Systematic Identification of Suitable Reference Genes for Quantitative Real-Time PCR Analysis in Melissa officinalis L
Abstract
1. Introduction
2. Results
2.1. Primer Efficiency and the Expression Profile of the Candidate Genes
2.2. Expression Stability of Candidate Reference Genes by Comparative ΔCt and Bestkeeper
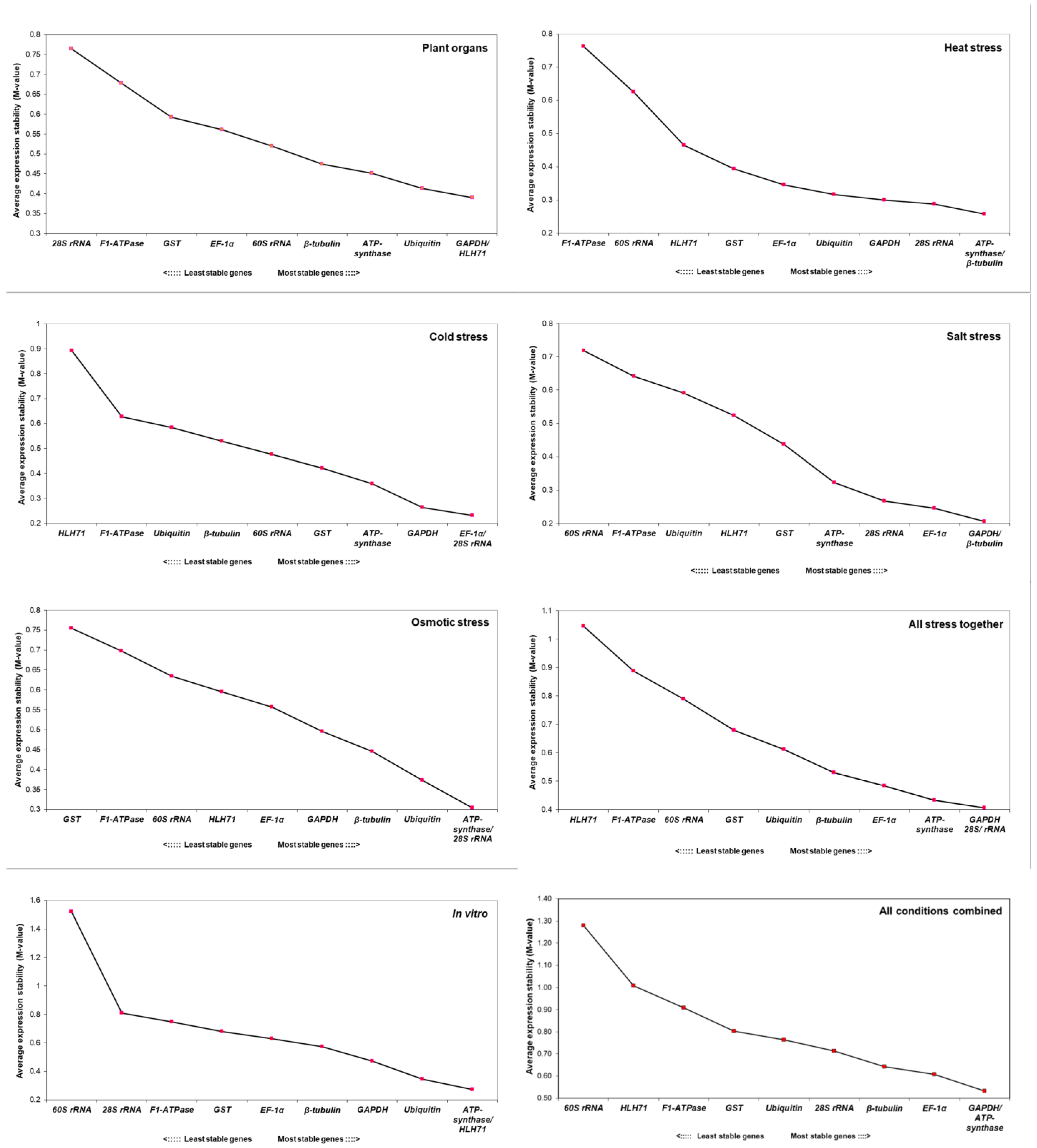
2.3. Expression Stability of Candidate Reference Genes by NormFinder
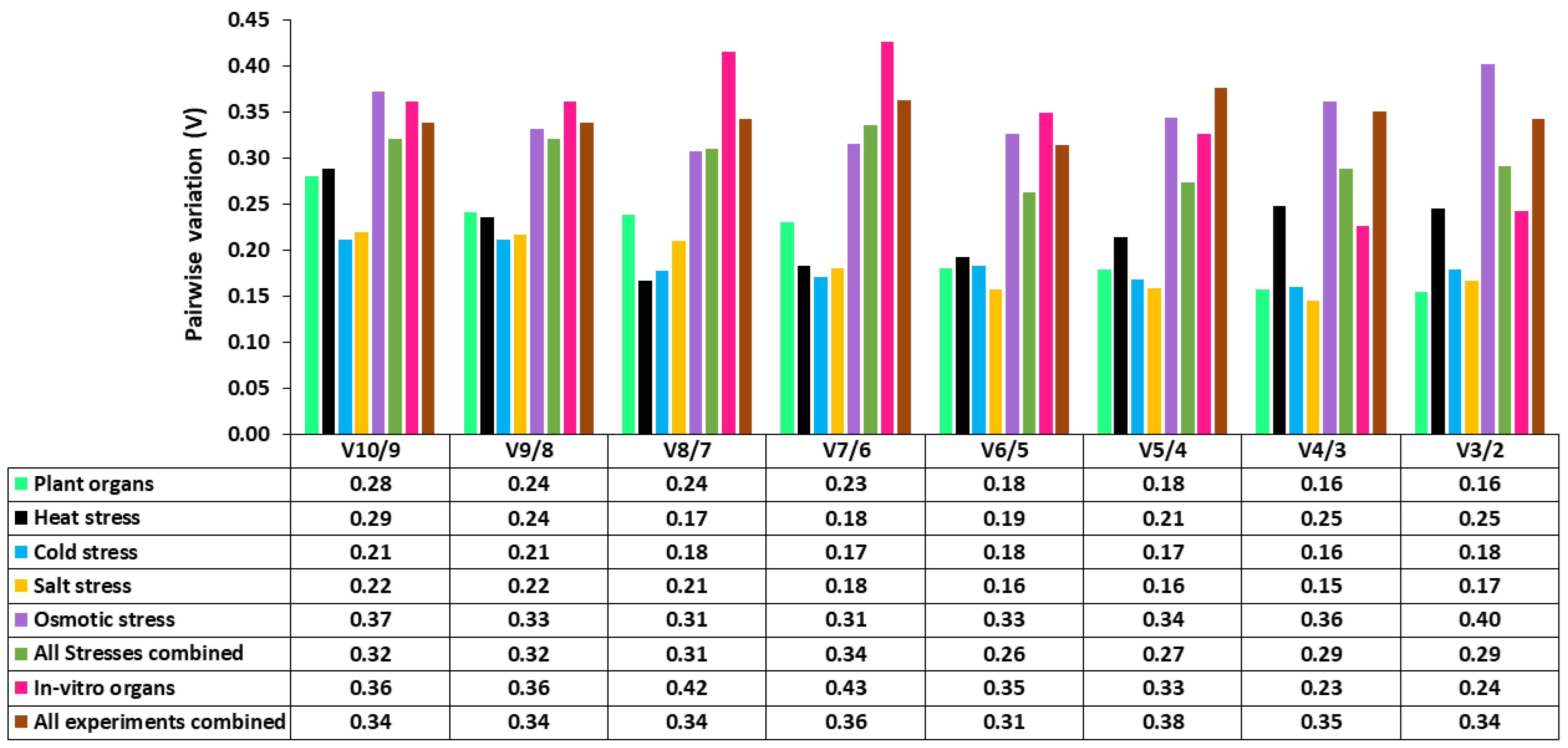
2.4. Expression Stability of Candidate Reference Genes by GeNorm
2.5. Comprehensive Ranking of the Candidate Reference Genes by RefFinder
2.6. Identification of the Most Suitable Reference Gene and Validation Experiments
3. Discussion
4. Materials and Methods
4.1. Plant Material Acquisition, Stress, and Elicitor Treatment
4.2. Total RNA Extraction, cDNA Synthesis, and qRT-PCR Experiment Conditions
4.3. Reference Gene Analysis
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shakeri, A.; Sahebkar, A.; Javadi, B. Melissa officinalis L.—A review of its traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2016, 188, 204–228. [Google Scholar] [CrossRef] [PubMed]
- Draginic, N.; Jakovljevic, V.; Andjic, M.; Jeremic, J.; Srejovic, I.; Rankovic, M.; Tomovic, M.; Nikolic Turnic, T.; Svistunov, A.; Bolevich, S.; et al. Melissa officinalis L. as a nutritional strategy for cardioprotection. Front. Physiol. 2021, 12, 661778. [Google Scholar] [CrossRef] [PubMed]
- Mahboubi, M.; Taghizadeh, M.; Talaei, S.A.; Takht Firozeh, S.M.; Rashidi, A.A.; Tamtaji, O.R. Combined administration of Melissa officinalis and Boswellia serrata extracts in an animal model of memory. Iran. J. Psychiatry Behav. Sci. 2016, 10, e681. [Google Scholar] [CrossRef] [PubMed]
- Heshmati, J.; Morvaridzadeh, M.; Sepidarkish, M.; Fazelian, S.; Rahimlou, M.; Omidi, A.; Palmowski, A.; Asadi, A.; Shidfar, F. Effects of Melissa officinalis (Lemon Balm) on cardio-metabolic outcomes: A systematic review and meta-analysis. Phytother. Res. 2020, 34, 3113–3123. [Google Scholar] [CrossRef] [PubMed]
- Božović, M.; Garzoli, S.; Baldisserotto, A.; Romagnoli, C.; Pepi, F.; Cesa, S.; Vertuani, S.; Manfredini, S.; Ragno, R. Melissa officinalis L. subsp. altissima (Sibth. & Sm.) Arcang. Essential Oil: Chemical composition and preliminary antimicrobial investigation of samples obtained at different harvesting periods and by fractionated extractions. Ind. Crops Prod. 2018, 117, 317–321. [Google Scholar] [CrossRef]
- Kianersi, F.; Amin Azarm, D.; Pour-Aboughadareh, A.; Poczai, P. Change in secondary metabolites and expression pattern of key rosmarinic acid related genes in iranian lemon balm (Melissa officinalis L.) ecotypes using methyl jasmonate treatments. Molecules 2022, 27, 1715. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, M.; Pellegrini, E.; D’Angiolillo, F.; Petersen, M.; Nali, C.; Pistelli, L.; Lorenzini, G. Ozone-elicited secondary metabolites in shoot cultures of Melissa officinalis L. Plant Cell Tissue Organ Cult. 2015, 120, 617–629. [Google Scholar] [CrossRef]
- Ramawat, K.G. An introduction to the process of cell, tissue, and organ differentiation, and production of secondary metabolites. In Plant Cell and Tissue Differentiation and Secondary Metabolites; Ramawat, K.G., Ekiert, H.M., Goyal, S., Eds.; Reference Series in Phytochemistry; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–22. ISBN 978-3-030-30184-2. [Google Scholar] [CrossRef]
- Bhaskar, R.; Xavier, L.S.E.; Udayakumaran, G.; Kumar, D.S.; Venkatesh, R.; Nagella, P. Biotic elicitors: A boon for the in-vitro production of plant secondary metabolites. Plant Cell Tissue Organ Cult. 2022, 149, 7–24. [Google Scholar] [CrossRef]
- Fooladi vanda, G.; Shabani, L.; Razavizadeh, R. Chitosan enhances rosmarinic acid production in shoot cultures of Melissa officinalis L. through the induction of methyl jasmonate. Bot. Stud. 2019, 60, 26. [Google Scholar] [CrossRef]
- Mansouri, M.; Mohammadi, F. Transcriptome analysis to identify key genes involved in terpenoid and rosmarinic acid biosynthesis in lemon balm (Melissa officinalis). Gene 2021, 773, 145417. [Google Scholar] [CrossRef]
- Sen, M.K.; Hamouzová, K.; Košnarová, P.; Roy, A.; Soukup, J. Identification of the most suitable reference gene for gene expression studies with development and abiotic stress response in Bromus sterilis. Sci. Rep. 2021, 11, 13393. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wang, B.; Li, Y.; Zeng, M.; Liu, J.; Ye, X.; Zhu, H.; Wen, Q. Reference gene selection for QRT-PCR Analyses of luffa (Luffa cylindrica) plants under abiotic stress conditions. Sci. Rep. 2021, 11, 3161. [Google Scholar] [CrossRef] [PubMed]
- Oneto, C.D.; Bossio, E.; Faccio, P.; Beznec, A.; Lewi, D. Validation of housekeeping genes for QPCR in maize during water deficit stress conditions at flowering time. Maydica 2017, 62(2), M13. [Google Scholar]
- Galli, V.; Borowski, J.M.; Perin, E.C.; Messias, R.d.S.; Labonde, J.; Pereira, I.d.S.; Silva, S.D.d.A.; Rombaldi, C.V. Validation of reference genes for accurate normalization of gene expression for real time-quantitative PCR in strawberry fruits using different cultivars and osmotic stresses. Gene 2015, 554, 205–214. [Google Scholar] [CrossRef]
- Joseph, J.T.; Poolakkalody, N.J.; Shah, J.M. Plant reference genes for development and stress response studies. J. Biosci. 2018, 43, 173–187. [Google Scholar] [CrossRef]
- Czechowski, T.; Stitt, M.; Altmann, T.; Udvardi, M.K.; Scheible, W.-R. Genome-wide identification and testing of superior reference genes for transcript normalization in arabidopsis. Plant Physiol. 2005, 139, 5–17. [Google Scholar] [CrossRef]
- Dong, X.-M.; Zhang, W.; Zhang, S.-B. Selection and validation of reference genes for quantitative real-time PCR analysis of development and tissue-dependent flower color formation in Cymbidium lowianum. Int. J. Mol. Sci. 2022, 23, 738. [Google Scholar] [CrossRef]
- Yin, H.; Yin, D.; Zhang, M.; Gao, Z.; Tuluhong, M.; Li, X.; Li, J.; Li, B.; Cui, G. Validation of appropriate reference genes for QRT–PCR normalization in oat (Avena sativa L.) under UV-B and high-light stresses. Int. J. Mol. Sci. 2022, 23, 11187. [Google Scholar] [CrossRef]
- Aminfar, Z.; Rabiei, B.; Tohidfar, M.; Mirjalili, M.H. Selection and validation of reference genes for quantitative real-time PCR in Rosmarinus officinalis L. in various tissues and under elicitation. Biocatal. Agric. Biotechnol. 2019, 20, 101246. [Google Scholar] [CrossRef]
- Pfaffl, M.W.; Tichopad, A.; Prgomet, C.; Neuvians, T.P. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper–Excel-based tool using pair-wise correlations. Biotechnol. Lett. 2004, 26, 509–515. [Google Scholar] [CrossRef]
- Silver, N.; Best, S.; Jiang, J.; Thein, S.L. Selection of housekeeping genes for gene expression studies in human reticulocytes using real-time PCR. BMC Mol. Biol. 2006, 7, 33. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, research0034.1. [Google Scholar] [CrossRef]
- Andersen, C.L.; Jensen, J.L.; Ørntoft, T.F. Normalization of real-time quantitative reverse transcription-PCR data: A model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004, 64, 5245–5250. [Google Scholar] [CrossRef]
- Xie, F.; Xiao, P.; Chen, D.; Xu, L.; Zhang, B. MiRDeepFinder: A MiRNA analysis tool for deep sequencing of plant small RNAs. Plant Mol. Biol. 2012, 80, 75–84. [Google Scholar] [CrossRef]
- El-Naggar, H.M.; Read, P.E. PAL Gene activity and rosmarinic acid production in rosemary genotypes. J. Herbs Spices Med. Plants 2010, 16, 83–89. [Google Scholar] [CrossRef]
- Vyas, P.; Mukhopadhyay, K. Elicitation of phenylpropanoids and expression analysis of PAL gene in suspension cell culture of Ocimum tenuiflorum L. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2018, 88, 1207–1217. [Google Scholar] [CrossRef]
- Kahila, M.M.H.; Najy, A.M.; Rahaie, M.; Mir-Derikvand, M. Effect of nanoparticle treatment on expression of a key gene involved in thymoquinone biosynthetic pathway in Nigella Sativa L. Nat. Prod. Res. 2018, 32, 1858–1862. [Google Scholar] [CrossRef]
- Jalali, S.; Salami, S.A.; Sharifi, M.; Sohrabi, S. Signaling compounds elicit expression of key genes in cannabinoid pathway and related metabolites in cannabis. Ind. Crops Prod. 2019, 133, 105–110. [Google Scholar] [CrossRef]
- Devi, K.; Mishra, S.K.; Sahu, J.; Panda, D.; Modi, M.K.; Sen, P. Genome wide transcriptome profiling reveals differential gene expression in secondary metabolite pathway of Cymbopogon winterianus. Sci. Rep. 2016, 6, 21026. [Google Scholar] [CrossRef] [PubMed]
- Aminfar, Z.; Rabiei, B.; Tohidfar, M.; Mirjalili, M.H. Identification of key genes involved in the biosynthesis of triterpenic acids in the mint family. Scietific Rep. 2019, 9, 15826. [Google Scholar] [CrossRef]
- Bolhassani, M.; Niazi, A.; Tahmasebi, A.; Moghadam, A. Identification of key genes associated with secondary metabolites biosynthesis by system network analysis in Valeriana officinalis. J. Plant Res. 2021, 134, 625–639. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi Mandoulakani, B.; Eyvazpour, E.; Ghadimzadeh, M. The effect of drought stress on the expression of key genes involved in the biosynthesis of phenylpropanoids and essential oil components in basil (Ocimum Basilicum L.). Phytochemistry 2017, 139, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Negrutskii, B.S.; El’skaya, A.V. Eukaryotic translation elongation factor 1α: Structure, expression, functions, and possible role in aminoacyl-TRNA channeling. In Progress in Nucleic Acid Research and Molecular Biology; Elsevier: Amsterdam, The Netherlands, 1998; Volume 60, pp. 47–78. ISBN 978-0-12-540060-2. [Google Scholar]
- Tristan, C.; Shahani, N.; Sedlak, T.W.; Sawa, A. The diverse functions of GAPDH: Views from different subcellular compartments. Cell. Signal. 2011, 23, 317–323. [Google Scholar] [CrossRef]
- Ashrafi, M.; Azimi Moqadam, M.R.; Moradi, P.; Mohsenifard, E.; Shekari, F. Evaluation and validation of housekeeping genes in two contrast species of thyme plant to drought stress using real-time PCR. Plant Physiol. Biochem. 2018, 132, 54–60. [Google Scholar] [CrossRef]
- Gopalam, R.; Rupwate, S.D.; Tumaney, A.W. Selection and validation of appropriate reference genes for quantitative real-time PCR analysis in Salvia hispanica. PLoS ONE 2017, 12, e0186978. [Google Scholar] [CrossRef]
- Lian, C.; Zhang, B.; Yang, J.; Lan, J.; Yang, H.; Guo, K.; Li, J.; Chen, S. Validation of suitable reference genes by various algorithms for gene expression analysis in isodon rubescens under different abiotic stresses. Sci. Rep. 2022, 12, 19599. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Hou, S.; Cui, G.; Chen, S.; Wei, J.; Huang, L. Characterization of reference genes for quantitative real-time PCR analysis in various tissues of Salvia miltiorrhiza. Mol. Biol. Rep. 2010, 37, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Borah, B.; Hussain, M.; Wann, S.B.; Bhau, B.S. Selection and Validation of suitable reference genes for quantitative real time PCR analysis of gene expression studies in patchouli under meloidogyne incognita attack and PGPR treatment. Gene Rep. 2020, 19, 100625. [Google Scholar] [CrossRef]
- Bharati, R.; Sen, M.K.; Kumar, R.; Gupta, A.; Sur, V.P.; Melnikovová, I.; Fernández-Cusimamani, E. Selection and validation of the most suitable reference genes for quantitative real-time PCR normalization in Salvia rosmarinus under in vitro conditions. Plants 2022, 11, 2878. [Google Scholar] [CrossRef]
- Wang, H.-L.; Chen, J.; Tian, Q.; Wang, S.; Xia, X.; Yin, W. Identification and validation of reference genes for populus euphratica gene expression analysis during abiotic stresses by quantitative real-time PCR. Physiol. Plant. 2014, 152, 529–545. [Google Scholar] [CrossRef]
- Valenzuela, F.; D’Afonseca, V.; Hernández, R.; Gómez, A.; Arencibia, A.D. Validation of reference genes in a population of blueberry (Vaccinium corymbosum) plants regenerated in colchicine. Plants 2022, 11, 2645. [Google Scholar] [CrossRef] [PubMed]
- Beranová, K.; Bharati, R.; Žiarovská, J.; Bilčíková, J.; Hamouzová, K.; Klíma, M.; Fernández-Cusimamani, E. Morphological, cytological, and molecular comparison between diploid and induced autotetraploids of Callisia fragrans (Lindl.) woodson. Agronomy 2022, 12, 2520. [Google Scholar] [CrossRef]
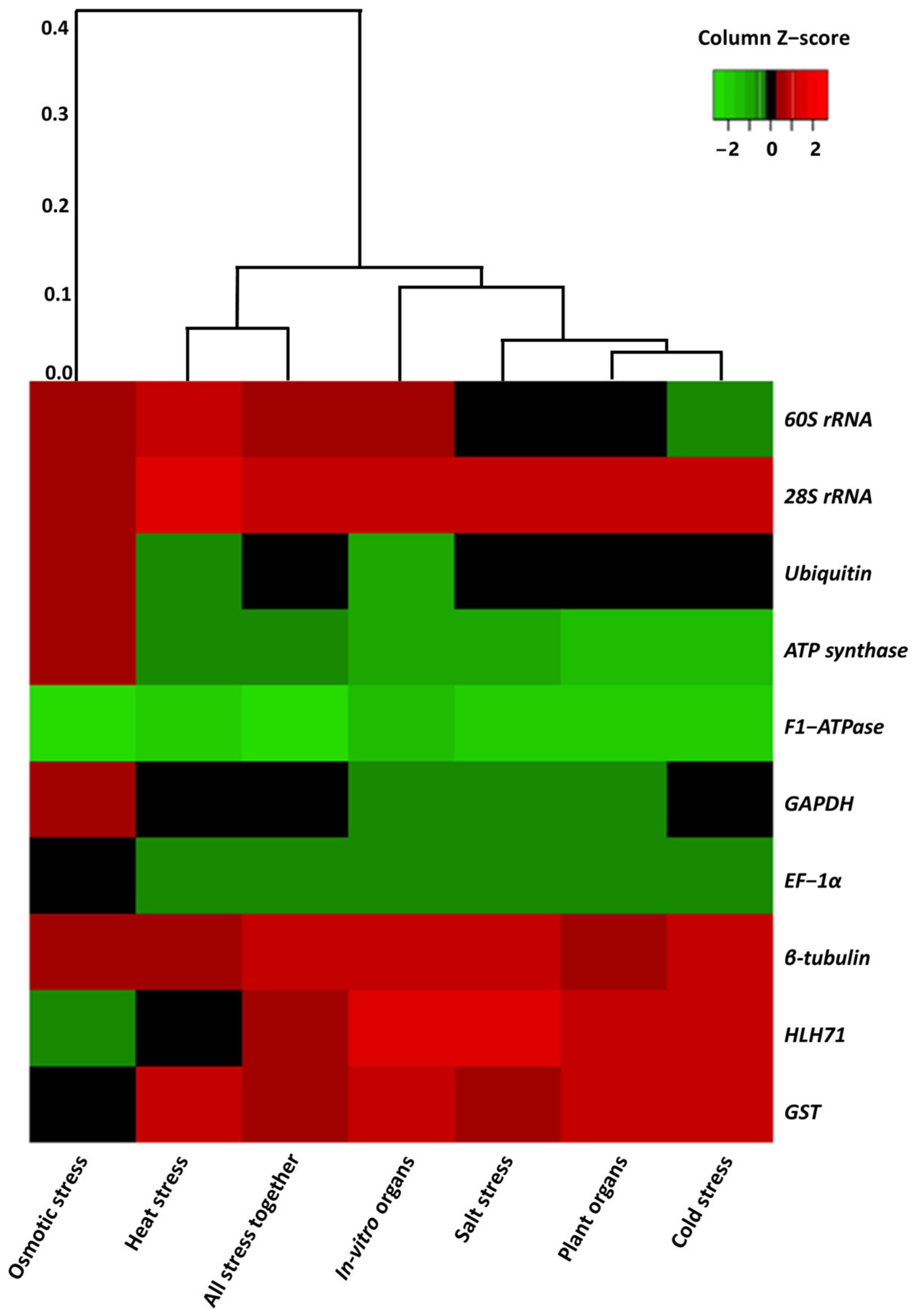

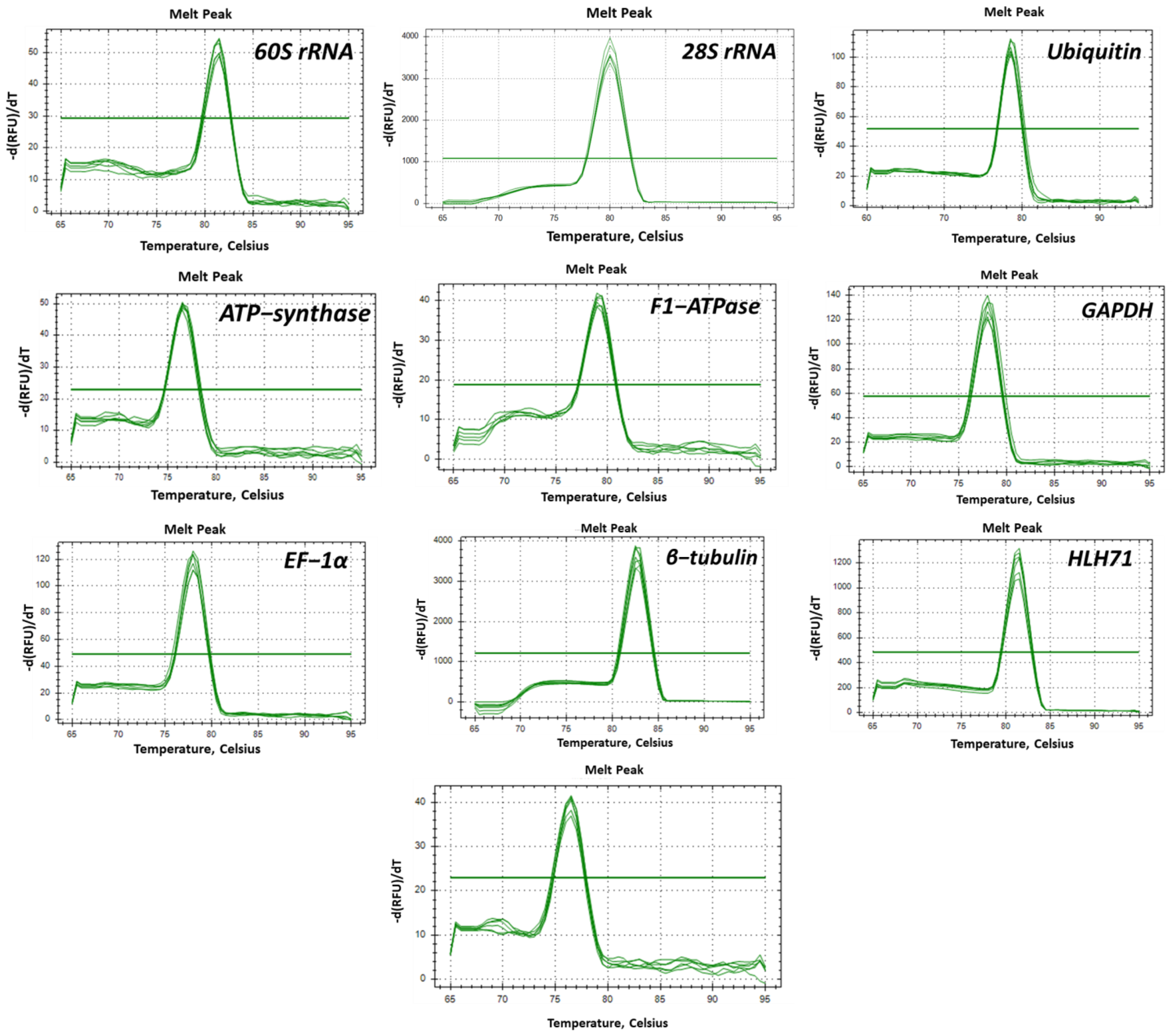

| Gene Name | Primer Sequence (5′-3′) | Annealing Temperature | Amplicon Size (bp) | Primer Efficiency | R2 Value | |
|---|---|---|---|---|---|---|
| Forward Primer | Reverse Primer | |||||
| 60S rRNA | TTGTCGGGAAATATGGCACC | CCTTCACTTTGCCACAGTCC | 59.1 °C | 175 | 101.73% | 1.00 |
| 28S rRNA | CAACTGGTCAAAGATCCGCC | CCTTCTGACGACGCTCTAAC | 59.1 °C | 170 | 104.06% | 0.99 |
| Ubiquitin | GAGCTCTCCACCTCCAAAGT | ACGCACTCTGGTTGACTACA | 56.8 °C | 152 | 100.87% | 0.99 |
| ATP synthase | GGCTTGAACGAAACGGAAGA | AGAGTTGGTTTGACTGCCCT | 59.5 °C | 150 | 101.35% | 0.91 |
| F1-ATPase | TATCTGTCAGTCGTGTCGGG | AAAGGCGGCTACTTCTCGAT | 59.1 °C | 150 | 101.14% | 0.99 |
| GAPDH | CCACTCCACTTTCGCTCTCT | TGAAGGGATTGTTGACAGCA | 59.5 °C | 181 | 97.00% | 1.00 |
| EF-1α | TGACAACGAAACGCAACACA | CATTGGGTACTTGACAGGCG | 59.5 °C | 180 | 97.33% | 1.00 |
| β-tubulin | AAAGGTCACTACACGGAGGG | GAGATCAGCAGGGTTCCCAT | 58.4 °C | 181 | 100.08% | 1.00 |
| HLH71 | CGAACGAAACCGCCGAAA | TGGCTTCTAGTGACTGCAGA | 59.1 °C | 181 | 97.61% | 0.97 |
| GST | TGGCCAGCACTCCTTTATCA | CTTGCATTAGGGTGGATGGC | 61 °C | 180 | 104.86% | 1.00 |
| Rank | Plant Organs | Heat Stress | ||||||
|---|---|---|---|---|---|---|---|---|
| Genes | Average of STDEV | Genes | Std dev [+/− CP] | Genes | Average of STDEV | Genes | Std dev [+/− CP] | |
| 1 | GAPDH | 1.78 | GST | 1.22 | 28S rRNA | 1.04 | HLH71 | 0.44 |
| 2 | F1-ATPase | 1.91 | 28S rRNA | 1.41 | Ubiquitin | 1.06 | GST | 1.5 |
| 3 | 28S rRNA | 1.92 | HLH71 | 1.95 | EF-1α | 1.07 | F1-ATPase | 1.53 |
| 4 | β-tubulin | 1.94 | ATP-synthase | 2.52 | β-tubulin | 1.08 | β-tubulin | 2.08 |
| 5 | 60SrRNA | 2.01 | β-tubulin | 2.58 | GAPDH | 1.14 | EF-1α | 2.33 |
| 6 | EF-1α | 2.01 | F1-ATPase | 2.62 | GST | 1.38 | 28S rRNA | 2.41 |
| 7 | GST | 2.01 | GAPDH | 2.64 | 60S rRNA | 1.65 | Ubiquitin | 2.6 |
| 8 | ATP-synthase | 2.12 | 60S rRNA | 2.78 | ATP-synthase | 1.73 | GAPDH | 2.82 |
| 9 | Ubiquitin | 3.11 | EF-1α | 2.98 | F1-ATPase | 1.83 | 60S rRNA | 3.25 |
| 10 | HLH71 | 3.19 | Ubiquitin | 3.65 | HLH71 | 2.5 | ATP-synthase | 3.57 |
| Rank | Cold stress | Salt stress | ||||||
| Genes | Average of STDEV | Genes | Std dev [+/− CP] | Genes | Average of STDEV | Genes | Std dev [+/− CP] | |
| 1 | EF-1α | 0.92 | 60S rRNA | 0.72 | GAPDH | 0.83 | GST | 0.79 |
| 2 | GST | 0.95 | F1-ATPase | 0.86 | β-tubulin | 0.85 | F1-ATPase | 1.57 |
| 3 | ATP-synthase | 0.95 | 28S rRNA | 1.34 | 28S rRNA | 0.89 | 60S rRNA | 1.82 |
| 4 | GAPDH | 0.99 | GST | 1.54 | EF-1α | 0.9 | 28S rRNA | 2.02 |
| 5 | 28S rRNA | 1.00 | ATP-synthase | 1.63 | HLH71 | 1.11 | GAPDH | 2.27 |
| 6 | β-tubulin | 1.31 | EF-1α | 1.91 | F1-ATPase | 1.29 | β-tubulin | 2.31 |
| 7 | HLH71 | 1.42 | GAPDH | 2.15 | 60S rRNA | 1.29 | HLH71 | 2.43 |
| 8 | Ubiquitin | 1.49 | HLH71 | 2.26 | Ubiquitin | 1.39 | EF-1α | 2.47 |
| 9 | F1-ATPase | 1.54 | β-tubulin | 2.44 | ATP-synthase | 1.43 | Ubiquitin | 3.21 |
| 10 | 60S rRNA | 1.58 | Ubiquitin | 2.72 | GST | 1.8 | ATP-synthase | 3.28 |
| Rank | Osmotic stress | All stress together | ||||||
| Genes | Average of STDEV | Genes | Std dev [+/− CP] | Genes | Average of STDEV | Genes | Std dev [+/− CP] | |
| 1 | 28S rRNA | 2.26 | HLH71 | 0.48 | 28S rRNA | 1.93 | GST | 1.19 |
| 2 | 60S rRNA | 2.27 | F1-ATPase | 0.76 | EF-1α | 2.01 | F1-ATPase | 1.29 |
| 3 | β-tubulin | 2.28 | GST | 0.87 | GAPDH | 2.03 | 28S rRNA | 1.42 |
| 4 | EF-1α | 2.43 | 28S rRNA | 2.02 | β-tubulin | 2.07 | β-tubulin | 1.44 |
| 5 | GAPDH | 2.49 | β-tubulin | 2.19 | Ubiquitin | 2.15 | EF-1α | 2.02 |
| 6 | Ubiquitin | 2.59 | 60S rRNA | 3.62 | F1-ATPase | 2.47 | GAPDH | 2.1 |
| 7 | F1-ATPase | 3.06 | EF-1α | 4.3 | 60S rRNA | 2.48 | HLH71 | 2.11 |
| 8 | GST | 3.11 | GAPDH | 4.44 | GST | 2.51 | Ubiquitin | 2.24 |
| 9 | HLH71 | 3.39 | Ubiquitin | 4.61 | ATP-synthase | 3.24 | 60S rRNA | 2.7 |
| 10 | ATP-synthase | 4.31 | ATP-synthase | 6.54 | HLH71 | 3.26 | ATP-synthase | 3.5 |
| Rank | In vitro organs | All conditions combined | ||||||
| Genes | Average of STDEV | Genes | Std dev [+/− CP] | Genes | Average of STDEV | Genes | Std dev [+/− CP] | |
| 1 | β-tubulin | 1.21 | HLH71 | 1.87 | 28SrRNA | 2.04 | F1-ATPase | 0.62 |
| 2 | Ubiquitin | 1.23 | F1-ATPase | 2.35 | GAPDH | 2.13 | GST | 0.74 |
| 3 | GAPDH | 1.26 | GST | 2.67 | β-tubulin | 2.15 | β-tubulin | 0.86 |
| 4 | ATP-synthase | 1.34 | 28S rRNA | 2.71 | EF-1α | 2.28 | 28S rRNA | 1.07 |
| 5 | GST | 1.36 | β-tubulin | 3.2 | GST | 2.32 | EF-1α | 1.21 |
| 6 | 28S rRNA | 1.49 | Ubiquitin | 3.35 | 60S rRNA | 2.55 | HLH71 | 1.27 |
| 7 | F1-ATPase | 1.58 | GAPDH | 3.38 | F1-ATPase | 2.58 | Ubiquitin | 1.55 |
| 8 | HLH71 | 1.78 | ATP-synthase | 3.71 | ATP-synthase | 2.91 | GAPDH | 1.56 |
| 9 | EF-1α | 2.27 | 60S rRNA | 3.94 | Ubiquitin | 2.98 | 60S rRNA | 1.72 |
| 10 | 60S rRNA | 2.4 | EF-1α | 4.91 | HLH71 | 3.34 | ATP-synthase | 2.32 |
| Rank | Plant Organs | Heat Stress | ||
|---|---|---|---|---|
| Gene Name | Stability Value | Gene Name | Stability Value | |
| 1 | GAPDH | 0.16 | GAPDH | 0.10 |
| 2 | ATP-synthase | 0.17 | β-tubulin | 0.14 |
| 3 | β-tubulin | 0.20 | 28S rRNA | 0.17 |
| 4 | GST | 0.21 | ATP-synthase | 0.18 |
| 5 | Ubiquitin | 0.22 | EF-1α | 0.18 |
| 6 | HLH71 | 0.23 | HLH71 | 0.23 |
| 7 | 60S rRNA | 0.30 | Ubiquitin | 0.24 |
| 8 | EF-1α | 0.30 | GST | 0.36 |
| 9 | F1-ATPase | 0.47 | F1-ATPase | 0.46 |
| 10 | 28S rRNA | 0.49 | 60S rRNA | 0.47 |
| Best combination | GAPDH and β-tubulin | 0.12 | GAPDH and β-tubulin | 0.06 |
| Rank | Cold stress | Salt stress | ||
| Gene name | Stability value | Gene name | Stability value | |
| 1 | GAPDH | 0.120 | GAPDH | 0.09 |
| 2 | 28S rRNA | 0.188 | 28S rRNA | 0.11 |
| 3 | EF-1α | 0.191 | EF-1α | 0.14 |
| 4 | Ubiquitin | 0.209 | β-tubulin | 0.14 |
| 5 | F1-ATPase | 0.269 | ATP-synthase | 0.18 |
| 6 | ATP-synthase | 0.286 | GST | 0.24 |
| 7 | GST | 0.333 | HLH71 | 0.26 |
| 8 | 60S rRNA | 0.358 | F1-ATPase | 0.29 |
| 9 | β-tubulin | 0.485 | Ubiquitin | 0.33 |
| 10 | HLH71 | 1.015 | 60S rRNA | 0.33 |
| Best combination | GAPDH and F1-ATPase | 0.125 | 28S rRNA and β-tubulin | 0.07 |
| Rank | Osmotic stress | All stress together | ||
| Gene name | Stability value | Gene name | Stability value | |
| 1 | 28S rRNA | 0.11 | GAPDH | 0.17 |
| 2 | ATP-synthase | 0.11 | 28S rRNA | 0.18 |
| 3 | Ubiquitin | 0.12 | ATP-synthase | 0.24 |
| 4 | GAPDH | 0.16 | EF-1α | 0.28 |
| 5 | HLH71 | 0.18 | β-tubulin | 0.32 |
| 6 | EF-1α | 0.19 | Ubiquitin | 0.39 |
| 7 | β-tubulin | 0.20 | GST | 0.46 |
| 8 | 60S rRNA | 0.22 | F1-ATPase | 0.49 |
| 9 | F1-ATPase | 0.26 | 60S rRNA | 0.54 |
| 10 | GST | 0.27 | HLH71 | 0.66 |
| Best combination | ATP-synthase and 28S rRNA | 0.07 | GAPDH and 28S rRNA | 0.13 |
| Rank | In vitro organs | All conditions combined | ||
| Gene name | Stability value | Gene name | Stability value | |
| 1 | EF-1α | 0.12 | GAPDH | 0.25 |
| 2 | β-tubulin | 0.22 | ATP-synthase | 0.29 |
| 3 | GST | 0.24 | EF-1α | 0.29 |
| 4 | HLH71 | 0.40 | β-tubulin | 0.34 |
| 5 | GAPDH | 0.49 | Ubiquitin | 0.43 |
| 6 | ATP-synthase | 0.52 | 28S rRNA | 0.45 |
| 7 | Ubiquitin | 0.62 | GST | 0.46 |
| 8 | F1-ATPase | 0.66 | HLH71 | 0.53 |
| 9 | 28S rRNA | 0.76 | F1-ATPase | 0.60 |
| 10 | 60S rRNA | 2.47 | 60S rRNA | 0.86 |
| Best combination | EF-1α and β-tubulin | 0.14 | EF-1α and GAPDH | 0.22 |
| Plant Organs | Heat Stress | |||
|---|---|---|---|---|
| Rank | Genes | Geomean of Ranking Values | Genes | Geomean of Ranking Values |
| 1 | GAPDH | 1.93 | 28S rRNA | 1.86 |
| 2 | 28S rRNA | 2.45 | EF-1α | 2.59 |
| 3 | GST | 3.48 | Ubiquitin | 2.74 |
| 4 | F1-ATPase | 3.94 | β-tubulin | 3.94 |
| 5 | EF-1α | 4.41 | GST | 4.9 |
| 6 | β-tubulin | 4.68 | GAPDH | 5.32 |
| 7 | 60S rRNA | 5.57 | HLH71 | 5.62 |
| 8 | ATP-synthase | 5.98 | F1-ATPase | 6.64 |
| 9 | HLH71 | 7.4 | 60SrRNA | 7.45 |
| 10 | Ubiquitin | 9.24 | ATP-synthase | 8.11 |
| Cold stress | Salt stress | |||
| Rank | Genes | Geomean of ranking values | Genes | Geomean of ranking values |
| 1 | GST | 1.68 | GAPDH | 1.5 |
| 2 | EF-1α | 2.63 | β-tubulin | 2.21 |
| 3 | 28S rRNA | 2.78 | 28S rRNA | 3.46 |
| 4 | ATP-synthase | 3.41 | EF-1α | 4.43 |
| 5 | GAPDH | 5.14 | F1-ATPase | 5.05 |
| 6 | 60S rRNA | 5.62 | HLH71 | 5.44 |
| 7 | F1-ATPase | 6.18 | GST | 5.62 |
| 8 | β-tubulin | 6.64 | 60S rRNA | 5.86 |
| 9 | HLH71 | 7.48 | Ubiquitin | 7.67 |
| 10 | Ubiquitin | 8.18 | ATP-synthase | 8.68 |
| Osmotic stress | All stress together | |||
| Rank | Genes | Geomean of ranking values | Genes | Geomean of ranking values |
| 1 | 28S rRNA | 2.91 | 28S rRNA | 1.86 |
| 2 | β-tubulin | 2.94 | EF-1α | 2.34 |
| 3 | 60S rRNA | 3.13 | GAPDH | 2.91 |
| 4 | EF-1α | 3.25 | β-tubulin | 3.56 |
| 5 | GAPDH | 3.76 | GST | 4.76 |
| 6 | F1-ATPase | 5.12 | F1-ATPase | 4.92 |
| 7 | HLH71 | 5.2 | Ubiquitin | 4.95 |
| 8 | Ubiquitin | 5.58 | 60S rRNA | 6.9 |
| 9 | GST | 6.26 | HLH71 | 8.91 |
| 10 | ATP-synthase | 10 | ATP-synthase | 9.49 |
| In vitro organs | All conditions combined | |||
| Rank | Genes | Geomean of ranking values | Genes | Geomean of ranking values |
| 1 | β-tubulin | 2.11 | 28S rRNA | 1.86 |
| 2 | Ubiquitin | 2.21 | EF-1α | 2.11 |
| 3 | ATP-synthase | 3.56 | β-tubulin | 3.22 |
| 4 | GAPDH | 3.71 | GAPDH | 3.36 |
| 5 | GST | 4.16 | GST | 4.33 |
| 6 | HLH71 | 4.76 | F1-ATPase | 4.6 |
| 7 | F1-ATPase | 5.12 | 60S rRNA | 6.64 |
| 8 | 28S rRNA | 5.42 | Ubiquitin | 6.65 |
| 9 | EF-1α | 9.24 | HLH71 | 8.8 |
| 10 | 60S rRNA | 9.74 | ATP-synthase | 9.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bharati, R.; Sen, M.K.; Kumar, R.; Gupta, A.; Žiarovská, J.; Fernández-Cusimamani, E.; Leuner, O. Systematic Identification of Suitable Reference Genes for Quantitative Real-Time PCR Analysis in Melissa officinalis L. Plants 2023, 12, 470. https://doi.org/10.3390/plants12030470
Bharati R, Sen MK, Kumar R, Gupta A, Žiarovská J, Fernández-Cusimamani E, Leuner O. Systematic Identification of Suitable Reference Genes for Quantitative Real-Time PCR Analysis in Melissa officinalis L. Plants. 2023; 12(3):470. https://doi.org/10.3390/plants12030470
Chicago/Turabian StyleBharati, Rohit, Madhab Kumar Sen, Ram Kumar, Aayushi Gupta, Jana Žiarovská, Eloy Fernández-Cusimamani, and Olga Leuner. 2023. "Systematic Identification of Suitable Reference Genes for Quantitative Real-Time PCR Analysis in Melissa officinalis L" Plants 12, no. 3: 470. https://doi.org/10.3390/plants12030470
APA StyleBharati, R., Sen, M. K., Kumar, R., Gupta, A., Žiarovská, J., Fernández-Cusimamani, E., & Leuner, O. (2023). Systematic Identification of Suitable Reference Genes for Quantitative Real-Time PCR Analysis in Melissa officinalis L. Plants, 12(3), 470. https://doi.org/10.3390/plants12030470






