Metabolite Profiling and Bioactivities of Leaves, Stems, and Flowers of Rumex usambarensis (Dammer) Dammer, a Traditional African Medicinal Plant
Abstract
1. Introduction
2. Results and Discussion
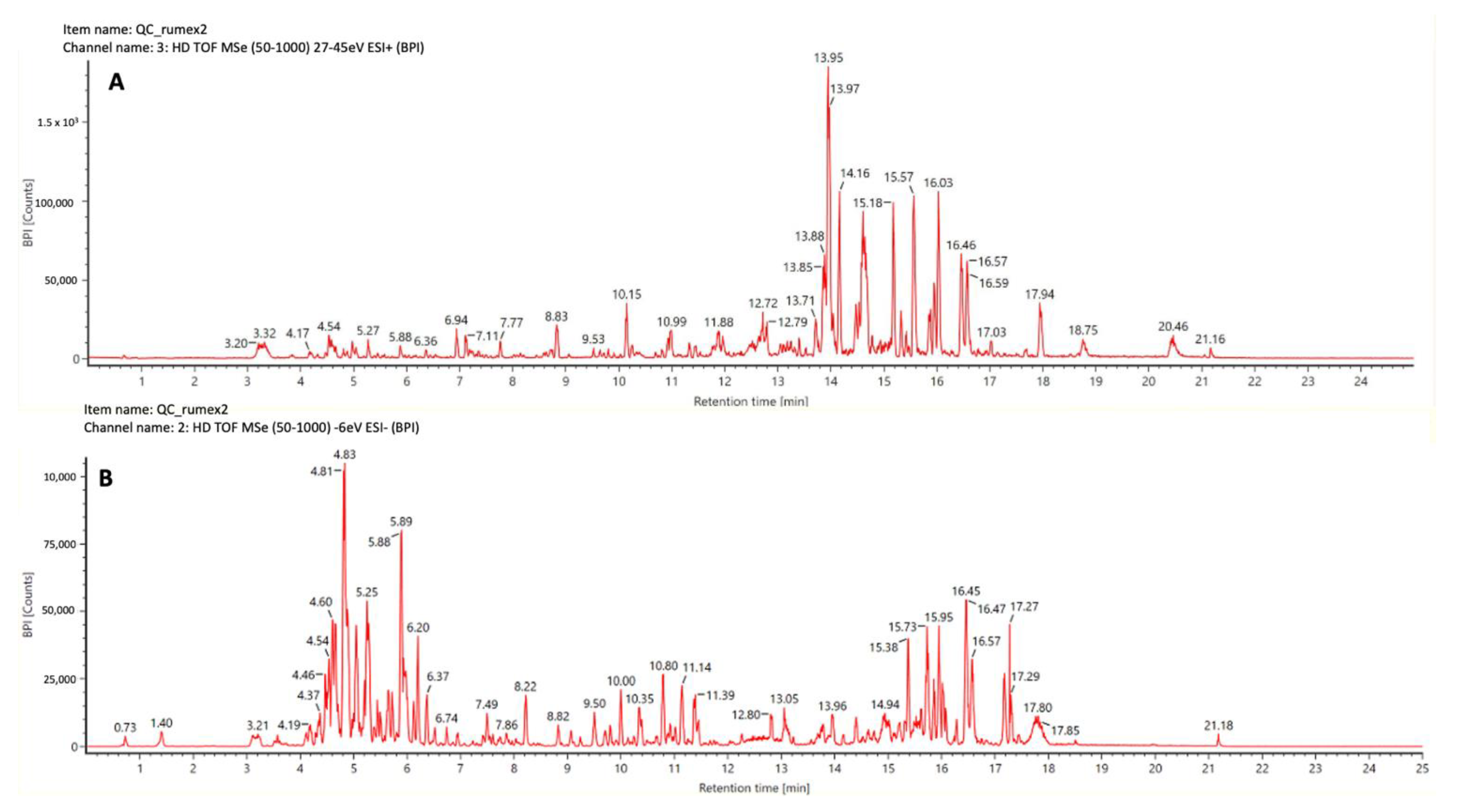
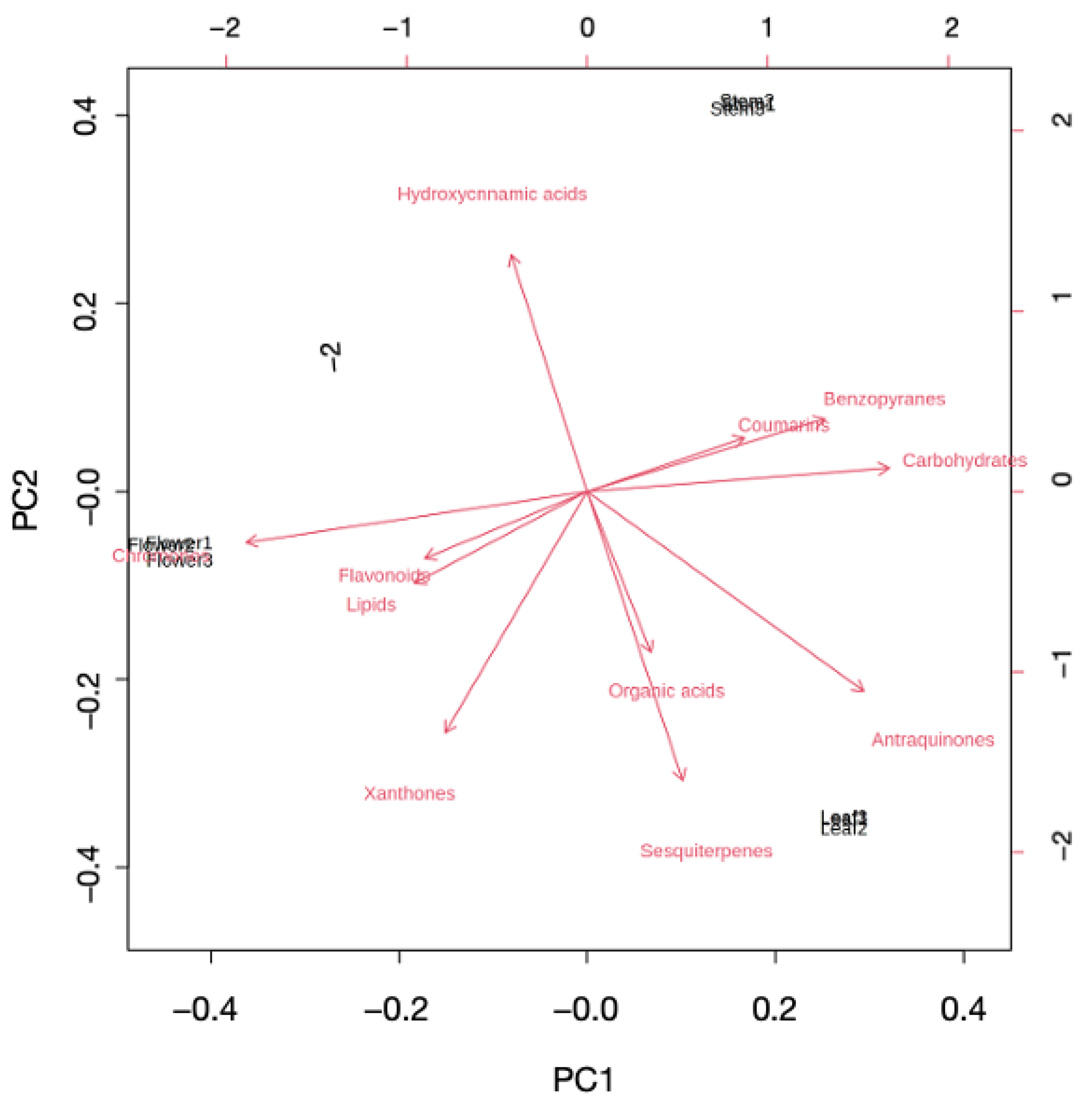
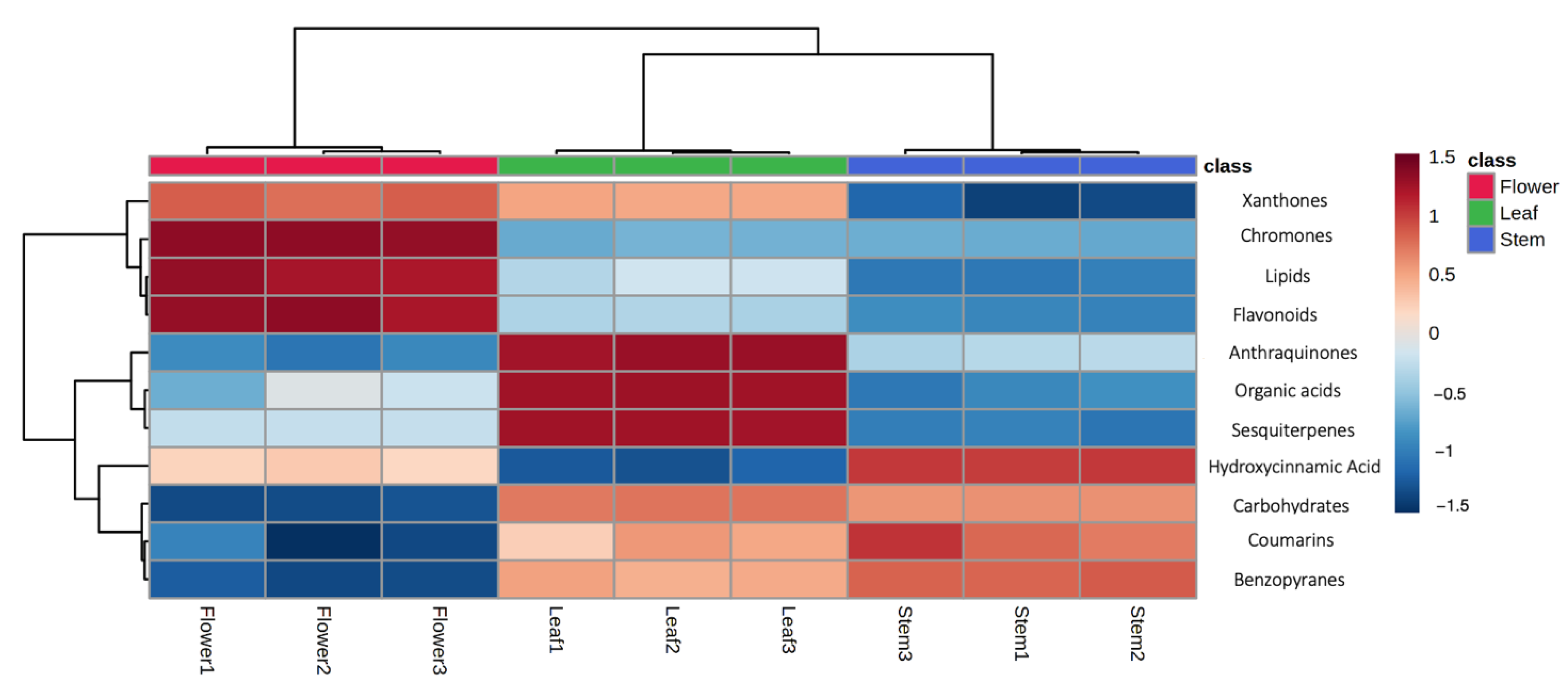
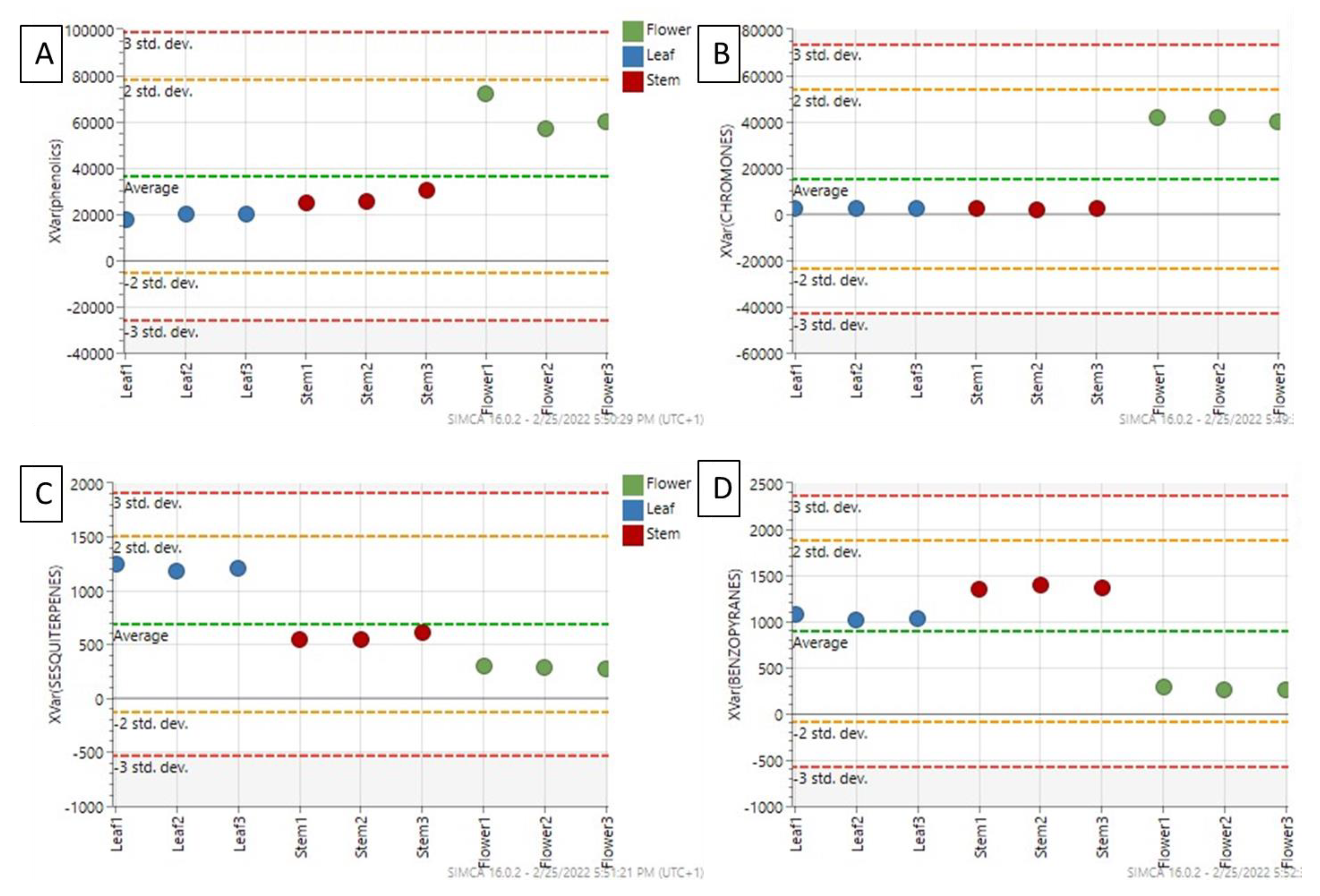
2.1. Multivariate Modeling
2.2. Investigating Metabolome Diversity of Stems, Leaves, and Flowers
2.3. Bioactivities Related to Traditional Use
2.3.1. Antioxidant Activity
2.3.2. Antimicrobial Activity
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Material
3.3. Sample Preparation
3.4. UHPLC-TWIMS-QTOF
Data Processing and Multivariate Modeling
3.5. Evaluation of Antimicrobial/Antifungal Activity
3.5.1. Inoculum Preparation
3.5.2. MIC Assay
3.6. Determination of Antioxidant Activity
3.7. Spectrophotometric Measurements
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rahman, S.; Haq, F.U.; Ali, A.; Khan, M.N.; Shah, S.M.Z.; Adhikhari, A.; El-Seedi, H.R.; Musharraf, S.G. Combining untargeted and targeted metabolomics approaches for the standardization of polyherbal formulations through UPLC–MS/MS. Metabolomics 2019, 15, 116. [Google Scholar] [CrossRef]
- Heinrich, M. Ethnopharmacy and natural product research—Multidisciplinary opportunities for research in the metabolomic age. Phytochem. Lett. 2008, 1, 1–5. [Google Scholar] [CrossRef]
- Smillie, T.J.; A Khan, I. A Comprehensive Approach to Identifying and Authenticating Botanical Products. Clin. Pharmacol. Ther. 2009, 87, 175–186. [Google Scholar] [CrossRef]
- Fabricant, D.S.; Farnsworth, N.R. The value of plants used in traditional medicine for drug discovery. Environ. Heal Perspect. 2001, 109, 69–75. [Google Scholar] [CrossRef]
- Gertsch, J. Botanical Drugs, Synergy, and Network Pharmacology: Forth and Back to Intelligent Mixtures. Planta Med. 2011, 77, 1086–1098. [Google Scholar] [CrossRef]
- Williamson, E.M. Synergy and other interactions in phytomedicines. Phytomedicine 2001, 8, 401–409. [Google Scholar] [CrossRef]
- Vasas, A.; Orbán-Gyapai, O.; Hohmann, J. The Genus Rumex: Review of traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2015, 175, 198–228. [Google Scholar] [CrossRef]
- Bello, O.M.; Fasinu, P.S.; Bello, O.E.; Ogbesejana, A.B.; Adetunji, C.O.; Dada, A.O.; Ibitoye, O.S.; Aloko, S.; Oguntoye, O.S. Wild vegetable Rumex acetosa Linn.: Its ethnobotany, pharmacology and phytochemistry—A review. S. Afr. J. Bot. 2019, 125, 149–160. [Google Scholar] [CrossRef]
- Gebrie, E.; Makonnen, E.; Debella, A.; Zerihun, L. Phytochemical screening and pharmacological evaluations for the antifertility effect of the methanolic root extract of Rumex steudelii. J. Ethnopharmacol. 2005, 96, 139–143. [Google Scholar] [CrossRef]
- Rouf, A.; Islam, M.; Rahman, M. Evaluation of antidiarrhoeal activity Rumex maritimus root. J. Ethnopharmacol. 2003, 84, 307–310. [Google Scholar] [CrossRef]
- Mishra, A.P.; Sharifi-Rad, M.; Shariati, M.A.; Mabkhot, Y.N.; Al-Showiman, S.S.; Rauf, A.; Salehi, B.; Župunski, M.; Sharifi-Rad, M.; Gusain, P.; et al. Bioactive compounds and health benefits of edible Rumex species-A review. Cell Mol. Biol. 2018, 64, 27–34. [Google Scholar] [CrossRef]
- Kumar, V.; Shaikh, S.; Shriram, V.; Srivastav, A.; Barve, P. A critical review on Nepal Dock (Rumex nepalensis): A tropical herb with immense medicinal importance. Asian Pac. J. Trop. Med. 2018, 11, 405. [Google Scholar] [CrossRef]
- Wambugu, S.N.; Mathiu, P.M.; Gakuya, D.W.; Kanui, T.I.; Kabasa, J.D.; Kiama, S.G. Medicinal plants used in the management of chronic joint pains in Machakos and Makueni counties, Kenya. J. Ethnopharmacol. 2011, 137, 945–955. [Google Scholar] [CrossRef]
- Van Puyvelde, L.; Geiser, I.; Rwangabo, P.-C.; Sebikali, B. Rwandese herbal remedies used against gonorrhoea. J. Ethnopharmacol. 1983, 8, 279–286. [Google Scholar] [CrossRef]
- Schlage, C.; Mabula, C.; Mahunnah, R.L.A.; Heinrich, M. Medicinal Plants of the Washambaa (Tanzania): Documentation and Ethnopharmacological Evaluation. Plant Biol. 2000, 2, 83–92. [Google Scholar] [CrossRef]
- Ammar, N.M.; El-Kassem, L.T.A.; Ayoub, N.A.; El-Ahmady, S.H.; Moharam, M.E.; AbouZeid, E.M. Anti-inflammatory and antimicrobial activities of the successive extracts of the aerial parts of Rumex pictus Forssk. growing in Egypt. J. Herbmed. Pharmacol. 2020, 10, 116–122. [Google Scholar] [CrossRef]
- Kisangau, D.P.; Hosea, K.M.; Lyaruu, H.V.M.; Joseph, C.C.; Mbwambo, Z.H.; Masimba, P.J.; Gwandu, C.B.; Bruno, L.N.; Devkota, K.P.; Sewald, N. Screening of traditionally used Tanzanian medicinal plants for antifungal activity. Pharm. Biol. 2009, 47, 708–716. [Google Scholar] [CrossRef]
- Muthee, J.; Gakuya, D.; Mbaria, J.; Kareru, P.; Mulei, C.; Njonge, F. Ethnobotanical study of anthelmintic and other medicinal plants traditionally used in Loitoktok district of Kenya. J. Ethnopharmacol. 2011, 135, 15–21. [Google Scholar] [CrossRef]
- Gahamanyi, N.; Munyaneza, E.; Dukuzimana, E.; Tuyiringire, N.; Pan, C.-H.; Komba, E.V.G. Ethnobotany, Ethnopharmacology, and Phytochemistry of Medicinal Plants Used for Treating Human Diarrheal Cases in Rwanda: A Review. Antibiotics 2021, 10, 1231. [Google Scholar] [CrossRef]
- Hilda, N.; David, N.; Engeu, O.P. Rumex usambarensis (Dammer) Leaf and Stem Extract Effect on Body Weight and Lipid Profile: A Study in Albino Rats. Br. J. Pharm. Res. 2016, 13, 1–7. [Google Scholar] [CrossRef]
- Midiwo, J. Distribution of anthraquinone pigments in Rumex species of Kenya. Phytochemistry 1985, 24, 1390–1391. [Google Scholar] [CrossRef]
- Tugizimana, F.; Piater, L.; Dubery, I. Plant metabolomics: A new frontier in phytochemical analysis. S. Afr. J. Sci. 2013, 109, 11. [Google Scholar] [CrossRef]
- Upton, R.; David, B.; Gafner, S.; Glasl, S. Botanical ingredient identification and quality assessment: Strengths and limitations of analytical techniques. Phytochem. Rev. 2019, 19, 1157–1177. [Google Scholar] [CrossRef]
- Wang, X.; Ren, J.; Zhang, A.; Sun, H.; Yan, G.; Han, Y.; Liu, L. Novel applications of mass spectrometry-based metabolomics in herbal medicines and its active ingredients: Current evidence. Mass Spectrom. Rev. 2019, 38, 380–402. [Google Scholar] [CrossRef]
- Caesar, L.K.; Cech, N.B. Synergy and antagonism in natural product extracts: When 1 + 1 does not equal 2. Nat. Prod. Rep. 2019, 36, 869–888. [Google Scholar] [CrossRef]
- Sharma, A.; Gupta, S.; Sarethy, I.P.; Dang, S.; Gabrani, R. Green tea extract: Possible mechanism and antibacterial activity on skin pathogens. Food Chem. 2012, 135, 672–675. [Google Scholar] [CrossRef]
- Reygaert, W.C. Green Tea Catechins: Their Use in Treating and Preventing Infectious Diseases. BioMed. Res. Int. 2018, 2018, 1–9. [Google Scholar] [CrossRef]
- Reygaert, W.C. The antimicrobial possibilities of green tea. Front Microbiol. 2014, 5, 434. [Google Scholar] [CrossRef]
- Abidi, J.; Ammar, S.; Ben Brahim, S.; Skalicka-Woźniak, K.; Ghrabi-Gammar, Z.; Bouaziz, M. Use of ultra-high-performance liquid chromatography coupled with quadrupole-time-of-flight mass spectrometry system as valuable tool for an untargeted metabolomic profiling of Rumex tunetanus flowers and stems and contribution to the antioxidant activity. J. Pharm. Biomed. Anal. 2018, 162, 66–81. [Google Scholar] [CrossRef]
- Pelzer, C.V.; Houriet, J.; Crandall, W.J.; Todd, D.A.; Cech, N.B.; Jones, D.D., Jr. More than Just a Weed: An Exploration of the Antimicrobial Activity of Rumex Crispus Using a Multivariate Data Analysis Approach. Planta Med. 2021, 88, 753–761. [Google Scholar] [CrossRef]
- Sharma, R.; Jandrotia, R.; Singh, B.; Sharma, U.; Kumar, D. Comprehensive Metabolomics Study of Traditionally Important Rumex Species Found in Western Himalayan Region. Nat. Prod. Commun. 2018, 13. [Google Scholar] [CrossRef]
- Sabo, V.A.; Svircev, E.; Mimica-Dukic, N.; Orcic, D.; Narancic, J.; Knezevic, P. Anti-Acinetobacter baumannii activity of Rumex crispus L. and Rumex sanguineus L. extracts. Asian Pac. J. Trop. Biomed. 2020, 10, 172–182. [Google Scholar]
- Negi, J.S.; Bisht, V.K.; Singh, P.; Rawat, M.S.M.; Joshi, G.P. Naturally Occurring Xanthones: Chemistry and Biology. J. Appl. Chem. 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Vieira, L.M.M.; Kijjoa, A. Naturally-occurring xanthones: Recent developments. Curr. Med. Chem. 2005, 12, 2413–2446. [Google Scholar] [CrossRef]
- Ruan, J.; Zheng, C.; Liu, Y.; Qu, L.; Yu, H.; Han, L.; Zhang, Y.; Wang, T. Chemical and Biological Research on Herbal Medicines Rich in Xanthones. Molecules 2017, 22, 1698. [Google Scholar] [CrossRef]
- Demirezer, L. Concentrations of Anthraquinone Glycosides of Rumex Crispus during Different Vegetation Stages. Z. Für Nat. C 1994, 49, 404–406. [Google Scholar] [CrossRef]
- European Commission. Commision Implementing Regulation (EU) 2018/784 of 29 May 2018. Off. J. Eur. Union 2018, 132, 35–39. [Google Scholar]
- Costa, G.; Ferreira, J.P.; Vitorino, C.; Pina, M.E.; Sousa, J.J.; Figueiredo, I.V.; Batista, M.T. Polyphenols from Cymbopogon citratus leaves as topical anti-inflammatory agents. J. Ethnopharmacol. 2016, 178, 222–228. [Google Scholar] [CrossRef]
- Nichols, J.A.; Katiyar, S.K. Skin photoprotection by natural polyphenols: Anti-inflammatory, antioxidant and DNA repair mechanisms. Arch. Dermatol. Res. 2009, 302, 71–83. [Google Scholar] [CrossRef]
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Leaper, D.; Georgopoulos, N.T. Reactive oxygen species (ROS) and wound healing: The functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int. Wound J. 2015, 14, 89–96. [Google Scholar] [CrossRef]
- Tsai, T.-H.; Tsai, T.-H.; Chien, Y.-C.; Lee, C.-W.; Tsai, P.-J. In vitro antimicrobial activities against cariogenic streptococci and their antioxidant capacities: A comparative study of green tea versus different herbs. Food Chem. 2008, 110, 859–864. [Google Scholar] [CrossRef]
- Fahy, E.; Subramaniam, S.; Murphy, R.C.; Nishijima, M.; Raetz, C.R.H.; Shimizu, T.; Spener, F.; van Meer, G.; Wakelam, M.J.O.; Dennis, E.A. Update of the LIPID MAPS comprehensive classification system for lipids. J. Lipid Res. 2009, 50, S9–S14. [Google Scholar] [CrossRef]
- Wishart, D.S.; Jewison, T.; Guo, A.C.; Wilson, M.; Knox, C.; Liu, Y.; Djoumbou, Y.; Mandal, R.; Aziat, F.; Dong, E.; et al. HMDB 3.0—The Human Metabolome Database in 2013. Nucleic Acids Res. 2013, 41, D801–D807. [Google Scholar] [CrossRef]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.-M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis. Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef]
- Stratil, P.; Klejdus, B.; Kubáň, V. Determination of Total Content of Phenolic Compounds and Their Antioxidant Activity in VegetablesEvaluation of Spectrophotometric Methods. J. Agric. Food Chem. 2006, 54, 607–616. [Google Scholar] [CrossRef]
- Rahman, M.; Islam, B.; Biswas, M.; Alam, A.H.M.K. In vitro antioxidant and free radical scavenging activity of different parts of Tabebuia pallida growing in Bangladesh. BMC Res. Notes 2015, 8, 621. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Fernandes, R.P.P.; Trindade, M.A.; Tonin, F.G.; Lima, C.G.; Pugine, S.M.P.; Munekata, P.E.S.; Lorenzo, J.M.; De Melo, M.P. Evaluation of antioxidant capacity of 13 plant extracts by three different methods: Cluster analyses applied for selection of the natural extracts with higher antioxidant capacity to replace synthetic antioxidant in lamb burgers. J. Food Sci. Technol. 2016, 53, 451–460. [Google Scholar] [CrossRef]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2018, 18, 241–272. [Google Scholar] [CrossRef]
- Taguri, T.; Tanaka, T.; Kouno, I. Antibacterial Spectrum of Plant Polyphenols and Extracts Depending upon Hydroxyphenyl Structure. Biol. Pharm. Bull. 2006, 29, 2226–2235. [Google Scholar] [CrossRef]
- Bouarab-Chibane, L.; Forquet, V.; Lantéri, P.; Clément, Y.; Léonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial Properties of Polyphenols: Characterization and QSAR (Quantitative Structure–Activity Relationship) Models. Front. Microbiol. 2019, 10, 829. [Google Scholar] [CrossRef]
| Compound Name | Chemical Class | Experimental m/z | RT (min) | q Value | Mass Error (ppm) | Highest Mean | Fragmentation Score |
|---|---|---|---|---|---|---|---|
| PG(14:0) | Lipid | 457.2542 | 9.7 | 3.1 × 10−10 | −4.06 | Flower | 97 |
| Hydroxy-phenyl--icosanone | Lipid | 371.3297 | 15.8 | 7.2 × 10−15 | −2.84 | Flower | 97.1 |
| Cedeodarin | Flavonoid | 317.0646 | 3.9 | 2.7 × 10−12 | −2.85 | Flower | 86.2 |
| Sylpin | Flavonoid | 337.0697 | 4.6 | 7.5 × 10−12 | 4.74 | Leaf | 77.9 |
| Eriocitrin | Flavonoid | 635.1382 | 3.4 | 8.9 × 10−8 | 1.60 | Leaf | 85.2 |
| Epicatechin-gallate | Flavonoid | 713.1484 | 3.9 | 3.6 × 10−10 | −2.33 | Stem | 85.4 |
| Xanthomicrol | Flavonoid | 345.0957 | 7.8 | 1.1 × 10−10 | −3.47 | Stem | 82 |
| Cinchonain Ia | Flavolignan | 451.1022 | 4.1 | 7.1 × 10−9 | −2.70 | Leaf | 72.7 |
| Silandrin | Flavolignan | 449.1229 | 7.6 | 5.2 × 10−15 | −0.47 | Stem | 68.2 |
| Microdiplodiasone | Chromone | 259.0602 | 3.8 | 3.3 × 10−9 | −3.59 | Flower | 82.1 |
| Botrallin | Chromone | 299.0552 | 6.5 | 1.3 × 10−10 | −2.95 | Leaf | 84.1 |
| Gynuraone | Chromone | 177.0540 | 7.9 | 1.0 × 10−15 | −3.18 | Stem | 87 |
| Isoscopoletin | Coumarin | 191.0344 | 3.4 | 1.2 × 10−7 | −2.76 | Leaf | 73 |
| Micromelin | Coumarin | 269.0454 | 7.6 | 3.1 × 10−9 | −0.37 | Flower | 69.3 |
| Pratenol A | Benzopyran | 241.0494 | 4.3 | 5.4 × 10−11 | −4.81 | Stem | 72.2 |
| Azanigerone E | Benzopyran | 273.0734 | 7.5 | 6.2 × 10−12 | 0.19 | Stem | 66.1 |
| Agnestin A | Xanthone | 269.0448 | 6.5 | 8.6 × 10−7 | −2.56 | Flower | 84 |
| Bellidifolin | Xanthone | 255.0290 | 5.3 | 7.7 × 10−7 | −3.25 | Flower | 70.4 |
| Nidulalin a | Xanthone | 285.0749 | 8.2 | 2.9 × 10−14 | −2.91 | Leaf | 84.6 |
| Phosphoglyceroinositol | Carbohydrate | 317.0641 | 0.7 | 7.3 × 10−14 | 2.61 | Flower | 82.8 |
| Methyl-fusarubinlactone | Carbohydrate | 333.0611 | 4.0 | 1.9 × 10−12 | −1.44 | Stem | 75.4 |
| Aloenin | Glycoside | 428.1538 | 3.3 | 1.8 × 10−10 | −3.34 | Leaf | 90.9 |
| Microlenin | Sesquiterpen | 477.2262 | 13.7 | 2.0 × 10−7 | −1.96 | Leaf | 68.5 |
| Hibiscoquinone A | Sesquiterpenoid | 241.0849 | 5.1 | 8.6 × 10−12 | −3.82 | Leaf | 68.8 |
| Lactucin-oxalate | Sesquerpen | 331.0820 | 6.0 | 1.0 × 10−13 | 2.20 | Leaf | 72.9 |
| Obtusifolin glucoside | Anthraquinone | 469.1110 | 4.3 | 2.3 × 10−11 | 1.07 | Leaf | 83.8 |
| Physcion-glucoside | Anthraquinone | 469.1117 | 4.2 | 5.2 × 10−12 | 2.73 | Leaf | 69.9 |
| Prosopinine | Alkaloid | 336.2521 | 8.2 | 1.1 × 10−11 | 3.79 | Flower | 83.9 |
| ABTS-TEAC µg/mL | DPPH-TEAC µg/mL | FRAP µM | TPC (mgGAE/mL) | ||
|---|---|---|---|---|---|
| Leaf | 1.17 ± 0.008 a | 0.059 ± 0.000 a | 2.5 ± 0.125 a | 1.1 ± 0 a | |
| Rumex usambarensis | Flower | 1.38 ± 0.01 b | 0.067 ± 0.002 b | 4.96 ± 0.07 b | 1.7 ± 0 b |
| Stem | 0.08 ± 0.001 c | 0.046 ± 0.001 c | 0.875 ± 0 c | 0.63 ± 0.029 c | |
| Camellia sinensis | Leaf | 11.34 ± 0.15 d | 0.07 ± 0.001 d | 8 ± 0 d | 2.22 ± 0.029 d |
| CONCENTRATION (µg/mL) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 256 | 128 | 64 | 32 | 16 | 8 | 4 | 2 | 1 | 0.5 | ||
| E. coli | RU flowers | 51% | 21% | −8% | −15% | −12% | −14% | −16% | −16% | −15% | −17% |
| RU stems | 36% | 29% | 18% | 13% | 12% | 8% | 3% | 3% | 1% | 0% | |
| RU leaves | 2% | −9% | −14% | −16% | −20% | −22% | −22% | −20% | −18% | −13% | |
| CS leaves | 33% | 34% | 34% | 22% | 22% | 15% | 11% | 8% | 9% | 7% | |
| S. aureus | flowers | 40% | 46% | 21% | −3% | −14% | −11% | −9% | −16% | 2% | −5% |
| stems | −152% | −69% | 10% | 10% | 3% | 43% | 27% | −11% | 18% | −3% | |
| leaves | −72% | 4% | 34% | 40% | 40% | 29% | 23% | 8% | 13% | 14% | |
| CS leaves | 84% | 6% | −2% | 15% | 9% | 28% | 15% | 11% | 15% | 0% | |
| C. albicans | flowers | 52% | 41% | 24% | 1% | 10% | −11% | −5% | −16% | −3% | −2% |
| stems | −147% | −84% | −42% | −16% | −23% | −23% | −18% | −26% | −26% | −21% | |
| leaves | −66% | −34% | −26% | −36% | −24% | −21% | −48% | −33% | −37% | −37% | |
| CS leaves | −65% | −85% | −95% | −76% | −26% | −14% | 6% | 0% | 0% | 0% | |
| M. pachydermatis | flowers | 91% | 92% | 66% | 26% | 39% | 32% | 25% | 30% | 48% | 28% |
| stems | 74% | 87% | 89% | 82% | 77% | 71% | 61% | 56% | 46% | 59% | |
| leaves | 90% | 92% | 86% | 70% | 67% | 56% | 57% | 64% | 56% | 44% | |
| CS leaves | −27% | −79% | 34% | 55% | 68% | 75% | 79% | 84% | 87% | 81% | |
| M. furfur | flowers | 58% | 80% | 76% | 57% | 45% | 35% | 28% | 11% | 18% | 21% |
| steams | 24% | 56% | 79% | 56% | 73% | 68% | 61% | 55% | 46% | 38% | |
| leaves | 29% | 68% | 73% | 63% | 43% | 53% | 34% | 32% | 23% | 22% | |
| CS leaves | −97% | −47% | 0% | 0% | 0% | 5% | 12% | 10% | 9% | 10% | |
| E. coli | S. aureus | C. albicans | M. pachydermatis | M. furfur | |
|---|---|---|---|---|---|
| µg/mL ± SD | |||||
| RU flowers | 256 | 256 | >256 | 112 ± 29.63 | 256 |
| RU steams | >256 | >256 | >256 | 64 | 256 |
| RU leaves | >256 | >256 | >256 | 120 ± 22.63 | >256 |
| CS leaves | >256 | 240 ± 45.25 | 192 ± 68.42 | ≤0.5 | >256 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spaggiari, C.; Righetti, L.; Spadini, C.; Annunziato, G.; Nsanzurwimo, A.; Cabassi, C.S.; Bruni, R.; Costantino, G. Metabolite Profiling and Bioactivities of Leaves, Stems, and Flowers of Rumex usambarensis (Dammer) Dammer, a Traditional African Medicinal Plant. Plants 2023, 12, 482. https://doi.org/10.3390/plants12030482
Spaggiari C, Righetti L, Spadini C, Annunziato G, Nsanzurwimo A, Cabassi CS, Bruni R, Costantino G. Metabolite Profiling and Bioactivities of Leaves, Stems, and Flowers of Rumex usambarensis (Dammer) Dammer, a Traditional African Medicinal Plant. Plants. 2023; 12(3):482. https://doi.org/10.3390/plants12030482
Chicago/Turabian StyleSpaggiari, Chiara, Laura Righetti, Costanza Spadini, Giannamaria Annunziato, Aimable Nsanzurwimo, Clotilde Silvia Cabassi, Renato Bruni, and Gabriele Costantino. 2023. "Metabolite Profiling and Bioactivities of Leaves, Stems, and Flowers of Rumex usambarensis (Dammer) Dammer, a Traditional African Medicinal Plant" Plants 12, no. 3: 482. https://doi.org/10.3390/plants12030482
APA StyleSpaggiari, C., Righetti, L., Spadini, C., Annunziato, G., Nsanzurwimo, A., Cabassi, C. S., Bruni, R., & Costantino, G. (2023). Metabolite Profiling and Bioactivities of Leaves, Stems, and Flowers of Rumex usambarensis (Dammer) Dammer, a Traditional African Medicinal Plant. Plants, 12(3), 482. https://doi.org/10.3390/plants12030482





