiz, E. Essential and beneficial trace elements in plants, and their transport in roots: A review. Appl. Biochem. Biotechnol. 2017, 181, 464–482. [Google Scholar] [CrossRef]
- Artyszak, A. Effect of silicon fertilization on crop yield quantity and quality—A literature review in Europe. Plants 2018, 7, 54. [Google Scholar] [CrossRef]
- Calero Hurtado, A.; Chiconato, D.A.; de Prado, R.M.; da Sousa Junior, G.S.; Felisberto, G. Silicon attenuates sodium toxicity by improving nutritional efficiency in sorghum and sunflower plants. Plant Physiol. Biochem. 2019, 142, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Coskun, D.; Deshmukh, R.; Sonah, H.; Menzies, J.G.; Reynolds, O.; Ma, J.F.; Kronzucker, H.J.; Belanger, R.R. The controversies of silicon’s role in plant biology. N. Phytol. 2019, 221, 67–85. [Google Scholar] [CrossRef] [PubMed]
- Joudmand, A.; Hajiboland, R. Silicon mitigates cold stress in barley plants via modifying the activity of apoplasmic enzymes and concentration of metabolites. Acta Physiol. Plant. 2019, 41, 29. [Google Scholar] [CrossRef]
- Ali, N.; Réthoré, E.; Yvin, J.-C.; Hosseini, S.A. The regulatory role of silicon in mitigating plant nutritional stresses. Plants 2020, 9, 1779. [Google Scholar] [CrossRef] [PubMed]
- Hajiboland, R.; Moradtalab, N.; Aliasgharzad, N.; Eshaghi, Z.; Feizy, J. Silicon influences growth and mycorrhizal responsiveness in strawberry plants. Physiol. Mol. Biol. Plants 2018, 24, 1103–1115. [Google Scholar] [CrossRef] [PubMed]
- Luyckx, M.; Hausman, J.-F.; Lutts, S.; Guerriero, G. Silicon and plants: Current knowledge and technological perspectives. Front. Plant Sci. 2017, 8, 411. [Google Scholar] [CrossRef] [PubMed]
- Sahebi, M.; Hanafi, M.M.; Nor Akmar, A.S.; Rafii, M.Y.; Azizi, P.; Tengoua, F.F.; Azwa, J.N.M.; Shabanimofrad, M. Importance of silicon and mechanisms of biosilica formation in plants. BioMed Res. Int. 2015, 2015, 396010. [Google Scholar] [CrossRef]
- Lomovsky, O.I.; Lomovskiy, I.O.; Orlov, D.V. Mechanochemical solid acid/base reactions for obtaining biologically active preparations and extracting plant materials. Green Chem. Lett. Rev. 2017, 10, 171–185. [Google Scholar] [CrossRef]
- Trofimova, E.G.; Podgorbunskikh, E.M.; Skripkina, T.S.; Bychkov, A.L.; Lomovsky, O.I. Scaling of the mechanochemical process of production of silicon chelates. Bulg. Chem. Commun. 2018, 50, 45–48. [Google Scholar]
- Tolisano, C.; Del Buono, D. Biobased: Biostimulants and biogenic nanoparticles enter the scene. Sci. Total Environ. 2023, 885, 163912. [Google Scholar] [CrossRef]
- Ambros, E.; Karpova, E.; Kotsupiy, O.; Zaytseva, Y.; Trofmova, E.; Novikova, T. Silicon chelates from plant waste promote in vitro shoot production and physiological changes in strawberry plantlets. Plant Cell Tissue Organ Cult. 2021, 145, 209–221. [Google Scholar] [CrossRef]
- Ambros, E.; Karpova, E.; Kotsupiy, O.; Trofimova, E.; Zakabluk, G.; Chernonosov, A.; Koval, V.; Novikova, T. A mechanocomposite based on biogenic silica and green tea flavonoids modulates adaptability of strawberry microclones to in vitro and ex vitro conditions. J. Soil Sci. Plant Nutr. 2023, 23, 612–627. [Google Scholar] [CrossRef]
- Liang, Y.; Urano, D.; Liao, K.L.; Hedrick, T.L.; Gao, Y.; Jones, A.M. A nondestructive method to estimate the chlorophyll content of Arabidopsis seedlings. Plant Methods 2017, 13, 26. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, N.S.H.; Kirana, I.A.P.; Abidin, A.S.; Jupri, A.; Widyastuti, S.; Hernawan, A.; Nikmatullah, A.; Sunarpi, H.; Prasedya, E.S. Analysis of leaf chlorophyll content of paddy plants during vegetative stage grown in soil media containing macroalgae organic fertilizer. IOP Conf. Ser. Earth Environ. Sci. 2021, 913, 012025. [Google Scholar] [CrossRef]
- Qian, X.; Liu, L.; Chen, X.; Zhang, X.; Chen, S.; Sun, Q. The global leaf chlorophyll content dataset over 2003–2012 and 2018–2020 derived from MERIS/OLCI satellite data (GLCC): Algorithm and validation. Earth Syst. Sci. 2022, in press. [Google Scholar] [CrossRef]
- Borghesi, E.; González-Miret, M.L.; Escudero-Gilete, M.L.; Malorgio, F.; Heredia, F.J.; Meléndez-Martínez, A.J. Effects of salinity stress on carotenoids, anthocyanins, and color of diverse tomato genotypes. J. Agric. Food Chem. 2011, 59, 11676–11682. [Google Scholar] [CrossRef]
- Motyka, O.; Štrbová, K.; Zinicovscaia, I. Chlorophyll content in two medicinal plant species following nano-TiO2 exposure. Bull. Environ. Contam. Toxicol. 2020, 104, 373–379. [Google Scholar] [CrossRef]
- Amri, M.; Abbes, Z.; Trabelsi, I.; Ghanem, M.E.; Mentag, R.; Kharrat, M. Chlorophyll content and fluorescence as physiological parameters for monitoring Orobanche foetida Poir. infection in faba bean. PLoS ONE 2021, 16, e0241527. [Google Scholar] [CrossRef]
- Jiroutova, P.; Kovalikova, Z.; Toman, J.; Dobrovolna, D.; Andrys, R. Complex analysis of antioxidant activity, abscisic acid level, and accumulation of osmotica in apple and cherry in vitro cultures under osmotic stress. Int. J. Mol. Sci. 2021, 22, 7922. [Google Scholar] [CrossRef]
- Vuolo, M.M.; Lima, V.S.; Maróstica Junior, M.R. Phenolic compounds: Structure, classification, and antioxidant power. In Bioactive Compounds: Health Benefits and Potential Applications Provides; Campos, M.R.S., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 33–50. [Google Scholar] [CrossRef]
- Zhang, J.; Kirkham, M.B. Drought-stress-induced changes in activities of superoxide dismutase, catalase, and peroxidase in wheat species. Plant Cell Physiol. 1994, 35, 785–791. [Google Scholar] [CrossRef]
- Leung, D.W.M. Studies of catalase in plants under abiotic stress. In Antioxidants and Antioxidant Enzymes in Higher Plants; Gupta, D.K., Palma, J.M., Corpas, F.J., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 27–39. [Google Scholar]
- Ranjith, H.V.; Sagar, D.; Kalia, V.K.; Dahuja, A.; Subramanian, S. Differential activities of antioxidant enzymes, superoxide dismutase, peroxidase, and catalase vis-à-vis phosphine resistance in field populations of lesser grain borer (Rhyzopertha dominica) from India. Antioxidants 2023, 12, 270. [Google Scholar] [CrossRef] [PubMed]
- Peleg, Z.; Blumwald, E. Hormone balance and abiotic stress tolerance in crop plants. Curr. Opin. Plant Biol. 2011, 14, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Sabagh, A.E.; Islam, M.S.; Hossain, A.; Iqbal, M.A.; Mubeen, M.; Waleed, M.; Reginato, M.; Battaglia, M.; Ahmed, S.; Rehman, A.; et al. Phytohormones as growth regulators during abiotic stress tolerance in plants. Front. Agron. 2022, 4, 765068. [Google Scholar] [CrossRef]
- Lim, C.W.; Baek, W.; Jung, J.; Kim, J.H.; Lee, S.C. Function of ABA in stomatal defense against biotic and drought stresses. Int. J. Mol. Sci. 2015, 16, 15251–15270. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Zhu, J.K. Regulation of abscisic acid biosynthesis. Plant Physiol. 2003, 133, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.Y.; Zhang, J.H. Role of abscisic acid in water stress induced antioxidant defense in leaves of maize seedlings. Free Radic. Res. 2002, 36, 1001–1015. [Google Scholar] [CrossRef] [PubMed]
- Ntatsi, G.; Savvas, D.; Schwarz, D. Role of abscisic acid in the adaptation of grafted tomato to moderately suboptimal temperature stress. Acta Hortic. 2012, 952, 295–302. [Google Scholar] [CrossRef]
- Tuteja, N.; Sopory, S.K. Chemical signaling under abiotic stress environment in plants. Plant Signal. Behav. 2008, 3, 525–536. [Google Scholar] [CrossRef]
- Ahmad, Z.; Abbasi, A.; Sultana, S.; Waraich, E.; Artyszak, A.; Iqbal, M.A.; Barutçular, C. Manipulation of silicon metabolism in plants for stress tolerance. In Silicon and Nano-Silicon in Environmental Stress Management and Crop Quality Improvement; Etesami, H., Saeedi, A.H.A., El-Ramady, H., Masayuki, F., Mohammad, P., Anwar, H.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 339–348. [Google Scholar] [CrossRef]
- Mir, R.A.; Bhat, B.A.; Yousuf, H.; Islam, S.T.; Raza, A.; Rizvi, M.A.; Charagh, S.; Albaqami, M.; Sofi, P.A.; Zargar, S.M. Multidimensional role of silicon to activate resilient plant growth and to mitigate abiotic stress. Front. Plant Sci. 2022, 13, 819658. [Google Scholar] [CrossRef]
- Currie, H.A.; Perry, C.C. Silica in plants: Biological, biochemical and chemical studies. Ann. Bot. 2007, 7, 1383–1389. [Google Scholar] [CrossRef]
- Ma, J.F.; Takahashi, E. Soil, Fertilizer, and Plant Silicon Research in Japan; Elsevier: Amsterdam, The Netherlands, 2002; p. 281. [Google Scholar] [CrossRef]
- Miyake, Y.; Takahashi, E. Effect of silicon on the growth and fruit production of strawberry plants in a solution culture. J. Soil Sci. Plant Nutr. 1986, 32, 321–326. [Google Scholar] [CrossRef]
- Ouellette, S.; Goyette, M.H.; Labbé, C.; Laur, J.; Gaudreau, L.; Gosselin, A.; Dorais, M.; Deshmukh, R.K.; Bélanger, R.R. Silicon transporters and effects of silicon amendments in strawberry under high tunnel and field conditions. Front. Plant Sci. 2017, 8, 949. [Google Scholar] [CrossRef] [PubMed]
- Kanto, T.; Miyoshi, A.; Ogawa, T.; Maekawa, K.; Aino, M. Suppressive effect of liquid potassium silicate on powdery mildew of strawberry in soil. J. Gen. Plant Pathol. 2006, 72, 137–142. [Google Scholar] [CrossRef]
- Hodson, M.J.; White, P.J.; Mead, A.; Broadley, M.R. Phylogenetic variation in the silicon composition of plants. Ann. Bot. 2005, 96, 1027–1046. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Nikolic, M.; Bélanger, R.; Gong, H.; Song, A. Silicon in Agriculture: From Theory to Practice; Springer: Dordrecht, The Netherlands, 2015; p. 235. [Google Scholar] [CrossRef]
- Singh, P.; Kumar, V.; Sharma, A. Interaction of silicon with cell wall components in plants: A review. J. Appl. Nat. Sci. 2023, 15, 480–497. [Google Scholar] [CrossRef]
- Chen, W.; Yao, X.; Cai, K.; Chen, J. Silicon alleviates drought stress of rice plants by improving plant water status, photosynthesis and mineral nutrient absorption. Biol. Trace Element Res. 2011, 142, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.X.; Gong, H.J.; Yin, J.L. Role of silicon in mediating salt tolerance in plants: A review. Plants 2019, 8, 147. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, J.; Hu, J.; Jeong, B.R. Method of silicon application affects quality of strawberry daughter plants during cutting propagation in hydroponic substrate system. Agronomy 2020, 10, 1753. [Google Scholar] [CrossRef]
- Pozo, J.; Urrestarazu, M.; Morales, I.; Sanchez, J.; Santos, M.; Dianez, F.; Alvaro, J.E. Effects of silicon in the nutrient solution for three horticultural plant families on the vegetative growth, cuticle, and protection against Botrytis cinerea. HortScience 2015, 50, 1447–1452. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, X.; Hu, Y.; Han, W.; Yin, J.; Li, H.; Gong, H.-J. Silicon improves salt tolerance by increasing root water uptake in Cucumis sativus L. Plant Cell Rep. 2015, 34, 1629–1646. [Google Scholar] [CrossRef]
- Chen, D.; Wang, S.; Yin, L.; Deng, X. How does silicon mediate plant water uptake and loss under water deficiency? Front. Plant Sci. 2018, 9, 281. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, R.; Mur, L.A.J.; Ruan, J.; Shen, Q.; Guo, S. Functions of silicon in plant drought stress responses. Hortic. Res. 2021, 8, 254. [Google Scholar] [CrossRef] [PubMed]
- Remus-Borel, W.; Menzies, J.G.; Bélanger, R.R. Silicon induces antifungal compounds in powdery mildew-infected wheat. Physiol. Mol. Plant Pathol. 2005, 66, 108–115. [Google Scholar] [CrossRef]
- Rahman, A.; Wallis, C.M.; Uddin, W. Silicon-induced systemic defense responses in perennial ryegrass against infection by Magnaporthe oryzae. Phytopathology 2015, 105, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Dubey, A.K.; Upadhyay, A.K.; Gautam, A.; Ranjan, R.; Srikishna, S.; Sahu, N.; Behera, S.K.; Mallick, S. GABA accretion reduces Lsi-1 and Lsi-2 gene expressions and modulates physiological responses in Oryza sativa to provide tolerance towards arsenic. Sci. Rep. 2017, 7, 8786. [Google Scholar] [CrossRef] [PubMed]
- Katz, O.; Puppe, D.; Kaczorek, D.; Prakash, N.B.; Schaller, J. Silicon in the soil–plant continuum: Intricate feedback mechanisms within ecosystems. Plants 2021, 10, 652. [Google Scholar] [CrossRef]
- Markovich, O.; Steiner, E.; Kouřil, Š.; Tarkowski, P.; Aharoni, A.; Elbaum, R. Silicon promotes cytokinin biosynthesis and delays senescence in Arabidopsis and Sorghum. Plant Cell Environ. 2017, 40, 1189–1196. [Google Scholar] [CrossRef]
- Gai, Z.; Wang, Y.; Ding, Y.; Qian, W.; Qiu, C.; Xie, H.; Sun, L.; Jiang, Z.; Ma, Q.; Wang, L.; et al. Exogenous abscisic acid induces the lipid and flavonoid metabolism of tea plants under drought stress. Sci. Rep. 2020, 10, 12275. [Google Scholar] [CrossRef]
- Hao, G.; Du, X.; Zhao, F.; Ji, H. Fungal endophytes-induced abscisic acid is required for flavonoid accumulation in suspension cells of Ginkgo biloba. Biotechnol. Lett. 2010, 32, 305–314. [Google Scholar] [CrossRef]
- Vega, I.; Rumpel, C.; Ruíz, A.; Mora, M.D.L.L.; Calderini, D.F.; Cartes, P. Silicon modulates the production and composition of phenols in barley under aluminum stress. Agronomy 2020, 10, 1138. [Google Scholar] [CrossRef]
- Pagassini, J.A.V.; de Godoy, L.J.G.; Campos, F.G.; Barzotto, G.R.; Vieira, M.A.R.; Boaro, C.S.F. Silicon and mechanical damage increase polyphenols and vitexin in Passiflora incarnata L. Sci. Rep. 2021, 11, 22064. [Google Scholar] [CrossRef] [PubMed]
- Carneiro-Carvalho, A.; Aires, A.; Anjos, R.; Martins, L.; Pinto, T.; Peixoto, F.; Gomes-Laranjo, J. The role of silicon fertilization in the synthesis of phenolic compounds on chestnut plants infected with P. cinnamomi and C. parasitica. J. Plant Dis. Prot. 2020, 127, 211–227. [Google Scholar] [CrossRef]
- Shahnaz, G.; Shekoofeh, E.; Kourosh, D.; Moohamadbagher, B. Interactive effects of Silicon and Aluminum on the malondialdehyde (MDA), proline, protein and phenolic compounds in Borago officinalis L. J. Med. Plants Res. 2011, 5, 5818–5827. [Google Scholar] [CrossRef]
- Fini, A.; Brunetti, M.D.F.C.; Tattini, M. Stress-induced flavonoid biosynthesis and the antioxidant machinery of plants. Plant Signal. Behav. 2011, 6, 709–711. [Google Scholar] [CrossRef] [PubMed]
- Astashenkov, A.Y.; Karpova, E.A.; Cheryomushkina, V.A. Diversity patterns of life forms and phenolic profiles of endemic Nepeta plants along an aridity gradient of a high-mountain zone in Central Asia. Taiwania 2021, 66, 541–556. [Google Scholar] [CrossRef]
- Hajiboland, R.; Moradtalab, N.; Eshaghi, Z.; Feizy, J. Effect of silicon supplementation on growth and metabolism of strawberry plants at three developmental stages. N. Z. J. Crop Hortic. Sci. 2018, 46, 144–161. [Google Scholar] [CrossRef]
- Shetty, R.; Fretté, X.; Jensen, B.; Shetty, N.P.; Jensen, J.D.; Jorgensen, H.J.L.; Newman, M.; Christensen, L.P. Silicon-induced changes in antifungal phenolic acids, flavonoids, and key phenylpropanoid pathway genes during the interaction between miniature roses and the biotrophic pathogen Podosphaera pannosa. Plant Physiol. 2011, 157, 2194–2205. [Google Scholar] [CrossRef]
- Heilemann, J.; Strack, D. Incorporation of kaempferol 3-O-glucoside into the cell walls of Norway spruce needles. Planta 1990, 181, 599–603. [Google Scholar] [CrossRef]
- Adato, A.; Mandel, T.; Mintz-Oron, S.; Venger, I.; Levy, D.; Yativ, M.; Domínguez, E.; Wang, Z.; De Vos, R.C.; Jetter, R.; et al. Fruit-surface flavonoid accumulation in tomato is controlled by a SlMYB12-regulated transcriptional network. PLoS Genet. 2009, 5, e1000777. [Google Scholar] [CrossRef]
- Xu, D.; Qu, S.; Tucker, M.R.; Zhang, D.; Liang, W.; Shi, J. Ostkpr1 functions in anther cuticle development and pollen wall formation in rice. BMC Plant Biol. 2019, 19, 104. [Google Scholar] [CrossRef]
- Cho, J.Y.; Moon, J.H.; Seong, K.Y.; Park, K.H. Antimicrobial activity of 4-hydroxybenzoic acid and trans 4-hydroxycinnamic acid isolated and identified from rice hull. Biosci. Biotechnol. Biochem. 1998, 62, 2273–2276. [Google Scholar] [CrossRef] [PubMed]
- Kępa, M.; Miklasińska-Majdanik, M.; Wojtyczka, R.D.; Idzik, D.; Korzeniowski, K.; Smoleń-Dzirba, J.; Wąsik, T.J. Antimicrobial potential of caffeic acid against Staphylococcus aureus clinical strains. Biomed. Res. Int. 2018, 2018, 7413504. [Google Scholar] [CrossRef]
- Li, S.; Pi, J.; Zhu, H.; Yang, L.; Zhang, X.; Ding, W. Caffeic acid in tobacco root exudate defends tobacco plants from infection by Ralstonia solanacearum. Front. Plant Sci. 2021, 12, 690586. [Google Scholar] [CrossRef] [PubMed]
- Nehela, Y.; Taha, N.A.; Elzaawely, A.A.; Xuan, T.D.; Amin, M.A.; Ahmed, M.E.; El-Nagar, A. Benzoic acid and its hydroxylated derivatives suppress early blight of tomato (Alternaria solani) via the induction of salicylic acid biosynthesis and enzymatic and nonenzymatic antioxidant defense machinery. J. Fungi. 2021, 7, 663. [Google Scholar] [CrossRef] [PubMed]
- Barbehenn, R.V.; Peter Constabel, C. Tannins in plant-herbivore interactions. Phytochemistry 2011, 72, 1551–1565. [Google Scholar] [CrossRef] [PubMed]
- Farha, A.K.; Yang, Q.; Kim, G.; Li, H.; Zhu, F.; Liu, H.; Gan, R.; Corke, H.; Li, K.; Liu, Z.; et al. Tannins as an alternative to antibiotics. Food Biosci. 2020, 38, 100751. [Google Scholar] [CrossRef]
- Kalisz, A.; Huska, D.; Jurkow, R.; Dvořák, M.; Caruso, G.; Sękara, A. Nanoparticles of cerium, iron, and silicon oxides change the metabolism of phenols and flavonoids in butterhead lettuce and sweet pepper seedlings. Environ. Sci. Nano 2021, 8, 1945–1959. [Google Scholar] [CrossRef]
- Weber, F.; Barrantes, A.; Tiainen, H. Silicic acid-mediated formation of tannic acid nanocoatings. Langmuir 2019, 35, 3327–3336. [Google Scholar] [CrossRef]
- Yamasaki, S.; Dillenbur, L.R. Measurements of leaf relative water content in Araucaria angustifolia. R. Bras. Fisiol. Veg. 1999, 11, 69–75. [Google Scholar]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV–Vis spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.2.1–F4.2.6. [Google Scholar] [CrossRef]
- Wolff, S.P. Ferrous ion oxidation in presence of ferric iron indicator xylenol orange for measurement of hydroperoxide. Methods Enzymol. 1994, 233, 182–189. [Google Scholar] [CrossRef]
- Bellincampi, D.; Dipperro, N.; Salvi, G.; Cervcone, F.; De Lorenzo, G. Extracellular H2O2 induced by oligogalacturonides is not involved in the inhibition of the auxin-regulated rolB gene expression in tobacco leaf explants. Plant Physiol. 2000, 122, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Mehl, K.A.; Davis, M.; Berger, F.G.; Carson, J.A. Myofber degeneration/regeneration is induced in the cachectic ApsMin/+ mouse. J. Appl. Physiol. 2005, 99, 2379–2387. [Google Scholar] [CrossRef] [PubMed]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutase I. occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Zhanaeva, T.A.; Lobanova, I.E.; Kukushkina, T.A. Flavonols and flavonol-oxidizing enzymes during buckwheat ontogenesis. Biol. Bull. Russ. Acad. Sci. 1999, 26, 105–108. [Google Scholar]
- Dobrev, P.I.; Hoyerová, K.; Petrášek, J. Analytical determination of auxins and cytokinins. Methods Mol. Biol. 2017, 1569, 31–39. [Google Scholar] [CrossRef]
- Goldenberg, B.G.; Gusev, I.S.; Zubavichus, Y.V. Synchrotron-radiation technological station at the VEPP-4M storage ring. J. Surf. Investig. 2023, 17, 1088–1093. [Google Scholar] [CrossRef]



| Parameter | Treatment Type | |
|---|---|---|
| (−MC) Group | (+MC) Group | |
| root length (cm) | 13.32 ± 0.75 b * | 18.00 ± 1.27 a * |
| roots (number per plant) | 13.30 ± 1.47 b * | 20.35 ± 1.49 a * |
| root fresh weight (g) | 3.18 ± 0.40 b * | 6.52 ± 0.92 a * |
| root dry weight (g) | 0.83 ± 0.08 b ** | 1.72 ± 0.16 a ** |
| shoot height (cm) | 10.88 ± 0.42 b ** | 14.26 ± 0.63 a ** |
| leaves (number per plant) | 10.17 ± 0.66 a | 14.13 ± 1.16 a |
| leaf blade area (cm2) | 39.57 ± 4.34 a | 43.43 ± 3.00 a |
| shoot fresh weight (g) | 14.18 ± 1.54 b * | 26.30 ± 3.11 a * |
| shoot dry weight (g) | 3.33 ± 0.25 b * | 5.35 ± 0.54 a * |
| Parameter | Treatment Type | |
|---|---|---|
| (−MC) Group | (+MC) Group | |
| RWC (%) | 55.92 ± 1.71 b | 64.62 ± 2.40 a |
| chlorophyll a (mg g−1 FW) | 1.31 ± 0.05 a | 1.38 ± 0.04 a |
| chlorophyll b (mg g−1 FW) | 0.33 ± 0.01 a | 0.30 ± 0.01 a |
| total chlorophyll (mg g−1 FW) | 1.64 ± 0.07 a | 1.67 ± 0.05 a |
| chlorophyll a/b ratio | 3.94 ± 0.07 b | 4.66 ± 0.13 a |
| carotenoids (mg g−1 FW) | 0.44 ± 0.02 a | 0.46 ± 0.02 a |
| total chlorophyll/carotenoids | 3.93 ± 0.31 a | 3.66 ± 0.06 a |
| H2O2 (μmol (g FW)–1) | 123.27 ± 24.19 a | 56.08 ± 14.40 b |
| SOD (U (g FW)–1) | 98.28 ± 1.21 b | 103.74 ± 0.22 a |
| CAT ((U H2O2)(g FW)−1 min−1) | 8.33 ± 2.85 b | 29.51 ± 10.14 a |
| POD (U (g FW)–1 min–1) | 2.78 ± 1.00 a | 3.13 ± 0.07 a |
| IAA (ng g−1 FW) | 6.60 ± 1.01 a | 9.37 ± 0.83 a |
| ABA (ng g−1 FW) | 3.57 ± 0.61 a | 1.63 ± 0.10 b |
| t-Z (ng g−1 FW) | 2.65 ± 0.49 a | 2.06 ± 0.74 a |
| Phenolic Compound, mg g−1 DW | tR, min | λmax, nm | Treatment Type | |
|---|---|---|---|---|
| (−MC) Group | (+MC) Group | |||
| Hydroxybenzoic acids: | ||||
| gallic acid | 2.4 | 216, 272 | 0.17 ± 0.01 a | 0.18 ± 0.01 a |
| p-hydroxybenzoic acid | 6.8 | 254 | 0.07 ± 0.00 b | 0.12 ± 0.01 a |
| vanillic acid | 9.4 | 260, 290 | 4.03 ± 0.18 b | 5.82 ± 0.28 a |
| syringic acid | 9.7 | 220, 270 | 0.51 ± 0.03 b | 0.69 ± 0.02 a |
| Total | 4.78 ± 0.21 b | 6.81 ± 0.26 a | ||
| Hydroxycinnamic acids: | ||||
| neochlorogenic acid | 3.8 | 240, 296 sh., 325 | 0.15 ± 0.01 a | 0.17 ± 0.01 a |
| caffeic acid | 8.7 | 218, 240, 298 sh., 324 | 1.19 ± 0.05 a | 1.26 ± 0.06 a |
| p-coumaric acid | 10.8 | 226, 310 | 0.11 ± 0.01 a | 0.11 ± 0.05 a |
| ferulic acid | 13.3 | 218, 236, 320 | 0.04 ± 0.00 a | 0.04 ± 0.00 a |
| Total | 1.49 ± 0.07 a | 1.58 ± 0.07 a | ||
| Catechins: | ||||
| (+)-catechin | 5.3 | 204, 230 sh., 280 | 0.55 ± 0.03 a | 0.49 ± 0.02 a |
| epigallocatechin gallate | 7.7 | 204, 230 sh., 280 | 0.09 ± 0.00 b | 0.06 ± 0.00 a |
| Total | 0.64 ± 0.04 a | 0.55 ± 0.02 a | ||
| Ellagic acid and its derivatives: | ||||
| ellagic acid | 17.7 | 254, 367 | 1.04 ± 0.05 a | 1.13 ± 0.06 a |
| ellagic acid derivative 1 | 8.0 | 230, 262 | 1.00 ± 0.04 a | 1.13 ± 0.06 a |
| ellagic acid derivative 2 | 11.2 | 230, 276 | 1.16 ± 0.05 b | 1.82 ± 0.05 a |
| ellagic acid derivative 3 | 12.4 | 254, 362 | 0.74 ± 0.03 a | 0.53 ± 0.02 b |
| ellagic acid derivative 4 | 16.3 | 254, 360 | 2.55 ± 0.14 a | 2.26 ± 0.14 a |
| ellagic acid derivative 5 | 16.8 | 254, 360 | 0.75 ± 0.04 a | 0.65 ± 0.04 a |
| Total | 7.24 ± 0.35 a | 7.52 ± 0.21 a | ||
| Flavonol glycosides: | ||||
| kaempferol rutinoside | 19.2 | 265, 356 | 0.23 ± 0.01 a | 0.13 ± 0.01 b |
| quercetin glycoside 1 | 13.8 | 270, 355 | 4.78 ± 0.27 a | 5.48 ± 0.35 a |
| quercetin glycoside 2 | 14.0 | 270, 355 | 1.54 ± 0.07 a | 1.61 ± 0.07 a |
| Total | 6.55 ± 0.36 a | 7.23 ± 0.41 a | ||
| Total phenolic compounds | 20.70 ± 1.03 a | 23.69 ± 0.81 a | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ambros, E.; Kotsupiy, O.; Karpova, E.; Panova, U.; Chernonosov, A.; Trofimova, E.; Goldenberg, B. A Biostimulant Based on Silicon Chelates Enhances Growth and Modulates Physiological Responses of In-Vitro-Derived Strawberry Plants to In Vivo Conditions. Plants 2023, 12, 4193. https://doi.org/10.3390/plants12244193
Ambros E, Kotsupiy O, Karpova E, Panova U, Chernonosov A, Trofimova E, Goldenberg B. A Biostimulant Based on Silicon Chelates Enhances Growth and Modulates Physiological Responses of In-Vitro-Derived Strawberry Plants to In Vivo Conditions. Plants. 2023; 12(24):4193. https://doi.org/10.3390/plants12244193
Chicago/Turabian StyleAmbros, Elena, Olga Kotsupiy, Evgeniya Karpova, Ulyana Panova, Alexander Chernonosov, Elena Trofimova, and Boris Goldenberg. 2023. "A Biostimulant Based on Silicon Chelates Enhances Growth and Modulates Physiological Responses of In-Vitro-Derived Strawberry Plants to In Vivo Conditions" Plants 12, no. 24: 4193. https://doi.org/10.3390/plants12244193
APA StyleAmbros, E., Kotsupiy, O., Karpova, E., Panova, U., Chernonosov, A., Trofimova, E., & Goldenberg, B. (2023). A Biostimulant Based on Silicon Chelates Enhances Growth and Modulates Physiological Responses of In-Vitro-Derived Strawberry Plants to In Vivo Conditions. Plants, 12(24), 4193. https://doi.org/10.3390/plants12244193








