Biostimulants for Sustainable Management of Sport Turfgrass
Abstract
1. Introduction
2. Results
2.1. Biostimulant Effects on the Plant
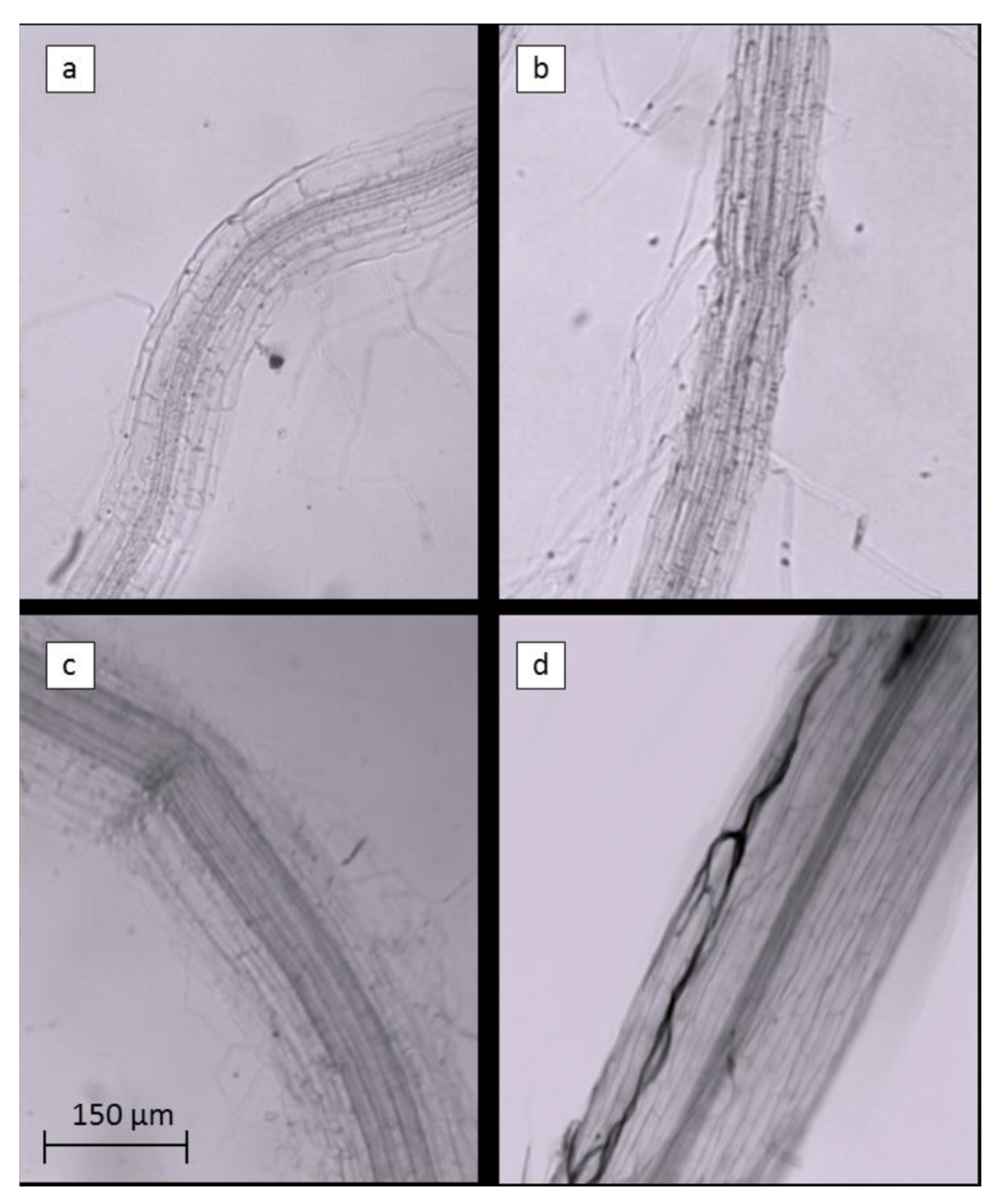
2.2. Biostimulant Effects on the Plant Rhizosphere
3. Discussion
4. Materials and Methods
4.1. Biostimulant Preparations
4.2. Experimental Field Design, Biostimulant Applications and Maintenance Activities
4.3. Biostimulant Effects on the Plant
4.3.1. Leaf Biomass
4.3.2. Leaf Pigment Analyses
- -
- Chlorophyll a (μg/mL) = 11.24 × A661.6 − 2.04 × A644.8;
- -
- Chlorophyll b (μg/mL) = 20.13 × A644.8 − 4.19 × A661.6;
- -
- Carotenoids (x + c) (μg/mL) = (1000 × A470 − 1.90 × Chl a − 63.14 × Chl b)/214.
4.3.3. Turfgrass Colour Analysis
4.3.4. Evapotranspiration
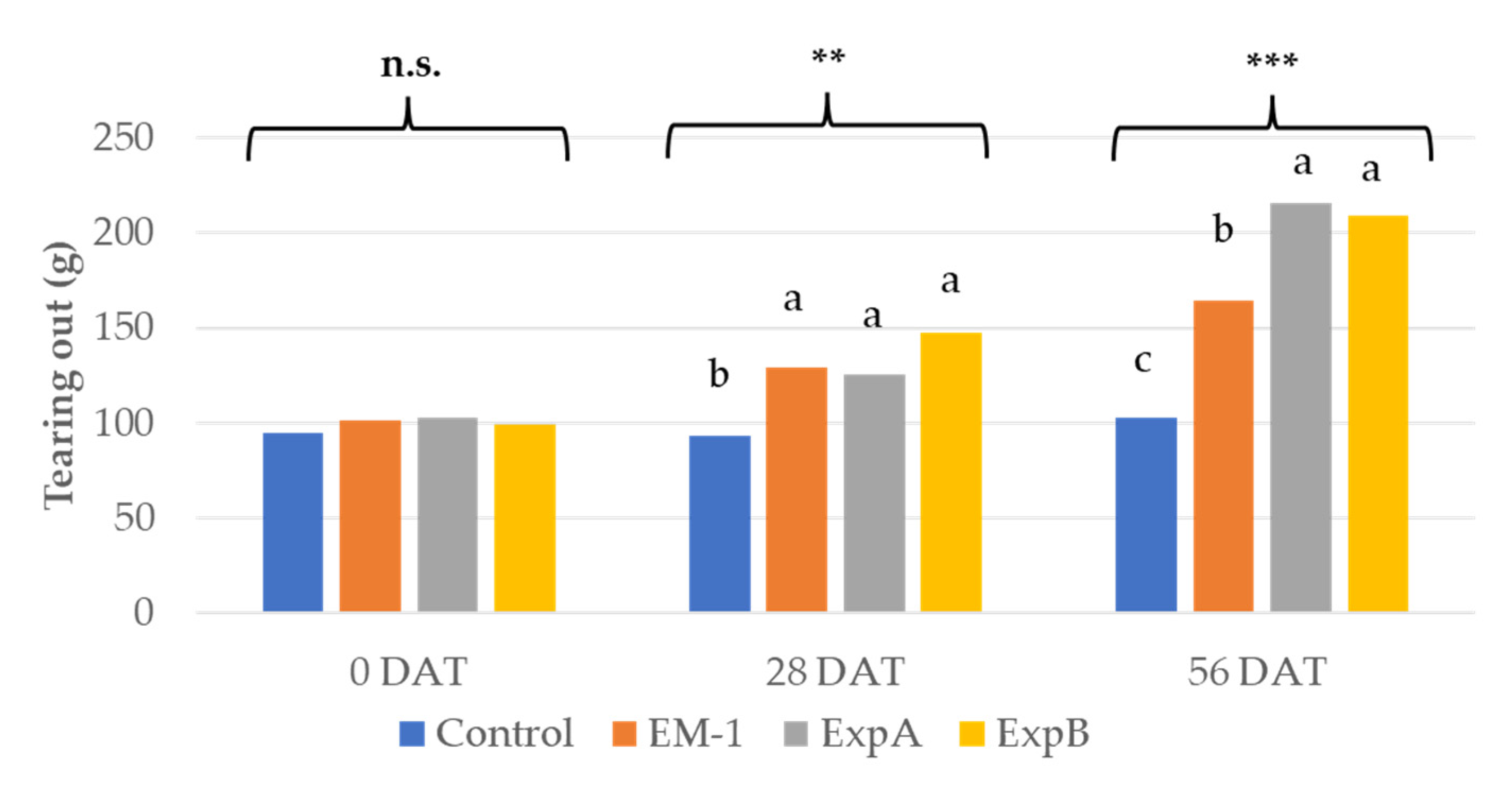
4.3.5. Tearing
4.4. Plant Rhizosphere Analyses
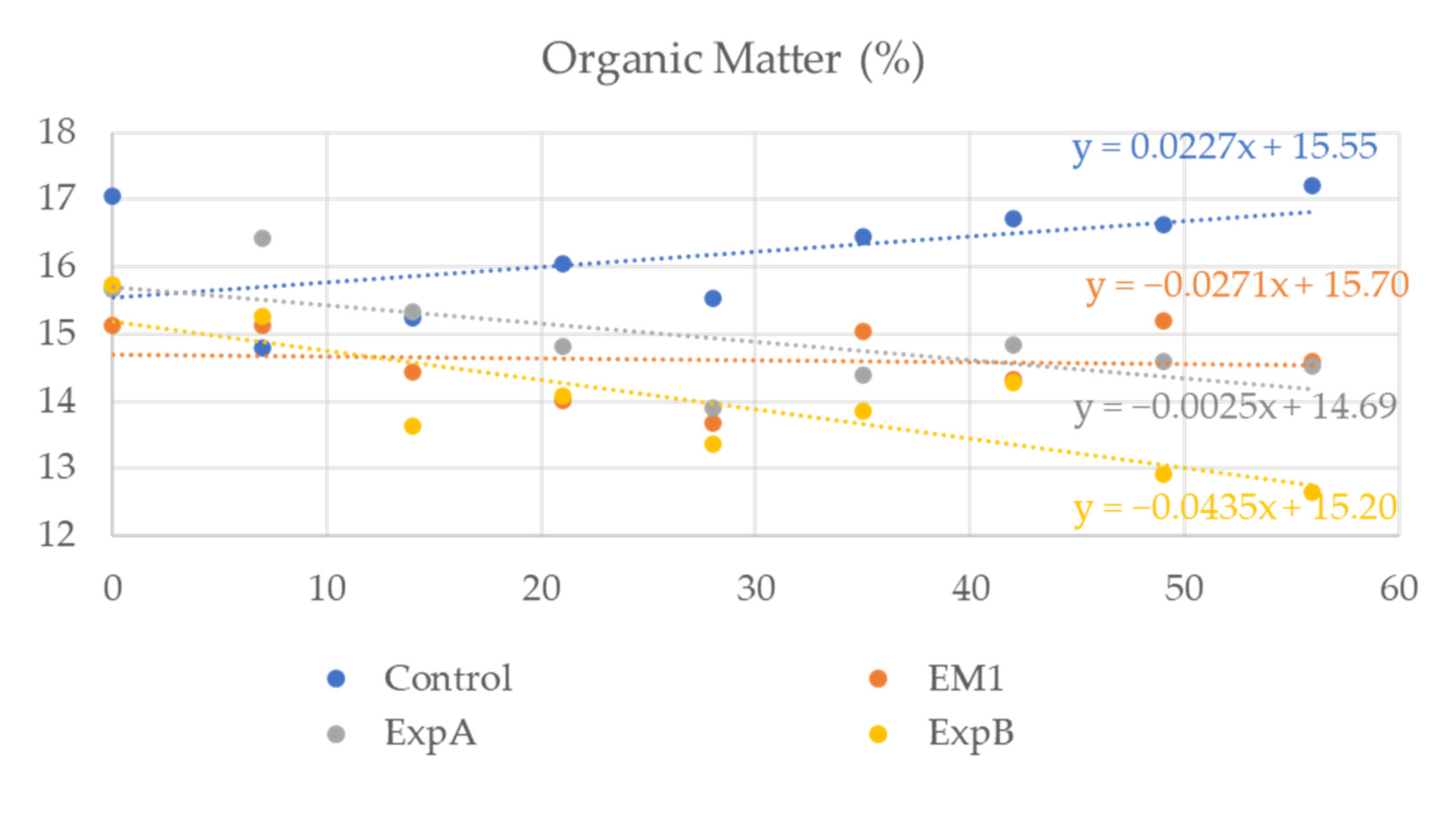
4.4.1. The Soil Profile
4.4.2. Thatch Biomass
4.4.3. Arbuscular Mycorrhiza Fungi Analysis
4.5. Statistical Analysis
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gaggìa, F.; Baffoni, L.; Di Gioia, D.; Accorsi, M.; Bosi, S.; Marotti, I.; Biavati, B.; Dinelli, G. Inoculation with microorganisms of Lolium perenne L.: Evaluation of plant growth parameters and endophytic colonization of roots. N. Biotechnol. 2013, 30, 695–704. [Google Scholar] [CrossRef]
- Ward, M. Reduce the cost of construction. In Technical Soil Biology; Pitchcare: Telford, UK, 2016; Available online: https://www.symbio.co.uk (accessed on 9 June 2021).
- Tidåker, P.; Wesström, T.; Kätterer, T. Energy use and greenhouse gas emissions from turf management of two Swedish golf courses. Urban For. Urban Green. 2017, 21, 80–87. [Google Scholar] [CrossRef]
- Talar-Krasa, M.; Wolski, K.; Biernacik, M. Biostimulants and possibilities of their usage in grassland. Grassl. Sci. 2019, 65, 205–209. [Google Scholar] [CrossRef]
- du Jardin, P. Plant biostimulants: Definition. concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Ward, M. Biostimulants—What are they and what do they do? Pitchcare Magazine, 10 February 2016. Available online: https://www.pitchcare.com/magazine/biostimulants.html (accessed on 9 June 2021).
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in plant science: A global perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, Y.; Colla, G. Synergistic biostimulatory action: Designing the next generation of plant biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1655. [Google Scholar] [CrossRef]
- Radkowski, A.; Radkowska, I.; Bocianowski, J.; Sladkovska, T.; Wolski, K. The effect of foliar application of an amino acid based biostimulant on lawn functional value. Agronomy 2020, 10, 1656. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Editorial: Biostimulants in agriculture. Front. Plant Sci. 2020, 11, 40. [Google Scholar] [CrossRef]
- Regulation (EU) 2019/1009. Regulation of the European Parliament and of the Council Laying down Rules on the Making Available on the Market of EU Fertilising Products and Amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and Repealing Regulation (EC) No 2003/2003. 2019. Available online: https://eur-lex.europa.eu/legalcontent/EN/TXT/?uri=OJ:L:2019:170:TOC (accessed on 24 June 2020).
- Kumar, H.D.; Aloke, P. Role of Biostimulant Formulations in Crop Production: An Overview. Int. J. Appl. Res. Vet. M 2020, 8, 38–46. [Google Scholar]
- De Luca, V.; de Barreda, D.G.; Lidón, A.; Lull, C. Effect of nitrogen-fixing microorganisms and amino acid-based biostimulants on perennial ryegrass. Horttechnology 2020, 30, 280–291. [Google Scholar] [CrossRef]
- Sidhu, S.S.; Huang, Q.; Carrow, R.N.; Raymer, P.L. Laccase mediated changes in physical and chemical composition properties of thatch layer in creeping bentgrass (Agrostis stolonifera L.). Soil Biol. Biochem. 2013, 64, 48–56. [Google Scholar] [CrossRef]
- Sidhu, S.S.; Huang, Q.; Carrow, R.N.; Raymer, P.L. Short-term and residual effects of laccase application on creeping bentgrass thatch layer. Hortscience 2019, 54, 1610–1620. [Google Scholar] [CrossRef]
- Weaver, J.R.; McCarty, L.B.; Quisenberry, V.L.; Hubbard, L.R., Jr.; Bridges, W.G.; Brown, P.J. Evaluating biological thatch control in turfgrass. Int. Turfgrass Soc. Res. J. 2021, 14, 539–543. [Google Scholar] [CrossRef]
- Weaver, J.R.; McCarty, L.B.; Quisenberry, V.L.; Hubbard, L.R., Jr.; Bridges, W.G.; Brown, P.J. Evaluating biological thatch control on Zoysia matrella (L.) Merr. golf greens. Int. Turfgrass Soc. Res. J. 2021, 14, 480–486. [Google Scholar] [CrossRef]
- Sidhu, S.; Huang, Q.; Carrow, R.N.; Jesperson, D.; Liu, J.; Raymer, P.L. A review of a novel enzyme system for the management of thatch and soil water repellence in turfgrass. Int. Turfgrass Soc. Res. J. 2022, 14, 450–461. [Google Scholar] [CrossRef]
- McCarty, L.B.; Gregg, M.F.; Toler, J.E. Thatch and mat management in an established creeping bentgrass golf green. Agron. J. 2007, 99, 1530–1537. [Google Scholar] [CrossRef]
- Cockerham, S.T. Culture of natural turf athletic fields. In Handbook of Turfgrass Management and Physiology; Pessarkli, M., Ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 151–170. [Google Scholar]
- Bhupenchandra, I.; Chongtham, S.; Devi, E.; Ramesh, R.; Choudhary, A.; Salam, M.; Sahoo, M.; Bhutia, T.; Devi, S.; Thounaojam, A.; et al. Role of biostimulants in mitigating the effects of climate change on crop performance. Front. Plant Sci. 2022, 13, 967665. [Google Scholar] [CrossRef]
- Research and Markets. Global Biostimulants Market Report 2022-2030—Compound Annual Growth of 10.4% with Market Set to Reach $6.79 Billion by 2030. 2022. Available online: https://www.globenewswire.com/en/news-release/2022/06/17/2464668/28124/en/Global-Biostimulants-Market-Report-2022-2030-Compound-Annual-Growth-of-10-4-with-Market-Set-to-Reach-6-79-Billion-by-2030.html (accessed on 13 November 2022).
- Mueller, S.R.; Kussow, W.R. Biostimulant influences on turfgrass microbial communities and creeping bentgrass putting green quality. Hort. Sci. 2005, 40, 1904–1910. [Google Scholar] [CrossRef]
- Roberts, J.A.; Ritchie, D.F.; Kerns, J.P. Plant growth regulator effects on bacterial etiolation of creeping Bentgrass putting green turf caused by Acidovorax avenae. Plant Dis. 2016, 100, 577–582. [Google Scholar] [CrossRef]
- Joshi, H.; Duttand, S.; Choudhary, P.; Mundra, S. Role of effective microorganisms (EM) in sustainable agriculture. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 172–181. [Google Scholar] [CrossRef]
- Maibodi, N.D.H.; Kafi, M.; Nikbakht, A.; Rejali, F. Effect of foliar applications of humic acid on growth. visual quality. nutrients content and root parameters of Perennial Ryegrass (Lolium perenne L.). J. Plant Nutr. 2015, 38, 224–236. [Google Scholar] [CrossRef]
- Zhang, X.; Goatley, M.; McCall, D.; Kosiarski, K.; Reith, F. Humic acids-based biostimulants impact on root viability and hormone metabolism in creeping bentgrass putting greens. Int. Turfgrass Soc. Res. J. 2021, 14, 288–294. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Liang, X.; Su, D.; Wang, Z.; Qiao, X. Effects of Turfgrass thatch on water infiltration, surface runoff, and evaporation. Water Resour. Prot. 2017, 9, 799–810. [Google Scholar] [CrossRef]
- Ricci, M.; Tilbury, L.; Daridon, B.; Sukalac, K. General principles to justify plant biostimulant claims. Front. Plant. Sci. 2019, 10, 494. [Google Scholar] [CrossRef]
- Hallet, P.D. An introduction to soil water repellency. In Proceedings of the 8th International Symposium on Adjuvants for Agrochemicals (ISAA), Columbus, OH, USA, 6–9 August 2007. [Google Scholar]
- Chamberlain, K.; Crawford, D.L. Thatch biodegradation and antifungal activities of two lignocellulolytic Streptomyces strains in laboratory cultures and in golf green turfgrass. Can. J. Microbiol. 2000, 46, 550–558. [Google Scholar] [CrossRef]
- Aalipour, H.; Nikbakht, A.; Ghasemi, M.; Amiri, R. Morpho-physiological and biochemical responses of two turfgrass species to arbuscular mycorrhizal fungi and humic acid under water stress condition. J. Soil Sci. Plant. Nutr. 2020, 20, 566–576. [Google Scholar] [CrossRef]
- Xu, Y.; Huan, B. Responses of creeping bentgrass to trinexapac-ethyl and biostimulants under summer stress. HortScience 2010, 45, 125–131. [Google Scholar] [CrossRef]
- Patent Cooperation Treaty (PCT). WO 2017/017633 A1, 2 February 2017. Applicant: ALMA MATER STUDIORUM-UNIVERSITA’ DI BOLOGNAUSGA Green Section Staff, 1993. USGA Recommendations for a Method of Putting Green Construction. USGA Green Record Mar/Apr: 1–3. USGA. NY. Available online: www.turfdrain.com (accessed on 15 June 2021).
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS Spectroscopy. In Current Protocols in Food Analytical Chemistry (CPFA); Wrolstad, R.E., Acree, T.E., An, H., Decker, E.A., Penner, M.H., Reid, D.S., Schwartz, S.J., Shoemaker, C.F., Sporns, P., Eds.; John Wiley and Sons: New York, NY, USA, 2001; pp. F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Karcher, D.G.; Richardson, M.D. Quantifying turfgrass color using digital image analysis. Crop. Sci. 2003, 43, 943–951. [Google Scholar] [CrossRef]
- Kool, D.; Agam, N.; Lazarovitch, N.; Heitman, J.L.; Sauer, T.J.; Ben-Gal, A. A review of approaches for evapotranspiration partitioning. Agric. For. Meteorol. 2014, 184, 56–70. [Google Scholar] [CrossRef]
- Brundrett, M.C.; Piché, Y.; Peterson, R.L. A new method for observing the morphology of vesicular-arbuscular mycorrhizae. Can. J. Bot. 1984, 62, 2128–2134. [Google Scholar] [CrossRef]
- Giovannetti, M.; Mosse, B. An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]

| Treatments | Fresh Leaf Weight (g) | Dry Leaf Weight (g) | |
|---|---|---|---|
| 0 DAT | ns | ns | |
| Control | 812.7 | 207.2 | |
| EM-1 | 767.5 | 218.9 | |
| ExpA | 810.4 | 217.4 | |
| ExpB | 751.8 | 227.3 | |
| 28 DAT | ns | ns | |
| Control | 850.5 | 236.5 | |
| EM-1 | 814.9 | 260.0 | |
| ExpA | 835.6 | 287.6 | |
| ExpB | 867.1 | 257.4 | |
| 56 DAT | ** | * | |
| Control | 821.9 (a) | 222.8 (b) | |
| EM-1 | 630.5 (b) | 209.8 (b) | |
| ExpA | 836.2 (a) | 252.1 (ab) | |
| ExpB | 947.8 (a) | 305.1 (a) |
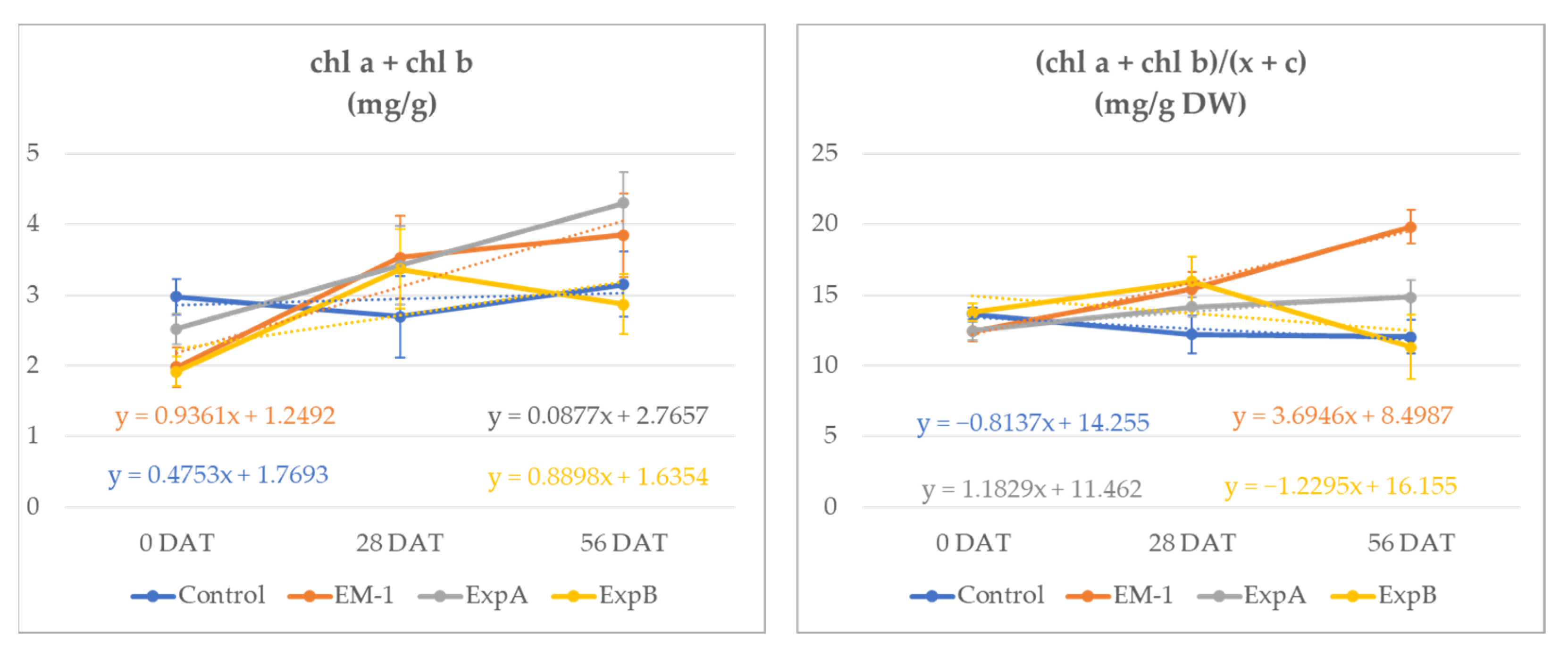
| Treatments | chl a + chl b (mg/g DW) | chl a/chl b (mg/g DW) | (chl a + chl b)/c + x (mg/g DW) | |
|---|---|---|---|---|
| 0 DAT | ** | ns | ns | |
| Control | 2.98 (a) | 2.01 | 13.65 | |
| EM-1 | 1.98 (b) | 2.13 | 12.42 | |
| ExpA | 2.52 (ab) | 2.06 | 12.48 | |
| ExpB | 1.92 (b) | 1.89 | 13.79 | |
| 28 DAT | ns | ns | ns | |
| Control | 2.69 | 1.96 | 12.20 | |
| EM-1 | 3.53 | 1.81 | 15.43 | |
| ExpA | 3.42 | 1.81 | 14.16 | |
| ExpB | 3.37 | 1.92 | 15.98 | |
| 56 DAT | * | * | * | |
| Control | 3.15 (ab) | 1.53 (bc) | 12.03 (b) | |
| EM-1 | 3.85 (ab) | 1.47 (c) | 19.81 (a) | |
| ExpA | 4.30 (a) | 1.81 (ab) | 14.85 (ab) | |
| ExpB | 2.87 (b) | 1.84 (a) | 11.33 (b) |
| Treatment | DGCI | |
|---|---|---|
| * | ||
| 56 DAT | Control | 0.36 (b) |
| EM-1 | 0.47 (ab) | |
| ExpA | 0.55 (a) | |
| ExpB | 0.55 (a) |
| Treatments | |||||
|---|---|---|---|---|---|
| DAFH | Control | EM-1 | ExpA | ExpB | Significance |
| 3 | 4.2 (b) | 4.4 (b) | 4.7 (b) | 5.6 (a) | ** |
| 6 | 10.5 (b) | 10.5 (b) | 11.4 (b) | 14.1 (a) | * |
| 9 | 17.3 (b) | 18.4 (ab) | 18.8 (ab) | 24.2 (a) | * |
| 12 | 26.0 (b) | 27.0 (b) | 28.1 (b) | 35.8 (a) | * |
| 15 | 35.0 | 36.3 | 38.8 | 46.0 | ns |
| 18 | 43.7 | 47.4 | 47.5 | 53.3 | ns |
| 21 | 50.9 | 54.1 | 54.0 | 56.5 | ns |
| 24 | 56.3 | 57.8 | 56.9 | 58.3 | ns |
| 27 | 58.5 | 59.9 | 58.6 | 59.5 | ns |
| 30 | 62.6 | 61.3 | 61.6 | 62.5 | ns |
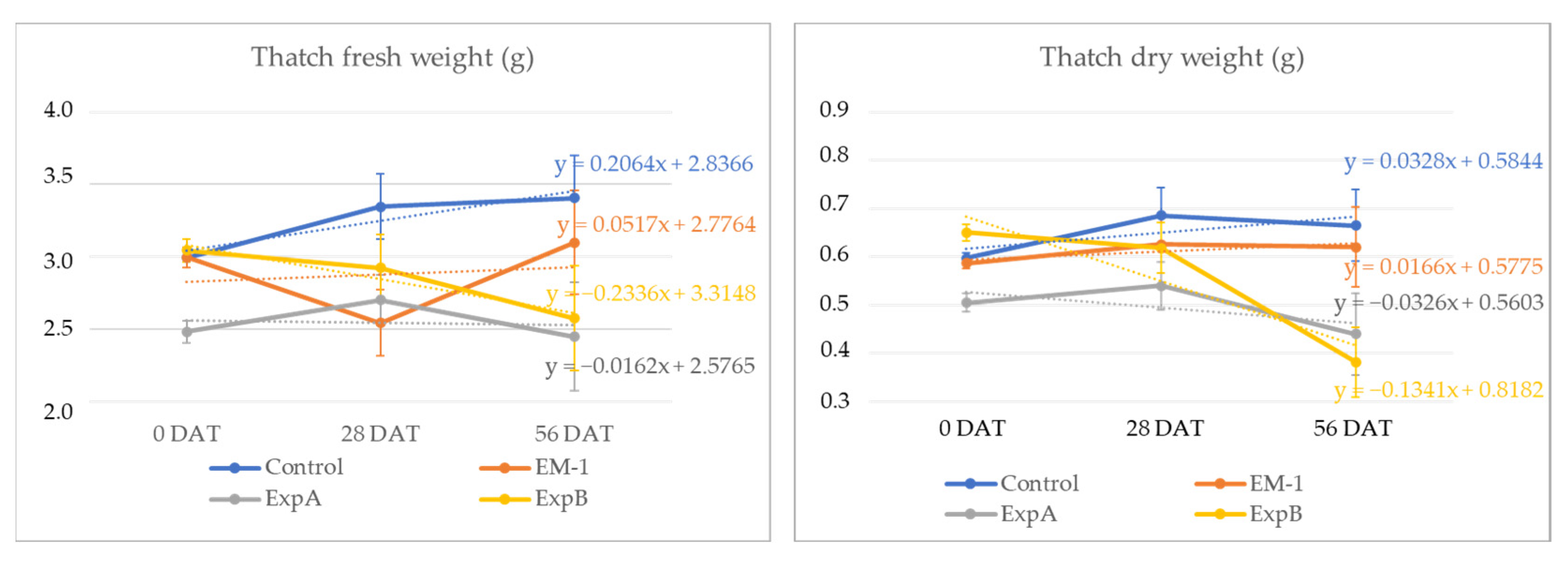
| Treatments | Humus Thickness (mm) | Thatch Thickness (mm) | Thatch Fresh Weight (g) | Thatch Dry Weight (g) | |
|---|---|---|---|---|---|
| 0 DAT | ns | ns | *** | ns | |
| Control | 18.4 | 18.30 | 3.00 (a) | 0.60 | |
| EM-1 | 18.1 | 20.70 | 3.00 (a) | 0.59 | |
| ExpA | 17.3 | 18.50 | 2.48 (b) | 0.51 | |
| ExpB | 18.3 | 21.10 | 3.04 (a) | 0.65 | |
| 28 DAT | ns | ns | * | ns | |
| Control | 17.9 | 19.70 | 3.35 (a) | 0.69 | |
| EM-1 | 18.9 | 21.10 | 2.54 (b) | 0.63 | |
| ExpA | 18.0 | 19.30 | 2.70 (b) | 0.54 | |
| ExpB | 20.5 | 19.90 | 2.92 (ab) | 0.62 | |
| 56 DAT | ** | * | * | * | |
| Control | 16.7 (c) | 21.70 (a) | 3.41 (a) | 0.67 (a) | |
| EM-1 | 22.0 (ab) | 19.40 (ab) | 3.10 (ab) | 0.62 (ab) | |
| ExpA | 19.0 (bc) | 17.80 (ab) | 2.45 (b) | 0.44 (bc) | |
| ExpB | 24.8 (a) | 15.50 (b) | 2.58 (b) | 0.38 (c) |
| Treatment | AMF % in Roots |
|---|---|
| Control | 5.5 ± 1.6 |
| EM-1 | 6.2 ± 1.4 |
| ExpA | 4.3 ± 3.7 |
| ExpB | 28.7 ± 5.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bosi, S.; Negri, L.; Accorsi, M.; Baffoni, L.; Gaggia, F.; Gioia, D.D.; Dinelli, G.; Marotti, I. Biostimulants for Sustainable Management of Sport Turfgrass. Plants 2023, 12, 539. https://doi.org/10.3390/plants12030539
Bosi S, Negri L, Accorsi M, Baffoni L, Gaggia F, Gioia DD, Dinelli G, Marotti I. Biostimulants for Sustainable Management of Sport Turfgrass. Plants. 2023; 12(3):539. https://doi.org/10.3390/plants12030539
Chicago/Turabian StyleBosi, Sara, Lorenzo Negri, Mattia Accorsi, Loredana Baffoni, Francesca Gaggia, Diana Di Gioia, Giovanni Dinelli, and Ilaria Marotti. 2023. "Biostimulants for Sustainable Management of Sport Turfgrass" Plants 12, no. 3: 539. https://doi.org/10.3390/plants12030539
APA StyleBosi, S., Negri, L., Accorsi, M., Baffoni, L., Gaggia, F., Gioia, D. D., Dinelli, G., & Marotti, I. (2023). Biostimulants for Sustainable Management of Sport Turfgrass. Plants, 12(3), 539. https://doi.org/10.3390/plants12030539












