Copper Uptake and Its Effects on Two Riparian Plant Species, the Native Urtica dioica, and the Invasive Fallopia japonica
Abstract
:1. Introduction
2. Results
2.1. Seasonal Plant Growth
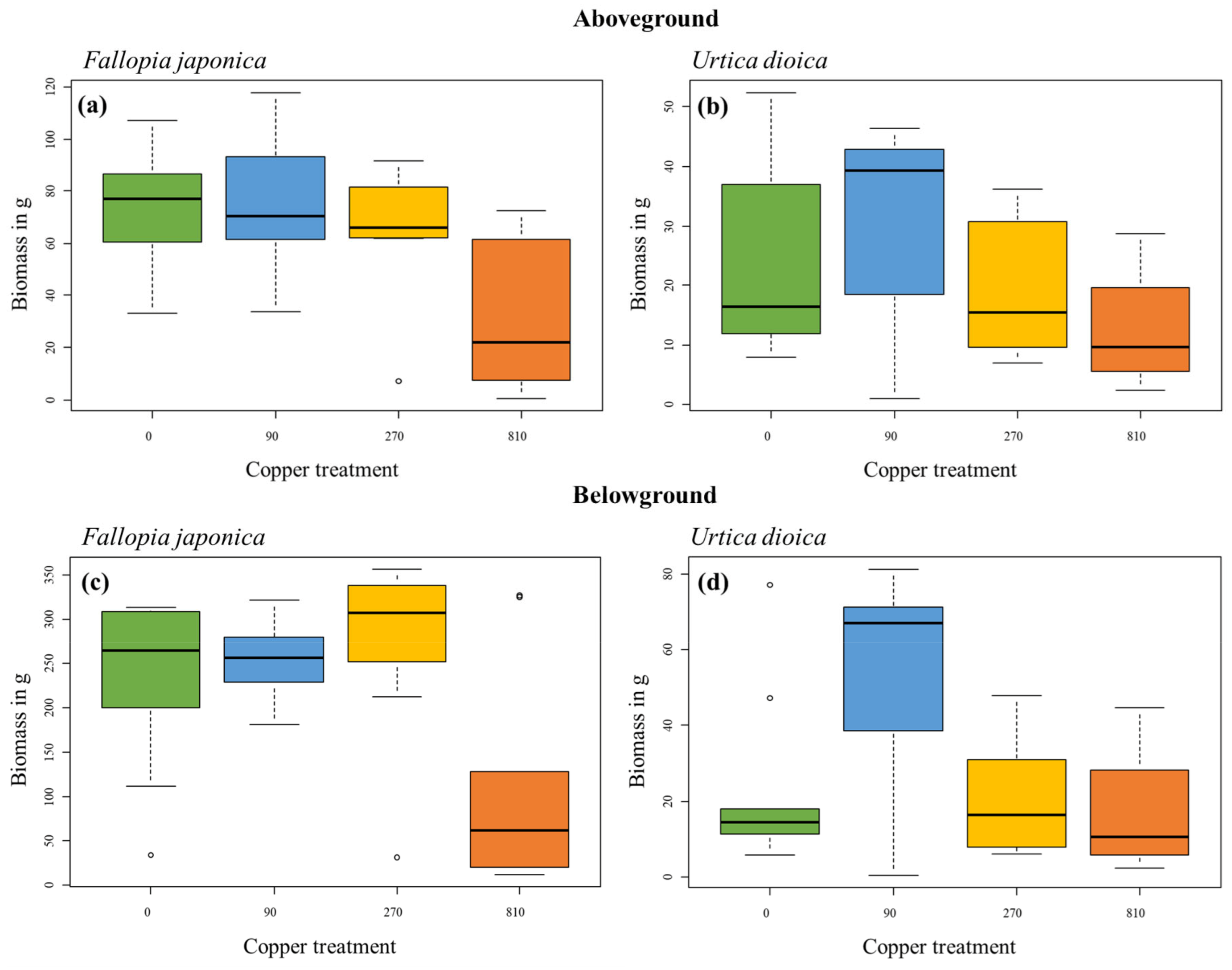
2.2. Biomass after a Two-Year Experiment
2.3. Copper Content of Plant Tissues
3. Discussion
3.1. Copper Contamination Negatively Impacts Plant Growth, but More Strongly in U. dioica Than F. japonica
3.2. Copper Content in Plant Tissue Is Higher in U. dioica Than in F. japonica
4. Materials and Methods
4.1. Experimental Setup
4.2. Plant Parameter Measurements
4.3. Plant Copper Content
4.4. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnson, G.F. The Early History of Copper Fungicides. Agric. Hist. 1935, 9, 67–79. [Google Scholar]
- Wightwick, A.M.; Mollah, M.R.; Partington, D.L.; Allinson, G. Copper fungicide residues in Australian vineyard soils. J. Agric. Food Chem. 2008, 56, 2457–2464. [Google Scholar] [CrossRef] [PubMed]
- Brunetto, G.; de Melo, G.W.B.; Terzano, R.; Del Buono, D.; Astolfi, S.; Tomasi, N.; Cesco, S. Copper accumulation in vineyard soils: Rhizosphere processes and agronomic practices to limit its toxicity. Chemosphere 2016, 162, 293–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruyters, S.; Salaets, P.; Oorts, K.; Smolders, E. Copper toxicity in soils under established vineyards in Europe: A survey. Sci. Total. Environ. 2013, 443, 470–477. [Google Scholar] [CrossRef]
- Ripke, F.O.; Warnecke-Busch, G.; Garrelts, J. Technische Lösungsansätze zur Minimierung des Eintrags von Pflanzenschutzmittel in Oberflächengewässer durch Abtrift. In Mitteilungen der Biologischen Bundesanstalt für Land und Forstwirtschaft; Parey: Berlin, Germany, 2001; Volume 381, pp. 82–96. ISBN 3-8263-3357-8. [Google Scholar]
- Han, F.X.; Hargreaves, J.A.; Kingery, W.L.; Huggett, D.B.; Schlenk, D.K. Accumulation, distribution, and toxicity of copper in sediments of catfish ponds receiving periodic copper sulfate applications. J. Environ. Qual. 2001, 30, 912–919. [Google Scholar] [CrossRef]
- Schulz, R.; Bundschuh, M.; Gergs, R.; Brühl, C.A.; Diehl, D.; Entling, M.H.; Fahse, L.; Frör, O.; Jungkunst, H.F.; Lorke, A.; et al. Review on environmental alterations propagating from aquatic to terrestrial ecosystems. Sci. Total. Environ. 2015, 538, 246–261. [Google Scholar] [CrossRef]
- Gilbert, F.A. Copper in Nutrition. In Advances in Agronomy; Norman, A.G., Ed.; Elsevier: Amsterdam, Netherlands, 1952; Volume 4, pp. 147–177. ISBN 978-0-12-000704-2. [Google Scholar]
- Shabbir, Z.; Sardar, A.; Shabbir, A.; Abbas, G.; Shamshad, S.; Khalid, S.; Murtaza, G.; Dumat, C.; Shahid, M. Copper uptake, essentiality, toxicity, detoxification and risk assessment in soil-plant environment. Chemosphere 2020, 259, 127436. [Google Scholar] [CrossRef]
- Reichman, S.M. The Responses of Plants to Metal Toxicity: A Review Forusing on Copper, Manganese & Zinc; Australian Minerals & Energy Environment Foundation: Melbourne, Australia, 2002; ISBN 1-876205-13-X. [Google Scholar]
- Tsay, C.C.; Wang, L.W.; Chen, Y.R. Plant in response to copper toxicity. Taiwania 1995, 40, 173–181. [Google Scholar] [CrossRef]
- Lombardi, L.; Sebastiani, L. Copper toxicity in Prunus cerasifera: Growth and antioxidant enzymes responses of in vitro grown plants. Plant Sci. 2005, 168, 797–802. [Google Scholar] [CrossRef]
- Kuhns, L.J.; Sydnor, T.D. Copper toxicity in woody ornamentals. J. Arboric. 1976, 90, 68–73. [Google Scholar] [CrossRef]
- Bouazizi, H.; Jouili, H.; Geitmann, A.; El Ferjani, E. Copper toxicity in expanding leaves of Phaseolus vulgaris L.: Antioxidant enzyme response and nutrient element uptake. Ecotoxicol. Environ. Saf. 2010, 73, 1304–1308. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K. Biological Flora of the British Isles: Urtica dioica L. J. Ecol. 2009, 97, 1436–1458. [Google Scholar] [CrossRef]
- Beerling, D.J.; Bailey, J.P.; Conolly, A.P. Fallopia Japonica (Houtt.) Ronse Decraene. J. Ecol. 1994, 82, 959. [Google Scholar] [CrossRef]
- Aguilera, A.G.; Alpert, P.; Dukes, J.S.; Harrington, R. Impacts of the invasive plant Fallopia japonica (Houtt.) on plant communities and ecosystem processes. Biol. Invasions 2010, 12, 1243–1252. [Google Scholar] [CrossRef]
- Li, J.; Leng, Z.; Wu, Y.; Du, Y.; Dai, Z.; Biswas, A.; Zheng, X.; Li, G.; Mahmoud, E.K.; Jia, H.; et al. Interactions between invasive plants and heavy metal stresses: A review. J. Plant Ecol. 2022, 15, 429–436. [Google Scholar] [CrossRef]
- Barberis, L.; Chevalier, W.; Toussaint, M.-L.; Binet, P.; Piola, F.; Michalet, S. Responses of the species complex Fallopia × bohemica to single-metal contaminations to Cd, Cr or Zn: Growth traits, metal accumulation and secondary metabolism. Environ. Monit. Assess. 2020, 192, 673. [Google Scholar] [CrossRef]
- Pedersen, M.B.; Kjaer, C.; Elmegaard, N. Toxicity and bioaccumulation of copper to black bindweed (Fallopia convolvulus) in relation to bioavailability and the age of soil contamination. Arch. Environ. Contam. Toxicol. 2000, 39, 431–439. [Google Scholar] [CrossRef] [Green Version]
- Berchová-Bímová, K.; Soltysiak, J.; Vach, M. Role of different taxa and cytotypes in heavy metals absorption in knotweeds (Fallopia). Sci. Agric. Bohem. 2014, 45, 11–18. [Google Scholar] [CrossRef]
- Rahmonov, O.; Czylok, A.; Orczewska, A.; Majgier, L.; Parusel, T. Chemical composition of the leaves of Reynoutria japonica Houtt. and soil features in polluted areas. Open Life Sci. 2014, 9, 320–330. [Google Scholar] [CrossRef] [Green Version]
- Sołtysiak, J. Heavy Metals Tolerance in an Invasive Weed (Fallopia japonica) under Different Levels of Soils Contamination. J. Ecol. Eng. 2020, 21, 81–91. [Google Scholar] [CrossRef]
- Sołtysiak, J.; Brej, T. Invasion of Fallopia genus plants in urban environment. Pol. J. Environ. Stud. 2014, 23, 449–458. [Google Scholar]
- Grubor, M. Lead uptake, tolerance, and accumulation exhibited by the plants Urtica dioica and Sedum spectabile in contaminated soil without additives. Arch. Biol. Sci. 2008, 60, 239–244. [Google Scholar] [CrossRef]
- Balabanova, B.; Stafilov, T.; Bačeva, K. Bioavailability and bioaccumulation characterization of essential and heavy metals contents in R. acetosa, S. oleracea and U. dioica from copper polluted and referent areas. J. Environ. Health Sci. Eng. 2015, 13, 2. [Google Scholar] [CrossRef] [Green Version]
- Štrba, T.; Turisová, I.; Aschenbrenner, Š. Flora and vegetation of a copper mine heap in Richtárová (The Starohorské Vrchy Mts., Slovakia). Ann. Univ. Mariae Curie Sklodowska Sect C Biologia 2015, 69, 29. [Google Scholar] [CrossRef] [Green Version]
- Bislimi, K.; Halili, J.; Sahiti, H.; Bici, M.; Mazreku, I. Effect of Mining Activity in Accumulation of Heavy Metals in Soil and Plant (Urtica dioica L). J. Ecol. Eng. 2021, 22, 1–7. [Google Scholar] [CrossRef]
- Poschenrieder, C.; Cabot, C.; Martos, S.; Gallego, B.; Barceló, J. Do toxic ions induce hormesis in plants? Plant Sci. 2013, 212, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Agathokleous, E. Accumulator plants and hormesis. Environ. Pollut. 2021, 274, 116526. [Google Scholar] [CrossRef]
- Nishizono, H.; Kubota, K.; Suzuki, S.; Ishii, F. Accumulation of Heavy Metals in Cell Walls of Polygonum cuspidatum Roots from Metalliferous Habitats. Plant Cell Physiol. 1989, 30, 595–598. [Google Scholar] [CrossRef]
- Kubota, K.; Nishizono, H.; Suzuki, S.; Ishii, F. A Copper-Binding Protein in Root Cytoplasm of Polygonum cuspidatum Growing in a Metalliferous Habitat. Plant Cell Physiol. 1988, 29, 1029–1033. [Google Scholar] [CrossRef]
- Barney, J.N.; Tharayil, N.; DiTommaso, A.; Bhowmik, P.C. The Biology of Invasive Alien Plants in Canada. 5. Polygonum cuspidatum Sieb. & Zucc [= Fallopia japonica (Houtt.) Ronse Decr.]. Can. J. Plant Sci. 2006, 86, 887–906. [Google Scholar] [CrossRef] [Green Version]
- Sołtysiak, J.; Brej, T. Characteristics that make the Fallopia genus (Polygonaceae) highly invasive. Ecol. Quest. 2012, 16, 23–28. [Google Scholar] [CrossRef] [Green Version]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant. 2016, 38, 102. [Google Scholar] [CrossRef]
- Sengar, R.S.; Pandey, M. Inhibition of chlorophyll biosynthesis by lead in greening Pisum sativum leaf segments. Biol. Plant. 1996, 38, 459–462. [Google Scholar] [CrossRef]
- Singh, D.; Agnihotri, A.; Seth, C.S. Interactive effects of EDTA and oxalic acid on chromium uptake, translocation and photosynthetic attributes in Indian mustard (Brassica juncea L. var. Varuna). Curr. Sci. 2017, 112, 2034–2042. [Google Scholar] [CrossRef]
- Gupta, S.; Seth, C.S. Salicylic acid alleviates chromium (VI) toxicity by restricting its uptake, improving photosynthesis and augmenting antioxidant defense in Solanum lycopersicum L. Physiol. Mol. Biol. Plants 2021, 27, 2651–2664. [Google Scholar] [CrossRef]
- Dey, S.; Mazumder, P.B.; Paul, S.B. Effect of copper on growth and chlorophyll content in tea plants (Camellia sinensis (L.) O. kuntze). Int. J. Res. Appl. Nat. Soc. Sci. 2014, 2, 223–230. [Google Scholar]
- Monni, S.; Uhlig, C.; Hansen, E.; Magel, E. Ecophysiological responses of Empetrum nigrum to heavy metal pollution. Environ. Pollut. 2001, 112, 121–129. [Google Scholar] [CrossRef]
- Shakya, K.; Chettri, M.K.; Sawidis, T. Impact of heavy metals (copper, zinc, and lead) on the chlorophyll content of some mosses. Arch. Environ. Contam. Toxicol. 2008, 54, 412–421. [Google Scholar] [CrossRef]
- Krystofova, O.; Adam, V.; Babula, P.; Zehnalek, J.; Beklova, M.; Havel, L.; Kizek, R. Effects of various doses of selenite on stinging nettle (Urtica dioica L.). Int. J. Environ. Res. Public Health 2010, 7, 3804–3815. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Chen, H.; He, D.; He, X.; Yan, Y.; Wu, K.; Wei, H. Effects of Exogenous Organic Acids on Cd Tolerance Mechanism of Salix variegata Franch. Under Cd Stress. Front. Plant Sci. 2020, 11, 594352. [Google Scholar] [CrossRef]
- Ali, B. Salicylic acid induced antioxidant system enhances the tolerance to aluminium in mung bean (Vigna radiata L. Wilczek) plants. Indian J. Plant Physiol. 2017, 22, 178–189. [Google Scholar] [CrossRef]
- Ekoja, E.E.; Pitan, O.O.R.; Ataiyese, M.O. Physiological Response of Okra to Flea Beetle Herbivory as Measured by Leaf Loss, Chlorophyll Disruption, and Dry Matter Yield. Int. J. Veg. Sci. 2012, 18, 171–181. [Google Scholar] [CrossRef]
- Thomson, V.P.; Cunningham, S.A.; Ball, M.C.; Nicotra, A.B. Compensation for herbivory by Cucumis sativus through increased photosynthetic capacity and efficiency. Oecologia 2003, 134, 167–175. [Google Scholar] [CrossRef]
- Hulina, N.; Ðumija, L. Ability of Reynoutria japonica Houtt. (Polygonaceae) to accumulate heavy metals. Period. Biol. 1999, 101, 233–235. [Google Scholar]
- Širka, V.H.; Jakovljević, K.; Mihailović, N.; Jovanović, S. Heavy metal accumulation in invasive Reynoutria × bohemica Chrtek & Chrtková in polluted areas. Environ. Earth Sci. 2016, 75, 1–12. [Google Scholar] [CrossRef]
- Lerch, S.; Sirguey, C.; Michelot-Antalik, A.; Jurjanz, S. Accumulation of metallic trace elements in Reynoutria japonica: A risk assessment for plant biomass valorization. Environ. Sci. Pollut. Res. Int. 2022, 29, 67390–67401. [Google Scholar] [CrossRef] [PubMed]
- Rahmonov, O.; Banaszek, J.; Pukowiec-Kurda, K. Relationships Between Heavy Metal Concentrations in Japanese Knotweed (Reynoutria Japonica Houtt.) Tissues and Soil in Urban Parks in Southern Poland. IOP Conf. Ser. Earth Environ. Sci. 2019, 221, 12145. [Google Scholar] [CrossRef] [Green Version]
- Shaw, J. Heavy Metal Tolerance in Plants: Evolutionary Aspects; CRC press: Boca Raton, FL. USA, 1989; ISBN 9780849368523. [Google Scholar]
- Agrarmeteorologie Rheinland-Pfalz, Wetterstation Siebeldingen. Available online: https://www.dlr.rlp.de/Agrarmeteorologie/Wetterdaten/Pfalz (accessed on 23 October 2022).
- DIN EN 16174:2012-11. Sludge, Treated Biowaste and Soil—Digestion of Aqua Regia Soluble Fractions of Elements; German Version; Beuth Verlag GmbH: Berlin, Germany, 2012. [Google Scholar] [CrossRef]
- Mikuła, B.; Puzio, B. Determination of trace metals by ICP-OES in plant materials after preconcentration of 1,10-phenanthroline complexes on activated carbon. Talanta 2007, 71, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Buhk, C.; Thielsch, A. Hybridisation boosts the invasion of an alien species complex: Insights into future invasiveness. Perspect. Plant Ecol. Evol. Syst. 2015, 17, 274–283. [Google Scholar] [CrossRef]
- Croft, H.; Chen, J.M.; Luo, X.; Bartlett, P.; Chen, B.; Staebler, R.M. Leaf chlorophyll content as a proxy for leaf photosynthetic capacity. Glob. Chang. Biol. 2017, 23, 3513–3524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ling, Q.; Huang, W.; Jarvis, P. Use of a SPAD-502 meter to measure leaf chlorophyll concentration in Arabidopsis thaliana. Photosynth. Res. 2011, 107, 209–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 23 October 2022).
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S. An {R} Companion to Applied Regression, 3rd ed; Sage publications: Thousand Oaks, CA, USA, 2019; pp. 1–608. [Google Scholar]



| Treatment | Time | Treatment * Time | ||||
|---|---|---|---|---|---|---|
| Chi2 | p | Chi2 | p | Chi2 | p | |
| Fallopia japonica | ||||||
| Plant height | 77.37 | <0.001 | 209.69 | <0.001 | 3.89 | 0.273 |
| Leaf number | 48.82 | <0.001 | 286.16 | <0.001 | 21.35 | <0.001 |
| Chlorophyll content | 40.49 | <0.001 | 220.73 | <0.001 | 8.65 | 0.034 |
| Urtica dioica | ||||||
| Plant height | 147.29 | <0.001 | 383.30 | <0.001 | 21.14 | <0.001 |
| Leaf number | 35.88 | <0.001 | 354.69 | <0.001 | 30.85 | <0.001 |
| Chlorophyll content | 14.73 | 0.002 | 232.31 | <0.001 | 7.01 | 0.072 |
| Treatment | ||
|---|---|---|
| Chi2 | p | |
| Fallopia japonica | ||
| Aboveground biomass | 34.92 | <0.001 |
| Belowground biomass | 19.00 | <0.001 |
| Urtica dioica | ||
| Aboveground biomass | 4.17 | 0.244 |
| Belowground biomass | 6.59 | 0.086 |
| Treatment | ||
|---|---|---|
| Chi2 | p | |
| Fallopia japonica | ||
| Aboveground samples | 168.89 | <0.001 |
| Root samples | 47.16 | <0.001 |
| Rhizome samples | 44.57 | <0.001 |
| Urtica dioica | ||
| Aboveground samples | 143.79 | <0.001 |
| Root samples | 41.56 | <0.001 |
| Rhizome samples | 65.84 | <0.001 |
| Treatment [mg kg−1] (April 2020) | Initial Soil Copper Content [mg kg−1] (April 2020) | Deviation from Treatment | Final Soil Copper Content [mg kg−1] (August 2021) | Deviation from Treatment |
|---|---|---|---|---|
| 0 | 8.18 ± 1.35 | - | 4.99 ± 1.32 | - |
| 90 | 87.63 ± 11.62 | −2.6% | 93.63 ± 9.58 | 4.0% |
| 270 | 238.99 ± 38.50 | −11.5% | 252.83 ± 35.89 | −6.4% |
| 810 | 781.64 ± 154.91 | −3.5% | 714.48 ± 85.42 | −11.8% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmitz, D.; Girardi, J.; Jamin, J.; Bundschuh, M.; Geng, B.; Feldmann, R.; Rösch, V.; Riess, K.; Schirmel, J. Copper Uptake and Its Effects on Two Riparian Plant Species, the Native Urtica dioica, and the Invasive Fallopia japonica. Plants 2023, 12, 481. https://doi.org/10.3390/plants12030481
Schmitz D, Girardi J, Jamin J, Bundschuh M, Geng B, Feldmann R, Rösch V, Riess K, Schirmel J. Copper Uptake and Its Effects on Two Riparian Plant Species, the Native Urtica dioica, and the Invasive Fallopia japonica. Plants. 2023; 12(3):481. https://doi.org/10.3390/plants12030481
Chicago/Turabian StyleSchmitz, Daniel, Johanna Girardi, Jellian Jamin, Mirco Bundschuh, Benedict Geng, Rico Feldmann, Verena Rösch, Kai Riess, and Jens Schirmel. 2023. "Copper Uptake and Its Effects on Two Riparian Plant Species, the Native Urtica dioica, and the Invasive Fallopia japonica" Plants 12, no. 3: 481. https://doi.org/10.3390/plants12030481
APA StyleSchmitz, D., Girardi, J., Jamin, J., Bundschuh, M., Geng, B., Feldmann, R., Rösch, V., Riess, K., & Schirmel, J. (2023). Copper Uptake and Its Effects on Two Riparian Plant Species, the Native Urtica dioica, and the Invasive Fallopia japonica. Plants, 12(3), 481. https://doi.org/10.3390/plants12030481






