Ascophyllum nodosum and Silicon-Based Biostimulants Differentially Affect the Physiology and Growth of Watermelon Transplants under Abiotic Stress Factors: The Case of Salinity
Abstract
:1. Introduction
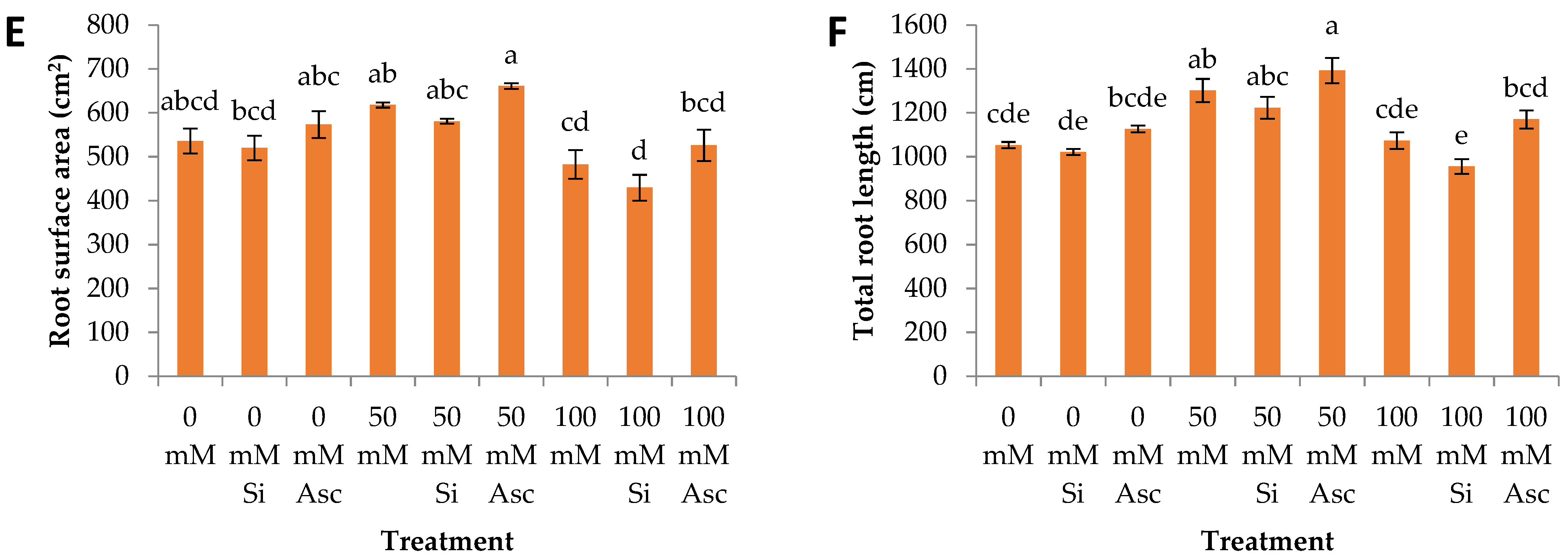
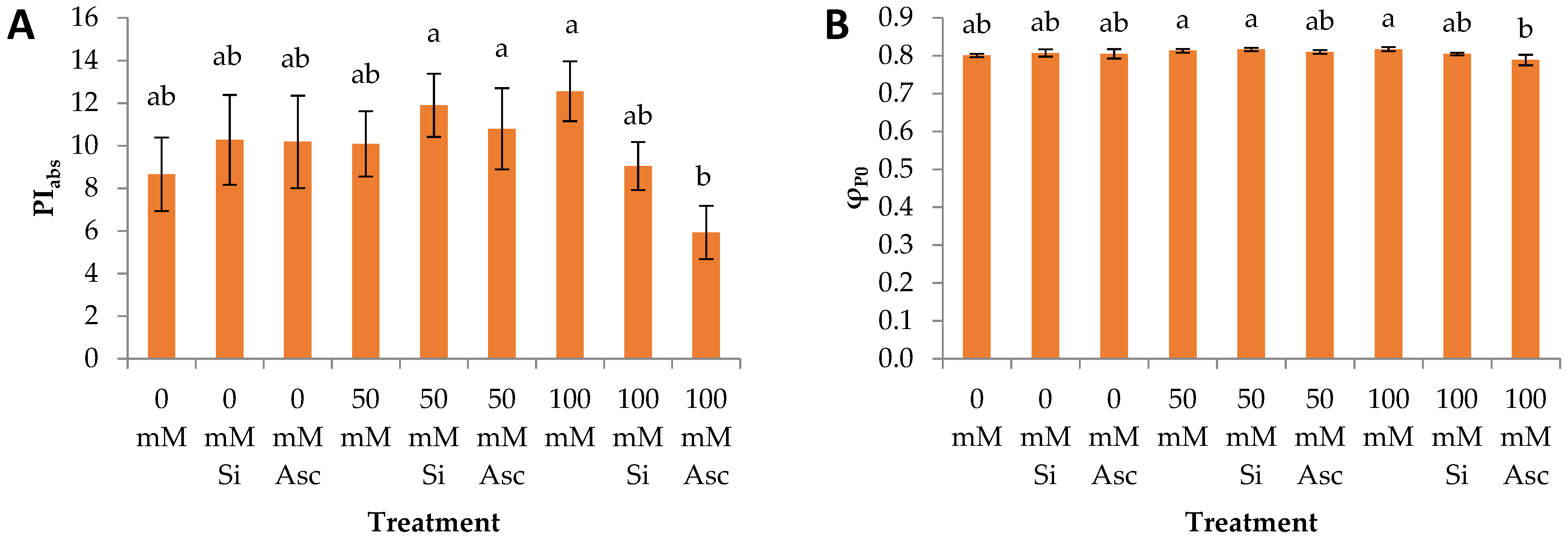
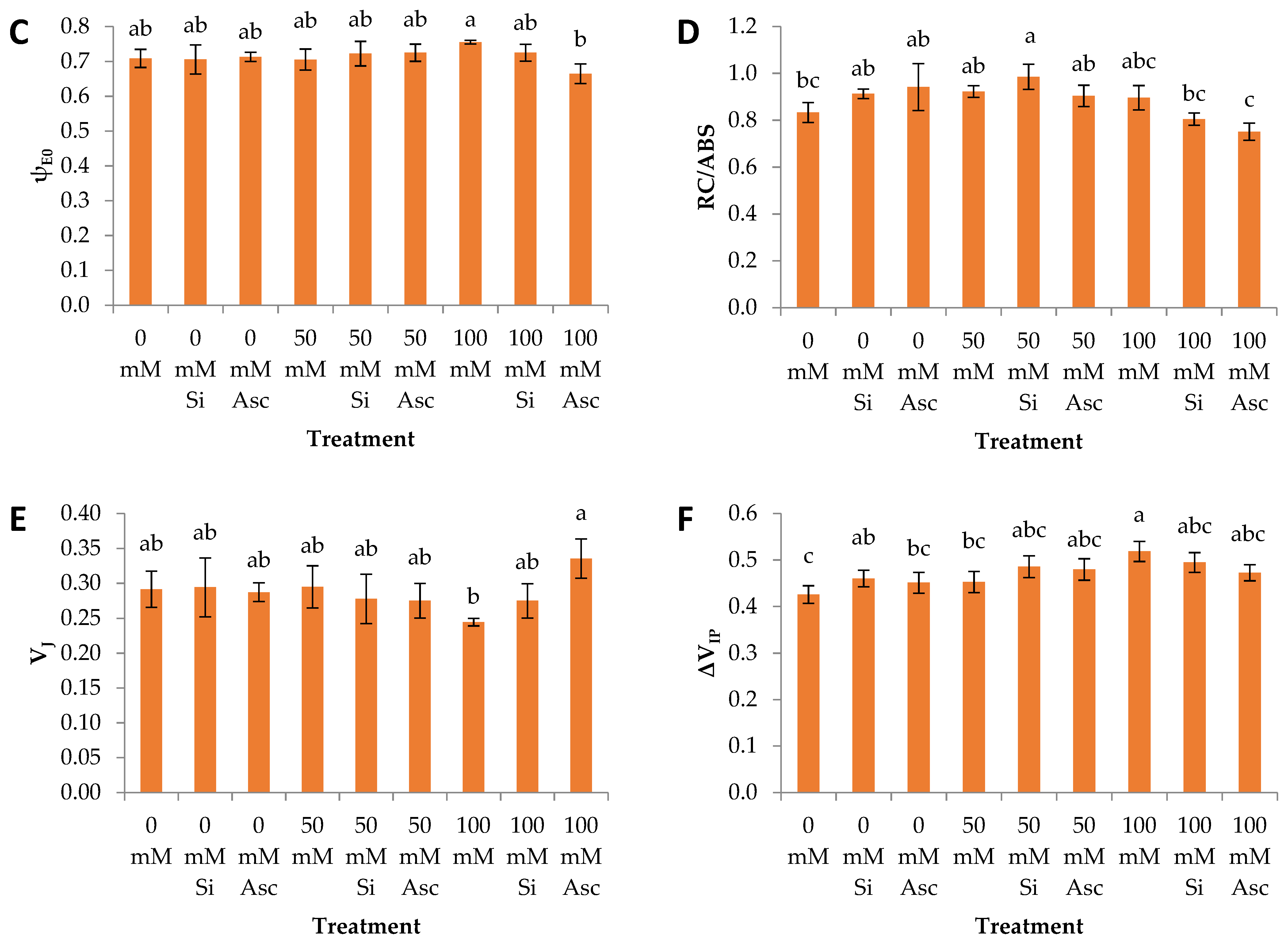
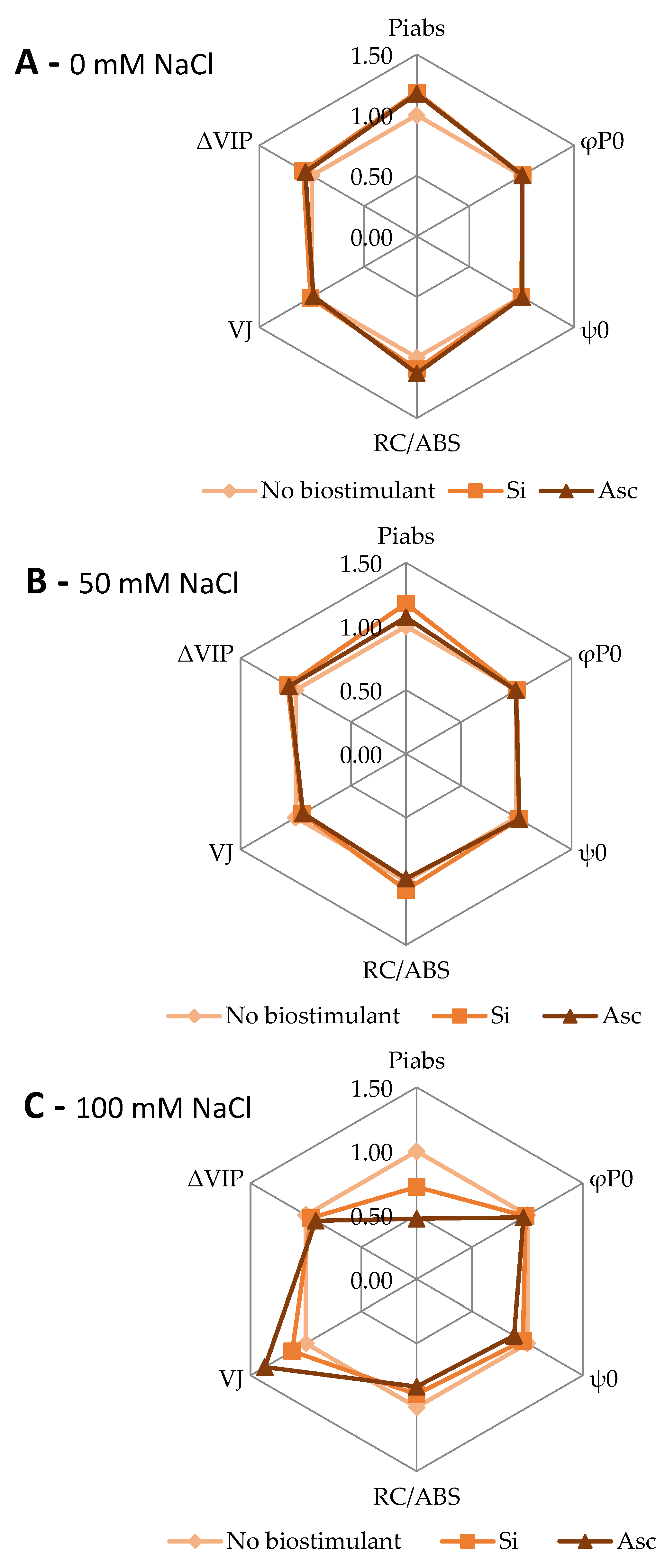
2. Results and Discussion
3. Materials and Methods
3.1. Plant Material and Cultivation
3.2. Salinity and Biostimulant Treatments
3.3. Measurements amd Analyses
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuartero, J.; Munoz, R.F. Tomato and salinity. Sci. Hortic. 1998, 78, 83–125. [Google Scholar] [CrossRef]
- Balliu, A.; Sallaku, G.; Rewald, B. AMF Inoculation Enhances Growth and Improves the Nutrient Uptake Rates of Transplanted, Salt-Stressed Tomato Seedlings. Sustainability 2015, 7, 15967–15981. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Li, J.-L.; Liu, L.-N.; Xie, Q.; Sui, N. Photosynthetic Regulation Under Salt Stress and Salt-Tolerance Mechanism of Sweet Sorghum. Front. Plant Sci. 2020, 10, 1722. [Google Scholar] [CrossRef] [Green Version]
- Rouphael, Y.; Kyriacou, M.C.; Colla, G. Vegetable grafting: A toolbox for securing yield stability under multiple stress conditions. Front. Plant Sci. 2018, 8, 2255. [Google Scholar] [CrossRef] [Green Version]
- Rouphael, Y.; Colla, G. Biostimulants in Agriculture. Front. Plant Sci. 2020, 11, 40. [Google Scholar] [CrossRef] [Green Version]
- Du Jardin, P. Plant Biostimulants: Definition, Concept, Main Categories and Regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef] [Green Version]
- EU. Regulation of the European Parliament and of the Council Laying Down Rules on the Making Available on the Market of EU Fertilising Products and Amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and Repealing Regulation (EC) No 2003/2003; EU: Brussels, Belgium, 2019. [Google Scholar]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural Uses of Plant Biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef] [Green Version]
- Ertani, A.; Francioso, O.; Tinti, A.; Schiavon, M.; Pizzeghello, D.; Nardi, S. Evaluation of Seaweed Extracts From Laminaria and Ascophyllum nodosum spp. as Biostimulants in Zea mays L. Using a Combination of Chemical, Biochemical and Morphological Approaches. Front. Plant Sci. 2018, 9, 428. [Google Scholar] [CrossRef]
- Zhu, Y.; Gong, H. Beneficial effects of silicon on salt and drought tolerance inplants. Agron. Sustain. Dev. 2014, 34, 455–472. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, Y.; Yao, H.; Wu, J.; Sun, H.; Gong, H. Silicon improves seed germination and alleviates oxidative stress of bud seedlings in tomato underwater deficit stress. Plant Physiol. Biochem. 2014, 78, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Savvas, D.; Giotis, D.; Chatzieustratiou, E.; Bakea, M.; Patakioutas, G. Silicon supply in soilless cultivations of zucchini alleviates stress induced by salinity and powdery mildew infections. Environ. Exp. Bot. 2009, 65, 11–17. [Google Scholar] [CrossRef]
- Hernandez-Apaolaza, L. Can silicon partially alleviate micronutrient deficiency in plants? A review. Planta 2014, 240, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Kubota, C.; Tsao, S.J.; Bie, Z.; Echevarria, P.H.; Morra, L.; Odag, M. Current status of vegetable grafting: Diffusion, grafting techniques, automation. Sci. Hortic. 2010, 127, 93–105. [Google Scholar] [CrossRef]
- Bantis, F.; Panteris, E.; Dangitsis, C.; Carrera, E.; Koukounaras, A. Blue light promotes hormonal induced vascular reconnection, while red light boosts the physiological response and quality of grafted watermelon seedlings. Sci. Rep. 2021, 11, 21754. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2021: The Physical Science Basis. In Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021; 2391p. [Google Scholar]
- Bantis, F.; Gkotzamani, A.; Dangitsis, C.; Koukounaras, A. A Light Recipe including Far-Red Wavelength during Healing of Grafted Watermelon Seedlings Enhances the Floral Development and Yield Earliness. Agriculture 2022, 12, 982. [Google Scholar] [CrossRef]
- Schwarz, D.; Rouphael, Y.; Colla, G.; Venema, J.H. Grafting as a tool to improve tolerance of vegetables to abiotic stresses: Thermal stress, water stress and organic pollutants. Sci. Hortic. 2010, 127, 162–171. [Google Scholar] [CrossRef]
- Zhang, H.; Irving, L.J.; McGill, C.; Matthew, C.; Zhou, D.; Kemp, P. The effects of salinity and osmotic stress on barley germination rate: Sodium as an osmotic regulator. Ann. Bot. 2010, 106, 1027–1035. [Google Scholar] [CrossRef] [Green Version]
- Ikuyinminu, E.; Goñi, O.; O’Connell, S. Enhancing Irrigation Salinity Stress Tolerance and Increasing Yield in Tomato Using a Precision Engineered Protein Hydrolysate and Ascophyllum nodosum-Derived Biostimulant. Agronomy 2022, 12, 809. [Google Scholar] [CrossRef]
- Romero-Aranda, M.R.; Jurado, O.; Cuartero, J. Silicon alleviates the deleterious salt effects on tomato plant growth by improving plant water status. J. Plant Physiol. 2006, 163, 847–855. [Google Scholar] [CrossRef]
- Savant, N.K.; Korndörfer, G.H.; Datnoff, L.E.; Snyder, G.H. Silicon nutrition and sugarcane production: A review. J. Plant Nutr. 1999, 22, 1853–1903. [Google Scholar] [CrossRef]
- Gong, H.J.; Chen, K.M.; Chen, G.C.; Wang, S.M.; Zhang, C.L. Effects of silicon on growth of wheat under drought. J. Plant Nutr. 2003, 26, 1055–1063. [Google Scholar] [CrossRef]
- Bantis, F.; Koukounaras, A.; Siomos, A.; Dangitsis, C. Impact of scion and rootstock seedling quality selection on the vigor of watermelon–interspecific squash grafted seedlings. Agriculture 2020, 10, 326. [Google Scholar] [CrossRef]
- Goreta, S.; Bucevic-Popovic, V.; Selak, G.; Pavela-Vrancic, M.; Perica, S. Vegetative growth, superoxide dismutase activity and ion concentration of salt-stressed watermelon as influenced by rootstock. J. Agric. Sci. 2008, 146, 695–704. [Google Scholar] [CrossRef]
- Ye, L.; Zhao, X.; Bao, E.; Cao, K.; Zou, Z. Effects of Arbuscular Mycorrhizal Fungi on Watermelon Growth, Elemental Uptake, Antioxidant, and Photosystem II Activities and Stress-Response Gene Expressions Under Salinity-Alkalinity Stresses. Front. Plant Sci. 2019, 10, 863. [Google Scholar] [CrossRef] [Green Version]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the chlorophyll a fluorescence transient. In Chlorophyll a Fluorescence: A Signature of Photosynthesis. Advances in Photosynthesis and Respiration; Papageorgiou, G.C., Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 321–362. [Google Scholar]
- Esmaeilizadeh, M.; Shamsabad, M.R.M.; Roosta, H.R.; Dąbrowski, P.; Rapacz, M.; Zieliński, A.; Wróbel, J.; Kalaji, H.M. Manipulation of light spectrum can improve the performance of photosynthetic apparatus of strawberry plants growing under salt and alkalinity stress. PLoS ONE 2021, 16, e0261585. [Google Scholar] [CrossRef]
- Dąbrowski, P.; Baczewska-Dąbrowska, A.H.; Bussotti, F.; Pollastrini, M.; Piekut, K.; Kowalik, W.; Wróbel, J.; Kalaji, H.M. Photosynthetic efficiency of Microcystis ssp. under salt stress. Environ. Exp. Bot. 2021, 186, 104459. [Google Scholar] [CrossRef]
- Zhu, Z.; Wei, G.; Li, J.; Qian, Q.; Yu, J. Silicon alleviates salt stress and increases antioxidant enzymes activity in leaves of salt-stressed cucumber (Cucumis sativus L.). Plant Sci. 2004, 167, 527–533. [Google Scholar] [CrossRef]
- Liang, Y.C.; Chen, Q.; Liu, Q.; Zhang, W.; Ding, R. Exogenous silicon (Si)increases antioxidant enzyme activity and reduces lipid peroxidation in rootsof salt-stressed barley (Hordeum vulgare L.). J. Plant Physiol. 2003, 160, 1157–1164. [Google Scholar] [CrossRef]
- Xu, C.; Leskovar, D.I. Effects of A. nodosum seaweed extracts on spinach growth, physiology and nutrition value under drought stress. Sci. Hortic. 2015, 183, 39–47. [Google Scholar] [CrossRef]
- Kumari, R.; Kaur, I.; Bhatnagar, A.K. Effect of aqueous extract of Sargassum john-stonii Setchell & Gardner on growth, yield and quality of Lycopersicon esculentum Mill. J. Appl. Phycol. 2011, 23, 623–633. [Google Scholar]
- Savvas, D.; Ntatsi, G. Biostimulant activity of silicon in horticulture. Sci. Hortic. 2015, 196, 66–81. [Google Scholar] [CrossRef]
- Hashemi, A.; Abdolzadeh, A.; Sadeghipour, H.R. Beneficial effects of silicon nutrition in alleviating salinity stress in hydroponically grown canola, Brassica napus L. plants. Soil Sci. Plant Nutr. 2010, 56, 244–253. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.T.; Ckurshumova, W.; Fefer, M.; Liu, J.; Uddin, W.; Rosa, C. A Plant Based Modified Biostimulant (Copper chlorophyllin), Mediates Defense Response in Arabidopsis thaliana under Salinity Stress. Plants 2021, 10, 625. [Google Scholar] [CrossRef]
- Yousefirad, S.; Soltanloo, H.; Ramezanpour, S.S.; Zaynali Nezhad, K.; Shariati, V. The RNA-Seq Transcriptomic Analysis Reveals Genes Mediating Salt Tolerance through Rapid Triggering of Ion Transporters in a Mutant Barley. PLoS ONE 2020, 15, e0229513. [Google Scholar] [CrossRef] [PubMed]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants without Soil, 2nd ed.; Circular; California Agricultural Experiment Station: Davis, CA, USA, 1950; p. 347. [Google Scholar]


| Treatments | RWC % | Male Flower Number | Female Flower Number | Male/Female Flower % | Stem Diameter (mm) | Rel. Chlorophyll Content |
|---|---|---|---|---|---|---|
| 0 mM NaCl | 73.8 ± 2.7 a | 2.33 ± 0.66 a | 0.67 ± 0.40 a | 78/22 | 6.98 ± 0.44 a | 81.7 ± 8.1 ab |
| 0 mM NaCl + Si | 73.3 ± 2.7 a | 2.17 ± 0.82 ab | 0.00 ± 0.00 a | 100/0 | 6.75 ± 0.26 a | 104.3 ± 10.4 a |
| 0 mM NaCl + Asc | 71.3 ± 0.7 a | 0.17 ± 0.16 ab | 0.33 ± 0.26 a | 34/66 | 6.41 ± 0.15 a | 78.1 ± 11.2 ab |
| 50 mM NaCl | 75.3 ± 1.7 a | 1.00 ± 0.52 ab | 0.17 ± 0.21 a | 85/15 | 6.42 ± 0.09 a | 65.1 ± 15.00 ab |
| 50 mM NaCl + Si | 74.8 ± 1.6 a | 1.50 ± 0.67 ab | 0.00 ± 0.17 a | 100/0 | 6.90 ± 0.18 a | 71.7 ± 9.5 ab |
| 50 mM NaCl + Asc | 77.5 ± 1.7 a | 1.00 ± 0.52 ab | 0.00 ± 0.00 a | 100/0 | 6.71 ± 0.18 a | 47.7 ± 7.2 b |
| 100 mM NaCl | 72.2 ± 0.7 a | 0.00 ± 0.00 b | 0.00 ± 0.00 a | 0/0 | 6.68 ± 0.16 a | 52.3 ± 10.3 b |
| 100 mM NaCl + Si | 72.7 ± 0.9 a | 0.17 ± 0.17 ab | 0.00 ± 0.00 a | 100/0 | 7.00 ± 0.17 a | 48.5 ± 4.9 b |
| 100 mM NaCl + Asc | 77.9 ± 1.2 a | 0.00 ± 0.00 b | 0.00 ± 0.00 a | 0/0 | 6.49 ± 0.21 a | 68.5 ± 4.8 ab |
| Parameter | 0 mM NaCl | 50 mM NaCl | 100 mM NaCl | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No PB | Si | Asc | No PB | Si | Asc | No PB | Si | Asc | |
| RWC | a | a | a | a | a | a | b | b | a |
| Male flowers | a | a | a | a | a | a | a | a | a |
| Female flowers | a | a | a | a | a | a | a | a | a |
| Stem diameter | a | a | a | a | a | a | a | a | a |
| Rel. chl. content | a | a | a | a | a | a | a | a | a |
| Plant area | a | a | a | a | a | a | a | a | a |
| Leaf number | a | a | a | a | a | a | a | a | a |
| Shoot dry weight | a | a | a | a | a | a | a | a | a |
| Root dry weight | a | a | a | ab | b | a | ab | b | a |
| Root surface area | a | a | a | b | c | a | a | a | a |
| Total root length | b | b | a | a | a | a | ab | b | a |
| VJ | a | a | a | a | a | a | b | ab | a |
| PIabs | a | a | a | a | a | a | a | ab | b |
| φP0 | a | a | a | a | a | a | a | a | a |
| ψE0 | a | a | a | a | a | a | a | ab | b |
| RC/ABS | a | a | a | a | a | a | a | a | a |
| ΔVIP | a | a | a | a | a | a | a | a | a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bantis, F.; Koukounaras, A. Ascophyllum nodosum and Silicon-Based Biostimulants Differentially Affect the Physiology and Growth of Watermelon Transplants under Abiotic Stress Factors: The Case of Salinity. Plants 2023, 12, 433. https://doi.org/10.3390/plants12030433
Bantis F, Koukounaras A. Ascophyllum nodosum and Silicon-Based Biostimulants Differentially Affect the Physiology and Growth of Watermelon Transplants under Abiotic Stress Factors: The Case of Salinity. Plants. 2023; 12(3):433. https://doi.org/10.3390/plants12030433
Chicago/Turabian StyleBantis, Filippos, and Athanasios Koukounaras. 2023. "Ascophyllum nodosum and Silicon-Based Biostimulants Differentially Affect the Physiology and Growth of Watermelon Transplants under Abiotic Stress Factors: The Case of Salinity" Plants 12, no. 3: 433. https://doi.org/10.3390/plants12030433
APA StyleBantis, F., & Koukounaras, A. (2023). Ascophyllum nodosum and Silicon-Based Biostimulants Differentially Affect the Physiology and Growth of Watermelon Transplants under Abiotic Stress Factors: The Case of Salinity. Plants, 12(3), 433. https://doi.org/10.3390/plants12030433








