5-Aminolevulinic Acid Induces Chromium [Cr(VI)] Tolerance in Tomatoes by Alleviating Oxidative Damage and Protecting Photosystem II: A Mechanistic Approach
Abstract
1. Introduction
2. Results
2.1. Chromium and 5-ALA Treatments on Phenotypic Appearance of Tomato Seedlings
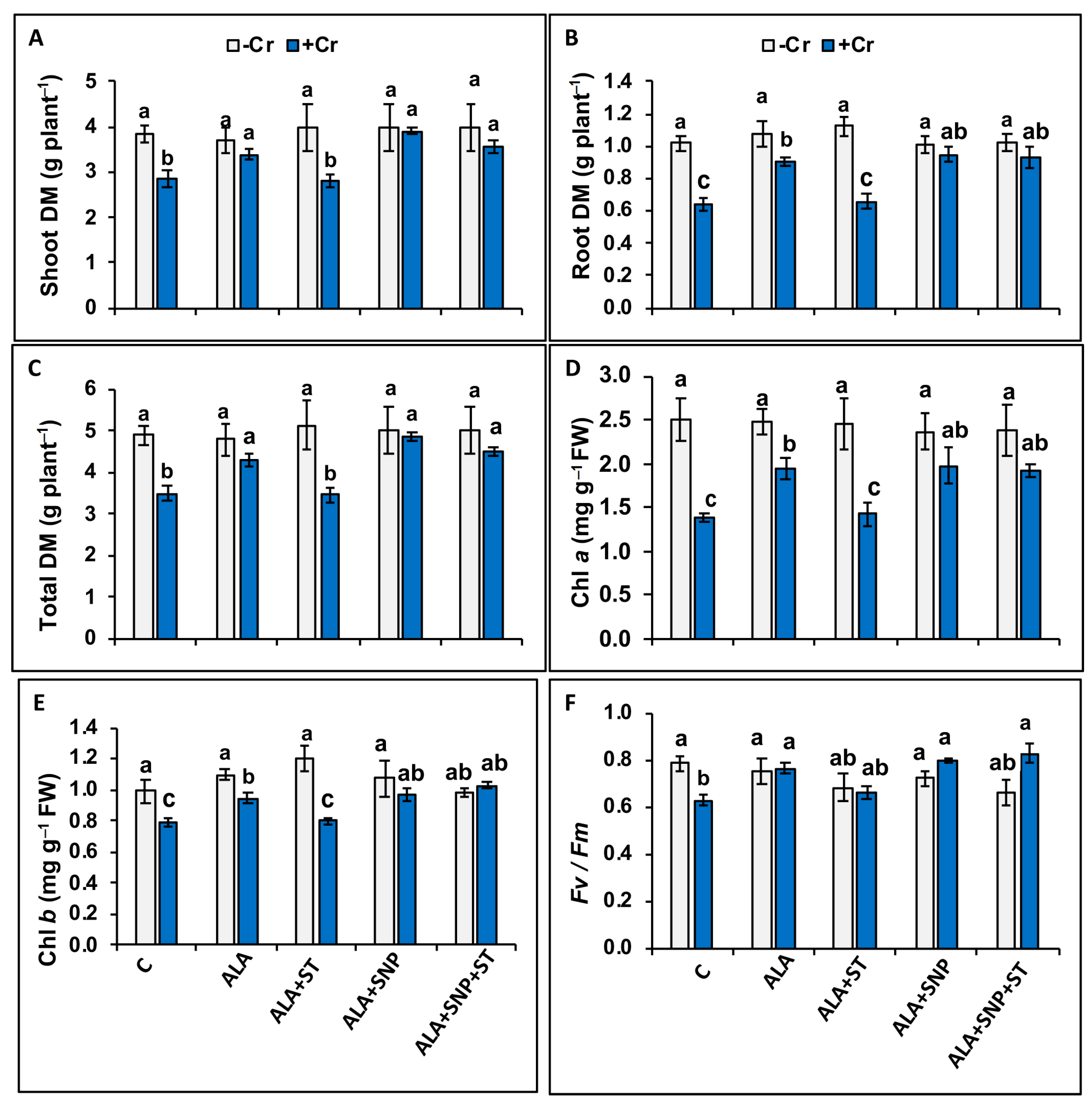
2.2. Chromium and 5-ALA Treatments on Tomato Biomass
2.3. Chromium and 5-ALA Treatments on Photosynthesis-Associated Traits
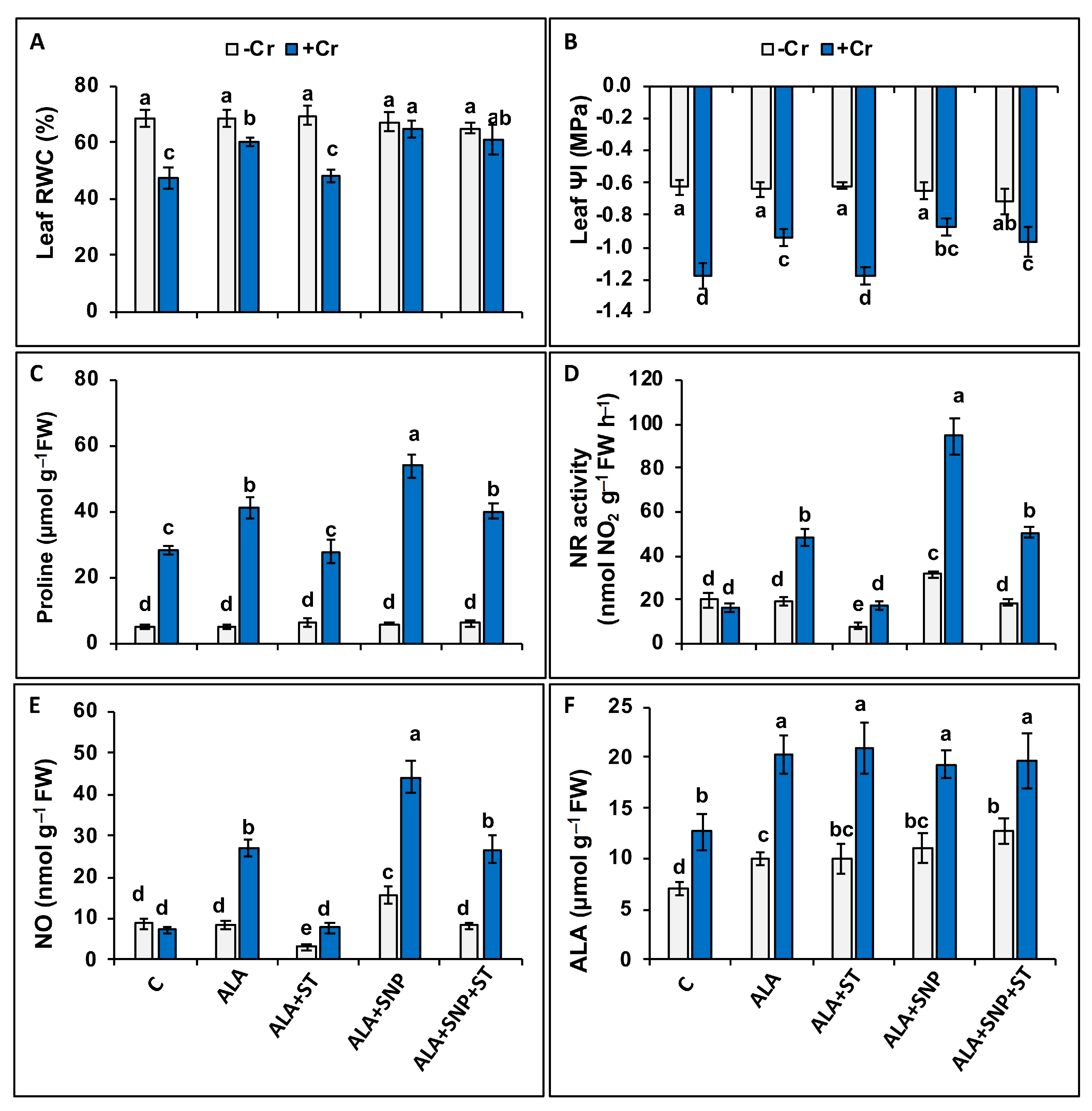
2.4. Chromium and 5-ALA Treatments on Leaf–Water Relations and Proline Content
2.5. Chromium and 5-ALA Treatments on Nitrate Reductase (NR) Activity and Nitric Oxide (NO) Synthesis
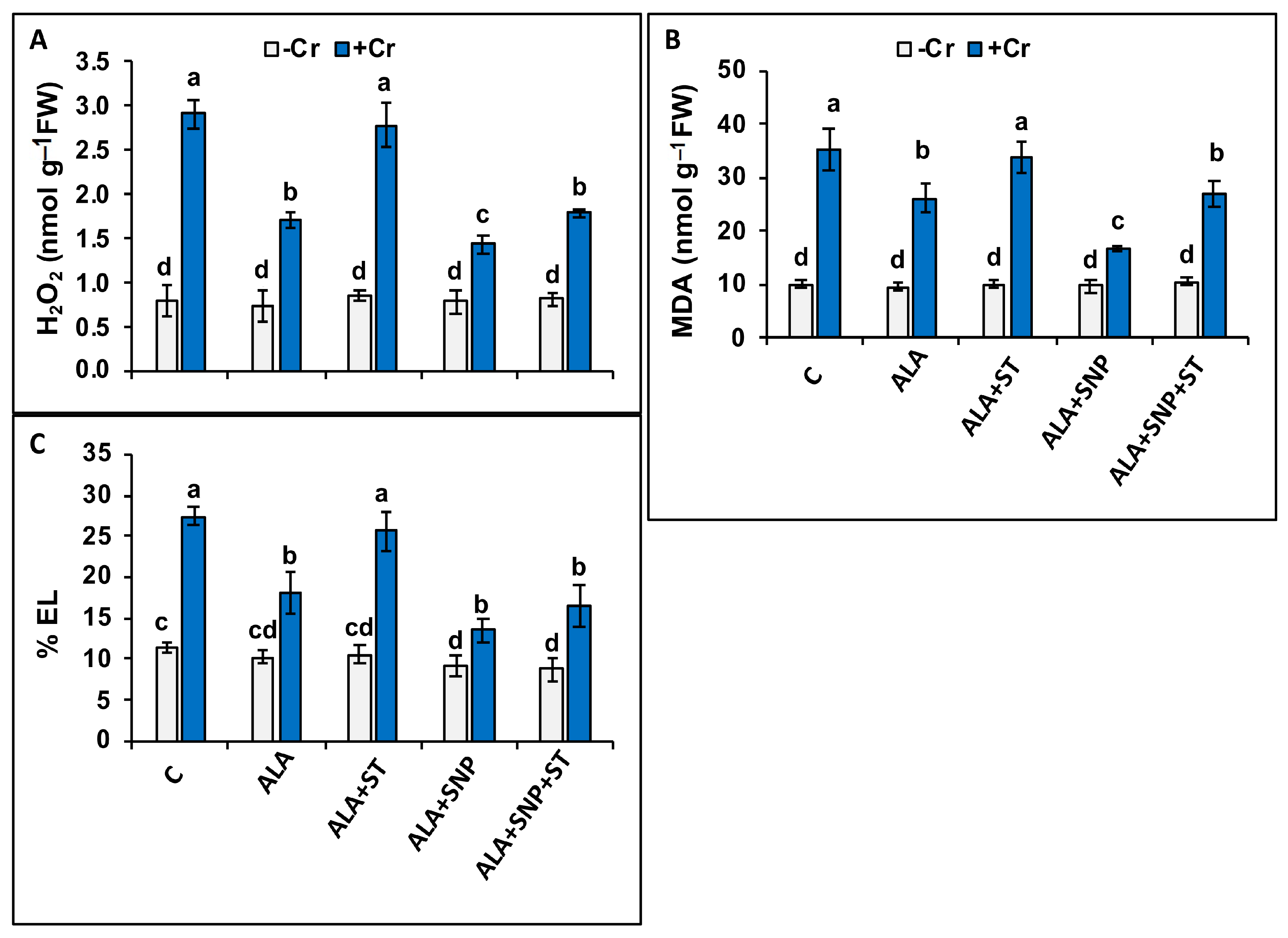
2.6. Chromium and 5-ALA Treatments on Oxidative Stress Markers
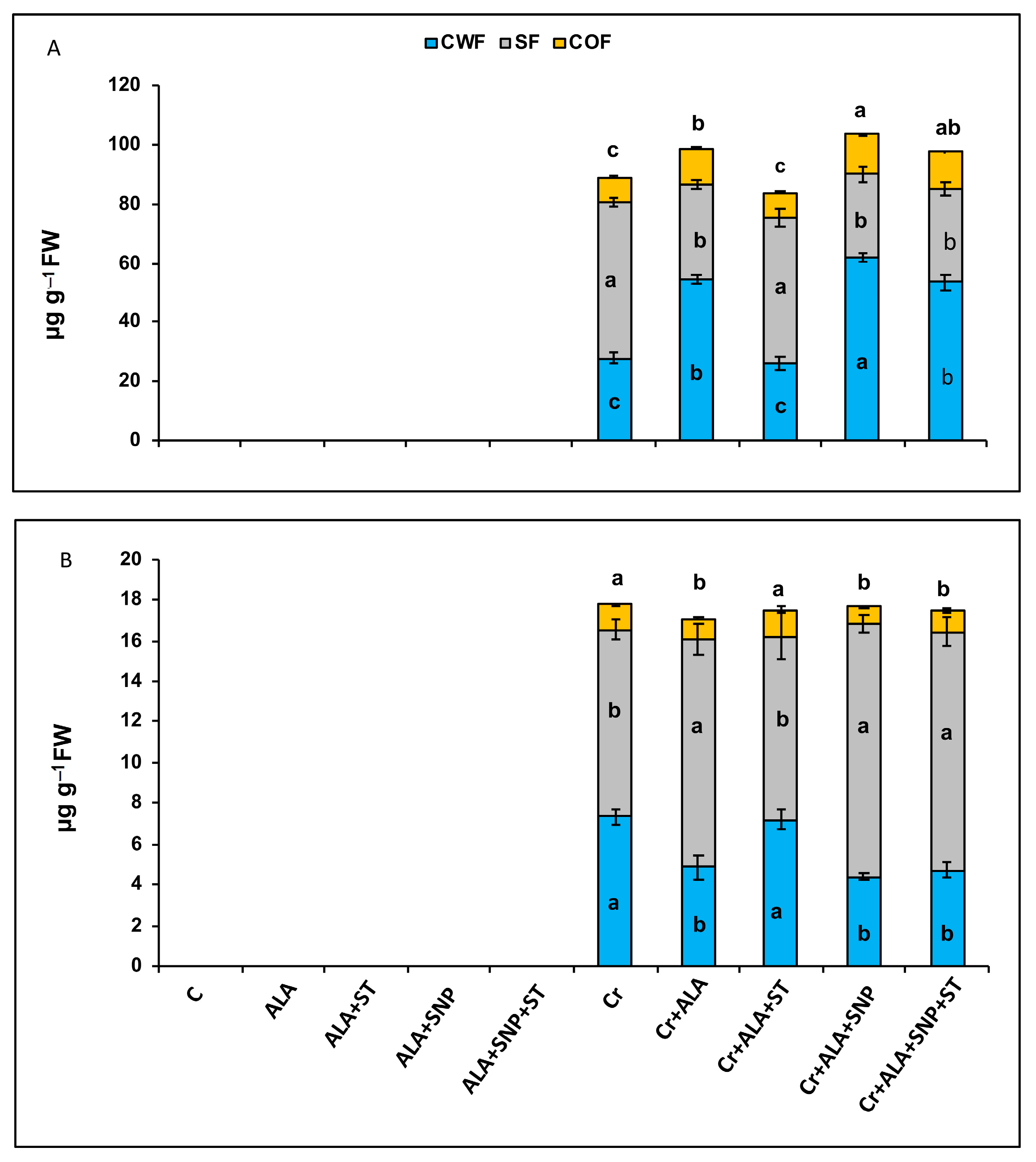
2.7. 5-ALA Regulated Subcellular Chromium Content in Tomatoes
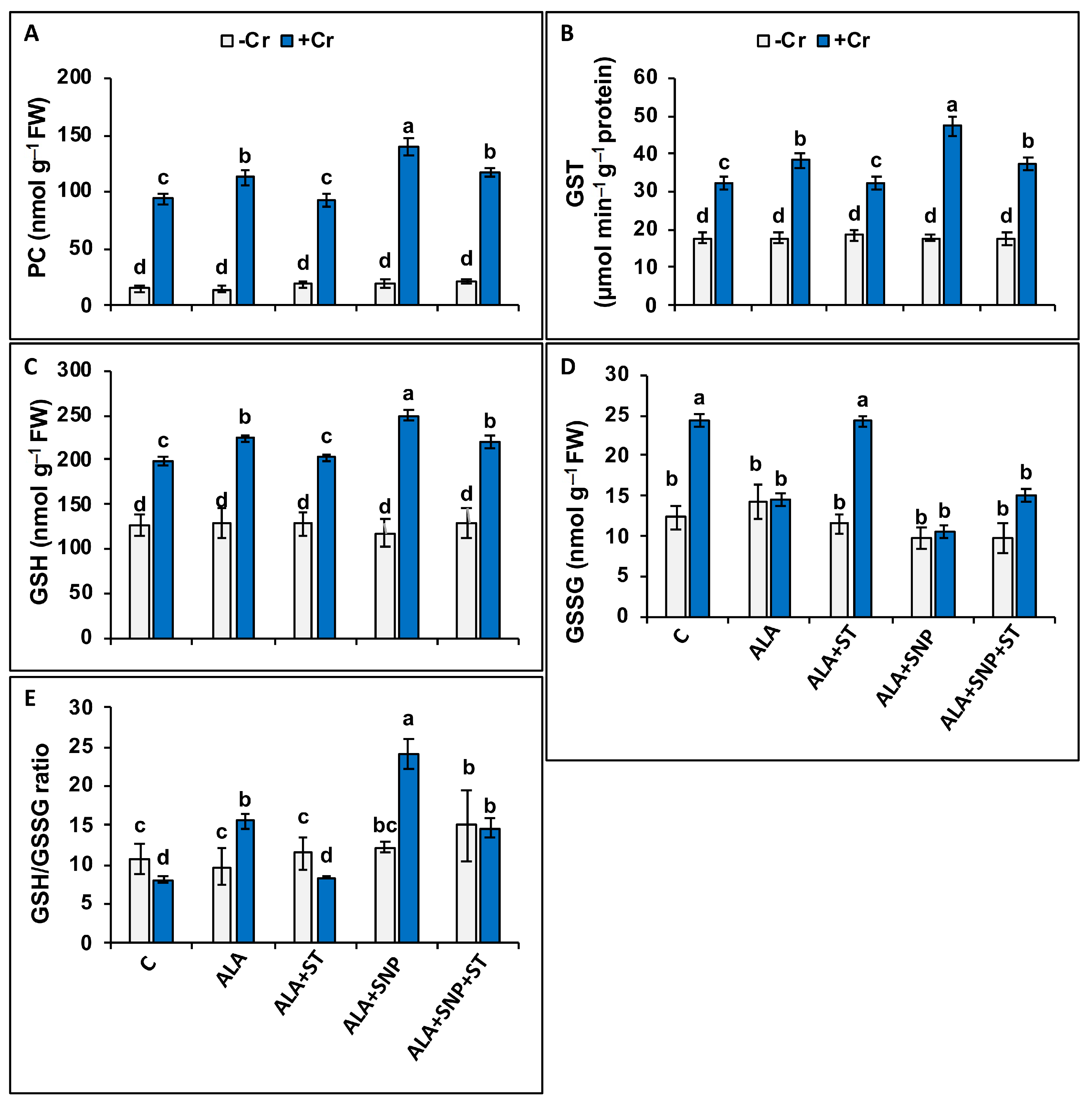
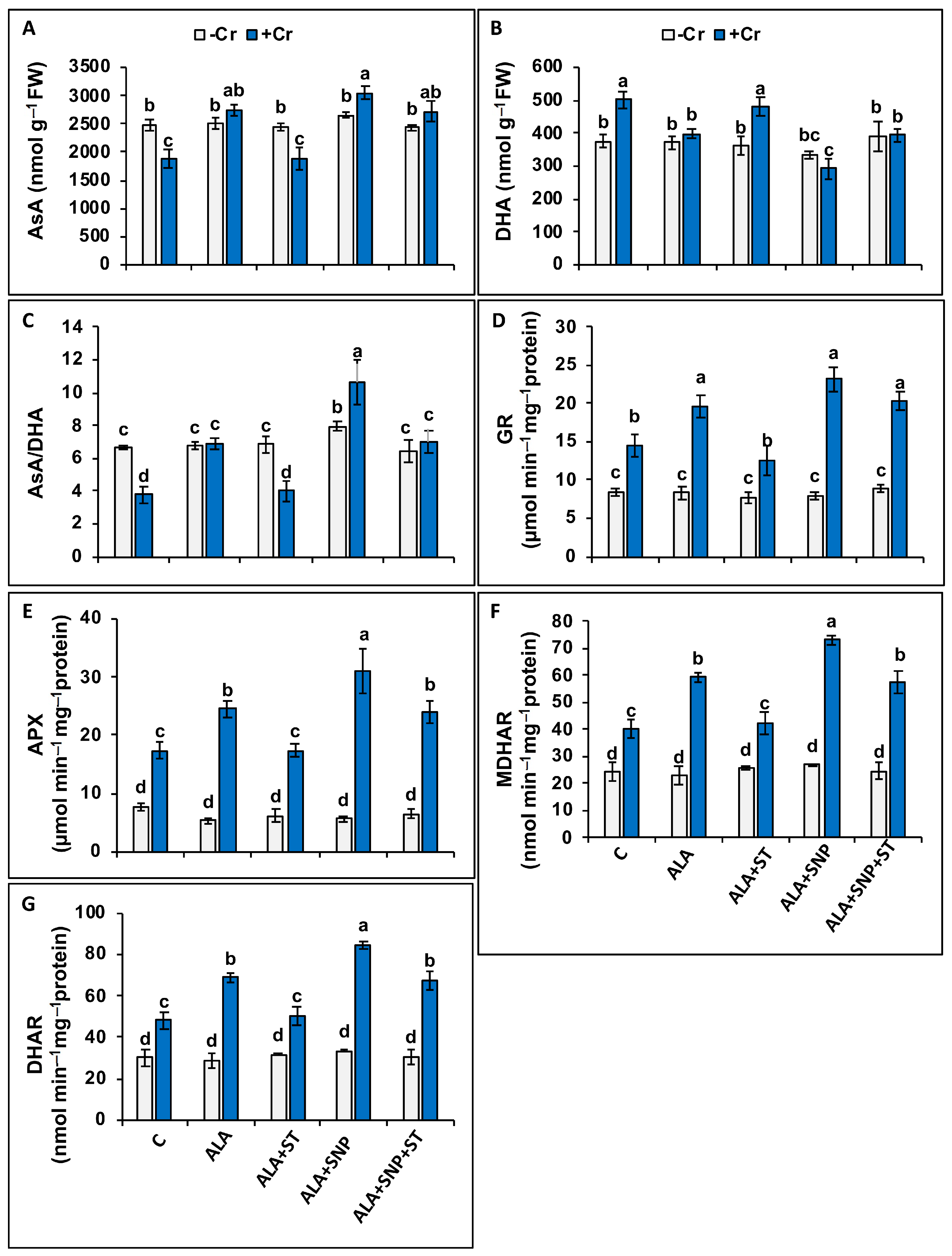
2.8. Phytochelatin, Glutathione, and Ascorbate Synthesis under Chromium and 5-ALA Treatments
2.9. Ascorbate-Glutathione Cycle Enzymes under Chromium and 5-ALA Treatments
3. Discussion
4. Materials and Methods
4.1. Plant Cultivation and Treatments
4.2. Estimation of Chlorophyll Content and Chlorophyll Fluorescence
4.3. Estimation of Relative Water Content (RWC) and Leaf Water Potential
4.4. Estimation of Leaf Proline
4.5. Estimation of NO Content
4.6. Quantification of Nitrate Reductase (NR)
4.7. Quantification of Chromium Distribution
4.8. Quantification of Leaf H2O2 and MDA
4.9. Electrolyte Leakage (EL)
4.10. Determination of 5-ALA
4.11. Determination of Ascorbic Acid and Glutathione
4.12. Extraction of Leaf Samples and Enzyme Assays
4.13. Statistical Treatment of the Data
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coetzee, J.J.; Bansal, N.; Chirwa, E.M. Chromium in environment, its toxic effect from chromite-mining and ferrochrome industries, and its possible bioremediation. Expo. Health 2020, 12, 51–62. [Google Scholar] [CrossRef]
- Prasad, S.; Yadav, K.K.; Kumar, S.; Gupta, N.; Cabral-Pinto, M.M.; Rezania, S.; Radwan, N. Chromium contamination and effect on environmental health and its remediation: A sustainable approaches. J. Environ. Manag. 2021, 285, 112174. [Google Scholar] [CrossRef]
- Ding, G.; Jin, Z.; Han, Y.; Sun, P.; Li, G.; Li, W. Mitigation of chromium toxicity in Arabidopsis thaliana by sulfur supplementation. Ecotoxicol. Environ. Saf. 2019, 182, 109379. [Google Scholar] [CrossRef]
- Sonone, S.S.; Jadhav, S.; Sankhla, M.S.; Kumar, R. Water contamination by heavy metals and their toxic effect on aquaculture and human health through food Chain. Lett. Appl. NanoBioScience 2020, 10, 2148–2166. [Google Scholar]
- Samrana, S.; Ali, A.; Muhammad, U.; Azizullah, A.; Ali, H.; Khan, M.; Naz, S.; Khan, M.D.; Zhu, S.; Chen, J. Physiological, ultrastructural, biochemical, and molecular responses of glandless cotton to hexavalent chromium (Cr6+) exposure. Environ. Pollut. 2020, 266, 115394. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kumar, S.; Panghal, V. Adsorption of chromium (Cr6+) on dead biomass of Salvinia molesta (Kariba weed) and Typha latifolia (broadleaf cattail): Isotherm, kinetic, and thermodynamic study. Appl. Water Sci. 2021, 11, 149. [Google Scholar] [CrossRef]
- Farouk, S.; Al-Amri, S. Ameliorative roles of melatonin and/or zeolite on chromium-induced leaf senescence in marjoram plants by activating antioxidant defense, osmolyte accumulation, and ultrastructural modification. Ind. Crops Prod. 2019, 142, 111823. [Google Scholar] [CrossRef]
- Goyal, D.; Yadav, A.; Prasad, M.; Singh, T.B.; Shrivastav, P.; Ali, A.; Dantu, P.K.; Mishra, S. Effect of heavy metals on plant growth: An overview. In Contaminants in Agriculture; Springer: Berlin/Heidelberg, Germany, 2020; pp. 79–101. [Google Scholar]
- Seleiman, M.F.; Ali, S.; Refay, Y.; Rizwan, M.; Alhammad, B.A.; El-Hendawy, S.E. Chromium resistant microbes and melatonin reduced Cr uptake and toxicity, improved physio-biochemical traits and yield of wheat in contaminated soil. Chemosphere 2020, 250, 126239. [Google Scholar] [CrossRef]
- Alamm, P.; Balawi, T.H.; Altalayan, F.H.; Hatamleh, A.A.; Ashraf, M.; Ahmad, P. Silicon attenuates the negative effects of chromium stress in tomato plants by modifying antioxidant enzyme activities, ascorbate–glutathione cycle and glyox5-ALAse system. Acta Physiol. Plant. 2021, 43, 110. [Google Scholar] [CrossRef]
- Kushwaha, B.K.; Singh, V.P. Mitigation of chromium (VI) toxicity by additional sulfur in some vegetable crops involves glutathione and hydrogen sulfide. Plant Physiol. Biochem. 2020, 155, 952–964. [Google Scholar] [CrossRef]
- Chaudhary, K.; Agarwal, S.; Khan, S. Role of phytochelatins (PCs), metallothioneins (MTs), and heavy metal ATPase (HMA) genes in heavy metal tolerance. In Mycoremediation and Environmental Sustainability; Springer: Berlin/Heidelberg, Germany, 2018; pp. 39–60. [Google Scholar]
- Al-Huqail, A.A.; Ali, H.M.; Kushwaha, B.K.; Al-Huqail, A.A.; Singh, V.P.; Siddiqui, M.H. Ascorbic acid is essential for inducing chromium (VI) toxicity tolerance in tomato roots. J. Biotechnol. 2020, 322, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Elansary, H.O.; El-Ansary, D.O.; Al-Mana, F.A. 5-aminolevulinic acid and soil fertility enhance the resistance of rosemary to Alternaria dauci and Rhizoctonia solani and modulate plant biochemistry. Plants 2019, 8, 585. [Google Scholar] [CrossRef]
- Tan, S.; Cao, J.; Xia, X.; Li, Z. Advances in 5-aminolevulinic acid priming to enhance plant tolerance to abiotic stress. Int. J. Mol. Sci. 2022, 23, 702. [Google Scholar] [CrossRef] [PubMed]
- Beale, S.I. Biosynthesis of the tetrapyrrole pigment precursor, δ-aminolevulinic acid, from glutamate. Plant Physiol. 1990, 93, 1273–1279. [Google Scholar] [CrossRef] [PubMed]
- El-Amier, Y.; Elhindi, K.; El-Hendawy, S.; Al-Rashed, S.; Abd-ElGawad, A. Antioxidant system and biomolecules alteration in Pisum sativum under heavy metal stress and possible alleviation by 5-aminolevulinic acid. Molecules 2019, 24, 4194. [Google Scholar] [CrossRef] [PubMed]
- Averina, N.; Yemelyanava, H.; Sherbakov, R.; Kozel, N.; Obukhovskaya, L.; Usatov, A. Photosynthesis and oxygen uptake rate in winter rape plants treated with 5-aminolevulinic acid. Russ. J. Plant Physiol. 2019, 66, 966–975. [Google Scholar] [CrossRef]
- Wu, Y.; Jin, X.; Liao, W.; Hu, L.; Dawuda, M.M.; Zhao, X.; Tang, Z.; Gong, T.; Yu, J. 5-Aminolevulinic acid (5-ALA) alleviated salinity stress in cucumber seedlings by enhancing chlorophyll synthesis pathway. Front. Plant Sci. 2018, 9, 635. [Google Scholar] [CrossRef]
- Anwar, A.; Wang, J.; Yu, X.; He, C.; Li, Y. Substrate application of 5-aminolevulinic acid enhanced low-temperature and weak-light stress tolerance in cucumber (Cucumis sativus L.). Agronomy 2020, 10, 472. [Google Scholar] [CrossRef]
- Cai, C.; He, S.; An, Y.; Wang, L. Exogenous 5-aminolevulinic acid improves strawberry tolerance to osmotic stress and its possible mechanisms. Physiol. Plant. 2020, 168, 948–962. [Google Scholar] [CrossRef]
- He, S.; An, Y.; Yang, H.; Cao, R.; Tang, Q.; Wang, L. 5-Aminolevulinic acid-induced salt tolerance in strawberry (cv. ‘Benihope’): Possible role of nitric oxide on interception of salt ions in roots. Sci. Hortic. 2022, 304, 111294. [Google Scholar] [CrossRef]
- Habiba, U.; Ali, S.; Rizwan, M.; Hussain, M.B.; Hussain, A.; 5-ALAm, P.; Alqarawi, A.A.; Hashem, A.; AbdAllah, E.F. The ameliorative role of 5-aminolevulinic acid (5-ALA) under Cr stress in two maize cultivars showing differential sensitivity to Cr stress tolerance. J. Plant Growth Regul. 2019, 38, 788–798. [Google Scholar] [CrossRef]
- Besson-Bard, A.; Pugin, A.; Wendehenne, D. New insights into nitric oxide signaling in plants. Annu. Rev. Plant Biol. 2008, 59, 21–39. [Google Scholar] [CrossRef] [PubMed]
- Tewari, R.K.; Horemans, N.; Watanabe, M. Evidence for a role of nitric oxide in iron homeostasis in plants. J. Exp. Bot. 2021, 72, 990–1006. [Google Scholar] [CrossRef] [PubMed]
- Beligni, M.V.; Fath, A.; Bethke, P.C.; Lamattina, L.; Jones, R.L. Nitric oxide acts as an antioxidant and delays programmed cell death in barley aleurone layers. Plant Physiol. 2002, 129, 1642–1650. [Google Scholar] [CrossRef] [PubMed]
- Sang, J.; Jiang, M.; Lin, F.; Xu, S.; Zhang, A.; Tan, M. Nitric oxide reduces hydrogen peroxide accumulation involved in water stress-induced subcellular anti-oxidant defense in maize plants. J. Integr. Plant Biol. 2008, 50, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Shah, F.; Ali, F.; Yun, B.W. Role of nitric oxide in plant senescence. Front. Plant Sci. 2022, 13, 851631. [Google Scholar] [CrossRef]
- Foyer, C.H. Reactive oxygen species, oxidative signaling and the regulation of photosynthesis. Environ. Exp. Bot. 2018, 154, 134–142. [Google Scholar] [CrossRef]
- Moustakas, M. Plant photochemistry, reactive oxygen species, and photoprotection. Photochem 2022, 2, 5–8. [Google Scholar] [CrossRef]
- Kataria, S.; Jain, M.; Tripathi, D.K.; Singh, V.P. Involvement of nitrate reductase-dependent nitric oxide production in magnetopriming-induced salt tolerance in soybean. Physiol. Plant. 2020, 168, 422–436. [Google Scholar] [CrossRef]
- Liang, Y.; Zheng, P.; Li, S.; Li, K.-z.; Xu, H.-n. Nitrate reductase-dependent NO production is involved in H2S-induced nitrate stress tolerance in tomato via activation of antioxidant enzymes. Sci. Hortic. 2018, 229, 207–214. [Google Scholar] [CrossRef]
- Kataria, S.; Anand, A.; Raipuria, R.K.; Kumar, S.; Jain, M.; Watts, A.; Brestic, M. Magnetopriming actuates nitric oxide synthesis to regulate phytohormones for ımproving germination of soybean seeds under salt stress. Cells 2022, 11, 2174. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, C.; Li, C.; Zhao, Z.; Wei, L.; Liu, Z.; Bozhkov, P. Nitric oxide is involved in hydrogen sulfide-induced adventitious rooting in tomato (Solanum lycopersicum). Funct. Plant Biol. 2022, 49, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Mondal, S.; Karmakar, S. Phytohormones and nitric oxide cross-talk in regulation of stress tolerance in plants. In Nitric Oxide in Plants: A Molecule with Dual Roles, 1st ed.; Ahanger, M.A., Ahmad, P., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2022; pp. 179–200. [Google Scholar]
- Dhal, S.; Pal, H. Role of nitric oxide in fruit ripening. In Nitric Oxide in Plant Biology. An Ancient Molecule with Emerging Roles, 1st ed.; Singh, V.P., Singh, S., Tripathi, D., Romero-Puertas, M., Sandalio, L., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 707–752. [Google Scholar]
- Bhat, J.A.; Ahmad, P.; Corpas, F.J. Main nitric oxide (NO) hallmarks to relieve arsenic stress in higher plants. J. Hazard. Mater. 2021, 406, 124289. [Google Scholar] [CrossRef] [PubMed]
- Kaya, C.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. Responses of nitric oxide and hydrogen sulfide in regulating oxidative defence system in wheat plants grown under cadmium stress. Physiol. Plant. 2020, 168, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Oku, H.; Nahar, K.; Bhuyan, M.; Mahmud, J.A.; Baluska, F.; Fujita, M. Nitric oxide-induced salt stress tolerance in plants: ROS metabolism, signaling, and molecular interactions. Plant Biotechnol. Rep. 2018, 12, 77–92. [Google Scholar] [CrossRef]
- Lau, S.-E.; Hamdan, M.F.; Pua, T.-L.; Saidi, N.B.; Tan, B.C. Plant nitric oxide signaling under drought stress. Plants 2021, 10, 360. [Google Scholar] [CrossRef]
- Saif, S.; Khan, M.S. Assessment of toxic impact of metals on proline, antioxidant enzymes, and biological characteristics of Pseudomonas aeruginosa inoculated Cicer arietinum grown in chromium and nickel-stressed sandy clay loam soils. Environ. Monit. Assess. 2018, 190, 1–18. [Google Scholar] [CrossRef]
- Mohammadi, H.; Amani-Ghadim, A.R.; Matin, A.A.; Ghorbanpour, M. Fe0 nanoparticles improve physiological and antioxidative attributes of sunflower (Helianthus annuus) plants grown in soil spiked with hexavalent chromium. 3 Biotech 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Naz, R.; Sarfraz, A.; Anwar, Z.; Yasmin, H.; Nosheen, A.; Keyani, R.; Roberts, T.H. Combined ability of salicylic acid and spermidine to mitigate the individual and interactive effects of drought and chromium stress in maize (Zea mays L.). Plant Physiol. Biochem. 2021, 159, 285–300. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Rasheed, R.; Hussain, I.; Iqbal, M.; Farooq, M.U.; Saleem, M.H.; Ali, S. Taurine modulates dynamics of oxidative defense, secondary metabolism, and nutrient relation to mitigate boron and chromium toxicity in Triticum aestivum L. plants. Environ. Sci. Pollut. Res. 2022, 29, 45527–45548. [Google Scholar] [CrossRef]
- Bashir, M.A.; Wang, X.; Naveed, M.; Mustafa, A.; Ashraf, S.; Samreen, T.; Nadeem, S.M.; Jamil, M. Biochar mediated-alleviation of chromium stress and growth improvement of different maize cultivars in tannery polluted soils. Int. J. Environ. Res. Public Health 2021, 18, 4461. [Google Scholar] [CrossRef] [PubMed]
- Farid, M.; Ali, S.; Saeed, R.; Rizwan, M.; Bukhari, S.A.H.; Abbasi, G.H.; Hussain, A.; Ali, B.; Zamir, M.S.I.; Ahmad, I. Combined application of citric acid and 5-aminolevulinic acid improved biomass, photosynthesis and gas exchange attributes of sunflower (Helianthus annuus L.) grown on chromium contaminated soil. Int. J. Phytoremediation 2019, 21, 760–767. [Google Scholar] [CrossRef]
- Farid, M.; Ali, S.; Rizwan, M.; Yasmeen, T.; Arif, M.S.; Riaz, M.; Saqib, M.; Ayub, M.A. Combined effects of citric acid and 5-aminolevulinic acid in mitigating chromium toxicity in sunflower (Helianthus annuus L.) grown in Cr spiked soil. Pak. J. Agric. Sci. 2020, 57, 477–488. [Google Scholar]
- Alamri, S.; Ali, H.M.; Khan, M.I.R.; Singh, V.P.; Siddiqui, M.H. Exogenous nitric oxide requires endogenous hydrogen sulfide to induce the resilience through sulfur assimilation in tomato seedlings under hexavalent chromium toxicity. Plant Physiol. Biochem. 2020, 155, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Kharbech, O.; Sakouhi, L.; Mahjoubi, Y.; Massoud, M.B.; Debez, A.; Zribi, O.T.; Djebali, W.; Chaoui, A.; Mur, L.A.J. Nitric oxide donor, sodium nitroprusside modulates hydrogen sulfide metabolism and cysteine homeostasis to aid the alleviation of chromium toxicity in maize seedlings (Zea mays L.). J. Hazard. Mater. 2022, 424, 127302. [Google Scholar] [CrossRef]
- Prakash, V.; Rai, P.; Sharma, N.C.; Singh, V.P.; Tripathi, D.K.; Sharma, S.; Sahi, S. Application of zinc oxide nanoparticles as fertilizer boosts growth in rice plant and alleviates chromium stress by regulating genes involved in regulating oxidative stress. Chemosphere 2022, 303, 134554. [Google Scholar] [CrossRef]
- Singh, S.; Husain, T.; Suhel, M.; Prasad, S.; Singh, V. Hydrogen sulphide ameliorates hexavalent chromium toxicity in two cereal crops: Role of antioxidant enzymes and proline metabolism. Plant Biol. 2022, 24, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Moustakas, M.; Sperdouli, I.; Kouna, T.; Antonopoulou, C.I.; Therios, I. Exogenous proline induces soluble sugar accumulation and alleviates drought stress effects on photosystem II functioning of Arabidopsis thaliana leaves. Plant Growth Regul. 2011, 65, 315–325. [Google Scholar] [CrossRef]
- Ghosh, U.; Islam, M.; Siddiqui, M.; Cao, X.; Khan, M. Proline, a multifaceted signalling molecule in plant responses to abiotic stress: Understanding the physiological mechanisms. Plant Biol. 2022, 24, 227–239. [Google Scholar] [CrossRef]
- Xiong, J.-L.; Wang, H.-C.; Tan, X.-Y.; Zhang, C.-L.; Naeem, M.S. 5-aminolevulinic acid improves salt tolerance mediated by regulation of tetrapyrrole and proline metabolism in Brassica napus L. seedlings under NaCl stress. Plant Physiol. Biochem. 2018, 124, 88–99. [Google Scholar] [CrossRef]
- Rasheed, R.; Yasmeen, H.; Hussain, I.; Iqbal, M.; Ashraf, M.A.; Parveen, A. Exogenously applied 5-aminolevulinic acid modulates growth, secondary metabolism and oxidative defense in sunflower under water deficit stress. Physiol. Mol. Biol. Plants 2020, 26, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.N.; 5-ALAmri, S.; Al-Amri, A.A.; Alsubaie, Q.D.; Al-Munqedi, B.; Ali, H.M.; Singh, V.P.; Siddiqui, M.H. Effect of nitric oxide on seed germination and seedling development of tomato under chromium toxicity. J. Plant Growth Regul. 2021, 40, 2358–2370. [Google Scholar] [CrossRef]
- Singh, S.; Prasad, S.M.; Singh, V.P. Additional calcium and sulfur manages hexavalent chromium toxicity in Solanum lycopersicum L. and Solanum melongena L. seedlings by involving nitric oxide. J. Hazard. Mater. 2020, 398, 122607. [Google Scholar] [CrossRef] [PubMed]
- Okant, M.; Kaya, C. The role of endogenous nitric oxide in melatonin-improved tolerance to lead toxicity in maize plants. Environ. Sci. Pollut. Res. 2019, 26, 11864–11874. [Google Scholar] [CrossRef]
- Kaya, C.; Ashraf, M. Nitric oxide is required for aminolevulinic acid-induced salt tolerance by lowering oxidative stress in maize (Zea mays). J. Plant Growth Regul. 2021, 40, 617–627. [Google Scholar] [CrossRef]
- Rhaman, M.S.; Imran, S.; Karim, M.M.; Chakrobortty, J.; Mahamud, M.A.; Sarker, P.; Tahjib-Ul-Arif, M.; Robin, A.H.K.; Ye, W.; Murata, Y.; et al. 5-aminolevulinic acid-mediated plant adaptive responses to abiotic stress. Plant Cell Rep. 2021, 40, 1451–1469. [Google Scholar] [CrossRef]
- Chen, G.; Fan, P.; Feng, W.; Guan, A.; Lu, Y.; Wan, Y. Effects of 5-aminolevulinic acid on nitrogen metabolism and ion distribution of watermelon seedlings under salt stress. Russ. J. Plant Physiol. 2017, 64, 116–123. [Google Scholar] [CrossRef]
- Gupta, D.; Prasad, S.M. 5-aminolevulinic acid (5-ALA) regulates photosynthetic performance and nitrogen metabolism status in UV-B challenged Cajanus cajan L. seedlings. J. Plant Biochem. Biotechnol. 2022, 31, 250–270. [Google Scholar] [CrossRef]
- Ghorbani, A.; Pishkar, L.; Roodbari, N.; Tavakoli, S.A.; Jahromi, E.M.; Wu, C. Nitrate reductase is needed for methyl jasmonate-mediated arsenic toxicity tolerance of rice by modulating the antioxidant defense system, glyox5-ALAse system and arsenic sequestration mechanism. J. Plant Growth Regul. 2022. [Google Scholar] [CrossRef]
- Kaya, C. Nitrate reductase is required for salicylic acid-induced water stress tolerance of pepper by upraising the AsA-GSH pathway and glyox5-ALAse system. Physiol. Plant. 2021, 172, 351–370. [Google Scholar] [CrossRef]
- Ahmad, R.; Ali, S.; Rizwan, M.; Dawood, M.; Farid, M.; Hussain, A.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P. Hydrogen sulfide alleviates chromium stress on cauliflower by restricting its uptake and enhancing antioxidative system. Physiol. Plant. 2020, 168, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, B.K.; Ali, H.M.; Siddiqui, M.H.; Singh, V.P. Nitric oxide-mediated regulation of sub-cellular chromium distribution, ascorbate–glutathione cycle and glutathione biosynthesis in tomato roots under chromium (VI) toxicity. J. Biotechnol. 2020, 318, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Ai, H.; Xu, X.; Chen, K.; Niu, H.; Zhu, H.; Sun, J.; Du, D.; Chen, L. Nitric oxide alleviates toxicity of hexavalent chromium on tall fescue and improves performance of photosystem II. Ecotoxicol. Environ. Saf. 2018, 164, 32–40. [Google Scholar] [CrossRef]
- Srivastava, D.; Tiwari, M.; Dutta, P.; Singh, P.; Chawda, K.; Kumari, M.; Chakrabarty, D. Chromium stress in plants: Toxicity, tolerance and phytoremediation. Sustainability 2021, 13, 4629. [Google Scholar] [CrossRef]
- Rani, P.; Arya, R.C.; Dwivedi, S. Chromium pollution: Impact on plants and its mitigation. In Innovations in Food Technology. Current Perspectives and Future Goals, 1st ed.; Mishra, P., Mishra, R.R., Oluwaseun Adetunji., C., Eds.; Springer Nature: Singapore, 2022; pp. 323–340. [Google Scholar]
- Samanta, S.; Banerjee, A.; Roychoudhury, A. Exogenous melatonin regulates endogenous phytohormone homeostasis and thiol-mediated detoxification in two indica rice cultivars under arsenic stress. Plant Cell Rep. 2021, 40, 1585–1602. [Google Scholar] [CrossRef]
- Ao, M.; Chen, X.; Deng, T.; Sun, S.; Tang, Y.; Morel, J.L.; Qiu, R.; Wang, S. Chromium biogeochemical behaviour in soil-plant systems and remediation strategies: A critical review. J. Hazard. Mater. 2022, 424, 127233. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Hu, L.; Liao, W.; Dawuda, M.M.; Lyu, J.; Xie, J.; Feng, Z.; Calderón-Urrea, A.; Yu, J. Foliar application of 5-aminolevulinic acid (5-ALA) alleviates NaCl stress in cucumber (Cucumis sativus L.) seedlings through the enhancement of ascorbate-glutathione cycle. Sci. Hortic. 2019, 257, 108761. [Google Scholar] [CrossRef]
- Dai, H.; Shan, C. Effects of lanthanum on the antioxidant capacity of chloroplasts and chlorophyll fluorescence parameters of maize seedlings under chromium stress. Photosynthetica 2019, 57, 27–31. [Google Scholar] [CrossRef]
- Hou, Y.; Li, Z.; Zheng, Y.; Jin, P. Effects of CaCl2 treatment alleviates chilling injury of loquat fruit (Eribotrya japonica) by modulating ROS homeostasis. Foods 2021, 10, 1662. [Google Scholar] [CrossRef]
- Ulhassan, Z.; Gill, R.A.; Huang, H.; Ali, S.; Mwamba, T.M.; Ali, B.; Huang, Q.; Hamid, Y.; Khan, A.R.; Wang, J. Selenium mitigates the chromium toxicity in Brassicca napus L. by ameliorating nutrients uptake, amino acids metabolism and antioxidant defense system. Plant Physiol. Biochem. 2019, 145, 142–152. [Google Scholar] [CrossRef]
- Anwar, A.; Yan, Y.; Liu, Y.; Li, Y.; Yu, X. 5-aminolevulinic acid improves nutrient uptake and endogenous hormone accumulation, enhancing low-temperature stress tolerance in cucumbers. Int. J. Mol. Sci. 2018, 19, 3379. [Google Scholar] [CrossRef] [PubMed]
- Faizan, M.; Bhat, J.A.; El-Serehy, H.A.; Moustakas, M.; Ahmad, P. Magnesium oxide nanoparticles (MgO-NPs) alleviate arsenic toxicity in soybean by modulating photosynthetic function, nutrient uptake and antioxidant potential. Metals 2022, 12, 2030. [Google Scholar] [CrossRef]
- Sperdouli, I.; Moustaka, J.; Antonoglou, O.; Adamakis, I.-D.S.; Dendrinou-Samara, C.; Moustakas, M. Leaf age-dependent effects of foliar-sprayed CuZn nanoparticles on photosynthetic efficiency and ROS generation in Arabidopsis thaliana. Materials 2019, 12, 2498. [Google Scholar] [CrossRef] [PubMed]
- Adamakis, I.D.S.; Sperdouli, I.; Eleftheriou, E.P.; Moustakas, M. Hydrogen peroxide production by the spot-like mode action of bisphenol A. Front. Plant Sci. 2020, 11, 1196. [Google Scholar] [CrossRef]
- Mittler, R. ROS are good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef]
- Romero-Puertas, M.C.; Terrón-Camero, L.C.; Peláez-Vico, M.Á.; Molina-Moya, E.; Sandalio, L.M. An update on redox signals in plant responses to biotic and abiotic stress crosstalk: Insights from cadmium and fungal pathogen interactions. J. Exp. Bot. 2021, 72, 5857–5875. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox signaling in plants. Antioxid. Redox Signal. 2013, 18, 2087–2090. [Google Scholar] [CrossRef]
- Moustakas, M. The role of metal ions in biology, biochemistry and medicine. Materials 2021, 14, 549. [Google Scholar] [CrossRef]
- Sperdouli, I.; Adamakis, I.D.S.; Dobrikova, A.; Apostolova, E.; Hanć, A.; Moustakas, M. Excess zinc supply reduces cadmium uptake and mitigates cadmium toxicity effects on chloroplast structure, oxidative stress, and photosystem II photochemical efficiency in Salvia sclarea plants. Toxics 2022, 10, 36. [Google Scholar] [CrossRef]
- Moustakas, M.; Moustaka, J.; Sperdouli, I. Hormesis in photosystem II: A mechanistic approach. Curr. Opin. Toxicol. 2022, 29, 57–64. [Google Scholar] [CrossRef]
- Sperdouli, I.; Andreadis, S.; Moustaka, J.; Panteris, E.; Tsaballa, A.; Moustakas, M. Changes in light energy utilization in photosystem II and reactive oxygen species generation in potato leaves by the pinworm Tuta absoluta. Molecules 2021, 26, 2984. [Google Scholar] [CrossRef] [PubMed]
- Sperdouli, I.; Andreadis, S.S.; Adamakis, I.-D.S.; Moustaka, J.; Koutsogeorgiou, E.I.; Moustakas, M. Reactive oxygen species initiate defence responses of potato photosystem II to sap-sucking insect feeding. Insects 2022, 13, 409. [Google Scholar] [CrossRef] [PubMed]
- Moustaka, J.; Tanou, G.; Adamakis, I.D.; Eleftheriou, E.P.; Moustakas, M. Leaf age dependent photoprotective and antioxidative mechanisms to paraquat-induced oxidative stress in Arabidopsis thaliana. Int. J. Mol. Sci. 2015, 16, 13989–14006. [Google Scholar] [CrossRef] [PubMed]
- Moustakas, M.; Sperdouli, I.; Adamakis, I.-D.S.; Moustaka, J.; İşgören, S.; Şaş, B. Harnessing the role of foliar applied salicylic acid in decreasing chlorophyll content to reassess photosystem II photoprotection in crop plants. Int. J. Mol. Sci. 2022, 23, 7038. [Google Scholar] [CrossRef] [PubMed]
- Moustaka, J.; Tanou, G.; Giannakoula, A.; Panteris, E.; Eleftheriou, E.P.; Moustakas, M. Anthocyanin accumulation in poinsettia leaves and its functional role in photo-oxidative stress. Environ. Exp. Bot. 2020, 175, 104065. [Google Scholar] [CrossRef]
- Stamelou, M.L.; Sperdouli, I.; Pyrri, I.; Adamakis, I.D.S.; Moustakas, M. Hormetic responses of photosystem II in tomato to Botrytis Cinerea. Plants 2021, 10, 521. [Google Scholar] [CrossRef]
- Elena-Real, C.A.; González-Arzola, K.; Pérez-Mejías, G.; Díaz-Quintana, A.; Velázquez-Campoy, A.; Desvoyes, B.; Gutiérrez, C.; De la Rosa, M.A.; Díaz-Moreno, I. Proposed mechanism for regulation of H2O2-induced programmed cell death in plants by binding of cytochrome c to 14-3-3 proteins. Plant J. 2021, 106, 74–85. [Google Scholar] [CrossRef]
- Ding, H.; Wang, B.; Han, Y.; Li, S. The pivotal function of dehydroascorbate reductase in glutathione homeostasis in plants. J. Exp. Bot. 2020, 71, 3405–3416. [Google Scholar] [CrossRef]
- Liu, T.; Hu, X.; Zhang, J.; Zhang, J.; Du, Q.; Li, J. H2O2 mediates 5-ALA-induced glutathione and ascorbate accumulation in the perception and resistance to oxidative stress in Solanum lycopersicum at low temperatures. BMC Plant Biol. 2018, 18, 34. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Z.; Li, J.; Luo, S.; Zhang, J.; Xie, J. Hydrogen sulfide interacts with 5-aminolevulinic acid to enhance the antioxidant capacity of pepper (Capsicum annuum L.) seedlings under chilling stress. Agronomy 2022, 12, 572. [Google Scholar] [CrossRef]
- Kamran, M.; Wang, D.; Alhaithloul, H.A.S.; Alghanem, S.M.; Aftab, T.; Xie, K.; Lu, Y.; Shi, C.; Sun, J.; Gu, W. Jasmonic acid-mediated enhanced regulation of oxidative, glyox5-ALAse defense system and reduced chromium uptake contributes to alleviation of chromium (VI) toxicity in choysum (Brassica parachinensis L.). Ecotoxicol. Environ. Saf. 2021, 208, 111758. [Google Scholar] [CrossRef] [PubMed]
- Sperdouli, I.; Moustakas, M. Differential response of photosystem II photochemistry in young and mature leaves of Arabidopsis thaliana to the onset of drought stress. Acta Physiol. Plant. 2012, 34, 1267–1276. [Google Scholar] [CrossRef]
- Moustakas, M.; Sperdouli, I.; Moustaka, J. Early drought stress warning in plants: Color pictures of photosystem II photochemistry. Climate 2022, 10, 179. [Google Scholar] [CrossRef]
- Bayçu, G.; Gevrek-Kürüm, N.; Moustaka, J.; Csatári, I.; Rognes, S.E.; Moustakas, M. Cadmium-zinc accumulation and photosystem II responses of Noccaea caerulescens to Cd and Zn exposure. Environ. Sci. Pollut. Res. 2017, 24, 2840–2850. [Google Scholar] [CrossRef] [PubMed]
- Moustakas, M.; Ouzounidou, G. Increased non-photochemical quenching in leaves of aluminium-stressed wheat plants is due to Al3+-induced elemental loss. Plant Physiol. Biochem. 1994, 32, 527–532. [Google Scholar]
- Ma, J.; Sun, M.; Qiu, L.; Xie, Y.; Ma, Y.; Liang, W. The 5-aminolevulinic acid (5-ALA) supplement enhances PSII photochemical activity and antioxidant activity in the late growth promotion of Pseudostellaria heterophylla. Plants 2022, 11, 3035. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circ. Calif. Agric. Exp. Stn. 1950, 347, 32. [Google Scholar]
- Strain, H.H.; Svec, W.A. Extraction, separation, estimation, and isolation of the chlorophylls. In The chlorophylls; Elsevier: Amsterdam, The Netherlands, 1966; pp. 21–66. [Google Scholar]
- Barrs, H.; Weatherley, P. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Zhou, B.; Guo, Z.; Xing, J.; Huang, B. Nitric oxide is involved in abscisic acid-induced antioxidant activities in Stylosanthes guianensis. J. Exp. Bot. 2005, 56, 3223–3228. [Google Scholar] [CrossRef]
- Sun, C.; Lu, L.; Liu, L.; Liu, W.; Yu, Y.; Liu, X.; Hu, Y.; Jin, C.; Lin, X. Nitrate reductase-mediated early nitric oxide burst alleviates oxidative damage induced by aluminum through enhancement of antioxidant defenses in roots of wheat (Triticum aestivum). New Phytol. 2014, 201, 1240–1250. [Google Scholar] [CrossRef] [PubMed]
- Sheng, H.; Zeng, J.; Liu, Y.; Wang, X.; Wang, Y.; Kang, H.; Fan, X.; Sha, L.; Zhang, H.; Zhou, Y. Sulfur mediated alleviation of Mn toxicity in polish wheat relates to regulating Mn allocation and improving antioxidant system. Front. Plant Sci. 2016, 7, 1382. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Liu, J.; Lu, Z.; Wang, X.; Zhang, Z.; Shi, G. Effects of iron deficiency on subcellular distribution and chemical forms of cadmium in peanut roots in relation to its translocation. Environ. Exp. Bot. 2014, 97, 40–48. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Weisany, W.; Sohrabi, Y.; Heidari, G.; Siosemardeh, A.; Ghassemi-Golezani, K. Changes in antioxidant enzymes activity and plant performance by salinity stress and zinc application in soybean (‘Glycine max’ L.). Plant Omics 2012, 5, 60–67. [Google Scholar]
- Dionisio-Sese, M.L.; Tobita, S. Antioxidant responses of rice seedlings to salinity stress. Plant Sci. 1998, 135, 1–9. [Google Scholar] [CrossRef]
- Harel, E.; Klein, S. Light dependent formation of δ-aminolevulinic acid in etiolated leaves of higher plants. Biochem. Biophys. Res. Commun. 1972, 49, 364–370. [Google Scholar] [CrossRef]
- Huang, C.; He, W.; Guo, J.; Chang, X.; Su, P.; Zhang, L. Increased sensitivity to salt stress in an ascorbate-deficient Arabidopsis mutant. J. Exp. Bot. 2005, 56, 3041–3049. [Google Scholar] [CrossRef]
- Yu, C.-W.; Murphy, T.M.; Lin, C.-H. Hydrogen peroxide-induced chilling tolerance in mung beans mediated through ABA-independent glutathione accumulation. Funct. Plant Biol. 2003, 30, 955–963. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Hasanuzzaman, M.; Hossain, M.A.; Fujita, M. Nitric oxide modulates antioxidant defense and the methylglyoxal detoxification system and reduces salinity-induced damage of wheat seedlings. Plant Biotechnol. Rep. 2011, 5, 353–365. [Google Scholar] [CrossRef]
- Hossain, M.A.; Hasanuzzaman, M.; Fujita, M. Up-regulation of antioxidant and glyox5-ALAse systems by exogenous glycinebetaine and proline in mung bean confer tolerance to cadmium stress. Physiol. Mol. Biol. Plants 2010, 16, 259–272. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaya, C.; Ugurlar, F.; Ashraf, M.; Alyemeni, M.N.; Moustakas, M.; Ahmad, P. 5-Aminolevulinic Acid Induces Chromium [Cr(VI)] Tolerance in Tomatoes by Alleviating Oxidative Damage and Protecting Photosystem II: A Mechanistic Approach. Plants 2023, 12, 502. https://doi.org/10.3390/plants12030502
Kaya C, Ugurlar F, Ashraf M, Alyemeni MN, Moustakas M, Ahmad P. 5-Aminolevulinic Acid Induces Chromium [Cr(VI)] Tolerance in Tomatoes by Alleviating Oxidative Damage and Protecting Photosystem II: A Mechanistic Approach. Plants. 2023; 12(3):502. https://doi.org/10.3390/plants12030502
Chicago/Turabian StyleKaya, Cengiz, Ferhat Ugurlar, Muhammed Ashraf, Mohammed Nasser Alyemeni, Michael Moustakas, and Parvaiz Ahmad. 2023. "5-Aminolevulinic Acid Induces Chromium [Cr(VI)] Tolerance in Tomatoes by Alleviating Oxidative Damage and Protecting Photosystem II: A Mechanistic Approach" Plants 12, no. 3: 502. https://doi.org/10.3390/plants12030502
APA StyleKaya, C., Ugurlar, F., Ashraf, M., Alyemeni, M. N., Moustakas, M., & Ahmad, P. (2023). 5-Aminolevulinic Acid Induces Chromium [Cr(VI)] Tolerance in Tomatoes by Alleviating Oxidative Damage and Protecting Photosystem II: A Mechanistic Approach. Plants, 12(3), 502. https://doi.org/10.3390/plants12030502







