The Chlorophyll Fluorescence Parameter Fv/Fm Correlates with Loss of Grain Yield after Severe Drought in Three Wheat Genotypes Grown at Two CO2 Concentrations
Abstract
:1. Introduction
2. Results
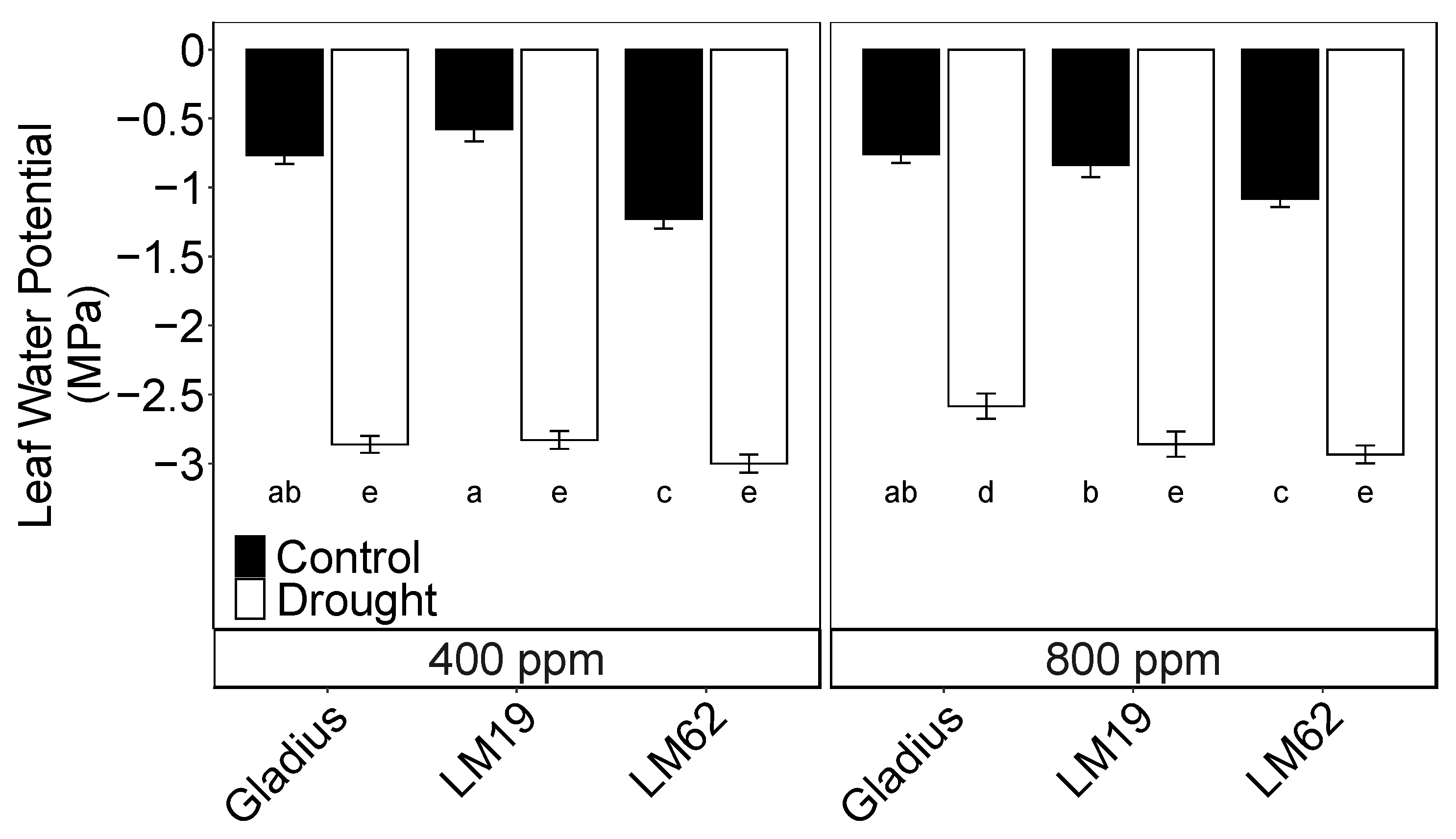
2.1. Leaf Water Potential
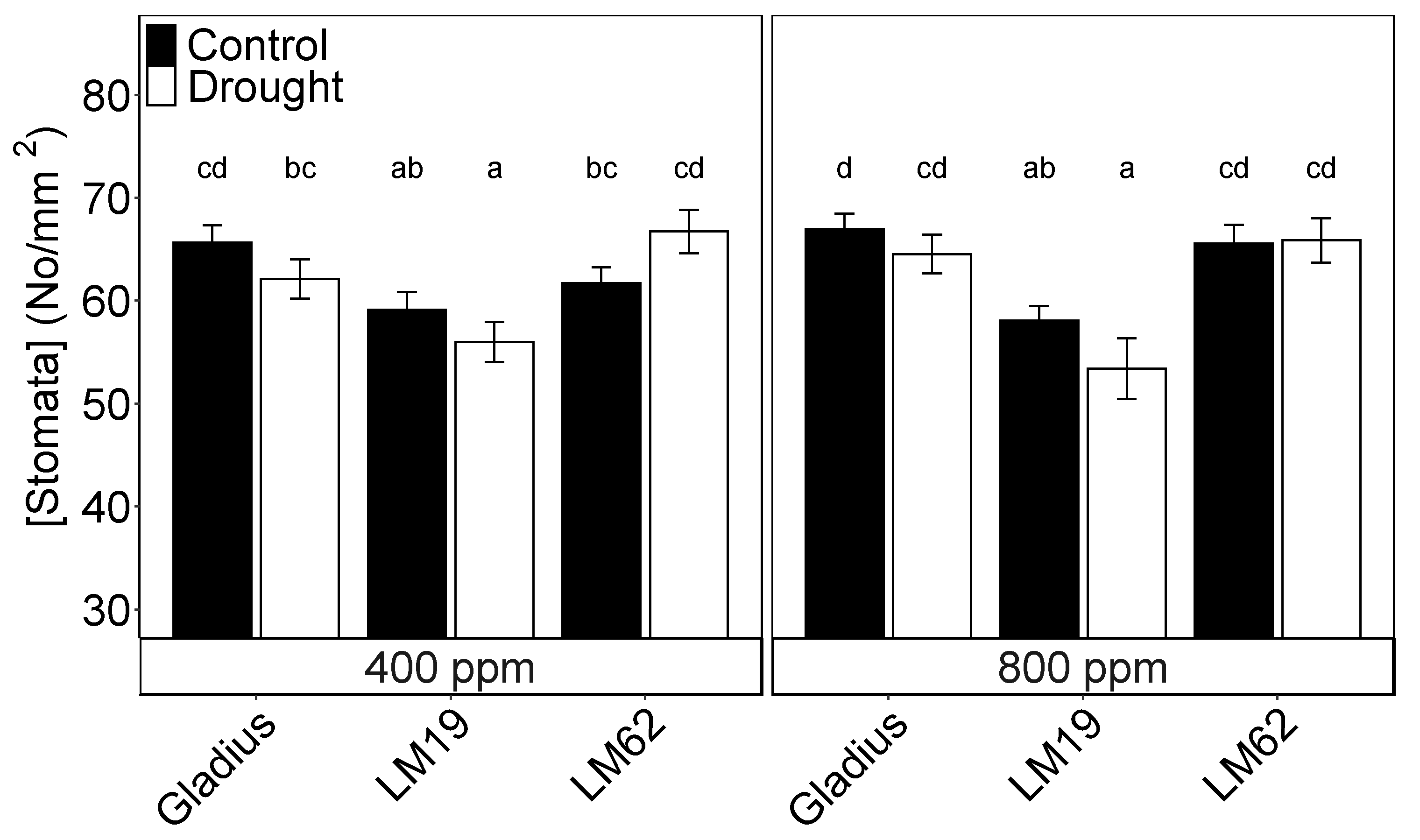
2.2. Stomatal Density
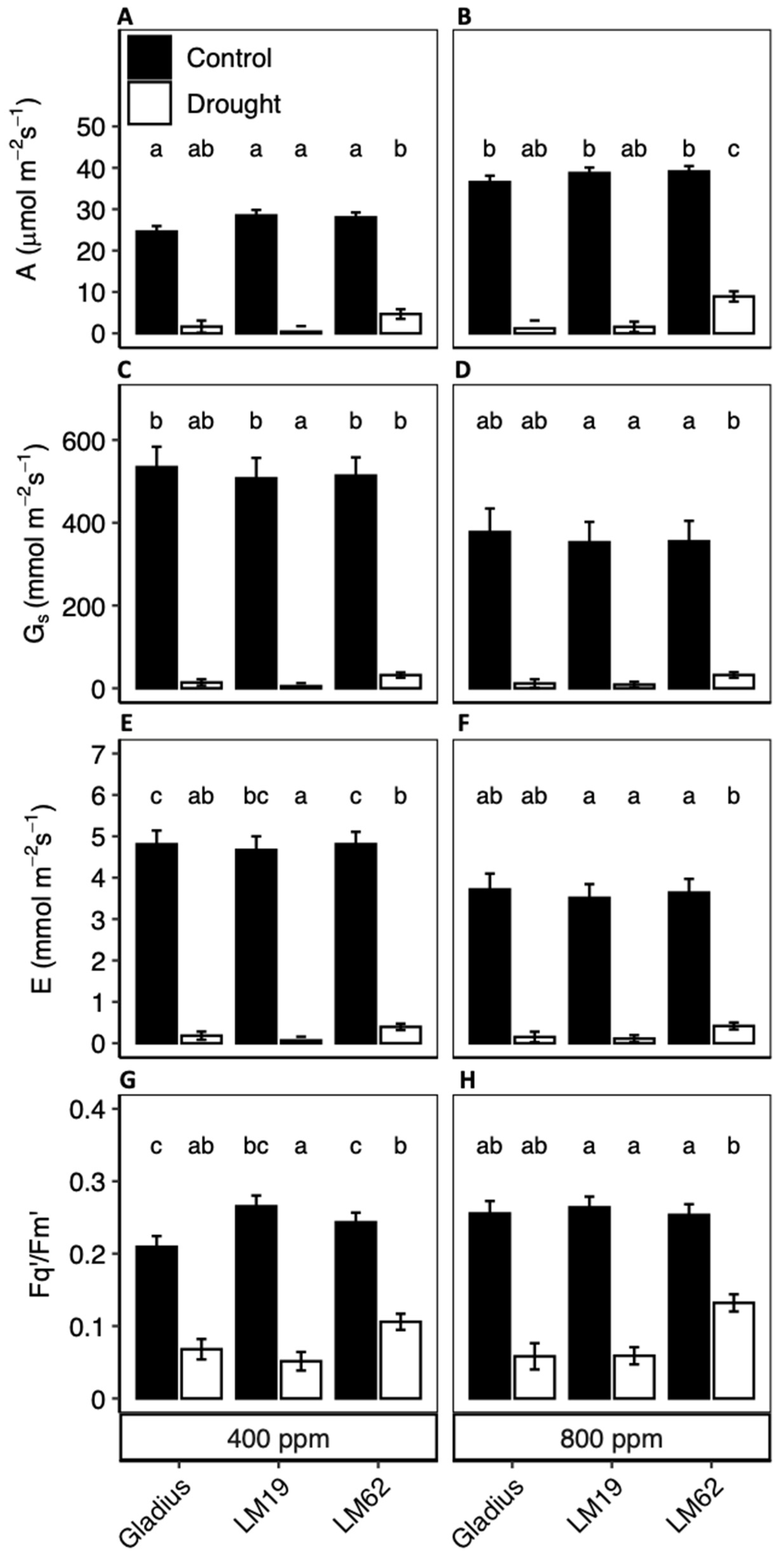
2.3. A, gs, Ci, E and Fq’/Fm’
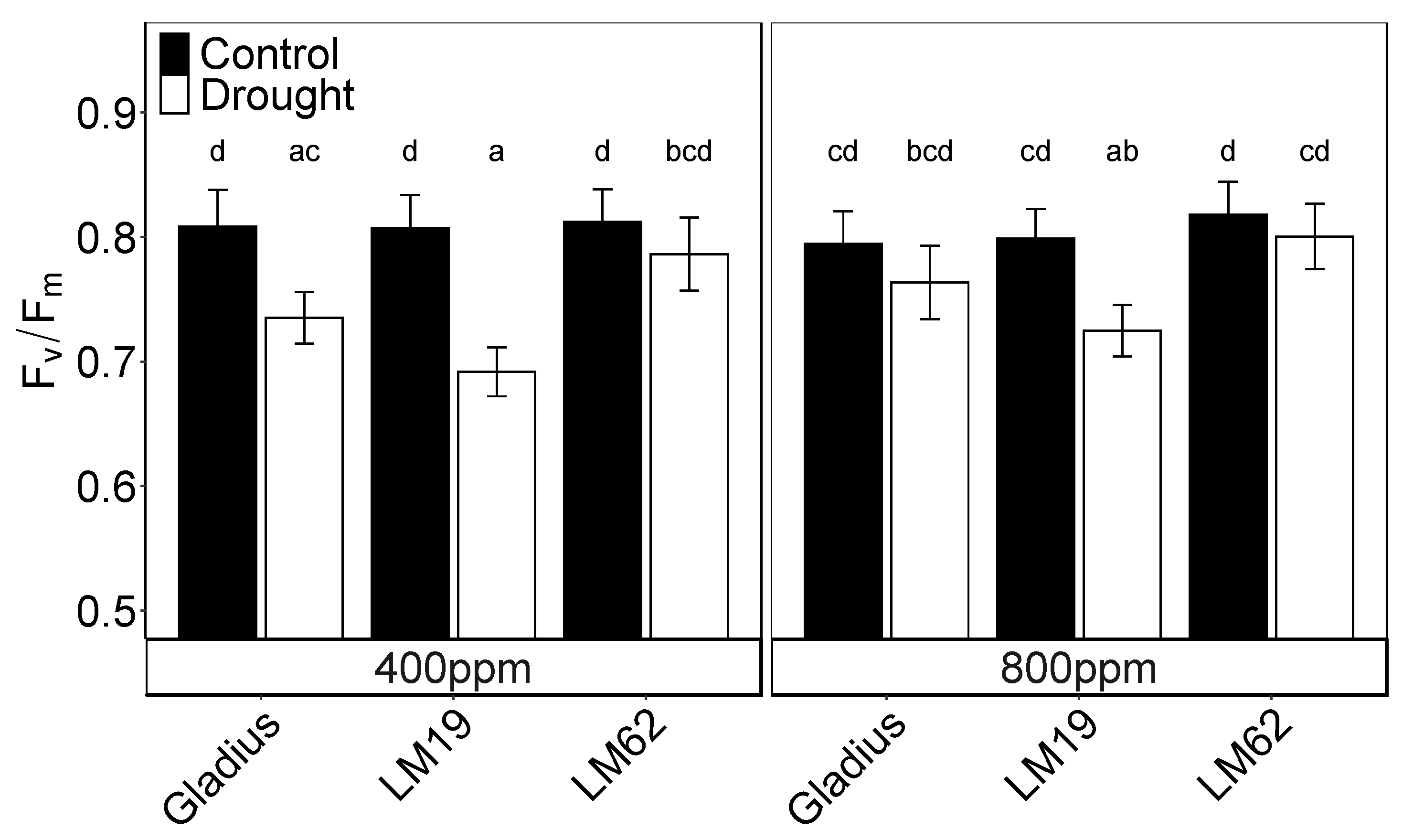
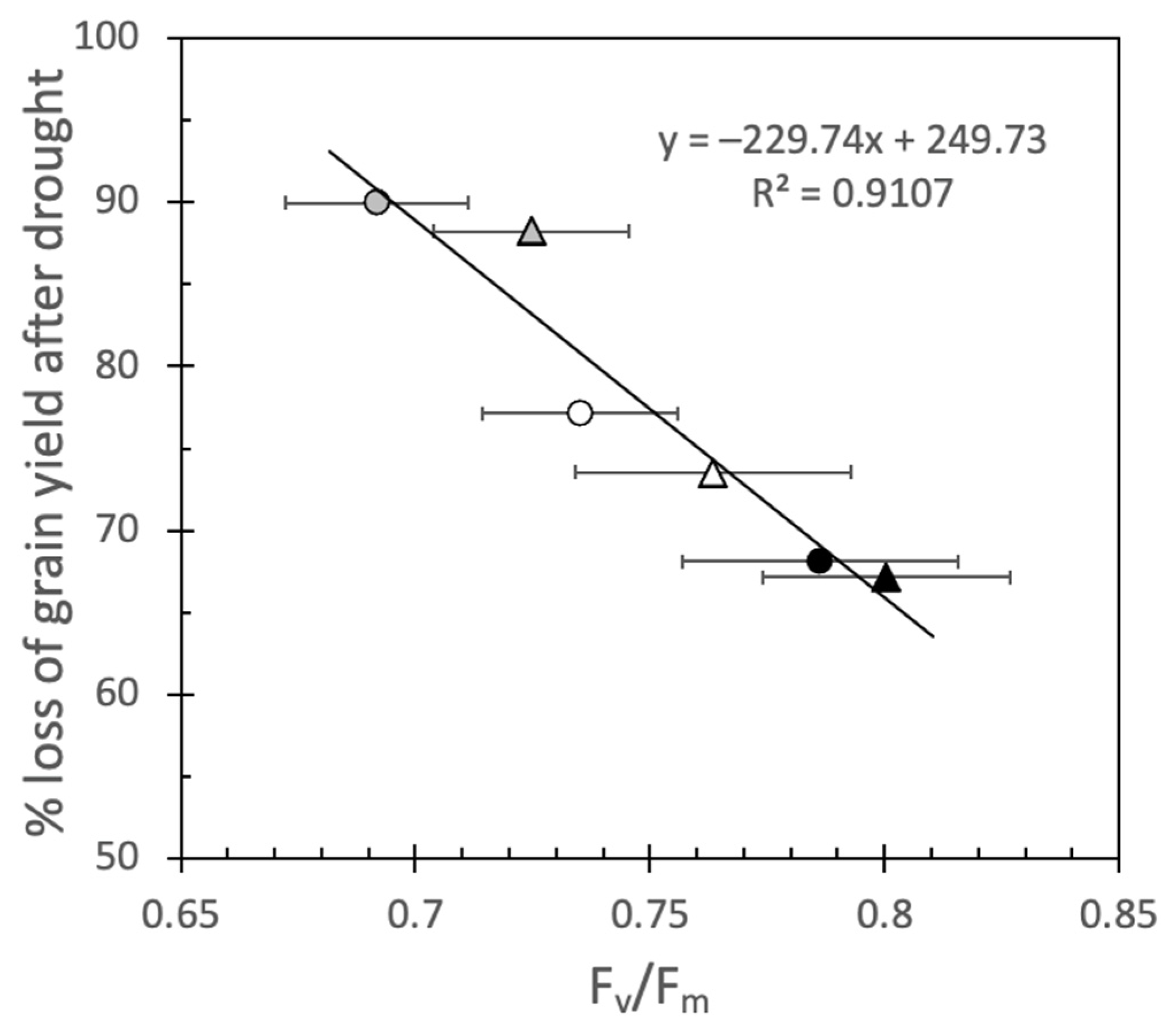
2.4. Fv/Fm
2.5. Pigments
2.6. Plant Size, Leaf Area and Water Use at Stress
2.7. Yields
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Plant Material
5.2. Growth Conditions and Treatments
5.3. Leaf Water Potential
5.4. Leaf Gas Exchange and Chlorophyll Fluorescence
5.5. Stomatal Imprints
5.6. Modulated Chlorophyll Fluorescence
5.7. Non-Destructive Pigment Measurements
5.8. Harvest and Yields
5.9. Statistics
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- OECD-FAO Agricultural Outlook 2020–2029; OECD-FAO Agricultural Outlook; OECD: Paris, France, 2020; ISBN 9789264317673.
- Alexandratos, N.; Bruinsma, J. World Agriculture Towards 2030/2050 The 2012 Revision PROOF COPY. ESA Work. Pap. 2012, 12, 146. [Google Scholar]
- Myhre, G.; Shindell, D.; Bréon, F.-M.; Collins, W.; Fuglestvedt, J.; Huang, J.; Koch, D.; Lamarque, J.-F.; Lee, D.; Mendoza, B.; et al. Anthropogenic and Natural Radiative Forcing. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2013; Volume 6. [Google Scholar]
- Masson-Delmotte, V.; Zhai, P.; Pirani, A.; Connors, S.L.; Péan, C.; Berger, S.; Caud, N.; Chen, Y.; Goldfarb, L.; Gomis, M.I.; et al. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Intergovernmental Panel on Climate Change (IPPC): Geneva, Switzerland, 2021. [Google Scholar]
- Wardlaw, I.F.; Blumenthal, C.; Larroque, O.; Wrigley, C.W. Contrasting Effects of Chronic Heat Stress and Heat Shock on Kernel Weight and Flour Quality in Wheat. Funct. Plant Biol. 2002, 29, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Richards, R.A.; Rebetzke, G.J.; Watt, M.; Condon, A.G.; Spielmeyer, W.; Dolferus, R. Breeding for Improved Water Productivity in Temperate Cereals: Phenotyping, Quantitative Trait Loci, Markers and the Selection Environment. Funct. Plant Biol. 2010, 37, 85–97. [Google Scholar] [CrossRef]
- Yu, Q.; Li, L.; Luo, Q.; Eamus, D.; Xu, S.; Chen, C.; Wang, E.; Liu, J.; Nielsen, D.C. Year Patterns of Climate Impact on Wheat Yields. Int. J. Climatol. 2014, 34, 518–528. [Google Scholar] [CrossRef]
- Pheloung, P.; Siddique, K. Contribution of Stem Dry Matter to Grain Yield in Wheat Cultivars. Funct. Plant Biol. 1991, 18, 53. [Google Scholar] [CrossRef]
- Nicolas, M.E.; Gleadow, R.M.; Dalling, M.J. Effect of Post-Anthesis Drought on Cell Division and Starch Accumulation in Developing Wheat Grains. Ann. Bot. 1985, 55, 433–444. [Google Scholar] [CrossRef]
- Sharma, D.K.; Andersen, S.B.; Ottosen, C.-O.; Rosenqvist, E. Wheat Cultivars Selected for High Fv/Fm under Heat Stress Maintain High Photosynthesis, Total Chlorophyll, Stomatal Conductance, Transpiration and Dry Matter. Physiol. Plant 2015, 153, 284–298. [Google Scholar] [CrossRef]
- Wilhelm, C.; Selmar, D. Energy Dissipation Is an Essential Mechanism to Sustain the Viability of Plants: The Physiological Limits of Improved Photosynthesis. J. Plant Physiol. 2011, 168, 79–87. [Google Scholar] [CrossRef]
- Baker, N.R.; Rosenqvist, E. Applications of Chlorophyll Fluorescence Can Improve Crop Production Strategies: An Examination of Future Possibilities. J. Exp. Bot. 2004, 55, 1607–1621. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.; Zhang, J. Effects of Water Stress on Photosystem II Photochemistry and Its Thermostability in Wheat Plants. J. Exp. Bot. 1999, 50, 1199–1206. [Google Scholar] [CrossRef]
- Živčák, M.; Brestič, M.; Olšovská, K.; Slámka, P. Performance Index as a Sensitive Indicator of Water Stress in Triticum aestivum L. Plant Soil Environ. 2008, 54, 133–139. [Google Scholar] [CrossRef]
- Driever, S.M.; Lawson, T.; Andralojc, P.J.; Raines, C.A.; Parry, M.A.J. Natural Variation in Photosynthetic Capacity, Growth, and Yield in 64 Field-Grown Wheat Genotypes. J. Exp. Bot. 2014, 65, 4959–4973. [Google Scholar] [CrossRef] [Green Version]
- Carmo-Silva, E.; Andralojc, P.J.; Scales, J.C.; Driever, S.M.; Mead, A.; Lawson, T.; Raines, C.A.; Parry, M.A.J. Phenotyping of Field-Grown Wheat in the UK Highlights Contribution of Light Response of Photosynthesis and Flag Leaf Longevity to Grain Yield. J. Exp. Bot. 2017, 68, 3473–3486. [Google Scholar] [CrossRef]
- Lopez, M.S.; Reynolds, M.P. Stay-Green in Spring Wheat Can Be Determined by Spectral Reflectance Measurements (Normalized Difference Vegetation Index) Independently from Phenology. J. Exp. Bot. 2012, 63, 3789–3798. [Google Scholar] [CrossRef] [Green Version]
- Christopher, J.T.; Christopher, M.J.; Borrell, A.K.; Fletcher, S.; Chenu, K. Stay-Green Traits to Improve Wheat Adaptation in Well-Watered and Water-Limited Environments. J. Exp. Bot. 2016, 67, 5159–5172. [Google Scholar] [CrossRef] [Green Version]
- Christopher, M.; Chenu, K.; Jennings, R.; Fletcher, S.; Butler, D.; Borrell, A.; Christopher, J. QTL for Stay-Green Traits in Wheat in Well-Watered and Water-Limited Environments. Field Crops Res. 2018, 217, 32–44. [Google Scholar] [CrossRef]
- Farquhar, G.D.; von Caemmerer, S.; Berry, J.A. A Biochemical Model of Photosynthetic CO2 Assimilation in Leaves of C3 Species. Planta 1980, 149, 78–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hikosaka, K.; Ishikawa, K.; Borjigidai, A.; Muller, O.; Onoda, Y. Temperature Acclimation of Photosynthesis: Mechanisms Involved in the Changes in Temperature Dependence of Photosynthetic Rate. J. Exp. Bot. 2006, 57, 291–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Feng, Z.; Schjoerring, J.K. Effects of Elevated Atmospheric CO2 on Physiology and Yield of Wheat (Triticum aestivum L.): A Meta-Analytic Test of Current Hypotheses. Agric. Ecosyst. Environ. 2013, 178, 57–63. [Google Scholar] [CrossRef]
- Kimball, B.A.; Pinter, P.J.J.; Garcia, R.L.; LaMorte, R.L.; Wall, G.W.; Hunsaker, D.J.; Wechsung, G.; Wechsung, F.; Kartschall, T. Productivity and Water Use of Wheat under Free-Air CO2 Enrichment. Glob. Chang. Biol. 1995, 1, 429–442. [Google Scholar] [CrossRef]
- Asseng, S.; Ewert, F.; Rosenzweig, C.; Jones, J.W.; Hatfield, J.L.; Ruane, A.C.; Boote, K.J.; Thorburn, P.J.; Rötter, R.P.; Cammarano, D.; et al. Uncertainty in Simulating Wheat Yields under Climate Change. Nat. Clim. Chang. 2013, 3, 627–632. [Google Scholar] [CrossRef]
- Franks, P.J.; Beerling, D.J. Maximum Leaf Conductance Driven by CO2 Effects on Stomatal Size and Density over Geologic Time. Proc. Natl. Acad. Sci. USA 2009, 106, 10343–10347. [Google Scholar] [CrossRef] [Green Version]
- Liao, J.X.; Chang, J.; Wang, G.X. Stomatal Density and Gas Exchange in Six Wheat Cultivars. Cereal Res. Commun. 2005, 33, 719–726. [Google Scholar] [CrossRef]
- Fleury, D.; Jefferies, S.; Kuchel, H.; Langridge, P. Genetic and Genomic Tools to Improve Drought Tolerance in Wheat. J. Exp. Bot. 2010, 61, 3211–3222. [Google Scholar] [CrossRef] [Green Version]
- Faralli, M.; Williams, K.S.; Han, J.; Corke, F.M.K.; Doonan, J.H.; Kettlewell, P.S. Water-Saving Traits Can Protect Wheat Grain Number Under Progressive Soil Drying at the Meiotic Stage: A Phenotyping Approach. J. Plant Growth Regul. 2019, 38, 1562–1573. [Google Scholar] [CrossRef]
- Li, X.; Kristiansen, K.; Rosenqvist, E.; Liu, F. Elevated CO2 Modulates the Effects of Drought and Heat Stress on Plant Water Relations and Grain Yield in Wheat. J. Agron. Crop Sci. 2019, 205, 362–371. [Google Scholar] [CrossRef]
- Oke, T.R. Boundary Layer Climates, 2nd ed.; Methuen Co.: London, UK, 1987; p. 464. [Google Scholar]
- Kobata, T.; Palta, J.A.; Turner, N.C. Rate of Development of Postanthesis Water Deficits and Grain Filling of Spring Wheat. Crop Sci. 1992, 32, 1238–1242. [Google Scholar] [CrossRef]
- Dunn, J.; Hunt, L.; Afsharinafar, M.; Al Meselmani, M.; Mitchell, A.; Howells, R.; Wallington, E.; Fleming, A.J.; Gray, J.E. Reduced Stomatal Density in Bread Wheat Leads to Increased Water-Use Efficiency. J. Exp. Bot. 2019, 70, 4737–4747. [Google Scholar] [CrossRef] [Green Version]
- Franks, P.J.; Drake, P.L.; Beerling, D.J. Plasticity in Maximum Stomatal Conductance Constrained by Negative Correlation between Stomatal Size and Density: An Analysis Using Eucalyptus Globulus. Plant Cell Environ. 2009, 32, 1737–1748. [Google Scholar] [CrossRef]
- Hughes, J.; Hepworth, C.; Dutton, C.; Dunn, J.A.; Hunt, L.; Stephens, J.; Waugh, R.; Cameron, D.D.; Gray, J.E. Reducing Stomatal Density in Barley Improves Drought Tolerance without Impacting on Yield. Plant Physiol. 2017, 174, 776–787. [Google Scholar] [CrossRef] [Green Version]
- Aisawi, K.A.B.; Reynolds, M.P.; Singh, R.P.; Foulkes, M.J. The Physiological Basis of the Genetic Progress in Yield Potential of CIMMYT Spring Wheat Cultivars from 1966 to 2009. Crop Sci. 2015, 55, 1749–1764. [Google Scholar] [CrossRef]
- Sharma, D.K.; Andersen, S.B.; Ottosen, C.O.; Rosenqvist, E. Phenotyping of Wheat Cultivars for Heat Tolerance Using Chlorophyll a Fluorescence. Funct. Plant Biol. 2012, 39, 936–947. [Google Scholar] [CrossRef] [PubMed]
- Poudyal, D.; Rosenqvist, E.; Ottosen, C.O. Phenotyping from Lab to Field -Tomato Lines Screened for Heat Stress Using Fv/Fm Maintain High Fruit Yield during Thermal Stress in the Field. Funct. Plant Biol. 2019, 46, 44–55. [Google Scholar] [CrossRef]
- Poorter, H.; Fiorani, F.; Pieruschka, R.; Wojciechowski, T.; van der Putten, W.H.; Kleyer, M.; Schurr, U.; Postma, J. Pampered inside, Pestered Outside? Differences and Similarities between Plants Growing in Controlled Conditions and in the Field. New Phytol. 2016, 212, 838–855. [Google Scholar] [CrossRef] [PubMed]
- Shahinnia, F.; Le Roy, J.; Laborde, B.; Sznajder, B.; Kalambettu, P.; Mahjourimajd, S.; Tilbrook, J.; Fleury, D. Genetic Association of Stomatal Traits and Yield in Wheat Grown in Low Rainfall Environments. BMC Plant Biol. 2016, 16, 150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lancashire, P.D.; Bleiholder, H.; Van Den Boom, T.; Langelüdekke, P.; Stauss, R.; Weber, E.; Witzenberger, A. A Uniform Decimal Code for Growth Stages of Crops and Weeds. Ann. Appl. Biol. 1991, 119, 561–601. [Google Scholar] [CrossRef]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A Decimal Code for the Growth Stages of Cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Jones, H.G. Monitoring Plant and Soil Water Status: Established and Novel Methods Revisited and Their Relevance to Studies of Drought Tolerance. J. Exp. Bot. 2006, 58, 119–130. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.G.; Han, E.; Petersen, J.; Olsen, N.A.F.; Giese, C.; Athmann, M.; Dresbøll, D.B.; Thorup-Kristensen, K. RootPainter: Deep Learning Segmentation of Biological Images with Corrective Annotation. New Phytol. 2022, 236, 774–791. [Google Scholar] [CrossRef]
- Han, E.; Smith, A.G.; Kemper, R.; White, R.; Kirkegaard, J.A.; Thorup-Kristensen, K.; Athmann, M. Digging Roots Is Easier with AI. J. Exp. Bot. 2021, 72, 4680–4690. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Bretz, F.; Hothorn, T.; Westfall, P. Multiple Comparisons Using R; Chapman and Hall/CRC: New York, NY, USA, 2011; ISBN 9781420010909. [Google Scholar]
- Wickham, H. The Split-Apply-Combine Strategy for Data Analysis. J. Stat. Softw. 2011, 40, 29. [Google Scholar] [CrossRef]

| A. Water Status, Gas Exchange and Chlorophyll Fluorescence | Water | A† | gs† | E† | Fq’/Fm’† | Fv/Fm | ||||
| Potential | Control | Drought | Control | Drought | Control | Drought | Control | Drought | ||
| Genotype | NS | NS | *** | NS | ** | NS | ** | NS | *** | * |
| CO2 | NS | *** | NS | *** | NS | *** | NS | NS | NS | NS |
| Treatment | *** | *** | – | *** | – | *** | – | *** | – | *** |
| Genotype + CO2 | NS | * | *** | NS | ** | NS | ** | NS | *** | NS |
| Genotype + Treatment | *** | – | – | – | – | – | – | – | – | * |
| CO2 + Treatment | NS | – | – | – | – | – | – | – | – | NS |
| Genotype + CO2 + Treatment | NS | – | – | – | – | – | – | – | – | NS |
| B. Leaf Properties and First Harvest after Drought Stress | Stomatal | Chlorophyll | Flavonols | Anthocyanin | Harvest 1 | Harvest 1 | Harvest 1 | Harvest 1 | ||
| Density | SDM | LA | WU | WU/LA | ||||||
| Genotype | *** | ** | * | NS | *** | * | *** | *** | ||
| CO2 | NS | NS | NS | NS | * | NS | NS | NS | ||
| Treatment | NS | *** | *** | *** | * | *** | – | – | ||
| Genotype + CO2 | NS | NS | NS | NS | * | NS | . | . | ||
| Genotype + Treatment | NS | *** | * | NS | *** | *** | – | – | ||
| CO2 + Treatment | NS | NS | NS | * | * | NS | – | – | ||
| Genotype + CO2 + Treatment | NS | NS | NS | NS | ** | – | – | – | ||
| C. Final Plant Harvest | SDM | Grain | Harvest | SN | Spikes/ | GNPS | TKW | |||
| Yield | Index | /Tillers | ||||||||
| Genotype | *** | *** | * | *** | * | *** | . | |||
| CO2 | NS | NS | NS | NS | NS | NS | NS | Statistical significance | ||
| Treatment | *** | *** | *** | *** | *** | ** | *** | *** | p ≤ 0.001 | |
| Genotype + CO2 | NS | NS | NS | NS | NS | NS | NS | ** | p ≤ 0.01 | |
| Genotype + Treatment | *** | *** | *** | *** | *** | *** | * | * | p ≤ 0.05 | |
| CO2 + Treatment | NS | *** | NS | NS | NS | NS | NS | . | p ≤ 0.1 | |
| Genotype + CO2 + Treatment | ** | NS | NS | * | . | NS | NS | NS (not significant) | p > 0.1 | |
| Genotype | CO2 | Treatment | DW | LA | WU | WU/LA | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| ppm | g | cm2 | g | g/cm2 | ||||||
| Gladius | 400 | Control | 11.1 ± 3.9 | a | 758 ± 147 | bc | 251 ± 22 | a | 0.33 ± 0.02 | d |
| Drought | 10.9 ± 3.9 | a | 134 ± 147 | a | ||||||
| 800 | Control | 20.5 ± 3.9 | ab | 1160 ± 147 | c | 235 ± 26 | a | 0.20 ± 0.02 | bc | |
| Drought | 23.7 ± 5.5 | abc | 187 ± 208 | a | ||||||
| LM19 | 400 | Control | 26.8 ± 3.9 | bc | 1881 ± 147 | d | 485 ± 30 | b | 0.26 ± 0.03 | c |
| Drought | 21.7 ± 3.9 | ab | 223 ± 147 | a | ||||||
| 800 | Control | 40.9 ± 3.9 | d | 2558 ± 147 | e | 447 ± 24 | b | 0.17 ± 0.02 | ab | |
| Drought | 23.3 ± 3.9 | b | 346 ± 147 | ab | ||||||
| LM62 | 400 | Control | 36.4 ± 3.9 | cd | 3052 ± 147 | f | 510 ± 26 | b | 0.17 ± 0.02 | ab |
| Drought | 25.6 ± 3.9 | bc | 362 ± 147 | ab | ||||||
| 800 | Control | 45.1 ± 3.9 | d | 3391 ± 147 | f | 454 ± 26 | b | 0.13 ± 0.02 | a | |
| Drought | 25.0 ± 3.9 | b | 720 ± 147 | b |
| Genotype | CO2, ppm | Treatment | SDM, (g/plant) ± SE | GY, (g) ± SE | GY Loss, % | HI, (Ratio) ± SE | SN, (n) ± SE | Spikes/Tillers, (Ratio) ± SE | GNPS, (n) ± SE | TKW, (g) ± SE | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gladius | 400 | Control | 38.5 ± 5.2 | b | 17.7 ± 2.9 | b | 0.46 ± 0.03 | g | 23 ± 2.5 | def | 0.99 ± 0.04 | d | 26.0 ± 3.1 | acd | 32.2 ± 2.9 | ce | |
| Drought | 14.4 ± 5.7 | a | 4.1 ± 3.1 | a | 77% | 0.27 ± 0.03 | cd | 7 ± 2.8 | a | 0.77 ± 0.05 | b | 22.6 ± 3.4 | ac | 23.7 ± 3.2 | ac | ||
| 800 | Control | 41.7 ± 6.4 | b | 20.2 ± 3.5 | b | 0.48 ± 0.04 | g | 17 ± 3.1 | bcd | 0.95 ± 0.05 | cd | 30.9 ± 3.8 | ce | 38.2 ± 3.5 | e | ||
| Drought | 15.6 ± 5.7 | a | 5.3 ± 3.1 | a | 74% | 0.34 ± 0.03 | de | 9 ± 2.8 | ab | 0.91 ± 0.05 | cd | 25.6 ± 3.4 | acd | 26.0 ± 3.2 | bcd | ||
| LM19 | 400 | Control | 73.2 ± 5.2 | c | 31.2 ± 2.9 | c | 0.43 ± 0.03 | fg | 23 ± 2.5 | def | 0.92 ± 0.04 | cd | 39.0 ± 3.1 | e | 34.7 ± 2.9 | e | |
| Drought | 29.7 ± 6.4 | ab | 3.1 ± 3.5 | a | 90% | 0.11 ± 0.04 | a | 7 ± 3.1 | a | 0.47 ± 0.05 | a | 34.8 ± 3.8 | de | 14.8 ± 3.5 | a | ||
| 800 | Control | 88.7 ± 5.2 | d | 38.9 ± 2.9 | c | 0.44 ± 0.03 | fg | 28 ± 2.5 | f | 0.90 ± 0.04 | cd | 39.7 ± 3.1 | e | 35.1 ± 2.9 | e | ||
| Drought | 36.3 ± 6.4 | b | 4.6 ± 3.5 | a | 88% | 0.13 ± 0.04 | ab | 13 ± 3.1 | ac | 0.61 ± 0.05 | a | 24.8 ± 3.8 | acd | 16.7 ± 3.5 | ab | ||
| LM62 | 400 | Control | 128.1 ± 5.2 | e | 47.7 ± 2.9 | d | 0.37 ± 0.03 | ef | 46 ± 2.5 | g | 0.87 ± 0.04 | bc | 29.7 ± 3.1 | bcd | 35.6 ± 2.9 | e | |
| Drought | 66.0 ± 6.4 | c | 15.2 ± 3.5 | b | 68% | 0.20 ± 0.04 | ac | 19 ± 3.1 | ce | 0.54 ± 0.05 | a | 19.9 ± 3.8 | ab | 28.6 ± 3.5 | ce | ||
| 800 | Control | 149.8 ± 5.7 | f | 50.7 ± 3.1 | d | 0.34 ± 0.03 | de | 57 ± 2.8 | h | 0.91 ± 0.05 | cd | 24.0 ± 3.4 | ac | 36.5 ± 3.2 | e | ||
| Drought | 78.2 ± 5.7 | cd | 16.6 ± 3.1 | b | 67% | 0.21 ± 0.03 | bc | 27 ± 2.8 | ef | 0.61 ± 0.05 | a | 18.4 ± 3.4 | a | 33.0 ± 3.2 | de | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sommer, S.G.; Han, E.; Li, X.; Rosenqvist, E.; Liu, F. The Chlorophyll Fluorescence Parameter Fv/Fm Correlates with Loss of Grain Yield after Severe Drought in Three Wheat Genotypes Grown at Two CO2 Concentrations. Plants 2023, 12, 436. https://doi.org/10.3390/plants12030436
Sommer SG, Han E, Li X, Rosenqvist E, Liu F. The Chlorophyll Fluorescence Parameter Fv/Fm Correlates with Loss of Grain Yield after Severe Drought in Three Wheat Genotypes Grown at Two CO2 Concentrations. Plants. 2023; 12(3):436. https://doi.org/10.3390/plants12030436
Chicago/Turabian StyleSommer, Søren Gjedde, Eusun Han, Xiangnan Li, Eva Rosenqvist, and Fulai Liu. 2023. "The Chlorophyll Fluorescence Parameter Fv/Fm Correlates with Loss of Grain Yield after Severe Drought in Three Wheat Genotypes Grown at Two CO2 Concentrations" Plants 12, no. 3: 436. https://doi.org/10.3390/plants12030436
APA StyleSommer, S. G., Han, E., Li, X., Rosenqvist, E., & Liu, F. (2023). The Chlorophyll Fluorescence Parameter Fv/Fm Correlates with Loss of Grain Yield after Severe Drought in Three Wheat Genotypes Grown at Two CO2 Concentrations. Plants, 12(3), 436. https://doi.org/10.3390/plants12030436









