Phenolic Compounds from Wild Plant and In Vitro Cultures of Ageratina pichichensis and Evaluation of Their Antioxidant Activity
Abstract
1. Introduction
2. Results and Discussion
2.1. Establishment of the In Vitro Cultures of A. pichichensis
2.2. Quantification of Phenolic Compounds
2.3. HPLC Analysis
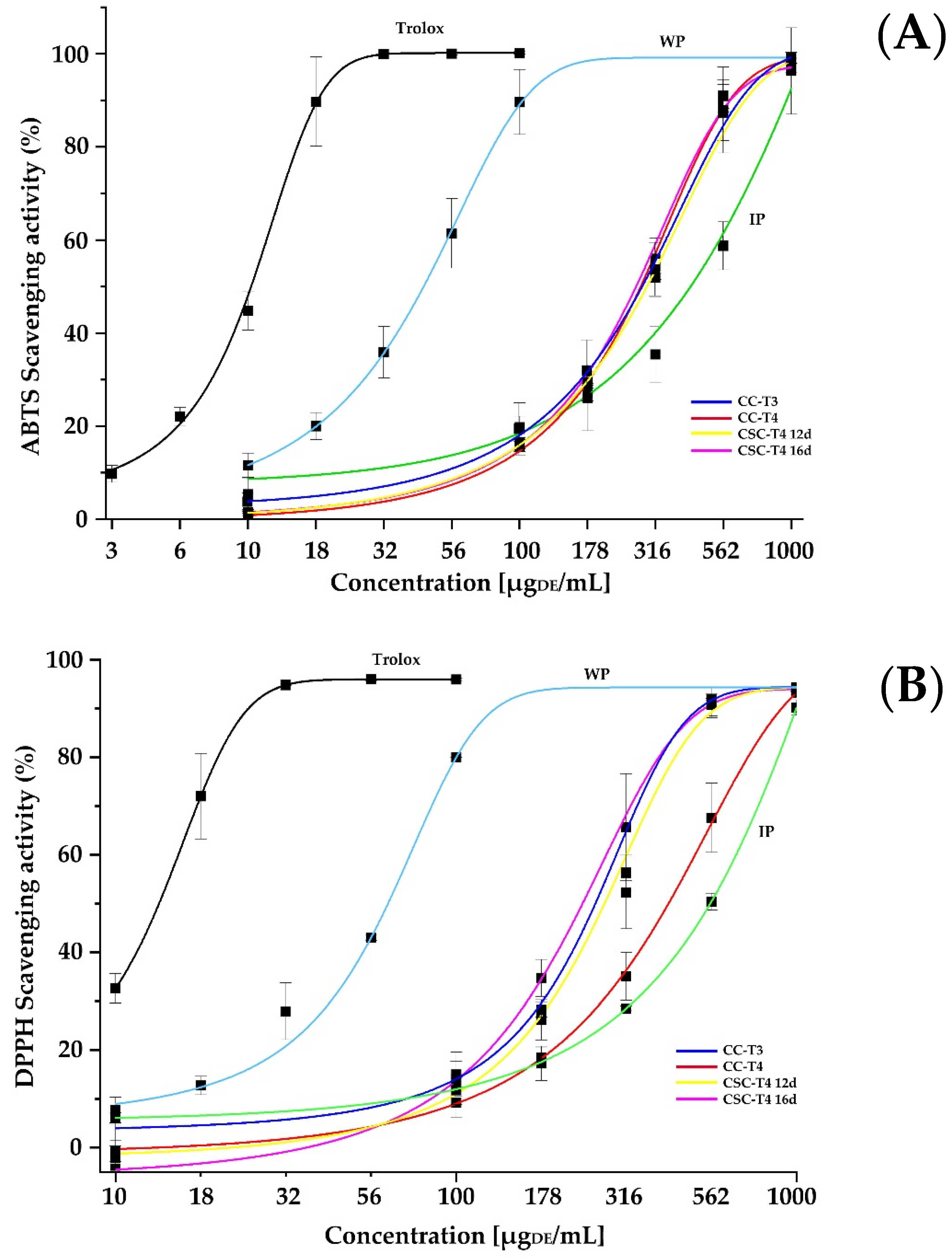
2.4. Antioxidant Activity
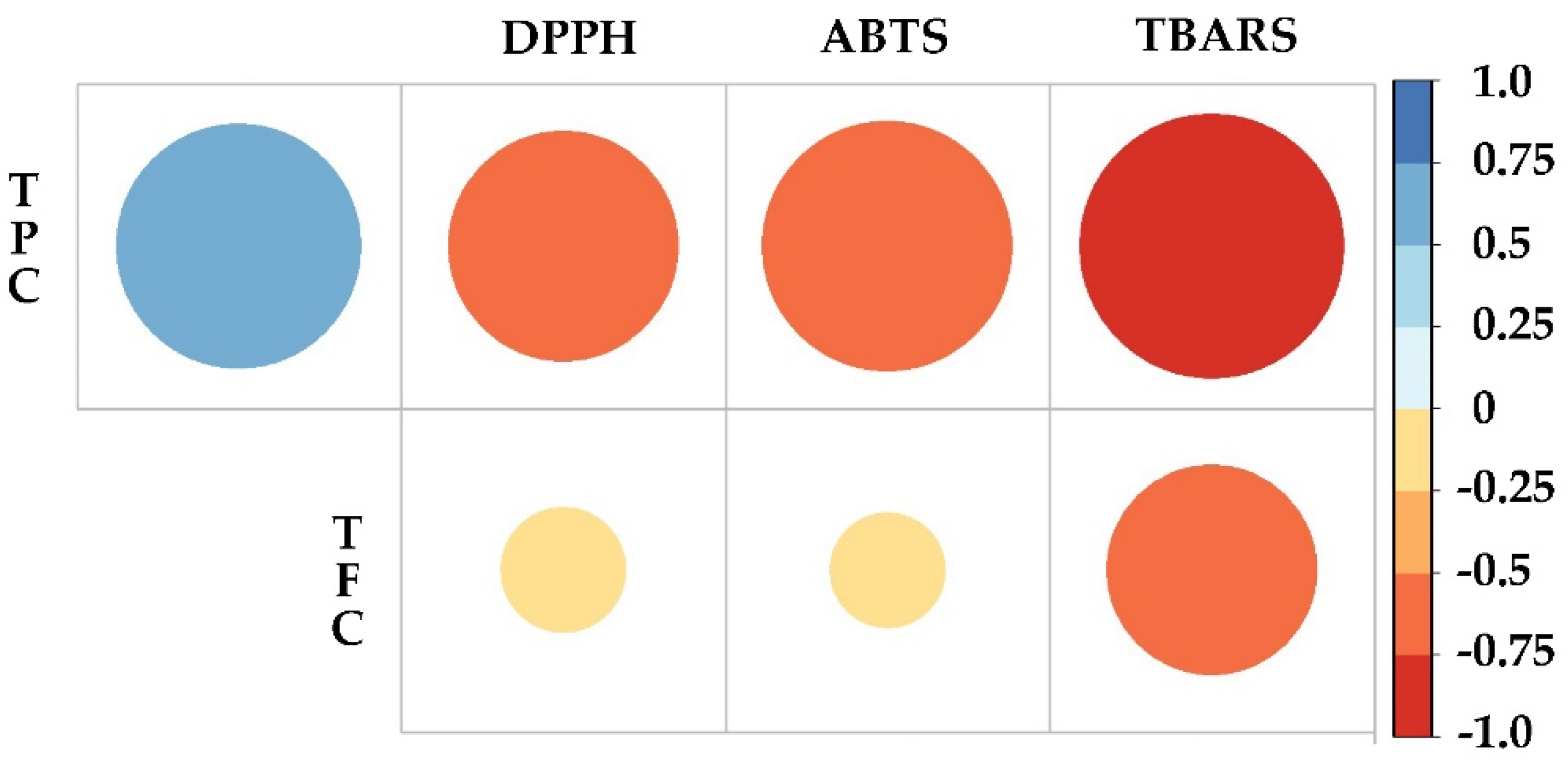
2.5. Correlation between Phenolic Content and Antioxidant Activity
3. Materials and Methods
3.1. Plant Material
3.2. In Vitro Cultures of A. pichichensis
3.2.1. Plant
3.2.2. Callus Induction
3.2.3. Cell Suspension Culture
3.3. Preparations of Extracts
3.4. Characterization of Extracts
3.4.1. Total Phenolic Content (TPC)
3.4.2. Total Flavonoid Content
3.5. High Performance Liquid Chromatography (HPLC)
3.6. Antioxidant Capacity Assays
3.6.1. DPPH Radical Scavenging Activity
3.6.2. ABTS Radical Scavenging Activity
3.6.3. Determination of TBARS
Animals
Rat Brain Homogenate Preparation
Induction of Lipid Peroxidation and Thiobarbituric Acid Reactive Substances (TBARS) Quantification
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Navarro García, V.M.; Gonzalez, A.; Fuentes, M.; Aviles, M.; Rios, M.Y.; Zepeda, G.; Rojas, M.G. Antifungal activities of nine traditional Mexican medicinal plants. J. Ethnopharmacol. 2003, 87, 85–88. [Google Scholar] [CrossRef]
- Sánchez-Mendoza, M.E.; Reyes-Trejo, B.; Sánchez-Gómez, P.; Rodríguez-Silverio, J.; Castillo-Henkel, C.; Cervantes-Cuevas, H.; Arrieta, J. Bioassay-guided isolation of an anti-ulcer chromene from Eupatorium aschenbornianum: Role of nitric oxide, prostaglandins and sulfydryls. Fitoterapia 2010, 81, 66–71. [Google Scholar] [CrossRef]
- Torres-Barajas, L.; Rojas-Vera, J.; Morales-Méndez, A.; Rojas-Fermín, L.; Lucena, M.; Buitrago, A. Chemical composition and evaluation of antibacterial activity of essential oils of Ageratina jahnii and Ageratina pichinchensis collected in Mérida, Venezuela. Boletín Latinoam. Caribe Plantas Med. Aromáticas 2013, 12, 92–98. [Google Scholar]
- Romero-Cerecero, O.; Zamilpa, A.; González-Cortazar, M.; Alonso-Cortés, D.; Jiménez-Ferrer, E.; Nicasio-Torres, P.; Aguilar-Santamaría, L.; Tortoriello, J. Pharmacological and chemical study to identify wound-healing active compounds in Ageratina pichinchensis. Planta Med. 2013, 79, 622–627. [Google Scholar] [CrossRef]
- Romero-Cerecero, O.; Zamilpa, A.; Díaz-García, E.R.; Tortoriello, J. Pharmacological effect of Ageratina pichinchensis on wound healing in diabetic rats and genotoxicity evaluation. J. Ethnopharmacol. 2014, 156, 222–227. [Google Scholar] [CrossRef]
- Romero-Cerecero, O.; Rojas, G.; Navarro, V.; Herrera-Arellano, A.; Zamilpa-Álvarez, A.; Tortoriello, J. Effectiveness and tolerability of a standardized extract from Ageratina pichinchensis on patients with tinea pedis: An explorative pilot study controlled with ketoconazole. Planta Med. 2006, 72, 1257–1261. [Google Scholar] [CrossRef]
- Romero-Cerecero, O.; Zamilpa, A.; Jiménez-Ferrer, J.E.; Rojas-Bribiesca, G.; Román-Ramos, R.; Tortoriello, J. Double-blind clinical trial for evaluating the effectiveness and tolerability of Ageratina pichinchensis extract on patients with mild to moderate onychomycosis. A comparative study with ciclopirox. Planta Med. 2008, 74, 1430–1435. [Google Scholar] [CrossRef]
- Romero-Cerecero, O.; Zamilpa-Álvarez, A.; Jiménez-Ferrer, E.; Tortoriello, J. Exploratory study on the effectiveness of a standardized extract from Ageratina pichinchensis in patients with chronic venous leg ulcers. Planta Med. 2012, 78, 304–310. [Google Scholar] [CrossRef]
- Romero-Cerecero, O.; Zamilpa, A.; Tortoriello, J. Effectiveness and tolerability of a standardized extract from Ageratina pichinchensis in patients with diabetic foot ulcer: A randomized, controlled pilot study. Planta Med. 2015, 81, 272–278. [Google Scholar] [CrossRef]
- Romero-Cerecero, O.; Islas-Garduño, A.L.; Zamilpa, A.; Tortoriello, J. Effectiveness of Ageratina pichinchensis Extract in Patients with Vulvovaginal Candidiasis. A Randomized, Double-Blind, and Controlled Pilot Study. Phyther. Res. 2017, 31, 885–890. [Google Scholar] [CrossRef]
- Romero-Cerecero, O.; Islas-Garduño, A.L.; Zamilpa, A.; Tortoriello, J. Effectiveness of an encecalin standardized extract of Ageratina pichinchensis on the treatment of onychomycosis in patients with diabetes mellitus. Phyther. Res. 2020, 34, 1678–1686. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Ramos, M.; Marquina-Bahena, S.; Alvarez, L.; Román-Guerrero, A.; Bernabé-Antonio, A.; Cruz-Sosa, F. Phytochemical, pharmacological, and biotechnological study of Ageratina pichinchensis: A native species of Mexico. Plants 2021, 10, 2225. [Google Scholar] [CrossRef] [PubMed]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Talukder, P.; Talapatra, S.; Ghoshal, N.; Sen Raychaudhuri, S. Antioxidant activity and high-performance liquid chromatographic analysis of phenolic compounds during in vitro callus culture of Plantago ovata Forsk. and effect of exogenous additives on accumulation of phenolic compounds. J. Sci. Food Agric. 2016, 96, 232–244. [Google Scholar] [CrossRef]
- Saeed, N.; Khan, M.R.; Shabbir, M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complement. Altern. Med. 2012, 12, 221. [Google Scholar] [CrossRef]
- Pereira Nunes, X.; Souza Silva, F.; Guedes da Almeida, J.R.S.; Tolentino de Lima, J.; de Araújo Ribeiro, L.A.; Quintans Júnior, L.J.; Barbosa-Filho, J.M. Biological Oxidations and Antioxidant Activity of Natural Products. In Phytochemicals as Nutraceuticals; IntechOpen: London, UK, 2012; pp. 1–22. ISBN 978-953-51-0203-8. [Google Scholar]
- Georgiev, M.I.; Eibl, R.; Zhong, J.J. Hosting the plant cells in vitro: Recent trends in bioreactors. Appl. Microbiol. Biotechnol. 2013, 97, 3787–3800. [Google Scholar] [CrossRef]
- Bhaskar, R.; Xavier, L.S.E.; Udayakumaran, G.; Kumar, D.S.; Venkatesh, R.; Nagella, P. Biotic elicitors: A boon for the in-vitro production of plant secondary metabolites. Plant Cell Tissue Organ Cult. 2021, 149, 7–24. [Google Scholar] [CrossRef]
- Castro, A.H.F.; Braga, K.D.Q.; de Sousa, F.M.; Coimbra, M.C.; Chagas, R.C.R. Callus induction and bioactive phenolic compounds production from Byrsonima verbascifolia (L.) DC. (Malpighiaceae). Rev. Cienc. Agron. 2016, 47, 143–151. [Google Scholar] [CrossRef]
- Mendoza, D.; Arias, J.P.; Cuaspud, O.; Arias, M. Phytochemical screening of callus and cell suspensions cultures of Thevetia peruviana. Braz. Arch. Biol. Technol. 2020, 63, 1–14. [Google Scholar] [CrossRef]
- Ruffoni, B.; Pistelli, L.; Bertoli, A.; Pistelli, L. Plant cell cultures: Bioreactors for industrial production. Adv. Exp. Med. Biol. 2010, 698, 203–221. [Google Scholar] [CrossRef]
- Motolinia-Alcántara, A.E.; Castillo-Araiza, C.O.; Rodríguez-Monroy, M.; Román-Guerrero, A.; Cruz-Sosa, F. Engineering Considerations to Produce Bioactive Compounds from Plant Cell Suspension Culture in Bioreactors. Plants 2022, 10, 2762. [Google Scholar] [CrossRef]
- Sánchez-Ramos, M.; Alvarez, L.; Romero-Estrada, A.; Bernabé-Antonio, A.; Marquina-Bahena, S.; Cruz-Sosa, F. Establishment of a cell suspension culture of Ageratina pichinchensis (Kunth) for the improved production of anti-inflammatory compounds. Plants 2020, 9, 1398. [Google Scholar] [CrossRef]
- Pandey, H.; Pandey, P.; Singh, S.; Gupta, R.; Banerjee, S. Production of anti-cancer triterpene (betulinic acid) from callus cultures of different Ocimum species and its elicitation. Protoplasma 2015, 252, 647–655. [Google Scholar] [CrossRef]
- Patel, S.R.; Joshi, A.G.; Pathak, A.R.; Shrivastava, N.; Sharma, S. Somatic embryogenesis in Leptadenia reticulata (Retz.) Wight and Arn along with assessment of shoot and callus cultures for HPTLC fingerprint and quantification of p-coumaric acid. Plant Cell Tissue Organ Cult. 2021, 145, 173–189. [Google Scholar] [CrossRef]
- Mariana, S.R.; Bahena, S.M.; Antonio, R.E.; Antonio, B.A.; Francisco, C.S.; Judith, G.C.; Juan, J.A.F.; Irene, P.A.; Alvarez, L. Establishment and phytochemical analysis of a callus culture from Ageratina pichinchensis (Asteraceae) and its anti-inflammatory activity. Molecules 2018, 23, 1258. [Google Scholar] [CrossRef]
- Patle, T.K.; Shrivas, K.; Kurrey, R.; Upadhyay, S.; Jangde, R.; Chauhan, R. Phytochemical screening and determination of phenolics and flavonoids in Dillenia pentagyna using UV–vis and FTIR spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 242, 118717. [Google Scholar] [CrossRef]
- Verma, N.; Shukla, S. Impact of various factors responsible for fluctuation in plant secondary metabolites. J. Appl. Res. Med. Aromat. Plants 2015, 2, 105–113. [Google Scholar] [CrossRef]
- Cicevan, R.; Sestras, A.F.; Plazas, M.; Boscaiu, M.; Vilanova, S.; Gramazio, P.; Vicente, O.; Prohens, J.; Sestras, R.E. Biological Traits and Genetic Relationships Amongst Cultivars of Three Species of Tagetes (Asteraceae). Plants 2022, 11, 760. [Google Scholar] [CrossRef]
- Nieto-Trujillo, A.; Buendía-González, L.; García-Morales, C.; Román-Guerrero, A.; Cruz-Sosa, F.; Estrada-Zúñiga, M.E. PHENOLIC COMPOUNDS AND PARTHENOLIDE PRODUCTION FROM in vitro CULTURES OF Tanacetum parthenium. Rev. Mex. Ing. Química 2017, 16, 371–383. [Google Scholar] [CrossRef]
- Giordano, A.; Morales-Tapia, P.; Moncada-Basualto, M.; Pozo-Martínez, J.; Olea-Azar, C.; Nesic, A.; Cabrera-Barjas, G. Polyphenolic Composition and Antioxidant Activity (ORAC, EPR and Cellular) of Different Extracts of Argylia radiata Vitroplants and Natural Roots. Molecules 2022, 27, 610. [Google Scholar] [CrossRef]
- Naranjo-Gómez, E.J.; Puertas-Mejía, M.A.; Mejía-Giraldo, J.C.; Amaya-Nieto, A.Z.; Atehortúa, L. Micropropagation of Baccharis antioquensis (Asteraceae) and photoinduction of polyphenols by UV radiation. Rev. Biol. Trop. 2018, 66, 754–764. [Google Scholar] [CrossRef]
- Mishra, M.K.; Pandey, S.; Niranjan, A.; Misra, P. Comparative analysis of phenolic compounds from wild and in vitro propagated plant Thalictrum foliolosum and antioxidant activity of various crude extracts. Chem. Pap. 2021, 75, 4873–4885. [Google Scholar] [CrossRef]
- Lugato, D.; Simão, M.J.; Garcia, R.; Mansur, E.; Pacheco, G. Determination of antioxidant activity and phenolic content of extracts from in vivo plants and in vitro materials of Passiflora alata Curtis. Plant Cell Tissue Organ Cult. 2014, 118, 339–346. [Google Scholar] [CrossRef]
- Gauchan, D.P.; Bhuju, S.; Lamichhane, J.; Shakya, R.; García-Gil, M.R. Establishment of Regenerative Callus, Cell Suspension System, and Molecular Characterization of Taxus wallichiana Zucc. For the In Vitro Production of Taxol. J. Appl. Pharm. Sci. 2021, 11, 022–034. [Google Scholar] [CrossRef]
- Coimbra, M.C.; Chagas, R.C.R.; Vilela, M.S.P.; Castro, A.H.F. Growth, morphology and bioactive phenolic compounds production in Pyrostegia venusta calli. Biocatal. Agric. Biotechnol. 2019, 18, 101036. [Google Scholar] [CrossRef]
- Arciniega-Carreón, I.Y.; Ramírez-Sotelo, M.G.; Ramos-Valdivia, A.C.; Salas, C.E.; Ortega, A.; Oliver-Salvador, C. Metabolites in cultured cells of Ibervillea sonorae (S. Watson) Greene display increased hypoglycemic activity compared to that seen in plant roots. Hortic. Environ. Biotechnol. 2020, 61, 1039–1049. [Google Scholar] [CrossRef]
- Coimbra, M.C.; Chagas, R.C.R.; Duarte-Almeida, J.M.; Castro, A.H.F. Influence of plant growth regulators and light on callus induction and bioactive phenolic compounds production in Pyrostegia venusta (Bignoniaceae). Indian J. Exp. Biol. 2017, 55, 584–590. [Google Scholar]
- Isah, T.; Umar, S.; Mujib, A.; Sharma, M.P.; Rajasekharan, P.E.; Zafar, N.; Frukh, A. Secondary metabolism of pharmaceuticals in the plant in vitro cultures: Strategies, approaches, and limitations to achieving higher yield. Plant Cell Tissue Organ Cult. 2018, 132, 239–265. [Google Scholar] [CrossRef]
- Ramezannezhad, R.; Aghdasi, M.; Fatemi, M. Enhanced production of cichoric acid in cell suspension culture of Echinacea purpurea by silver nanoparticle elicitation. Plant Cell Tissue Organ Cult. 2019, 139, 261–273. [Google Scholar] [CrossRef]
- Mendoza, D.; Cuaspud, O.; Arias, J.P.; Ruiz, O.; Arias, M. Effect of salicylic acid and methyl jasmonate in the production of phenolic compounds in plant cell suspension cultures of Thevetia peruviana. Biotechnol. Rep. 2018, 19, e00273. [Google Scholar] [CrossRef]
- Modarres, M.; Esmaeilzadeh Bahabadi, S.; Taghavizadeh Yazdi, M.E. Enhanced production of phenolic acids in cell suspension culture of Salvia leriifolia Benth. using growth regulators and sucrose. Cytotechnology 2018, 70, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Szopa, A.; Kubica, P.; Snoch, A.; Ekiert, H. High production of bioactive depsides in shoot and callus cultures of Aronia arbutifolia and Aronia × prunifolia. Acta Physiol. Plant. 2018, 40, 48. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Nasibi, F.; Kalantari, K.M.; Benakashani, F. Callogenesis optimization and cell suspension culture establishment of Dracocephalum polychaetum Bornm. and Dracocephalum kotschyi Boiss.: An in vitro approach for secondary metabolite production. South Afr. J. Bot. 2020, 132, 79–86. [Google Scholar] [CrossRef]
- Kikowska, M.; Thiem, B.; Szopa, A.; Ekiert, H. Accumulation of valuable secondary metabolites: Phenolic acids and flavonoids in different in vitro systems of shoot cultures of the endangered plant species—Eryngium alpinum L. Plant Cell Tissue Organ Cult. 2020, 141, 381–391. [Google Scholar] [CrossRef]
- Ali, M.; Abbasi, B.H. Ihsan-ul-haq. Production of commercially important secondary metabolites and antioxidant activity in cell suspension cultures of Artemisia absinthium L. Ind. Crop. Prod. 2013, 49, 400–406. [Google Scholar] [CrossRef]
- Mlcek, J.; Jurikova, T.; Skrovankova, S.; Sochor, J. Quercetin and its anti-allergic immune response. Molecules 2016, 21, 623. [Google Scholar] [CrossRef]
- Urbaniak, A.; Kujawski, J.; Czaja, K.; Szelag, M. Antioxidant properties of several caffeic acid derivatives: A theoretical study. Comptes Rendus Chim. 2017, 20, 1072–1082. [Google Scholar] [CrossRef]
- Zeng, N.; Hongbo, T.; Xu, Y.; Wu, M.; Wu, Y. Anticancer activity of caffeic acid n-butyl ester against A431 skin carcinoma cell line occurs via induction of apoptosis and inhibition of the mTOR/PI3K/AKT signaling pathway. Mol. Med. Rep. 2018, 17, 5652–5657. [Google Scholar] [CrossRef]
- Lima, V.N.; Oliveira-Tintino, C.D.M.; Santos, E.S.; Morais, L.P.; Tintino, S.R.; Freitas, T.S.; Geraldo, Y.S.; Pereira, R.L.S.; Cruz, R.P.; Menezes, I.R.A.; et al. Antimicrobial and enhancement of the antibiotic activity by phenolic compounds: Gallic acid, caffeic acid and pyrogallol. Microb. Pathog. 2016, 99, 56–61. [Google Scholar] [CrossRef]
- Liu, Y.L.; Hsu, C.C.; Huang, H.J.; Chang, C.J.; Sun, S.H.; Lin, A.M.Y. Gallic Acid Attenuated LPS-Induced Neuroinflammation: Protein Aggregation and Necroptosis. Mol. Neurobiol. 2020, 57, 96–104. [Google Scholar] [CrossRef]
- Esmaeili, A.K.; Taha, R.M.; Mohajer, S.; Banisalam, B. Antioxidant Activity and Total Phenolic and Flavonoid Content of Various Solvent Extracts from In Vivo and In Vitro Grown Trifolium pratense L. (Red Clover). Biomed Res. Int. 2015, 2015, 643285. [Google Scholar] [CrossRef]
- Martysiak-Żurowska, D.; Wenta, W. A comparison of ABTS and DPPH methods for assessing th total antioxidant capacity of human milk. Acta Sci. Pol. Technol. Aliment. 2012, 1, 83–89. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Costa, P.; Gonçalves, S.; Valentão, P.; Andrade, P.B.; Romano, A. Accumulation of phenolic compounds in in vitro cultures and wild plants of Lavandula viridis L’Hér and their antioxidant and anti-cholinesterase potential. Food Chem. Toxicol. 2013, 57, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 474–497. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Barreira, J.C.M.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P.; Pereira, J.A. Antioxidant activities of the extracts from chestnut flower, leaf, skins and fruit. Food Chem. 2008, 107, 1106–1113. [Google Scholar] [CrossRef]
- Ramirez-Lopez, L.M.; DeWitt, C.A.M. Analysis of phenolic compounds in commercial dried grape pomace by high-performance liquid chromatography electrospray ionization mass spectrometry. Food Sci. Nutr. 2014, 2, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, M.; Nieto, A.; Marin, J.C.; Keck, A.S.; Jeffery, E.; Céspedes, C.L. Antioxidant activities of extracts from Barkleyanthus salicifolius (Asteraceae) and Penstemon gentianoides (Scrophulariaceae). J. Agric. Food Chem. 2005, 53, 5889–5895. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Development and characterisation of carbon nanotube-reinforced polyurethane foams. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Rossato, J.I.; Ketzer, L.A.; Centurião, F.B.; Silva, S.J.N.; Lüdtke, D.S.; Zeni, G.; Braga, A.L.; Rubin, M.A.; Da Rocha, J.B.T. Antioxidant properties of new chalcogenides against lipid peroxidation in rat brain. Neurochem. Res. 2002, 27, 297–303. [Google Scholar] [CrossRef] [PubMed]
- LOWRY, O.H.; ROSEBROUGH, N.J.; FARR, A.L.; RANDALL, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.B.; Liu, F.; Wang, Z.T. Antioxidative activity of natural products from plants. Life Sci. 2000, 66, 709–723. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Esterbauer, H.; Cheeseman, K.H. Determination of aldehydic lipid peroxidation products: Malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990, 186, 407–421. [Google Scholar] [CrossRef] [PubMed]
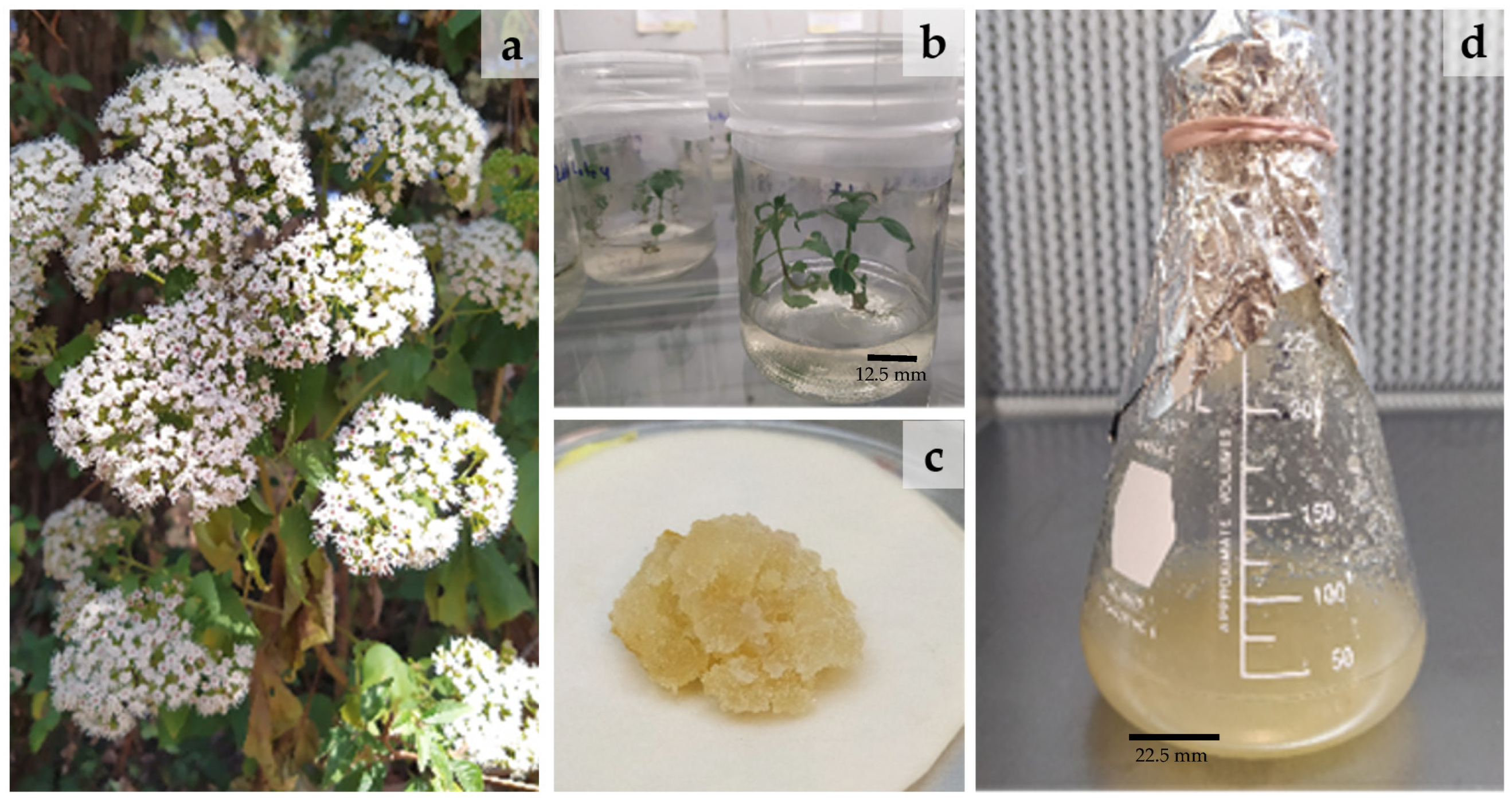



| Treatment | PGR | Callus Induction Response | Callus and Roots Induction Response | Callus and Plants Induction Response | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (μM) | (%) | Fresh Weight (g) | Dried Weight (g) | (%) | Fresh Weight (g) | Dried Weight (g) | (%) | Fresh Weight (g) | Dried Weight (g) | ||
| NAA | KIN | ||||||||||
| Control | 0 | 0 | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| T1 | 0.45 | 0.46 | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| T2 | 2.26 | 0.46 | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| T3 | 4.52 | 0.46 | 93.75 ± 12.50 a | 5.25 ± 1.69 a | 0.19 ± 0.06 a | ND | ND | ND | 37.50 ± 25.00 a | 6.72 ± 1.63 a | 0.23 ± 0.08 a |
| T4 | 6.79 | 0.46 | 81.25 ± 12.51 a | 6.42 ± 2.00 a | 0.24 ± 0.09 a | ND | ND | ND | 16.66 ± 14.43 b | 16.75 ± 3.17 b | 1.85 ± 0.73 b |
| T5 | 0.45 | 1.39 | 37.50 ± 14.43 b | 3.29 ± 0.76 b | 2.31 ± 0.3 b | ND | ND | ND | ND | ND | ND |
| T6 | 2.26 | 1.39 | 18.75 ± 12.50 c | 1.46 ± 0.24 c | 0.97 ± 0.15 c | ND | ND | ND | ND | ND | ND |
| T7 | 4.52 | 1.39 | 68.75 ± 12.50 b | 1.53 ± 0.18 c | 0.70 ± 0.08 d | 18.75 ± 12.52 | 3.83 ± 0.84 | 1.75 ± 0.83 | ND | ND | ND |
| T8 | 6.79 | 1.39 | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| T9 | 0.45 | 2.32 | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| T10 | 2.26 | 2.32 | 45.83 ± 29.46 b | 4.70 ± 1.43 b | 3.00 ± 0.48 b | ND | ND | ND | ND | ND | ND |
| T11 | 4.52 | 2.32 | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| T12 | 6.79 | 2.32 | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Treatment | TPC | TFC | ABTS | DPPH | TBARS |
|---|---|---|---|---|---|
| (mgGAE/gDW) | (mgQCT/gDW) | IC50 (µg DE/mL) | |||
| WP | 100.56 ± 6.83 bc | 77.26 ± 4.16 c | 45.09 ± 6.29 a | 60.24 ± 2.66 a | 27.85 ± 0.29 a |
| IP | 14.24 ± 1.71 e | 29.72 ± 3.78 d | 459.68 ± 59.32 c | 557.29 ± 16.49 d | 783.99 ± 28.06 e |
| CC-T3 | 122.12 ± 3.44 a | 209.93 ± 0.57 a | 284.77 ± 24.52 b | 242.58 ± 23.24 b | 260.49 ± 9.48 c |
| CC-T4 | 113.33 ± 4.46 ab | 155.60 ± 12.49 b | 282.81 ± 9.01 b | 422.67 ± 49.48 c | 362.72 ± 12.22 d |
| CSC-T4 12d | 91.89 ± 4.83 c | 196.93 ± 2.08 a | 282.2 ± 3.28 b | 229.96 ±30.81b | 157.01 ± 5.27 b |
| CSC-T4 16d | 61.47 ± 6.37 d | 88.5 ± 9.19 c | 285.04 ± 45.12 b | 276 ± 15.53 b | 234.31 ± 6.52 c |
| Treatment | Identified Phenolic Compounds | |||||
|---|---|---|---|---|---|---|
| µg GA/gDW | µg CAT/gDW | µg CfA/gDW | µg EPI/gDW | µg pCA/gDW | µg RUT/gDW | |
| WP | 1.75 a ± 0.06 | 2.58 a ± 0.08 | Ni | Ni | Ni | 3.36 ± 2.20 |
| IP | Ni | Ni | Ni | 34.24 a ± 6.18 | Ni | Ni |
| CC-T3 | 2.46 b ± 0.04 | Ni | 7.14 a ± 0.28 | 97.91 b ± 8.73 | 3.53 a ± 0.02 | Ni |
| CC-T4 | 3.92 c ± 0.09 | Ni | 8.11 b ± 0.03 | 91.17 b ± 4.99 | 1.53 b ± 0.14 | Ni |
| CSC-T4 12d | 0.57 d ± 0.04 | 9.97 b ± 1.33 | Ni | 145.10 c ± 3.36 | 2.45 c ± 0.07 | Ni |
| CSC-T4 16d | 1.17 e ± 0.04 | 6.56 c ± 1.16 | 14.73 c ± 0.51 | 195.87 d ± 3.77 | 5.06 d ± 0.31 | Ni |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motolinia-Alcántara, E.A.; Franco-Vásquez, A.M.; Nieto-Camacho, A.; Arreguín-Espinosa, R.; Rodríguez-Monroy, M.; Cruz-Sosa, F.; Román-Guerrero, A. Phenolic Compounds from Wild Plant and In Vitro Cultures of Ageratina pichichensis and Evaluation of Their Antioxidant Activity. Plants 2023, 12, 1107. https://doi.org/10.3390/plants12051107
Motolinia-Alcántara EA, Franco-Vásquez AM, Nieto-Camacho A, Arreguín-Espinosa R, Rodríguez-Monroy M, Cruz-Sosa F, Román-Guerrero A. Phenolic Compounds from Wild Plant and In Vitro Cultures of Ageratina pichichensis and Evaluation of Their Antioxidant Activity. Plants. 2023; 12(5):1107. https://doi.org/10.3390/plants12051107
Chicago/Turabian StyleMotolinia-Alcántara, Elizabeth Alejandra, Adrián Marcelo Franco-Vásquez, Antonio Nieto-Camacho, Roberto Arreguín-Espinosa, Mario Rodríguez-Monroy, Francisco Cruz-Sosa, and Angelica Román-Guerrero. 2023. "Phenolic Compounds from Wild Plant and In Vitro Cultures of Ageratina pichichensis and Evaluation of Their Antioxidant Activity" Plants 12, no. 5: 1107. https://doi.org/10.3390/plants12051107
APA StyleMotolinia-Alcántara, E. A., Franco-Vásquez, A. M., Nieto-Camacho, A., Arreguín-Espinosa, R., Rodríguez-Monroy, M., Cruz-Sosa, F., & Román-Guerrero, A. (2023). Phenolic Compounds from Wild Plant and In Vitro Cultures of Ageratina pichichensis and Evaluation of Their Antioxidant Activity. Plants, 12(5), 1107. https://doi.org/10.3390/plants12051107









