Abstract
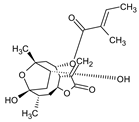
Medicinal herbs have long been utilized to treat various diseases or to relieve the symptoms of some ailments for extended periods. The present investigation demonstrates the phytochemical profile, molecular docking, anti-Candida activity, and anti-viral activity of the Saussurea costus acetic acid extract. GC-MS analysis of the extract revealed the presence of 69 chemical compounds. The chemical compounds were alkaloids (4%), terpenoids (79%), phenolic compounds (4%), hydrocarbons (7%), and sterols (6%). Molecular docking was used to study the inhibitory activity of 69 identified compounds against SARS-CoV-2. In total, 12 out of 69 compounds were found to have active properties exhibiting SARS-CoV-2 inhibition. The binding scores of these molecules were significantly low, ranging from −7.8 to −5.6 kcal/mol. The interaction of oxatricyclo [20.8.0.0(7,16)] triaconta-1(22),7(16),9,13,23,29-hexaene with the active site is more efficient. Furthermore, the extract exhibited significant antimicrobial activity (in vitro) against Candida albicans, which was the most susceptible microorganism, followed by Bacillus cereus, Salmonella enterica, Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa, respectively. On the other hand, its antiviral activity was evaluated against HSV-1 and SARS-CoV-2, and the results showed a significant positive influence against HSV-1 (EC50 = 82.6 g/mL; CC50 = 162.9 g/mL; selectivity index = 1.9). In spite of this, no impact could be observed in terms of inhibiting the entry of SARS-CoV-2 in vitro.
Keywords:
phytochemicals; Candida albicans; antibacterial; SARS-CoV-2; GC-MS; Saussurea costus; HSV-1 1. Introduction
Since antiquity, what we now refer to as folk, traditional, or alternative medicine has been the major source of remedies, which were mostly reliant on medicinal plants. On the other hand, modern medicine is now confronting a problem as a result of its inability to prevent the onset of disease. As a result, there is a pressing need to return to Mother Nature and its wealth of natural medicines [1,2].
In the scientific literature, numerous studies on the bioactive properties of medicinal plants have been published. For example, there are claims that Allium sativum, Trigonella foenum-graecum, Ferula assa-foetida, Carthamus tinctorius, and Mangifera indica are all plants with antidiabetic activity [3]. Pistacia lentiscus, Diospyros abyssinica, Sargentodoxa cuneata, Acacia auriculiformis, Ficus microcarpa, Salvia officinalis, and the Lamiaceae species contain potent antioxidants [4]. Aloe vera, Curcuma longa, Emblica officinalis, Stevia rebaudiana, and Gymnema sylvestre are suggested for possible anticancer activity [5]. Stephania glabra, Woodfordia fruticosa, Betula utilis, Nelumbo nucifera, and Calotropis gigantean have been identified as effective antimicrobial plants (in vitro) [6]. Recently, some medicinal plants have been reported to possibly possess anti-SARS-CoV-2 activity (in vitro), such as Lycoris radiate, Artemisia annua, Pyrrosia lingua, Nigella sativa, and Houttuynia cordata [7]. It is important to mention that clinical trials are required to demonstrate all these possible activities, particularly regarding SARS-CoV-2, for which no effective drug based on natural products has been approved yet.
Saussurea costus (Falc.) Lipschitz, also known as Saussurea lappa C.B. Clarke, is a well-known and valuable medicinal plant that is used in many indigenous medical systems to treat asthma, inflammatory diseases, ulcers, and stomach disorders, among other conditions [8]. Moreover, some studies have reported that Saussurea costus exhibits antiviral activity [9,10].
In January 2020, the World Health Organization (WHO) declared the outbreak of the COVID-19 pandemic due to the global spread of the disease and the novel emergency alarm raised by the WHO [11]. Electronic health records with easily accessible characteristics in combination with a COVID-19 mortality risk prediction model may allow rapid and precise risk categorization of COVID-19 patients upon admission.[12]. Coronaviruses are a broad group of respiratory viruses that are responsible for various disorders, including the common cold [13]. The new virus is unique due to its complex envelope protein, which resulted from mutations [14]. A coronavirus is an enveloped RNA virus that is covered by the spike protein. This protein assists the virus in entering the host and determining the properties of the host [15]. The protein has two parts: a membrane protein and an envelope protein, both of which are considered to have a role in the progression of the sickness [16]. In addition, hemagglutinin esterase is a glycoprotein that is present in bovine coronaviruses and is attached to their envelopes [17].
According to WHO statistics, the number of infected cases of COVID-19 has reached more than four hundred million globally, with a 1.3% death rate and 86.4% of patients treated [18]. The severe conditions have motivated global researchers to organize research teams to cover broad research areas in different fields, including drug discovery [16], mathematical modeling and data management, sociopolitical analysis, and education. Furthermore, various types of medicinal plants and herbs have been utilized for their possible antiviral activity. Several common ailments, including malaria, cholera, and asthma, are treated using medicinal herbs [19]. Although medicinal herbs are adjuvant treatments, the protocols to treat these diseases are still based on synthetic medicines. Heteromorpha spp. medicinal plants have demonstrated antiviral activity against HCV-229E (in vitro) [20]. Eleutherococcus senticosus extract has proven to be an RNA viral inhibitor [21]. A polar water-soluble natural compound extracted from Sannicolas europium was found to inhibit an RNA virus [22]. Medicinal natural compounds extracted from Rosa nutkana and Amelanchier alnifolia exhibited vigorous activity towards enteric coronavirus [23].
On the other hand, the crisis of drug-resistant microbes is growing globally. It has been reported that approximately 13 million deaths per year are attributed to microbial infections (other than viral infections), despite efforts to prevent and manage microbial pathogens that have been ongoing for more than a century and which have been largely successful with the aid of antibiotics. However, these antibiotics are currently failing to control newly emerging and re-emerging bacterial contagious diseases [24]. Bacteria, fungi, and parasites develop the ability to avoid the effects of antimicrobial drugs, survive, and, in some circumstances, grow extremely virulent. The process underpinning the development of drug-sensitive and drug-resistant microorganisms is a phenomenon of great complexity. Implementing an antimicrobial stewardship program is vital to controlling this growing phenomenon, and more efforts are needed to develop new antimicrobial medications [25].
Many medicinal herbs such as Allium sativum [26], Azadirachta indica [27], Tinospora Cordifolia [28], Syzygium aromaticum [29], Panax quinquefolius [30], Piper nigrum [31], Withania somnifera [32], Curcuma longa [33], Sambucus nigra [34],and Tinospora Cordifolia [35,36] have been approved as sources of minerals, steroids, and alkaloids and have been suggested for possible antiviral activities (in vitro). Saussurea Costus, a medicinal plant, is grown in vast regions worldwide, including India, Pakistan, and some parts of the Himalayas [37].The taxonomic details of Saussurea costus are as follows: its Plantae phylum is Trichophyte, its class is Magnoliopsidas, its order is Astral, and it belongs to the Asteraceae family [38]. Saussurea costus, in terms of morphology, is a 1–2 m tall upright herbaceous plant with a strong and hairy seedhead. Leaves are narrow, rough, glabrous, and auriculate and have irregularly formed teeth in general. At 0.50 to 1.25 m in length, the bottom leaves are somewhat large and feature long petioles with wings. Upper leaves are tiny and virtually sessile, with two small lobes at the base that almost wrap around the stem. The flowers are stemless and range in color from bluish-purple to nearly black. Their width varies from 2.4 to 3.9 cm, and they are spherical. The roots are dark brown or gray and reach a length of 40 cm [38]. The roots of Saussurea costus have been used for treating dysentery, rheumatism, stomachache, ulcer, and toothache [39]. The roots of Saussurea costus are widely used in Islamic culture and are famous in Arab regions, although it is not cultivated there. Moreover, traditional healers claim that it can control COVID-19 [40]. Studies on the potential anti-COVID-19 activity and the antimicrobial potential of Saussurea costus on specific fractions are scarce. Therefore, the main objective of the current study was to determine the phytochemical compounds in the acetic extract of Saussurea costus and investigate the possible in vitro antibacterial and anti-Candida, anti-herpes, and anti-SARS-CoV-2 potential, as well as its molecular docking to understand the possible interactions of these bioactive molecules at the atomic level.
2. Results and Discussion
2.1. Analysis of the Organic Chemical Compounds in Saussurea costus
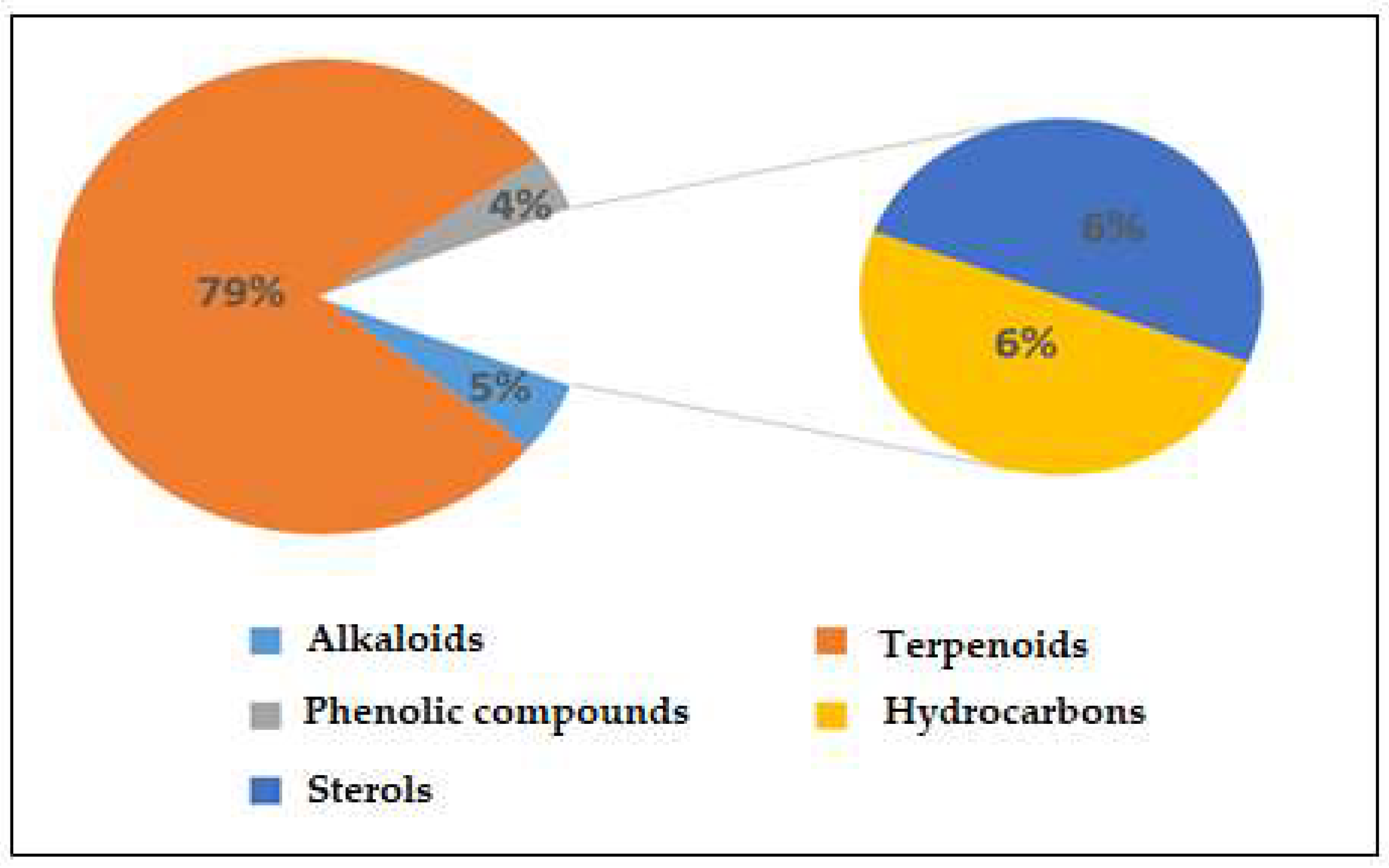
Figure S1 displays the GC-MS chromatograms of Saussurea costus extracted using acetic acid. We identified 109 peaks from the chromatograms that correspond to chemical substances by comparing their peak retention times, peak area percentages, height percentages, and mass spectral fragmentation features to those of known compounds in the National Institute of Standards and Technology (NIST) library [41]. As indicated in Table S2, phytochemical studies of the acetic acid extraction of Saussurea costus roots indicated the existence of 69 compounds. The percentages of the phytochemicals were as follows: alkaloids, 5%; terpenoids, 79%; phenolic compounds, 4%; hydrocarbons, 7%; and sterols, 6%. According to these findings, terpenoids accounted for the highest percentage of organic chemical compounds (78%), whereas alkaloids and phenolic compounds accounted for the lowest percentages (4%) of chemical compounds, as depicted in Figure 1. Terpenoids included 0.02% (4-Terpinenyl acetate) and 13.23% Vanillosmin. Phenolic compounds ranged between 0.01 (Phenol, 2-methoxy-4-(2-propenyl)-, acetate) and 0.06% (Phenol, 2,6-dimethoxy). Alkaloids ranged from 0.51% (Piperine) to 0.04% (Di(1,2,5-oxadiazolo)[3,4-b:3,4-E]pyrazine, 4,8-diacetyl). Hydrocarbons varied from 0.05% (1-Heptadecene) to 0.37 (9-Methyl-10,12-hexadecadien-1-ol acetate). Sterols ranged from 0.26% (Ergost-5-en-3-ol, (3.beta.)) to 3.51% (9,19-Cycloergost-24(28)-en-3-ol, 4,14-dimethyl-, acetate (3.beta.,4.alpha.,5.alpha)). Through a comparison of the chemical profile obtained in the current study with other published data, we found a difference in the nature and number of compounds and their concentrations [42,43]. Various studies have shown that chemical compound concentrations vary depending on different factors, which include the solvent used in plant extracts, the area of plants grown, and the quantity of the plant used [44]. The active constituents of this well-known medicinal plant are mostly terpenes, with various levels of flavonoids, anthraquinones, alkaloids, tannins, and inulin, as described in earlier research [45]. Several laboratory experiments and animal model studies have indicated that Saussurea costus possesses anti-inflammatory, antitrypanosomal, and anti-malignant tumor effects, confirming its extended use in medicine [46,47]. Costsunolide, dehydrocostus lactone, and cynaropicrin are some of the phytochemicals found in this plant that have shown promise in producing bioactive molecules [48]. Because Saussurea costus has displayed solid anticancer, anti-inflammatory, antibacterial, antifungal, and antiviral action, it will be possible to improve its use as a medication soon. According to several studies, sterol compounds act as cancer inhibitors, anti-inflammatory medications, immunomodulatory agents, and antiviral agents [49,50]. Various investigations have shown that terpenoids have a high proclivity for acting as SARS-CoV-2 blockers [51]. Based on a new study integrating quantum chemistry, molecular docking, and dynamic dynamics, phenolic chemicals have potential therapeutic implications for SARS-CoV-2. As a result, the mixture of active components present in each plant may be more effective than a single separated molecule in terms of treatment efficacy [52]. As reported in some investigations, researchers have estimated that the quinoline-2-carboxylic acids identified in Ephedra sinica might be employed as COVID-19 medicinal agents [53].
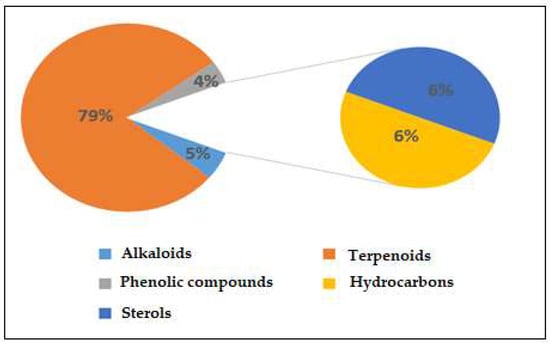
Figure 1.
Percentages of chemical compounds extracted by acetic acid from Saussurea costus.
2.2. Molecular Docking
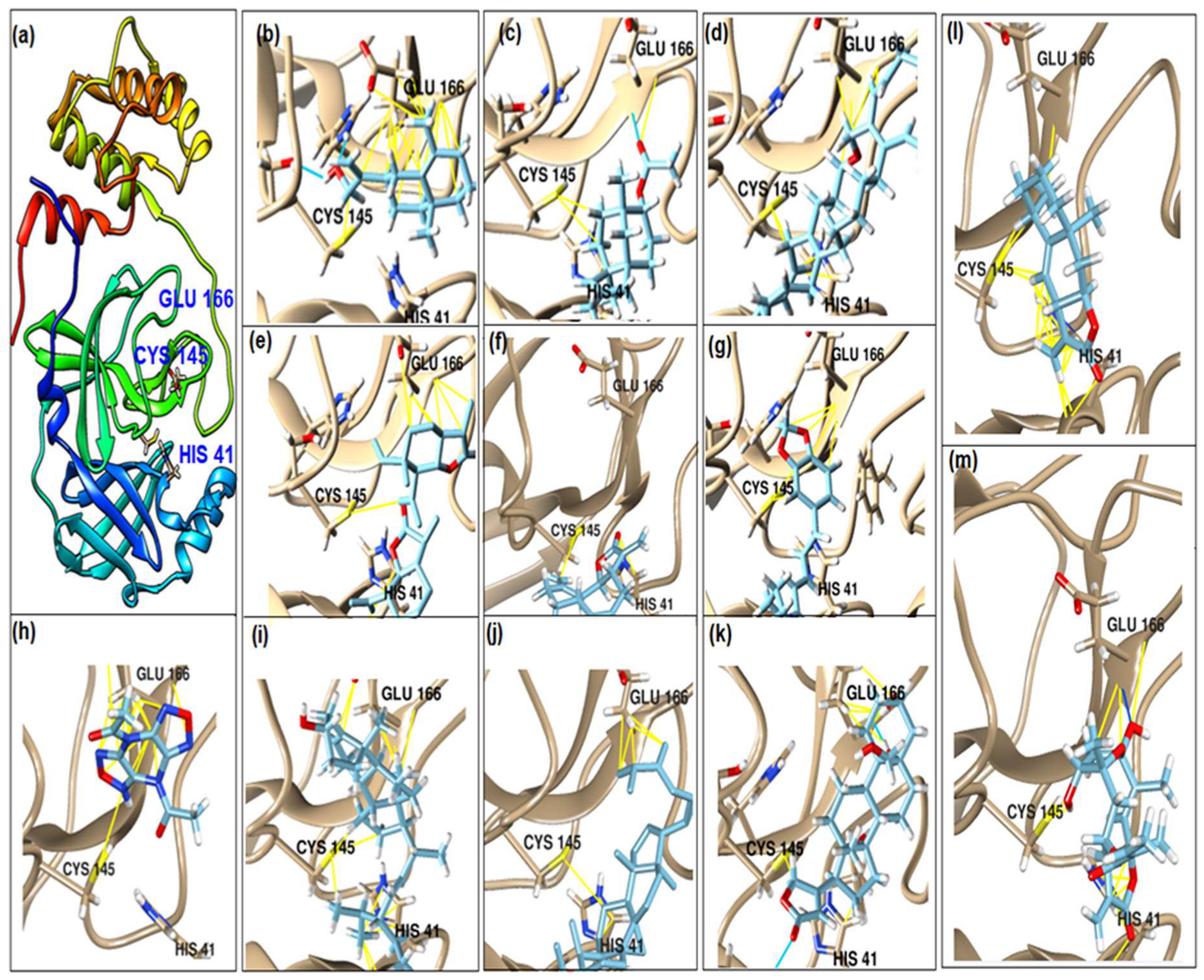
This study had a particular focus on the important residue GLU 166 and the catalytic dyad CYS 145, as well as HIS 41 of the main protease (Mpro). All of the 69 compounds identified were tested against Mpro. Among these, 12 were docked to the active site of the Mpro (Figure 2). As shown in Table 1, they had low energy scores and interacted with the active sites in the pocket of the Mpro enzyme. The binding scores of these molecules were significantly low, ranging from −7.8 to −5.6 kcal/mol. The interaction of oxatricyclo [20.8.0.0(7,16)]triaconta-1(22),7(16),9,13,23,29-hexaene with the active site has generally been shown to be more efficient. As sterols interact with the active site, they can be promising candidates, particularly oxygenous tetrahydroxy sterol, which has a high tendency to form hydrogen bonds. Schiff base ligands have been shown to inhibit Mpro in previous studies [54] as well as caffeine [54,55], nethylxanthines [56], natural product isolates [57], glycyrrhizin [58], ML188 [59], pyrimidonic and pyridonic pharmaceuticals [60,61], kaempferol [62], fungal natural products [63], marine natural compounds [64], Ceftazidime [65], hepatitis C virus protease drugs [66], and other inhibitors [67,68,69,70,71,72]. In our molecular docking study, these natural compounds were identified as potential Mpro inhibitors.
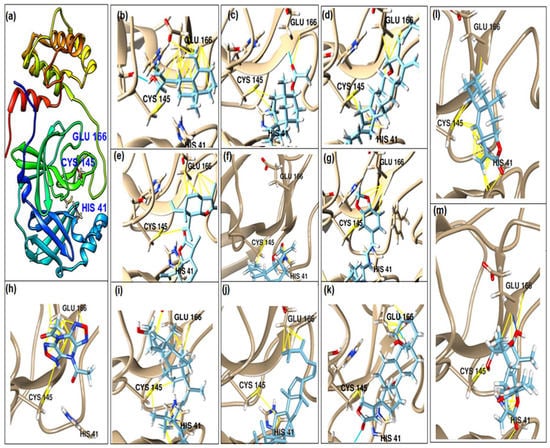
Figure 2.
(a) The main protease of the SARS-CoV-2 crystal structure, with a particular focus on GLU 166, CYS 145, and HIS 41 active residues (PDB ID: 6Y2E). Amplified picture of docked compound (a–m) with the active site of Mpro.

Table 1.
The binding affinity of the active compounds docked with the active site of the main protease of SARS-CoV-2 with a particular focus on the GLU 166, HIS 41, and CYS 145 residues.
2.3. Antimicrobial Activity
In the present study, the antimicrobial activity of Saussurea costus extracts (aqueous and acetic acid extracts) was analyzed qualitatively and quantitatively. The results of the disc-diffusion test are represented in Table 2. According to the results, the acetic acid extract revealed significant antimicrobial activity against all tested microorganisms. The most susceptible microorganism was Candida albicans with 38.5 ± 1.5 mm, followed by Bacillus cereus with 25.0 ± 2.0 mm, Salmonella enterica with 18.0 ± 0.0 mm, Staphylococcus aureus with 16.5 ± 0.5 mm, Escherichia coli with 14.0 ± 1.0 mm, and Pseudomonas aeruginosa with 13.5 ± 0.5 mm. Our results are in agreement with previous published studies which showed that the methanol and ethanol extracts of Saussurea lappa showed significant antibacterial and antifungal activity [73]. Essential oils of Saussurea costus roots were also cited to have high antimicrobial efficacy [74]. Our results revealed noticeable anti-Candida activity higher than the reference antibiotic (clotrimazole, 5 mg/mL), which was in agreement with previously published reports showing that Candida albicans was highly susceptible to the semi-polar and non-polar extracts [75]. The antimicrobial activity can be attributed to some phytochemical molecules in the acetic acid extract.

Table 2.
The zones of inhibition (in mm) of Saussurea costus aqueous and acetic acid extracts against examined microorganisms (mean ± standard deviation).
The results of MIC, MBC, MFC, MBC/MIC, and MFC/MIC analyses are represented in Table 3. These results support those of the disc-diffusion test, which showed that the MIC, which was the lowest concentration of Saussurea costus acetic acid root extract that inhibited the visible growth of microorganisms after overnight incubation, was 25 mg/mL for the bacterial stains and 6.25 mg/mL for Candida albicans. The results suggest that this yeast is highly susceptible to Saussurea costus and indicate that this plant contains significant organic active ingredients for treating pathogenic Candida spp. Diseases caused by Candida have become much more common around the world, and in some patient groups, the death rate is over 70% [76]. Candida spp. causes cutaneous, gastrointestinal, and vaginal infections, as well as severe vaginitis, endophthalmitis, and candidiasis for medically compromised patients [77]. Moreover, previous research showed that dehydro-costus lactone is the major anti-candida component of this plant [54]. The values of MBC (for bacteria) and MFC (for the fungus), which show the minimum concentrations of Saussurea costus acetic acid extract that killed 99.9% of the examined microorganisms, were 50 mg/mL for all bacteria except for 25 mg/mL for Staphylococcus aureus and 12.5 mg/mL for Candida albicans (MFC). Additionally, the MBC/MIC was less than four, leading us to conclude that the extract had a bactericidal effect on the bacterial strains, and the MFC/MIC ratio was also less than four, which means that it demonstrated fungicidal activity against Candida albicans. When an extract’s MBC/MIC ratio is less than four, the extract is regarded as bactericidal, and if the ratio is more than or equal to four, the extract is deemed bacteriostatic [78]. Furthermore, a compound is classified as fungistatic when the MFC/MIC ratio is greater than or equal to four and as fungicidal when the MFC/MIC ratio is less than four [79]. Therefore, we concluded that Saussurea costus acetic acid extract has bactericidal and fungicidal properties.

Table 3.
The MIC, MBC, MFC, MBC/MIC, and MFC/MIC values of the acetic acid extract of Saussurea costus against the examined microorganisms.
2.4. Antiviral Activity by Saussurea costus Acetic Extract
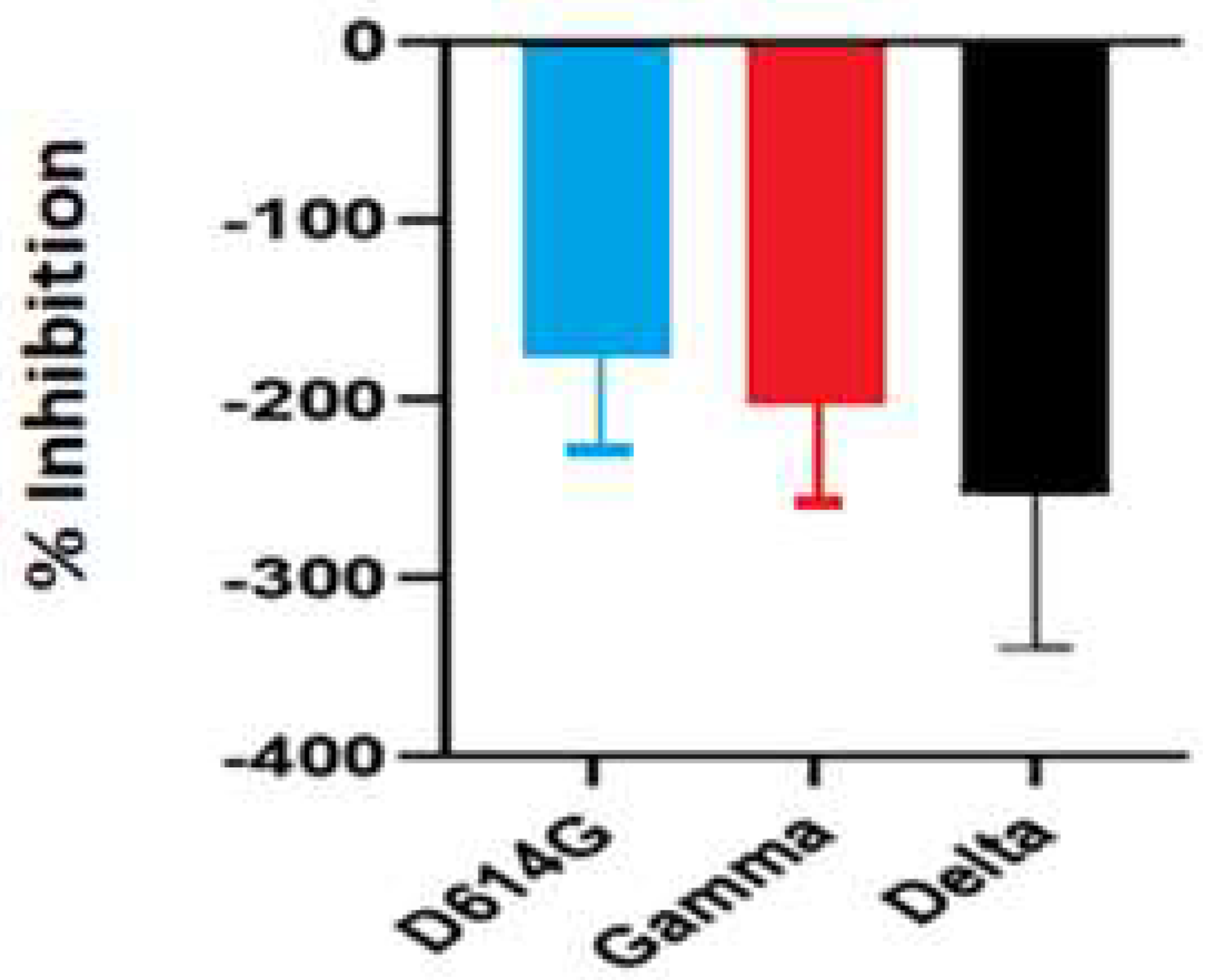
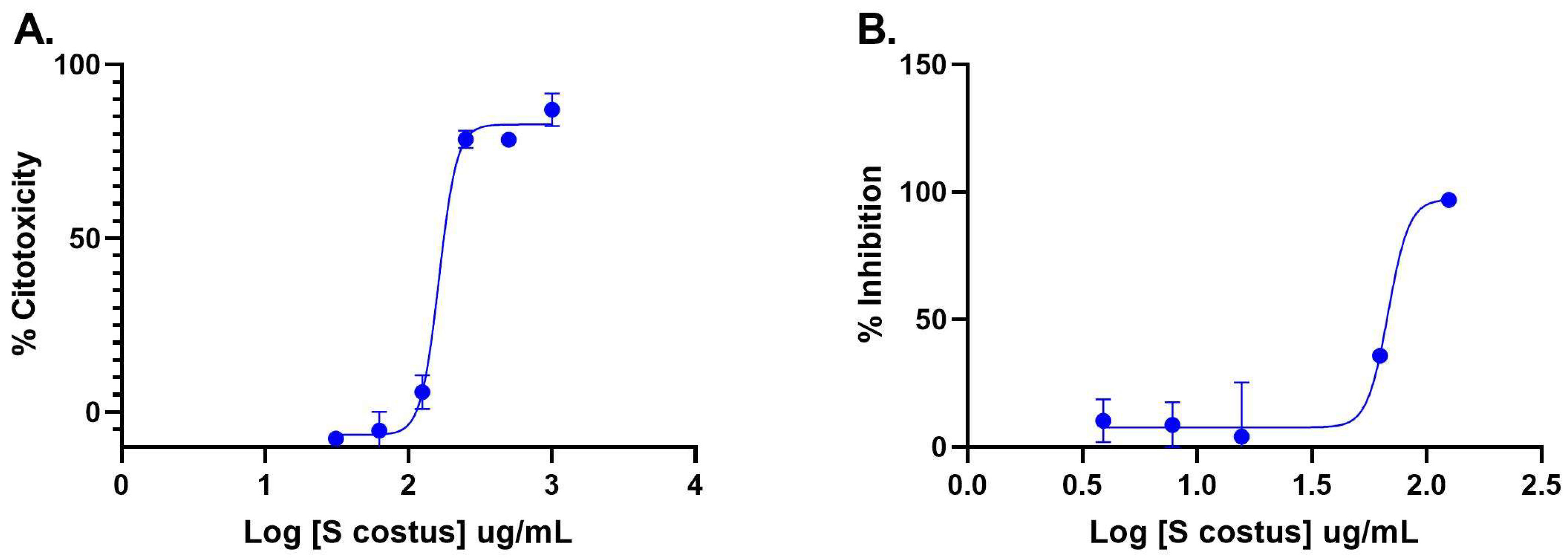
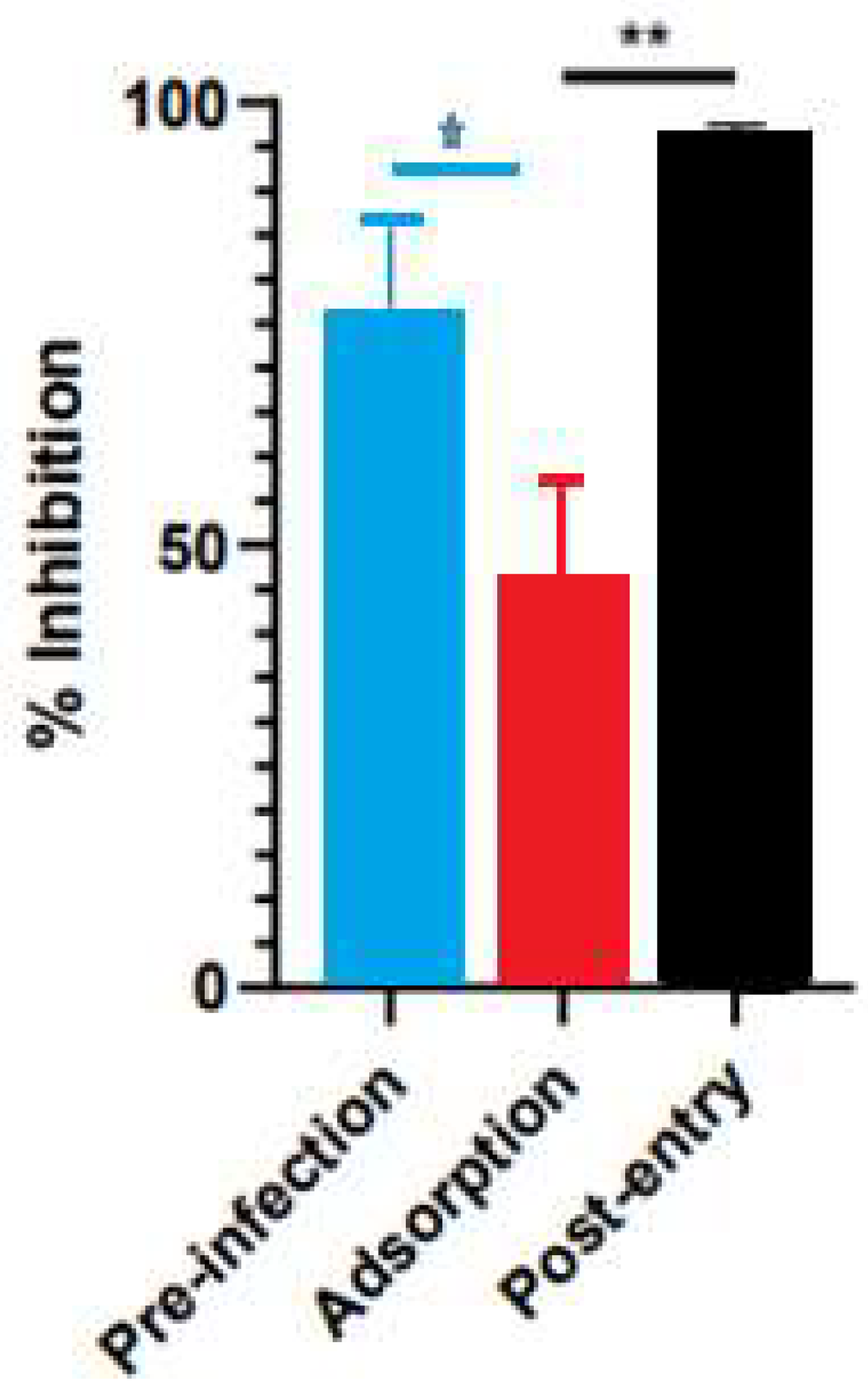
The inhibitory activity of the S. costus acetic extract against SARS-CoV2 was evaluated using the pseudovirus method. This system allows the investigation of inhibitors of virus entry into the cell [80]. To evaluate the effect of the extract on variants of concern (VOCs) of SARS-CoV2, the antiviral activity was observed using D614G, Gamma, and Delta variants of the pseudotyped virus. No antiviral effect of the acetic extract from Saussurea costus was observed with any SAR-CoV2 variants (Figure 3), and no differences between different variants were observed using one-way ANOVA. These results are in agreement with previous results indicating that the aqueous extract of S. costus lacks entry inhibitory activity against SARS-CoV2 [54]. Furthermore, the antiviral activity of S. costus acetic extract against HSV-1, another enveloped virus, was evaluated. For this purpose, serial dilutions of the extract were added post-virus-adsorption, and the amount of virus produced was evaluated by qPCR. The S. costus acetic extract showed antiviral activity with an effective concentration 50 (EC50) of 82.6 µg/mL, a cytotoxic concentration (CC50) of 162.9 µg/mL, and a selectivity index of 1.9 (Figure 4 and Figure 5).
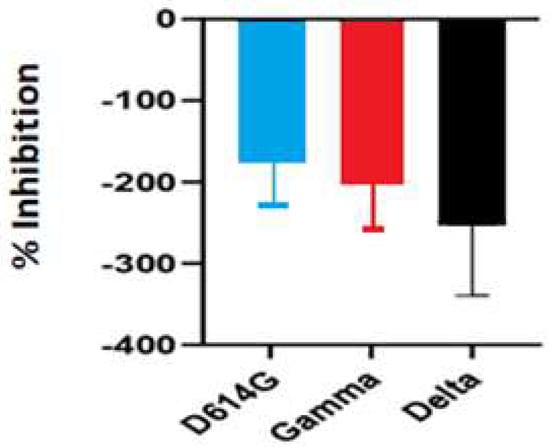
Figure 3.
Antiviral activity of Saussurea costus acetic extract against SAR-CoV2. HEK-293T ACE2 cells were infected with the corresponding pseudotyped virus in the presence or absence of the extract. After 48 h, the luciferase activity was measured. The percentage of inhibition was determined as the ratio between treated and untreated cells.
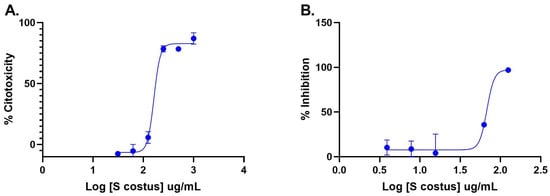
Figure 4.
Anti-herpetic activity and cytotoxicity of Saussurea costus extract. (A) Vero cells were incubated with increasing concentrations of the acetic extract, and after 72 h, the cytotoxicity was measured as described in the Materials and Methods. (B) Vero cells were infected at MOI 1.5 with HSV-1 and incubated with increasing concentrations of the extract. After 48 h, the virus genome was quantitated in the supernatant via qPCR. The percentage of inhibition was determined as the ratio between treated and untreated infected cells. Data are expressed as mean +/− SD for n = 2.
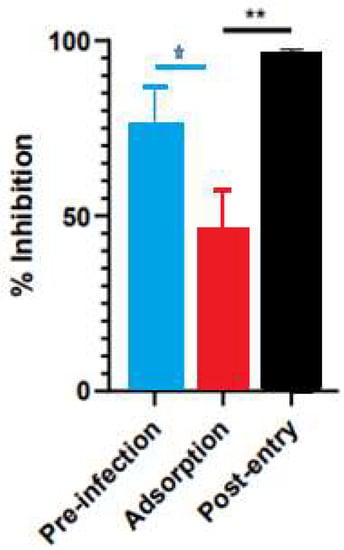
Figure 5.
Time-of-addition assay results for Saussurea costus acetic extract. Vero cells were infected at MOI 1.5 and incubated with the extract at different times during the infection, as described in the Materials and Methods. After 48 h post-infection, the virus genome was quantitated in the supernatant via qPCR. The percentage of inhibition was determined as the ratio between treated and untreated infected cells. Data are expressed as mean +/− SD for n = 3. Statistical analysis was performed using one-way ANOVA, followed by multiple comparisons testing with significance indicated as * p < 0.05, ** p < 0.01.
A time-of-infection study was performed to identify the extract’s effect on the viral cycle. The extract was added at different times during infection. In the pre-infection condition, the acetic extract was added 2 h before the addition of the virus, then it was removed, and the cells were infected. In the adsorption condition, the virus was added at the same time as the extract and incubated for one hour and then replaced with the medium. Under the post-entry condition, the extract was added after the virus entered the cell. The Saussurea costus acetic extract showed pre-infection and post-entry antiviral activities, indicating that its compounds act by different antiviral mechanisms.
In a previous study, the antiviral activity of the aqueous extract of S. costus (EC50 = 1.35 mg/mL, CC50 = 4.92 mg/mL, and SI = 3.6) was reported. In the present work, a similar behavior was observed with the S. costus acetic extract (EC50 = 82.6 mg/mL, CC50 = 162.9 mg/mL, and SI = 1.9). However, a difference in the mechanisms of action of the extracts was observed. The aqueous extract only showed post-entry antiviral activity, whereas the acetic extract also inhibited the virus in the pre-infection condition. This result suggests that the acetic extract also induced an antiviral state in cells.
3. Materials and Methods
3.1. Chemicals
All chemicals used (acetic acid, dimethyl sulfoxide, ethanol, anhydrous sodium sulfite) were of analytical grade, were used as received without any further purification.
3.2. Sample Preparation and Extraction
Roots of Saussurea costus of high quality were purchased from “the attar shop”, which is a trusted herbal store in Riyadh, Saudi Arabia, with a license from the government to sell herbs; the source of Saussurea costus is Kashmir in the northwestern region of India. The root of Saussurea costus was air-dried at room temperature for many days. The dried roots were ground using an electric grinder. Dried and powdered samples of Saussurea costus acetic acid solvent (99.8%) extract (Scharlau Sentmenat, Barcelona, Spain) underwent filtration, and the filtrate was allowed to dry on a glass Petri dish at room temperature. Fifty grams of dried Saussurea roots were extracted in 150 milliliters of acetic acid at room temperature for four days with an Orbital Shaker (BioSan PSU-20i, Riga Latvia).
3.3. Gas Chromatography–Mass Spectrometry (GC/MS) Analysis
A GM/MS system from Shimadzu Kyoto Japan, with serial number 020525101565SA and a capillary column (Rtx-5MS 30 m 0.25 mm × 0.25 mm), was employed for the analysis of samples. The solution of the samples was passed through a 0.45 mm syringe filter and into a 1.5 mL GC-MS vial, where it was prepared for injection. The specimen was injected utilizing split mode with helium serving as the carrier gas, which had a 1.61 mL/min flow rate and moved inside the injector. According to the manufacturer’s instructions, the temperature program started at 50 °C at a rate of 10 °C per minute and ended at 300 °C with a hold duration of 10 min. The injection port temperature was 300 degrees Celsius; the ion source was 200 °C, and the interface was 250 °C. The sample required 35 min for the analysis in scan mode in the m/z 40–500 mass-to-charge ratio range, with a 40-min run time. (GC-MS analysis conditions are shown in Table S1). The sample’s contents were identified by comparing the retention index and mass fragmentation patterns of the sample to those in the National Institute of Standards and Technology library (NIST). The relative quantities of the different components were determined without correction factors based on the peak area of the GC (FID response).
3.4. Molecular Docking
As described in PubChem, Canonical SMILES was used to prepare the 3D models for molecular docking, along with Open Babel. Using the Protein Data Bank database, we downloaded the Mpro of SARS-CoV-2’s crystal structure (PDB ID: 6Y2E). Then, water residues were removed, and their energy was minimized using the Molecular Modeling Toolkit plugin UCSF Chimera [60,81,82,83]. Molecular docking was accomplished using AutoDock Vina with a grid box of (−16.5 × −24.0 × 16.5) Å, and it was centered at (35.0, 65.0, 65.0) Å. UCSF Chimera was used for the visualization of images.
3.5. Microorganisms
In this investigation, six American type culture collection (ATCC) microbial isolates were utilized, including Gram-positive bacteria (Staphylococcus aureus ATCC BAA 1026 and Bacillus cereus ATCC 10876), Gram-negative bacteria (Pseudomonas aeruginosa ATCC 10145, Salmonella enterica ATCC 14028, and Escherichia coli ATCC 9637), and a yeast (Candida albicans ATCC 10231). All of the isolated microorganisms were provided by the Department of Laboratory Sciences at Al-Rass, Qassim University in Saudi Arabia.
3.6. Disc-Diffusion Test
The disc-diffusion test was used to evaluate the initial antimicrobial activities of the extract against the selected microbial strains, using the previously reported procedure with minor modifications [84]. Briefly, the dried acetic acid extract of Saussurea costus roots was re-constituted by dissolving 500 mg of the dry extract in 10% dimethyl sulfoxide (DMSO) and mixed well using a shaker for up to 1 h. According to our pre-experimental evaluation and the literature, 10% DMSO has no inhibitory effect on microbial growth [85,86,87]. A fresh microbial suspension adjusted to 0.5 McFarland turbidity (108 CFU/mL) (CFU: colony-forming units) was streaked over sterile plates containing Mueller–Hinton Agar for bacteria or Sabouraud Dextrose Agar for the yeast (microbial media were obtained from Oxoid, UK). Under aseptic conditions, sterile paper discs (6 mm) were carefully saturated with 10 µL of the reconstituted extract (100 mg/mL) using an Eppendorf pipette. Saturated papers were immediately loaded over the streaked plates and left to settle for up to 15 min, and then the seeded plates were incubated upside-down at about 37 °C for 24 hours for bacteria and for 48 hours for Candida albicans. A disc containing 10 µL of 10% DMSO served as a negative control, whereas a disc containing chloramphenicol (2.5 mg/mL) for bacteria and clotrimazole (5 mg/mL) for yeast served as a reference drug (positive control). The inhibitory diameter was then measured in millimeters (disc included) and reported as the mean ± standard deviation for two trials.
3.7. Minimum Inhibitory Concentration MIC Assay
The minimum inhibitory concentration (MIC) values were determined using the micro-well dilution method for the microorganisms determined to be sensitive to the extract in the disc diffusion experiment [88]. The microorganism inoculum was taken from stock culture (in slant tubes containing general-purpose nutrient agar, Oxoid, UK) for bacteria or Sabouraud dextrose agar (Oxoid, UK) for the yeast and inoculated in nutrient broth (general-purpose growth medium, Oxoid, UK for bacteria or Sabouraud dextrose broth for the yeast and incubated for up to 12 h and up to 24 h for the yeast to reach the exponential phase. Then, microbiological suspensions were adjusted to a turbidity standard of 0.5 McFarland units. The dry crude extract was diluted in 10% dimethyl sulfoxide (DMSO) to obtain a concentration of 100 mg/mL, and then two-fold dilutions were prepared in test tubes containing nutrient broth over a concentration range of 1.5 to 100 mg/mL. In order to prepare the 96-well plates, 100 µL of the previously prepared serial dilutions were transferred into each of seven successive wells. Then, 95 µL of nutrient broth for bacteria or Sabouraud dextrose broth for the yeast and 5 µL of microbial inoculum were added to each well, bringing the total amount to 200 µL. As a negative control in another well series, nutrient broth or Sabouraud dextrose broth with sterile normal saline instead of extract and 5 mL of the inoculum were applied to each well. Additionally, successive dilutions of the standard antimicrobial drugs (chloramphenicol or clotrimazole) were used as positive controls. The 96-well plates were covered using a sterile sealant. The contents of each well were shaken on a plate shaker at 300 rpm for 20 s, before being incubated for 24 h for bacteria of 48 h for yeast at the specified temperatures. Microbial growth was estimated by measuring the absorbance at 595 nm using an iMark Absorbance microplate reader (BIO-RAD Inc., Hercules, California, USA). In this investigation, the extract was screened twice against each organism. The MIC is the lowest concentration of a substance that inhibits the growth of microorganisms.
3.8. Minimum Bactericidal Concentration or Minimum Fungicidal Concentration MBC or MFC Assay
The minimum bactericidal concentration (MBC) or minimum fungicidal concentration (MFC) can be defined as the lowest concentration at which an antimicrobial agent will eradicate a certain microorganism. The agar diffusion test was used here with minor modifications [89]. After the MIC test was completed, the MBC or MFC test was conducted. In a nutshell, 50 µL from each MIC tube was taken using an Eppendorf pipette and spotted onto nutritional agar plates for bacteria or Sabouraud dextrose agar for the yeast. These plates were then incubated overnight at 30 °C –35 °C. MBC was defined as the lowest MIC that showed no detectable growth. Additionally, MBC/MIC values for bacteria and MBC/MFC values for Candida albicans were calculated to classify the extract as bactericidal/fungicidal (4 or less) or bacteriostatic/fungistatic (more than 4).
3.9. Virological Assessments
3.9.1. Cytotoxicity Assays
Cytotoxicity assays were performed as previously described [90,91]. A stock solution of 100 mg/mL of the re-constituted acetic acid extract of Saussurea costus in DMSO was made. Human ACE2 Stable Cell Line and HEK293T: is a popular derivative of the original HEK293 parent cell line (HEK293T-ACE2) or Vero cells were cultured at 1 × 104 cells/well in a 96-well plate in the presence of different concentrations of the S. costus extract in DMEM 10% FBS (Foetal Bovine Serum) for HEK293T-ACE2 cells and in DMEM 2% FBS in the case of Vero cells. DMSO-treated cells were used as a control and normalizer. After 48 h, resazurin was added to a final concentration of 0.0015% per well. Absorbance was measured on the Multiskan TM GO (Thermo Scientific, USA) at 570 and 630 nm.
3.9.2. HIV-1-Based SARS-CoV-2 Pseudotyped Particles
The plasmids used were as follows: pNL4.3-ΔEnv-FLuc, spike-G614-Δ19 (10.1126/sciadv.abe6855), pcDNA3.3_CoV2_P1 (Addgene plasmid #170450; http://n2t.net/addgene:170450; RRID: Addgene_170450) [92], and pcDNA3.3-SARS2-B.1.617.2 (Addgene plasmid # 172320; http://n2t.net/addgene:172320; RRID: Addgene_172320) [93]. The plasmids pcDNA3.3_CoV2_P1 and pcDNA3.3-SARS2-B.1.617.2 were a gift from David Nemazee.
Pseudotyped viral particles were generated by transfecting the plasmids (20 µg, molar ratio 3:2) pNL4.3-ΔEnv-FLuc and spike-G614-Δ19 or pcDNA3.3_CoV2_P1 (spike-GammaΔ18) or pcDNA3.3-SARS2-B.1.617.2 (spike-DeltaΔ18) with 2.5 M calcium chloride, and HEPES-buffered saline 2X (0.28 M NaCl, 0.05 M HEPES, 1.5 mM Na2HPO4, pH 7.05) [94]. The pseudotyped virus-containing supernatant was collected 48 h after transfection and centrifuged at 3500 rpm for 5 min at room temperature and stored at −80 °C. Stocks were titrated with HEK293T-ACE2, and firefly luciferase activity was measured 48 h later using the Dual-Luciferase Reporter Assay System kit (Promega, Madison, WI, USA) and Fluoroskan FL (Thermo Scientific). HEK293T (lacking the ACE2 receptor) cells transduced with the pseudotyped particles were used as a negative control.
3.9.3. Inhibition of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Entry Assay
The assay was performed in HEK293-ACE2 cells, as described previously [95]. Briefly, 1 × 104 cells in suspension were added to each well of 96-well plates and infected in the presence of 62.5 µg/mL of the re-constituted acetic acid extract of Saussurea costus in DMEM 10% FBS. After 48 h, the firefly luciferase activity was measured using Dual-Luciferase Reporter Assay System kit (Promega, USA) and Fluoroskan FL (Thermo Scientific). HEK293T-ACE2 cells transduced with the pseudotyped virus without the extract were used as control untreated cells. The following formula was used to calculate the percentage of inhibition: 100 − (RLUs of treated cells/RLUs of control untreated cells) × 100.
3.9.4. Antiviral Activity against Herpes Simplex Virus Type-1 (HSV-1)
Antiviral activity assays were performed as previously described [96]. Briefly, Vero cells were seeded in 96-well plates at 1 × 104 cells/well in DMEM 7.5% FBS. The next day, the cells were infected at MOI 1.5 in DMEM 2% FBS. After 1 h of virus adsorption, the medium was replaced, and dilutions of the extract in DMEM 2% FBS were added. Control-infected cells were incubated with DMSO. Forty-eight hours later, the virus in the supernatant was quantified via qPCR, using 5 µL of SYBR® Green Master Mix buffer (Bio-Rad) 2X, 500 pM of each primer, and 1 µL of sample as a template. Amplifications were carried out on a StepOnePlus™ thermocycler (Applied Biosystems). A two-step program of 10 min at 95 °C, 35 cycles of 15 s at 95 °C, and 30 s at 60 °C, and a final melting curve was used. The viral genome copies (VGC) were calculated using a calibration curve.
3.9.5. Time-of-Addition Assay of HSV-1
The time-of-addition assay was carried out under three different experimental conditions according to the time at which the extract was added. In the pre-infection condition, the extract was added two hours before viral adsorption. Subsequently, cells were washed with PBS, the virus was added, and the infection was performed as described previously. Under the adsorption conditions, the extract was added with the virus and was incubated for 1 h to 37 °C. After that, the viral inoculum was removed, cells were washed with PBS and replaced with DMEM 2% FBS, and the infection was performed as previously described. In the post-entry condition, the viral inoculum was added and incubated for 1 h at 37 °C and washed with PBS, and the extract in DMEM 2%FBS was added. Forty-eight hours later, the virus genome in the supernatant was quantitated via qPCR, and the antiviral activity was quantified as previously described [96].
3.10. Statistical Analysis
In this study, the data are shown as the mean standard error of the mean. The one-way ANOVA (analysis of variance) method and SPSS 14.0 (SPSS Inc., Chicago, IL, USA) were used to determine whether there were statistically significant differences between the microorganisms that were tested.
3.11. Research Limitations
Experiments carried out on Saussurea costus roots grown in India and those carried out on the same plant grown in other tropical and subtropical regions may show some variations in results. The antimicrobial experiments were repeated twice, and the mean was calculated.
4. Conclusions
In summary, GC-MS analytical screening of Saussurea costus extract using acetic acid was investigated. The survey outcomes resulted in the detection of 69 phytochemical substances in the acetic acid extract. The order of phytochemicals showed the following trend: terpenoids > hydrocarbons > sterols > alkaloids = phenolic compounds. The crude root extract of Saussurea costus obtained using acetic acid displayed significant antibacterial activity. The inhibitory activity of sterols and terpenoids against SARS-CoV-2 was studied using molecular docking. The examined sterols and terpenoids were found to be active. Furthermore, sterols generally exhibited a more efficient interaction with the active site. Candida albicans was the most vulnerable organism, followed by Bacillus cereus, Salmonella enterica, Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa, respectively. Evaluations of antiviral activity against HSV-1 and SARS-CoV2 revealed a considerable beneficial effect against HSV-1 (EC50 = 82.6 g/mL; CC50 = 162.9 g/mL; selectivity index = 1.9). However, no effect was detected in terms of the inhibition of SARS-CoV2 entry.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/plants12030460/s1, Figure S1: GC-MS chromatograms of acetic acid extract of Saussurea costus, Table S1: GC-MS analysis conditions, Table S2: Phytochemical in acetic acid extraction of Saussurea costus roots.
Author Contributions
Conceptualization, H.I., E.M.A., P.H.S. and A.O.E.; methodology, H.I., B.S., H.M.E., A.I.A., E.M.A., P.G.-M., P.H.S. and A.O.E.; GC-MS analysis, B.S.; antibacterial analysis, E.M.A.; antiviral analysis, P.G.-M. and P.H.S.; molecular docking and software, H.M.E. and A.O.E.; formal analysis and investigation, H.I. and A.O.E.; writing—original draft preparation, H.I., B.S. and A.O.E.; writing—review and editing H.I., E.M.A., P.G.-M., P.H.S. and A.O.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Deanship of Scientific Research, Imam Mohammad Ibn Saud Islamic University (IMSIU), Saudi Arabia, Grant No. (21-13-18-031).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data will be available upon suitable request.
Acknowledgments
The authors thank the laboratories of Ricardo Soto-Rifo and Fernando Valiente-Echeverría for providing the pseudoviral SARS-CoV-2 system.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Abdallah, E.M. Plants: An alternative source for antimicrobials. J. Appl. Pharm. Sci. 2011, 1, 16–20. [Google Scholar]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef] [PubMed]
- Moradi, B.; Abbaszadeh, S.; Shahsavari, S.; Alizadeh, M.; Beyranvand, F. The most useful medicinal herbs to treat diabetes. Biomed. Res. Ther. 2018, 5, 2538–2551. [Google Scholar] [CrossRef]
- Krishnaiah, D.; Sarbatly, R.; Nithyanandam, R. A review of the antioxidant potential of medicinal plant species. Food Bioprod. Process. 2011, 89, 217–233. [Google Scholar] [CrossRef]
- Shukla, S.; Mehta, A. Anticancer potential of medicinal plants and their phytochemicals: A review. Braz. J. Bot. 2015, 38, 199–210. [Google Scholar] [CrossRef]
- Vashist, H.; Jindal, A. Antimicrobial activities of medicinal plants–Review. Int. J. Res. Pharm. Biomed. Sci. 2012, 3, 222–230. [Google Scholar]
- Umeta Chali, B.; Melaku, T.; Berhanu, N.; Mengistu, B.; Milkessa, G.; Mamo, G.; Alemu, S.; Mulugeta, T. Traditional medicine practice in the context of COVID-19 pandemic: Community claim in jimma zone, oromia, Ethiopia. Infect Drug Resist. 2021, 14, 3773–3783. [Google Scholar] [CrossRef]
- Gwari, G.; Bhandari, U.; Andola, H.C.; Lohani, H.; Chauhan, N. Volatile constituents of Saussurea costus roots cultivated in Uttarakhand Himalayas, India. Pharmacogn. Res. 2013, 5, 179–182. [Google Scholar]
- Ansari, S.; Siddiqui, M.; Malhotra, S.; Maaz, M. Antiviral Efficacy of Qust (Saussurea lappa) and Afsanteen (Artemisia absinthium) for Chronic Hepatitis B: A Prospective Single-Arm Pilot Clinical Trial. Pharmacogn. Res. 2018, 10, 282. [Google Scholar] [CrossRef]
- Hassan, R.; Masoodi, M.H. Saussurea lappa: A comprehensive review on its pharmacological activity and phytochemistry. Curr. Tradit. Med. 2020, 6, 13–23. [Google Scholar] [CrossRef]
- WHO. WHO COVID-19 Preparedness and Response Progress Report-1 February to 30 June 2020; WHO: Geneva, Switzerland, 2020.
- Gao, Y.; Cai, G.-Y.; Fang, W.; Li, H.-Y.; Wang, S.-Y.; Chen, L.; Yu, Y.; Liu, D.; Xu, S.; Cui, P.-F.; et al. Machine learning based early warning system enables accurate mortality risk prediction for COVID-19. Nat. Commun. 2020, 11, 5033. [Google Scholar] [CrossRef] [PubMed]
- Nickbakhsh, S.; Mair, C.; Matthews, L.; Reeve, R.; Johnson, P.C.D.; Thorburn, F.; von Wissmann, B.; Reynolds, A.; McMenamin, J.; Gunson, R.N.; et al. Virus–virus interactions impact the population dynamics of influenza and the common cold. Proc. Natl. Acad. Sci. 2019, 116, 27142–27150. [Google Scholar] [CrossRef] [PubMed]
- Fatkin, D.; Macrae, C.; Sasaki, T.; Wolff, M.R.; Porcu, M.; Frenneaux, M.; Atherton, J.; Vidaillet, H.J., Jr.; Spudich, S.; De Girolami, U.; et al. Missense mutations in the rod domain of the lamin A/C gene as causes of dilated cardiomyopathy and conduction-system disease. New Engl. J. Med. 1999, 341, 1715–1724. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zhang, Z.; Chen, W.; Cai, Z.; Ge, X.; Zhu, H.; Jiang, T.; Tan, W.; Peng, Y. Predicting the receptor-binding domain usage of the coronavirus based on kmer frequency on spike protein. Infect. Genet. Evol. 2018, 61, 183. [Google Scholar] [CrossRef]
- Hutchison, C.J.; Alvarez-Reyes, M.; Vaughan, O.A. Lamins in disease: Why do ubiquitously expressed nuclear envelope proteins give rise to tissue-specific disease phenotypes? J. Cell Sci. 2001, 114, 9–19. [Google Scholar] [CrossRef]
- Zhang, X.; Kousoulas, K.G.; Storz, J. The hemagglutinin/esterase glycoprotein of bovine coronaviruses: Sequence and functional comparisons between virulent and avirulent strains. Virology 1991, 185, 847–852. [Google Scholar] [CrossRef]
- Salazar, E.; Christensen, P.A.; Graviss, E.A.; Nguyen, D.T.; Castillo, B.; Chen, J.; Lopez, B.V.; Eagar, T.N.; Yi, X.; Zhao, P.; et al. Treatment of coronavirus disease 2019 patients with convalescent plasma reveals a signal of significantly decreased mortality. Am. J. Pathol. 2020, 190, 2290–2303. [Google Scholar] [CrossRef]
- Holmes, E.A.; O’Connor, R.C.; Perry, V.H.; Tracey, I.; Wessely, S.; Arseneault, L.; Ballard, C.; Christensen, H.; Silver, R.C.; Everall, I.; et al. Multidisciplinary research priorities for the COVID-19 pandemic: A call for action for mental health science. Lancet Psychiatry 2020, 7, 547–560. [Google Scholar] [CrossRef]
- Mintah, S.O.; Asafo-Agyei, T.; Archer, M.A.; Junior, P.A.A.; Boamah, D.; Kumadoh, D.; Appiah, A.; Ocloo, A.; Boakye, Y.D.; Agyare, C. Medicinal plants for treatment of prevalent diseases. In Pharmacognosy-Medicinal Plants; IntechOpen: Rijeka, Croatia, 2019. [Google Scholar]
- Tahir, A.H.; Javed, M.M.; Hussain, Z. Nutraceuticals and herbal extracts: A ray of hope for COVID-19 and related infections. Int. J. Funct. Nutr. 2020, 1, 6. [Google Scholar] [CrossRef]
- Glatthaar-Saalmüller, B.; Sacher, F.; Esperester, A. Antiviral activity of an extract derived from roots of Eleutherococcus senticosus. Antivir. Res. 2001, 50, 223–228. [Google Scholar] [CrossRef]
- Karagöz, A.; Arda, N.; Gören, N.; Nagata, K.; Kuru, A. Antiviral activity of Sanicula europaea L. extracts on multiplication of human parainfluenza virus type 2. Phytother. Res. 1999, 13, 436–438. [Google Scholar] [PubMed]
- Al-Mijalli, S.H.; Mrabti, N.N.; Ouassou, H.; Sheikh, R.; Abdallah, E.; Assaggaf, H.; Bakrim, S.; Alshahrani, M.M.; Al Awadh, A.A.; Qasem, A.; et al. Phytochemical Variability, In Vitro and In Vivo Biological Investigations, and In Silico Antibacterial Mechanisms of Mentha piperita Essential Oils Collected from Two Different Regions in Morocco. Foods 2022, 11, 3466. [Google Scholar] [CrossRef]
- Razzaque, M.S. Commentary: Microbial resistance movements: An overview of global public health threats posed by antimicrobial resistance, and how best to counter. Front. Public Health 2021, 8, 629120. [Google Scholar] [CrossRef] [PubMed]
- Jassim, S.A.; Naji, M.A. Novel antiviral agents: A medicinal plant perspective. J. Appl. Microbiol. 2003, 95, 412–427. [Google Scholar] [CrossRef] [PubMed]
- Harazem, R.; El Rahman, S.A.; El-Kenawy, A. Evaluation of Antiviral Activity of Allium Cepa and Allium Sativum Extracts Against Newcastle Disease Virus. Alex. J. Vet. Sci. 2019, 61, 108. [Google Scholar] [CrossRef]
- Badam, L.; Joshi, S.; Bedekar, S. ‘In vitro’ antiviral activity of neem (Azadirachta indica A. Juss) leaf extract against group B coxsackieviruses. J. Commun. Dis. 1999, 31, 79–90. [Google Scholar]
- Sharma, P.; Dwivedee, B.P.; Bisht, D.; Dash, A.K.; Kumar, D. The chemical constituents and diverse pharmacological importance of Tinospora cordifolia. Heliyon 2019, 5, e02437. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, P.; Raghu, C.; Ashok, G.; A Dhanaraj, S.; Suresh, B. Antiviral activity of medicinal plants of Nilgiris. Indian J. Med. Res. 2004, 120, 24–29. [Google Scholar]
- Kochan, E.; Wasiela, M.; Sienkiewicz, M. The production of ginsenosides in hairy root cultures of American Ginseng, Panax quinquefolium L. and their antimicrobial activity. Vitr. Cell. Dev. Biol. Plant 2013, 49, 24–29. [Google Scholar] [CrossRef]
- Maideen, N.M.P. Prophetic medicine-Nigella Sativa (Black cumin seeds)–potential herb for COVID-19? J. Pharmacopunct. 2020, 23, 62. [Google Scholar] [CrossRef]
- Kim, H.J.; Yoo, H.S.; Kim, J.C.; Park, C.S.; Choi, M.S.; Kim, M.; Choi, H.; Min, J.S.; Kim, Y.S.; Yoon, S.W.; et al. Antiviral effect of Curcuma longa Linn extract against hepatitis B virus replication. J. Ethnopharmacol. 2009, 124, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Torabian, G.; Valtchev, P.; Adil, Q.; Dehghani, F. Anti-influenza activity of elderberry (Sambucus nigra). J. Funct. Foods 2019, 54, 353–360. [Google Scholar] [CrossRef]
- Kumar, D.V.; Geethanjali, B.; Avinash, K.O.; Kumar, J.R.; Basalingappa, K.M. Tinospora cordifolia: The antimicrobial property of the leaves of amruthaballi. J. Bacteriol. Mycol. Open Access 2017, 5, 363–371. [Google Scholar]
- Saha, S.; Ghosh, S. Tinospora cordifolia: One plant, many roles. Anc. Sci. Life 2012, 31, 151. [Google Scholar]
- Panday, J.; Singh, L.; Saxena, G.; Devkota, H.P. Environmental Challenges for Himalayan Medicinal Plants. In Environmental Challenges and Medicinal Plants; Aftab, T., Ed.; Springer: Cham, Switzerland; pp. 29–47.
- Pandey, M.M.; Rastogi, S.; Rawat, A. Saussurea costus: Botanical, chemical and pharmacological review of an ayurvedic medicinal plant. J. Ethnopharmacol. 2007, 110, 379–390. [Google Scholar] [CrossRef]
- Rathore, S.; Debnath, P.; Kumar, R. Kuth Saussurea costus (Falc.) Lipsch.: A critically endangered medicinal plant from Himalaya. J. Appl. Res. Med. Aromat. Plants 2021, 20, 100277. [Google Scholar]
- Singh, D.K.; Tóth, R.; Gácser, A. Mechanisms of pathogenic Candida species to evade the host complement attack. Front. Cell. Infect. Microbiol. 2020, 10, 94. [Google Scholar] [CrossRef]
- Simón-Manso, Y.; Lowenthal, M.S.; Kilpatrick, L.E.; Sampson, M.L.; Telu, K.H.; Rudnick, P.A.; Mallard, W.G.; Bearden, D.W.; Schock, T.B.; Tchekhovskoi, D.V.; et al. Metabolite profiling of a NIST Standard Reference Material for human plasma (SRM 1950): GC-MS, LC-MS, NMR, and clinical laboratory analyses, libraries, and web-based resources. Anal. Chem. 2013, 85, 11725–11731. [Google Scholar] [CrossRef]
- Khan, A.; Shah, A.H.; Ali, N. In-vitro propagation and phytochemical profiling of a highly medicinal and endemic plant species of the Himalayan region (Saussurea costus). Sci. Rep. 2021, 11, 23575. [Google Scholar] [CrossRef]
- Kumar, J.; Pundir, M. Phytochemistry and pharmacology of Saussurea genus (Saussurea lappa, Saussurea costus, Saussurea obvallata, Saussurea involucrata). Mater. Today Proc. 2022, 56, 1173–1181. [Google Scholar] [CrossRef]
- Zahnit, W.; Smara, O.; Bechki, L.; Bensouici, C.; Messaoudi, M.; Benchikha, N.; Larkem, I.; Awuchi, C.G.; Sawicka, B.; Simal-Gandara, J. Phytochemical Profiling, Mineral Elements, and Biological Activities of Artemisia campestris L. Grown in Algeria. Horticulturae 2022, 8, 914. [Google Scholar] [CrossRef]
- Mammate, N.; El oumari, F.E.; Imtara, H.; Belchkar, S.; Lahrichi, A.; Alqahtani, A.S.; Noman, O.M.; Tarayrah, M.; Houssaini, T.S. Antioxidant and Anti-Urolithiatic Activity of Aqueous and Ethanolic Extracts from Saussurea costus (Falc) Lispich Using Scanning Electron Microscopy. Life 2022, 12, 1026. [Google Scholar] [CrossRef]
- Ali, S.I.; Venkatesalu, V. Botany, traditional uses, phytochemistry and pharmacological properties of Saussurea costus–An endangered plant from Himalaya-A review. Phytochem. Lett. 2022, 47, 140–155. [Google Scholar] [CrossRef]
- Nadda, R.K.; Ali, A.; Goyal, R.C.; Khosla, P.K.; Goyal, R. Aucklandia costus (Syn. Saussurea costus): Ethnopharmacology of an endangered medicinal plant of the himalayan region. J. Ethnopharmacol. 2020, 263, 113199. [Google Scholar] [CrossRef] [PubMed]
- Madhuri, K.; Elango, K.; Ponnusankar, S. Saussurea lappa (Kuth root): Review of its traditional uses, phytochemistry and pharmacology. Orient. Pharm. Exp. Med. 2012, 12, 1–9. [Google Scholar] [CrossRef]
- Savadi, S.; Vazifedoost, M.; Didar, Z.; Nematshahi, M.M.; Jahed, E. Phytochemical analysis and antimicrobial/antioxidant activity of Cynodon dactylon (L.) Pers. rhizome methanolic extract. J. Food Qual. 2020, 2020, 5946541. [Google Scholar] [CrossRef]
- Dolmatova, L.S.; Dolmatov, I.Y. Tumor-Associated Macrophages as Potential Targets for Anti-Cancer Activity of Marine Invertebrate-Derived Compounds. Curr. Pharm. Des. 2021, 27, 3139–3160. [Google Scholar] [CrossRef]
- Quimque, M.T.J.; Notarte, K.I.R.; de Leon, V.N.O.; Manzano, J.A.H.; Munoz, J.E.R.; Pilapil IV, D.Y.H.; Lim, J.A.K.; Macabeo, A.P.G. Computationally Repurposed Natural Products Targeting SARS-CoV-2 Attachment and Entry Mechanisms. In Frontiers of COVID-19; Adibi, S., Griffin, P., Sanicas, M., Rashidi, M., Lanfranchi, F., Eds.; Springer: Cham, Switzerland, 2022; pp. 505–537. [Google Scholar]
- Khan, J.; Sakib, S.A.; Mahmud, S.; Khan, Z.; Islam, M.N.; Sakib, M.A.; Emran, T.B.; Simal-Gandara, J. Identification of potential phytochemicals from Citrus Limon against main protease of SARS-CoV-2: Molecular docking, molecular dynamic simulations and quantum computations. J. Biomol. Struct. Dyn. 2021, 40, 10741–10752. [Google Scholar] [CrossRef]
- Mej, J.; Zhou, Y.; Yang, X.; Zhang, F.; Liu, X.; Yu, B. Active components in Ephedra sinica stapf disrupt the interaction between ACE2 and SARS-CoV-2 RBD: Potent COVID-19 therapeutic agents. J. Ethnopharmacol. 2021, 278, 114303. [Google Scholar]
- Idriss, H.; Siddig, B.; Maldonado, P.G.; Elkhair, H.M.; Alakhras, A.I.; Abdallah, E.M.; Torres, P.H.S.; Elzupir, A.O. Phytochemical Discrimination, Biological Activity and Molecular Docking of Water-Soluble Inhibitors from Saussurea costus Herb against Main Protease of SARS-CoV-2. Molecules 2022, 27, 4908. [Google Scholar] [CrossRef]
- Romero-Martínez, B.S.; Montaño, L.M.; Solís-Chagoyán, H.; Sommer, B.; Ramírez-Salinas, G.L.; Pérez-Figueroa, G.E.; Flores-Soto, E. Possible beneficial actions of caffeine in SARS-CoV-2. Int. J. Mol. Sci. 2021, 22, 5460. [Google Scholar] [CrossRef] [PubMed]
- Rolta, R.; Salaria, D.; Sharma, B.; Awofisayo, O.; Fadare, O.; Sharma, S.; Patel, C.; Kumar, V.; Sourirajan, A.; Baumler, D.; et al. Methylxanthines as Potential Inhibitor of SARS-CoV-2: An In Silico Approach. Curr. Pharmacol. Rep. 2022, 8, 149–170. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, H.M.; El-Halawany, A.; Sirwi, A.; El-Araby, A.; Mohamed, G.; Ibrahim, S.; Koshak, A.; Asfour, H.; Awan, Z.; Elfaky, M.A. Repurposing of some natural product isolates as SARS-COV-2 main protease inhibitors via in vitro cell free and cell-based antiviral assessments and molecular modeling approaches. Pharmaceuticals 2021, 14, 213. [Google Scholar] [CrossRef]
- van de Sand, L.; Bormann, M.; Alt, M.; Schipper, L.; Heilingloh, C.S.; Steinmann, E.; Todt, D.; Dittmer, U.; Elsner, C.; Witzke, O.; et al. Glycyrrhizin effectively inhibits SARS-CoV-2 replication by inhibiting the viral main protease. Viruses 2021, 13, 609. [Google Scholar] [CrossRef] [PubMed]
- Lockbaum, G.J.; Reyes, A.; Lee, J.M.; Tilvawala, R.; Nalivaika, E.; Ali, A.; Yilmaz, N.K.; Thompson, P.; Schiffer, C. Crystal structure of SARS-CoV-2 main protease in complex with the non-covalent inhibitor ML188. Viruses 2021, 13, 174. [Google Scholar] [CrossRef]
- Elzupir, A.O. Inhibition of SARS-CoV-2 main protease 3CLpro by means of α-ketoamide and pyridone-containing pharmaceuticals using in silico molecular docking. J. Mol. Struct. 2020, 1222, 128878. [Google Scholar] [CrossRef]
- Elzupir, A.O. Molecular Docking and Dynamics Investigations for Identifying Potential Inhibitors of the 3-Chymotrypsin-like Protease of SARS-CoV-2: Repurposing of Approved Pyrimidonic Pharmaceuticals for COVID-19 Treatment. Molecules 2021, 26, 7458. [Google Scholar] [CrossRef]
- Khan, A.; Heng, W.; Wang, Y.; Qiu, J.; Wei, X.; Peng, S.; Saleem, S.; Khan, M.; Ali, S.S.; Wei, D.-Q. In silico and in vitro evaluation of kaempferol as a potential inhibitor of the SARS-CoV-2 main protease (3CLpro). Phytother. Res. 2021, 35, 2841–2845. [Google Scholar] [CrossRef]
- Quimque, M.T.J.; Notarte, K.I.; Fernandez, R.A.; Mendoza, M.A.; Liman, R.A.; Lim, J.A.; Pilapil, L.A.; Ong, J.K.; Pastrana, A.; Khan, A.; et al. Virtual screening-driven drug discovery of SARS-CoV2 enzyme inhibitors targeting viral attachment, replication, post-translational modification and host immunity evasion infection mechanisms. J. Biomol. Struct. Dyn. 2021, 39, 4316–4333. [Google Scholar] [CrossRef]
- Khan, M.T.; Ali, A.; Wang, Q.; Irfan, M.; Khan, A.; Zeb, M.T.; Zhang, Y.-J.; Chinnasamy, S.; Wei, D.-Q. Marine natural compounds as potents inhibitors against the main protease of SARS-CoV-2—A molecular dynamic study. J. Biomol. Struct. Dyn. 2021, 39, 3627–3637. [Google Scholar] [CrossRef]
- Koulgi, S.; Jani, V.; Uppuladinne, M.; Sonavane, U.; Nath, A.K.; Darbari, H.; Joshi, R. Drug repurposing studies targeting SARS-CoV-2: An ensemble docking approach on drug target 3C-like protease (3CLpro). J. Biomol. Struct. Dyn. 2021, 39, 5735–5755. [Google Scholar] [CrossRef] [PubMed]
- Gammeltoft, K.A.; Zhou, Y.; Hernandez, C.D.; Galli, A.; Offersgaard, A.; Costa, R.; Pham, L.; Fahnøe, U.; Feng, S.; Scheel, T.; et al. Hepatitis C virus protease inhibitors show differential efficacy and interactions with remdesivir for treatment of SARS-CoV-2 in vitro. Antimicrob. Agents Chemother. 2021, 65, e02680–20. [Google Scholar] [CrossRef] [PubMed]
- Hamed, M.I.; Darwish, K.; Soltane, R.; Chrouda, A.; Mostafa, A.; Shama, N.A.; Elhady, S.; Abulkhair, H.; Khodir, A.; Elmaaty, A.A.; et al. β-Blockers bearing hydroxyethylamine and hydroxyethylene as potential SARS-CoV-2 Mpro inhibitors: Rational based design, in silico, in vitro, and SAR studies for lead optimization. RSC Adv. 2021, 11, 35536–35558. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Yeh, C.-T.; Hsu, C.-W.; Lin, Y.-H. Reduction of ACE2 Serum Concentrations by Telbivudine in Chronic Hepatitis B Patients. Curr. Mol. Med. 2022, 23, 420–424. [Google Scholar] [CrossRef]
- Hussein, R.K.; Khouqeer, G.; Alkaoud, A.M.; El-Khayatt, A.M. Probing the Action of Screened Anticancer Triazole–Tetrazole Derivatives Against COVID-19 Using Molecular Docking and DFT Investigations. Nat. Prod. Commun. 2022, 17, 1934578X221093915. [Google Scholar] [CrossRef]
- Baig, A.; Srinivasan, H. SARS-CoV-2 Inhibitors from Nigella Sativa. Appl. Biochem. Biotechnol. 2022, 194, 1051–1090. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, P.; Bhardwaj, V.K.; Chauhan, M.; Chauhan, V.; Kumar, A.; Purohit, R.; Kumar, A.; Kumar, S. A ricin-based peptide BRIP from Hordeum vulgare inhibits Mpro of SARS-CoV-2. Sci. Rep. 2022, 12, 12802. [Google Scholar] [CrossRef] [PubMed]
- Mercorelli, B.; Desantis, J.; Celegato, M.; Bazzacco, A.; Siragusa, L.; Benedetti, P.; Eleuteri, M.; Croci, F.; Cruciani, G.; Goracci, L.; et al. Discovery of novel SARS-CoV-2 inhibitors targeting the main protease Mpro by virtual screenings and hit optimization. Antivir. Res. 2022, 204, 105350. [Google Scholar] [CrossRef]
- Silva, S.C.; Rodrigues, C.F.; Araújo, D.; Rodrigues, M.E.; Henriques, M. Candida species biofilms’ antifungal resistance. J. Fungi 2017, 3, 8. [Google Scholar] [CrossRef]
- Abdellatif, A.A.H.; Alhathloul, S.S.; Aljohani, A.S.M.; Maswadeh, H.; Abdallah, E.M.; Musa, K.H.; El Hamd, M.A. Green Synthesis of Silver Nanoparticles Incorporated Aromatherapies Utilized for Their Antioxidant and Antimicrobial Activities against Some Clinical Bacterial Isolates. Bioinorg. Chem. Appl. 2022, 2022, 1–14. [Google Scholar] [CrossRef]
- Abdallah, E.; Qureshi, K.; Ali, A.M.H.; Elhassan, G.O. Evaluation of some biological properties of Saussurea costus crude root extract. Biosci. Biotechnol. Res. Commun. 2017, 10, 601–611. [Google Scholar] [CrossRef]
- Siddiqui, Z.N.; Farooq, F.; Musthafa, T.M.; Ahmad, A.; Khan, A.U. Synthesis, characterization and antimicrobial evaluation of novel halopyrazole derivatives. J. Saudi Chem. Soc. 2013, 17, 237–243. [Google Scholar] [CrossRef]
- Vazquez, J.A.; Sobel, J.D. Candidiasis. In Essentials of clinical mycology; Kauffman, C., Pappas, P., Sobel, J., Dismukes, W., Eds.; Springer: New York, NY, USA, 2011; pp. 167–206. [Google Scholar]
- Canillac, N.; Mourey, A. Antibacterial activity of the essential oil of Picea excelsa on Listeria, Staphylococcus aureus and coliform bacteria. Food Microbiol. 2001, 18, 261–268. [Google Scholar] [CrossRef]
- Hazen, K.C. Fungicidal versus fungistatic activity of terbinafine and itraconazole: An in vitro comparison. J. Am. Acad. Dermatol. 1998, 38, 37–41. [Google Scholar] [CrossRef] [PubMed]
- González-Maldonado, P.; Alvarenga, N.; Burgos-Edwards, A.; Flores-Giubi, M.E.; Barúa, J.; Romero-Rodríguez, M.C.; Soto-Rifo, R.; Valiente-Echeverría, F.; Langjahr, P.; Cantero-González, G.; et al. Screening of Natural Products Inhibitors of SARS-CoV-2 Entry. Molecules 2022, 27, 1743. [Google Scholar] [CrossRef] [PubMed]
- Al-Janabi, A.S.; Elzupir, A.O.; Yousef, T.A. Synthesis, anti-bacterial evaluation, DFT study and molecular docking as a potential 3-chymotrypsin-like protease (3CLpro) of SARS-CoV-2 inhibitors of a novel Schiff bases. J. Mol. Struct. 2021, 1228, 129454. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Wang, J.; Wang, W.; Kollman, P.A.; Case, D.A. Automatic atom type and bond type perception in molecular mechanical calculations. J. Mol. Graph. Model. 2006, 25, 247–260. [Google Scholar] [CrossRef]
- Al-Mijalli, S.H.; Assaggaf, H.; Qasem, A.; El-Shemi, A.G.; Abdallah, E.M.; Mrabti, H.N.; Bouyahya, A. Antioxidant, Antidiabetic, and Antibacterial Potentials and Chemical Composition of Salvia officinalis and Mentha suaveolens Grown Wild in Morocco. Adv. Pharmacol. Pharm. Sci. 2022, 2022, 1–10. [Google Scholar] [CrossRef]
- Ansel, H.C.; Norred, W.P.; Roth, I.L. Antimicrobial activity of dimethyl sulfoxide against Escherichia coli, Pseudomonas aeruginosa, and Bacillus megaterium. J. Pharm. Sci. 1969, 58, 836–839. [Google Scholar] [CrossRef]
- Hassan, A.S. The antibacterial activity of dimethyl sulfoxide (DMSO) with and without of some ligand complexes of the transitional metal ions of ethyl coumarin against bacteria isolate from burn and wound infection. J. Nat. Sci. Res. 2014, 4, 426–435. [Google Scholar]
- Czuprynski, C.J.; Henson, P.M.; Campbell, P.A. Effect of dimethyl sulfoxide on the in vitro and in vivo bactericidal activity of human and mouse neutrophils and mononuclear phagocytes. Inflammation 1984, 8, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Gabaglio, S.; Alvarenga, N.; Cantero-González, G.; Degen, R.; Ferro, E.A.; Langjahr, P.; Chnaiderman, J.; Sotelo, P.H. A quantitative PCR assay for antiviral activity screening of medicinal plants against Herpes simplex 1. Nat. Prod. Res. 2021, 35, 2926–2930. [Google Scholar] [CrossRef] [PubMed]
- Gulluce, M.; Aslan, A.; Sokmen, M.; Sahin, F.; Adiguzel, A.; Agar, G.; Sokmen, A. Screening the antioxidant and antimicrobial properties of the lichens Parmelia saxatilis, Platismatia glauca, Ramalina pollinaria, Ramalina polymorpha and Umbilicaria nylanderiana. Phytomedicine 2006, 13, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Gonzales-Wartz, K.K.; Huang, D.; Yuan, M.; Peterson, M.; Liang, J.; Beutler, N.; Torres, J.; Cong, Y.; Postnikova, E.; et al. Bispecific antibodies targeting distinct regions of the spike protein potently neutralize SARS-CoV-2 variants of concern. Sci. Transl. Med. 2021, 13, eabj5413. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Huang, D.; Lee, C.-C.; Wu, N.; Jackson, A.; Zhu, X.; Liu, H.; Peng, L.; van Gils, M.; Sanders, R.; et al. Structural and functional ramifications of antigenic drift in recent SARS-CoV-2 variants. Science 2021, 373, 818–823. [Google Scholar] [CrossRef]
- Kingston, R.E.; Chen, C.A.; Rose, J.K. Calcium phosphate transfection. Curr. Protoc. Mol. Biol. 2003, 63, 9. [Google Scholar] [CrossRef]
- Telu, K.H.; Yan, X.; Wallace, W.E.; Stein, S.E.; Simón-Manso, Y. Analysis of human plasma metabolites across different liquid chromatography/mass spectrometry platforms: Cross-platform transferable chemical signatures. Rapid Commun. Mass Spectrom. 2016, 30, 581–593. [Google Scholar] [CrossRef]
- Khan, M.A.; Alam, A.; Husain, S.; Ahmed, S.; Nazamuddin, M.; Ahmed, Z. A potent herb of Unani medicine: A review. Int. J. Curr. Pharm. Res. 2013, 5, 1–4. [Google Scholar]
- Tag, H.M.; Khaled, H.E.; Ismail, H.A.; El-Shenawy, N.S. Evaluation of anti-inflammatory potential of the ethanolic extract of the Saussurea lappa root (costus) on adjuvant-induced monoarthritis in rats. J. Basic Clin. Physiol. Pharmacol. 2016, 27, 71–78. [Google Scholar] [CrossRef]
- Okubo, S.; Ohta, T.; Fujita, H.; Shoyama, Y.; Uto, T. Costunolide and dehydrocostuslactone from Saussurea lappa root inhibit autophagy in hepatocellular carcinoma cells. J. Nat. Med. 2021, 75, 240–245. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).