Do Tree Size and Tree Shade Tolerance Affect the Photosynthetic Capacity of Broad-Leaved Tree Species?
Abstract
1. Introduction
2. Results
2.1. Variations in Leaf Traits of Different Tree Types
2.2. Relationship between Leaf Structural Traits and Leaf Net Photosynthetic Rate
3. Discussion
3.1. Variations in Leaf Traits between Different Tree Size Groups or Shade-Tolerant Groups
3.2. Effects of Tree Size and Shade Tolerance on Pn
4. Materials and Methods
4.1. Research Site
4.2. Sampling
4.3. Leaf Trait Measures
4.3.1. Leaf Morphological Traits
4.3.2. Leaf Chemical Traits
4.3.3. Leaf Anatomical Traits
4.4. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gibert, A.; Gray, E.F.; Westoby, M.; Wright, I.J.; Falster, D.S.; Wilson, S. On the link between functional traits and growth rate: Meta-analysis shows effects change with plant size, as predicted. J. Ecol. 2016, 104, 1488–1503. [Google Scholar] [CrossRef]
- Falster, D.S.; Duursma, R.A.; FitzJohn, R.G. How functional traits influence plant growth and shade tolerance across the life cycle. Proc. Natl. Acad. Sci. USA 2018, 115, E6789–E6798. [Google Scholar] [CrossRef] [PubMed]
- Coble, A.P.; Cavaleri, M.A. Vertical leaf mass per area gradient of mature sugar maple reflects both height-driven increases in vascular tissue and light-driven increases in palisade layer thickness. Tree Physiol. 2017, 37, 1337–1351. [Google Scholar] [CrossRef] [PubMed]
- Ellsworth, D.S.; Crous, K.Y.; Lambers, H.; Cooke, J. Phosphorus recycling in photorespiration maintains high photosynthetic capacity in woody species. Plant Cell Environ. 2015, 38, 1142–1156. [Google Scholar] [CrossRef] [PubMed]
- Onoda, Y.; Wright, I.J.; Evans, J.R.; Hikosaka, K.; Kitajima, K.; Niinemets, U.; Poorter, H.; Tosens, T.; Westoby, M. Physiological and structural tradeoffs underlying the leaf economics spectrum. New Phytol. 2017, 214, 1447–1463. [Google Scholar] [CrossRef] [PubMed]
- Mo, Q.F.; Li, Z.A.; Sayer, E.J.; Lambers, H.; Li, Y.W.; Zou, B.; Tang, J.W.; Heskel, M.; Ding, Y.Z.; Wang, F.; et al. Foliar phosphorus fractions reveal how tropical plants maintain photosynthetic rates despite low soil phosphorus availability. Funct. Ecol. 2019, 33, 503–513. [Google Scholar] [CrossRef]
- Woodruff, D.R.; Meinzer, F.C. Water stress, shoot growth and storage of non-structural carbohydrates along a tree height gradient in a tall conifer. Plant Cell Environ. 2011, 34, 1920–1930. [Google Scholar] [CrossRef]
- Salgado-Luarte, C.; Gianoli, E. Shade tolerance and herbivory are associated with RGR of tree species via different functional traits. Plant Biol. 2017, 19, 413–419. [Google Scholar] [CrossRef]
- Zhang, X.S.; Jin, G.Z.; Liu, Z.L. Contribution of leaf anatomical traits to leaf mass per area among canopy layers for five coexisting broadleaf species across shade tolerances at a regional scale. For. Ecol. Manag. 2019, 452, 117569. [Google Scholar] [CrossRef]
- Evans, J.R. Nitrogen and photosynthesis in the flag leaf of wheat (Triticum aestivum L.). Plant Physiol. 1983, 72, 297–302. [Google Scholar] [CrossRef]
- Sun, J.; Yao, F.; Wu, J.; Zhang, P.; Xu, W. Effect of nitrogen levels on photosynthetic parameters, morphological and chemical characters of saplings and trees in a temperate forest. J. For. Res. 2017, 29, 1481–1488. [Google Scholar] [CrossRef]
- Xiong, D.; Flexas, J. Leaf anatomical characteristics are less important than leaf biochemical properties in determining photosynthesis responses to nitrogen top-dressing. J. Exp. Bot. 2021, 72, 5709–5720. [Google Scholar] [CrossRef] [PubMed]
- Rogers, A. The use and misuse of Vc,max in Earth System Models. Photosynth. Res. 2014, 119, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Friend, A.D. Terrestrial plant production and climate change. J. Exp. Bot. 2010, 61, 1293–1309. [Google Scholar] [CrossRef]
- Pierce, S.; Maffi, D.; Faoro, F.; Cerabolini, B.E.L.; Spada, A. The leaf anatomical trade-offs associated with plant ecological strategy variation. Plant Ecol. 2022, 223, 1233–1246. [Google Scholar] [CrossRef]
- Flexas, J.; Carriqui, M. Photosynthesis and photosynthetic efficiencies along the terrestrial plant’s phylogeny: Lessons for improving crop photosynthesis. Plant J. 2020, 101, 964–978. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Liu, Z.L.; Hikosaka, K.; Li, F.R.; Jin, G.Z.; Ostertag, R. Variations in leaf economics spectrum traits for an evergreen coniferous species: Tree size dominates over environment factors. Funct. Ecol. 2020, 34, 458–467. [Google Scholar] [CrossRef]
- Visakorpi, K.; Block, S.; Pellissier, L.; Levine, J.M.; Alexander, J. Eco-physiological and morphological traits explain alpine plant species’ response to warming. Funct. Ecol. 2022. early view. [Google Scholar] [CrossRef]
- Vogelmann, T.C.; Martin, G. The functional significance of palisade tissue: Penetration of directional versus diffuse light. Plant Cell Environ. 1993, 16, 65–72. [Google Scholar] [CrossRef]
- Knapp, A.K.; Carter, G.A. Variability in leaf optical properties among 26 species from a broad range of habitats. Am. J. Bot. 1998, 85, 940–946. [Google Scholar] [CrossRef]
- Terashima, I.; Hanba, Y.T.; Tholen, D.; Niinemets, U. Leaf functional anatomy in relation to photosynthesis. Plant Physiol. 2011, 155, 108–116. [Google Scholar] [CrossRef]
- Ryan, M.G.; Bond, B.J.; Law, B.E.; Hubbard, R.M.; Woodruff, D.; Cienciala, E.; Kucera, J. Transpiration and whole-tree conductance in ponderosa pine trees of different heights. Oecologia 2000, 124, 553–560. [Google Scholar] [CrossRef]
- Gago, J.; Carriqui, M.; Nadal, M.; Clemente-Moreno, M.J.; Coopman, R.E.; Fernie, A.R.; Flexas, J. Photosynthesis Optimized across Land Plant Phylogeny. Trends Plant Sci. 2019, 24, 947–958. [Google Scholar] [CrossRef]
- Liu, Z.; Jiang, F.; Li, F.; Jin, G. Coordination of intra and inter-species leaf traits according to leaf phenology and plant age for three temperate broadleaf species with different shade tolerances. For. Ecol. Manag. 2019, 434, 63–75. [Google Scholar] [CrossRef]
- Tredennick, A.T.; Teller, B.J.; Adler, P.B.; Hooker, G.; Ellner, S.P. Size-by-environment interactions: A neglected dimension of species’ responses to environmental variation. Ecol. Lett. 2018, 21, 1757–1770. [Google Scholar] [CrossRef]
- Mencuccini, M.; Martinez-Vilalta, J.; Vanderklein, D.; Hamid, H.A.; Korakaki, E.; Lee, S.; Michiels, B. Size-mediated ageing reduces vigour in trees. Ecol. Lett. 2005, 8, 1183–1190. [Google Scholar] [CrossRef]
- Falster, D.S.; Brännström, Å.; Dieckmann, U.; Westoby, M. Influence of four major plant traits on average height, leaf-area cover, net primary productivity, and biomass density in single-species forests: A theoretical investigation. J. Ecol. 2011, 99, 148–164. [Google Scholar] [CrossRef]
- Niinemets, U. Stomatal conductance alone does not explain the decline in foliar photosynthetic rates with increasing tree age and size in Picea abies and Pinus sylvestris. Tree Physiol. 2002, 22, 515–535. [Google Scholar] [CrossRef] [PubMed]
- Sterner, R.W.; Elser, J.J. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere; Princeton University Press: Princeton, NJ, USA, 2002. [Google Scholar]
- Mensah, S.; Glèlè Kakaï, R.; Seifert, T. Patterns of biomass allocation between foliage and woody structure: The effects of tree size and specific functional traits. Ann. For. Res. 2016, 59, 1–12. [Google Scholar] [CrossRef]
- Carranca, C.; Brunetto, G.; Tagliavini, M. Nitrogen nutrition of fruit trees to reconcile productivity and environmental concerns. Plants 2018, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Clare, S.; Mack, M.C.; Brooks, M. A direct test of nitrogen and phosphorus limitation to net primary productivity in a lowland tropical wet forest. Ecology 2013, 94, 1540–1551. [Google Scholar] [CrossRef]
- Augspurger, C.K.; Bartlett, E.A. Differences in leaf phenology between juvenile and adult trees in a temperate deciduous forest. Tree Physiol. 2003, 23, 517–525. [Google Scholar] [CrossRef]
- Marchand, L.J.; Dox, I.; Gricar, J.; Prislan, P.; Leys, S.; Van den Bulcke, J.; Fonti, P.; Lange, H.; Matthysen, E.; Penuelas, J.; et al. Inter-individual variability in spring phenology of temperate deciduous trees depends on species, tree size and previous year autumn phenology. Agric. For. Meteorol. 2020, 290, 108031. [Google Scholar] [CrossRef]
- Ryan, M.G.; Binkley, D.; Fownes, J.H. Age-related decline in forest productivity: Pattern and process. Adv. Ecol. Res. 1997, 27, 213–262. [Google Scholar] [CrossRef]
- Ryan, M.G.; Phillips, N.; Bond, B.J. The hydraulic limitation hypothesis revisited. Plant Cell Environ. 2006, 29, 367–381. [Google Scholar] [CrossRef]
- Portsmuth, A.; Niinemets, Ü. Structural and physiological plasticity in response to light and nutrients in five temperate deciduous woody species of contrasting shade tolerance. Funct. Ecol. 2007, 21, 61–77. [Google Scholar] [CrossRef]
- Koleszar, G.; Lukacs, B.A.; Nagy, P.T.; Szabo, S. Shade tolerance as a key trait in invasion success of submerged macrophyte Cabomba caroliniana over Myriophyllum spicatum. Ecol. Evol. 2022, 12, e9306. [Google Scholar] [CrossRef]
- Kaber, Y.; Meyer, P.; Stillhard, J.; De Lombaerde, E.; Zell, J.; Stadelmann, G.; Bugmann, H.; Bigler, C. Tree recruitment is determined by stand structure and shade tolerance with uncertain role of climate and water relations. Ecol. Evol. 2021, 11, 12182–12203. [Google Scholar] [CrossRef]
- Yu, Z.C.; Lin, W.; Zheng, X.T.; Cai, M.L.; Zhang, T.J.; Luo, Y.N.; Peng, C.L. Interpretation of the difference in shade tolerance of two subtropical forest tree species of different successional stages at the transcriptome and physiological levels. Tree Physiol. 2021, 41, 1669–1684. [Google Scholar] [CrossRef]
- Seiwa, K.; Kikuzawa, K.; Kadowaki, T.; Akasaka, S.; Ueno, N. Shoot life span in relation to successional status in deciduous broad-leaved tree species in a temperate forest. New Phytol. 2006, 169, 537–548. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Wright, I.J.; Zhu, S.; Onoda, Y.; Liu, H.; Li, R.; Liu, X.; Hua, L.; Oyanoghafo, O.O.; Ye, Q. Leaf mechanical strength and photosynthetic capacity vary independently across 57 subtropical forest species with contrasting light requirements. New Phytol. 2019, 223, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Chmura, D.J.; Modrzynski, J.; Chmielarz, P.; Tjoelker, M.G. Plasticity in seedling morphology, biomass allocation and physiology among ten temperate tree species in response to shade is related to shade tolerance and not leaf habit. Plant Biol. 2017, 19, 172–182. [Google Scholar] [CrossRef] [PubMed]
- He, N.; Liu, C.; Tian, M.; Li, M.; Yang, H.; Yu, G.; Guo, D.; Smith, M.D.; Yu, Q.; Hou, J.; et al. Variation in leaf anatomical traits from tropical to cold-temperate forests and linkage to ecosystem functions. Funct. Ecol. 2017, 32, 10–19. [Google Scholar] [CrossRef]
- Ollinger, S.V. Sources of variability in canopy reflectance and the convergent properties of plants. New Phytol. 2011, 189, 375–394. [Google Scholar] [CrossRef] [PubMed]
- Krober, W.; Heklau, H.; Bruelheide, H. Leaf morphology of 40 evergreen and deciduous broadleaved subtropical tree species and relationships to functional ecophysiological traits. Plant Biol. 2015, 17, 373–383. [Google Scholar] [CrossRef]
- Binks, O.; Meir, P.; Rowland, L.; da Costa, A.C.; Vasconcelos, S.S.; de Oliveira, A.A.; Ferreira, L.; Mencuccini, M. Limited acclimation in leaf anatomy to experimental drought in tropical rainforest trees. Tree Physiol. 2016, 36, 1550–1561. [Google Scholar] [CrossRef]
- Fu, P.L.; Zhu, S.D.; Zhang, J.L.; Finnegan, P.M.; Jiang, Y.J.; Lin, H.; Fan, Z.X.; Cao, K.F. The contrasting leaf functional traits between a karst forest and a nearby non-karst forest in south-west China. Funct. Plant Biol. 2019, 46, 907–915. [Google Scholar] [CrossRef]
- Sterck, F.; Markesteijn, L.; Schieving, F.; Poorter, L. Functional traits determine trade-offs and niches in a tropical forest community. Proc. Natl. Acad. Sci. USA 2011, 108, 20627–20632. [Google Scholar] [CrossRef]
- Chen, B.; Wang, C.; Tian, Y.; Chu, Q.; Hu, C. Anatomical characteristics of young stems and mature leaves of dwarf pear. Sci. Hortic. 2015, 186, 172–179. [Google Scholar] [CrossRef]
- Woods, D.B.; Turner, N.C. Stomatal Response to Changing Light by Four Tree Species of Varying Shade Tolerance. New Phytol. 1971, 70, 77–84. [Google Scholar] [CrossRef]
- Janse-Ten Klooster, S.H.; Thomas, E.J.P.; Sterck, F.J. Explaining interspecific differences in sapling growth and shade tolerance in temperate forests. J. Ecol. 2007, 95, 1250–1260. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Wong, S.C. An empirical model of stomatal conductance. Aust. J. Plant Physiol. 1984, 11, 191–210. [Google Scholar] [CrossRef]
- Rowland, L.; da Costa, A.C.; Galbraith, D.R.; Oliveira, R.S.; Binks, O.J.; Oliveira, A.A.; Pullen, A.M.; Doughty, C.E.; Metcalfe, D.B.; Vasconcelos, S.S.; et al. Death from drought in tropical forests is triggered by hydraulics not carbon starvation. Nature 2015, 528, 119–122. [Google Scholar] [CrossRef]
- Stovall, A.E.L.; Shugart, H.; Yang, X. Tree height explains mortality risk during an intense drought. Nat. Commun. 2019, 10, 4385. [Google Scholar] [CrossRef]
- Rijkers, T.; Pons, T.L.; Bongers, F. The effect of tree height and light availability on photosynthetic leaf traits of four neotropical species differing in shade tolerance. Funct. Ecol. 2000, 14, 77–86. [Google Scholar] [CrossRef]
- Sala, A.; Hoch, G. Height-related growth declines in ponderosa pine are not due to carbon limitation. Plant Cell Environ. 2009, 32, 22–30. [Google Scholar] [CrossRef]
- Oguchi, R.; Hikosaka, K.; Hirose, T. Leaf anatomy as a constraint for photosynthetic acclimation: Differential responses in leaf anatomy to increasing growth irradiance among three deciduous trees. Plant Cell Environ. 2005, 28, 916–927. [Google Scholar] [CrossRef]
- Xu, H.; Wang, H.; Prentice, I.C.; Harrison, S.P.; Wright, I.J. Coordination of plant hydraulic and photosynthetic traits: Confronting optimality theory with field measurements. New Phytol. 2021, 232, 1286–1296. [Google Scholar] [CrossRef]
- Aasamaa, K.; Sõber, A. Stomatal sensitivities to changes in leaf water potential, air humidity, CO2 concentration and light intensity, and the effect of abscisic acid on the sensitivities in six temperate deciduous tree species. Environ. Exp. Bot. 2011, 71, 72–78. [Google Scholar] [CrossRef]
- Poorter, L. Leaf traits show different relationships with shade tolerance in moist versus dry tropical forests. New Phytol. 2009, 181, 890–900. [Google Scholar] [CrossRef]
- Liu, Z.; Li, B.; Jin, G. Scale-dependent changes in the contributions of biotic and abiotic factors to leaf area index in a natural forest in northeast China. For. Ecol. Manag. 2021, 479, 118540. [Google Scholar] [CrossRef]
- Pu, X.; Weemstra, M.; Jin, G.; Umana, M.N. Tree mycorrhizal type mediates conspecific negative density dependence effects on seedling herbivory, growth, and survival. Oecologia 2022, 199, 907–918. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.L. Heilongjiang Tree Records; Heilongjiang Science & Technology Press: Heilongjiang, China, 1986. [Google Scholar]
- Niinemets, Ü.; Valladares, F. Tolerance To shade, drought, and waterlogging of femperate northern hemisphere trees and shrubs. Ecol. Monogr. 2006, 76, 521–547. [Google Scholar] [CrossRef]
- Fletcher, L.R.; Cui, H.; Callahan, H.; Scoffoni, C.; John, G.P.; Bartlett, M.K.; Burge, D.O.; Sack, L. Evolution of leaf structure and drought tolerance in species of Californian Ceanothus. Am. J. Bot. 2018, 105, 1672–1687. [Google Scholar] [CrossRef]
- Nally, R.M.; Walsh, C.J. Hierarchical partitioning public-domain software. Biodivers. Conserv. 2004, 13, 659–660. [Google Scholar] [CrossRef]
- Rosseel, Y. lavaan: An R Package for Structural Equation Modeling. J. Stat. Softw. 2012, 48, 1–36. [Google Scholar] [CrossRef]
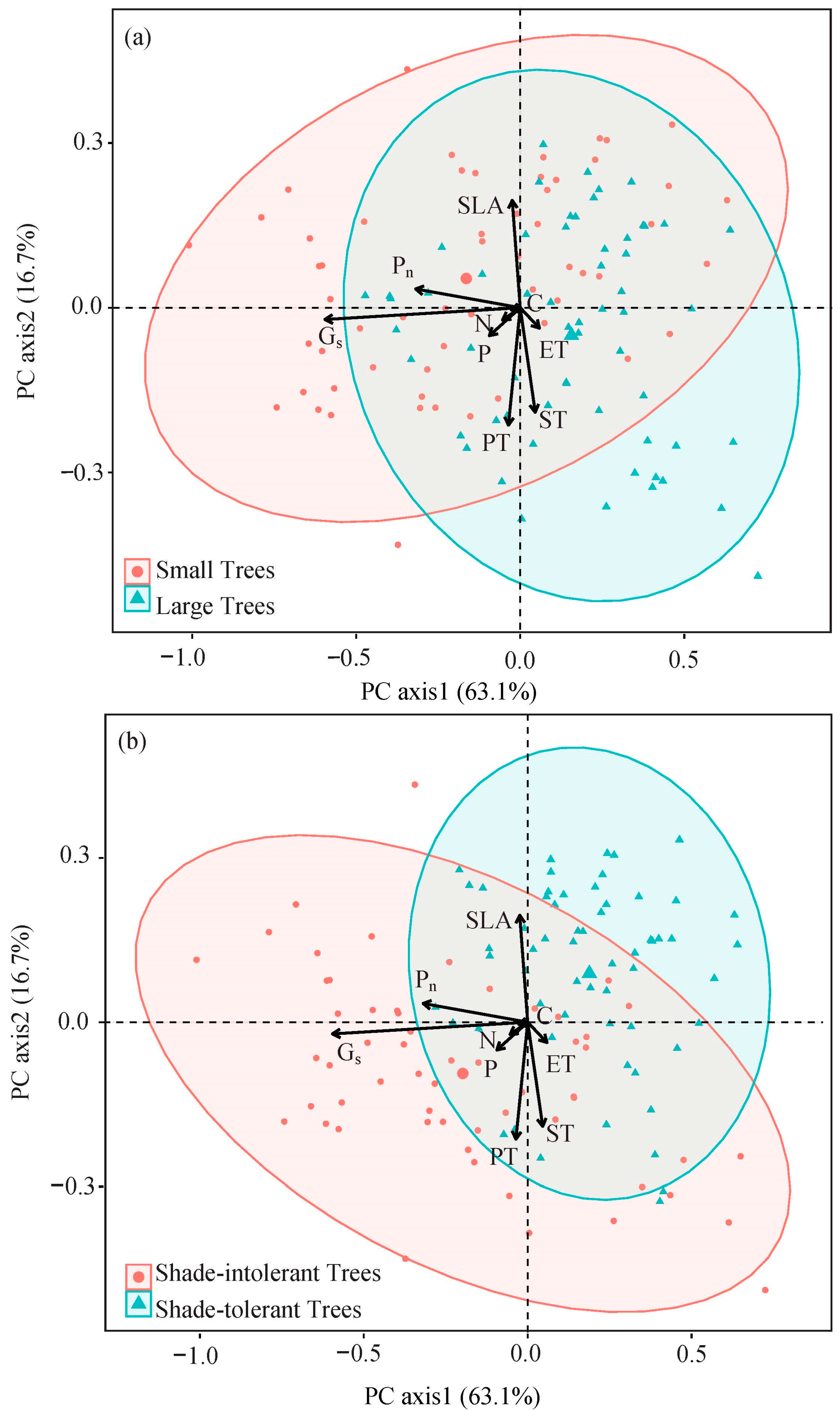
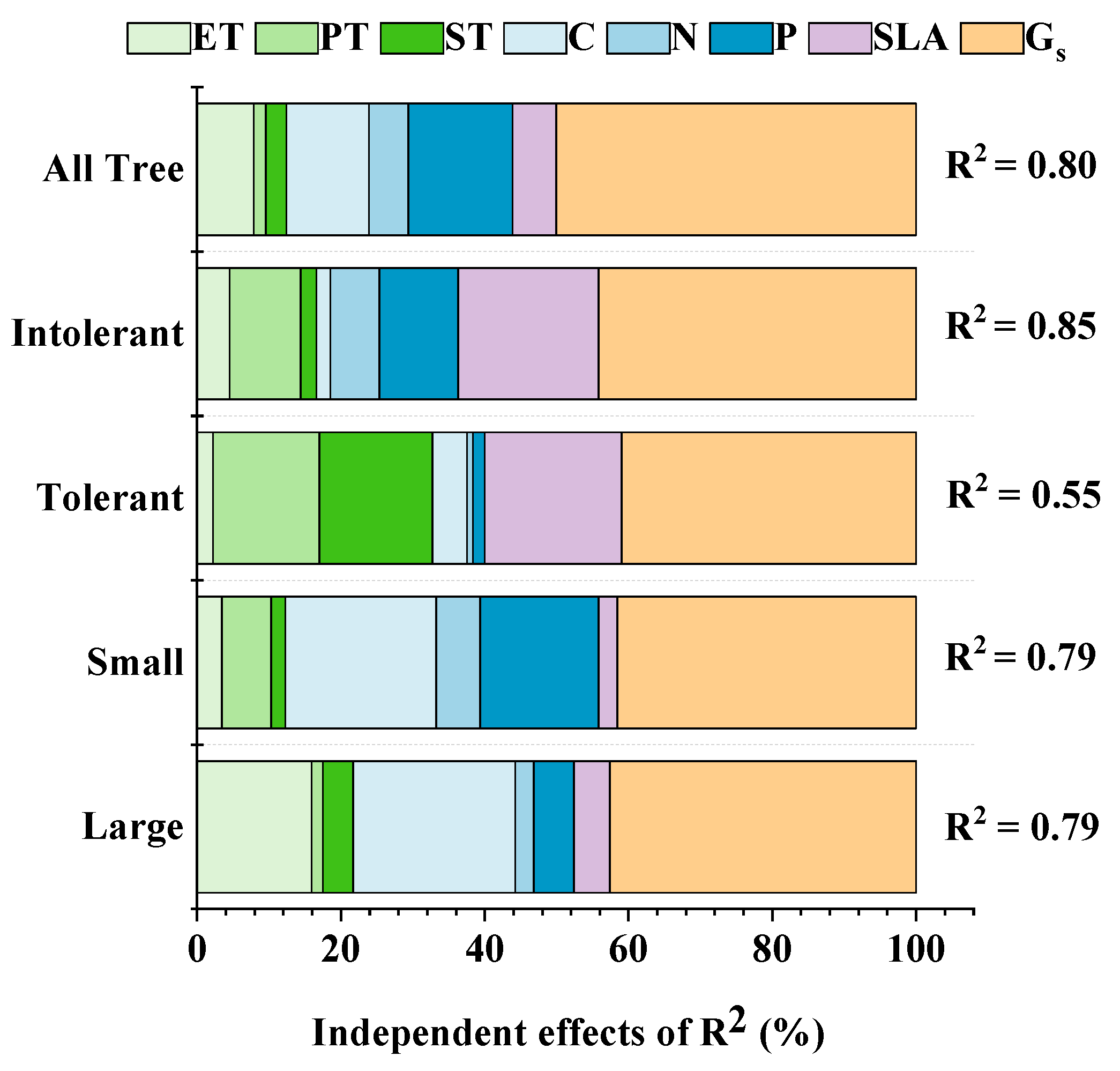
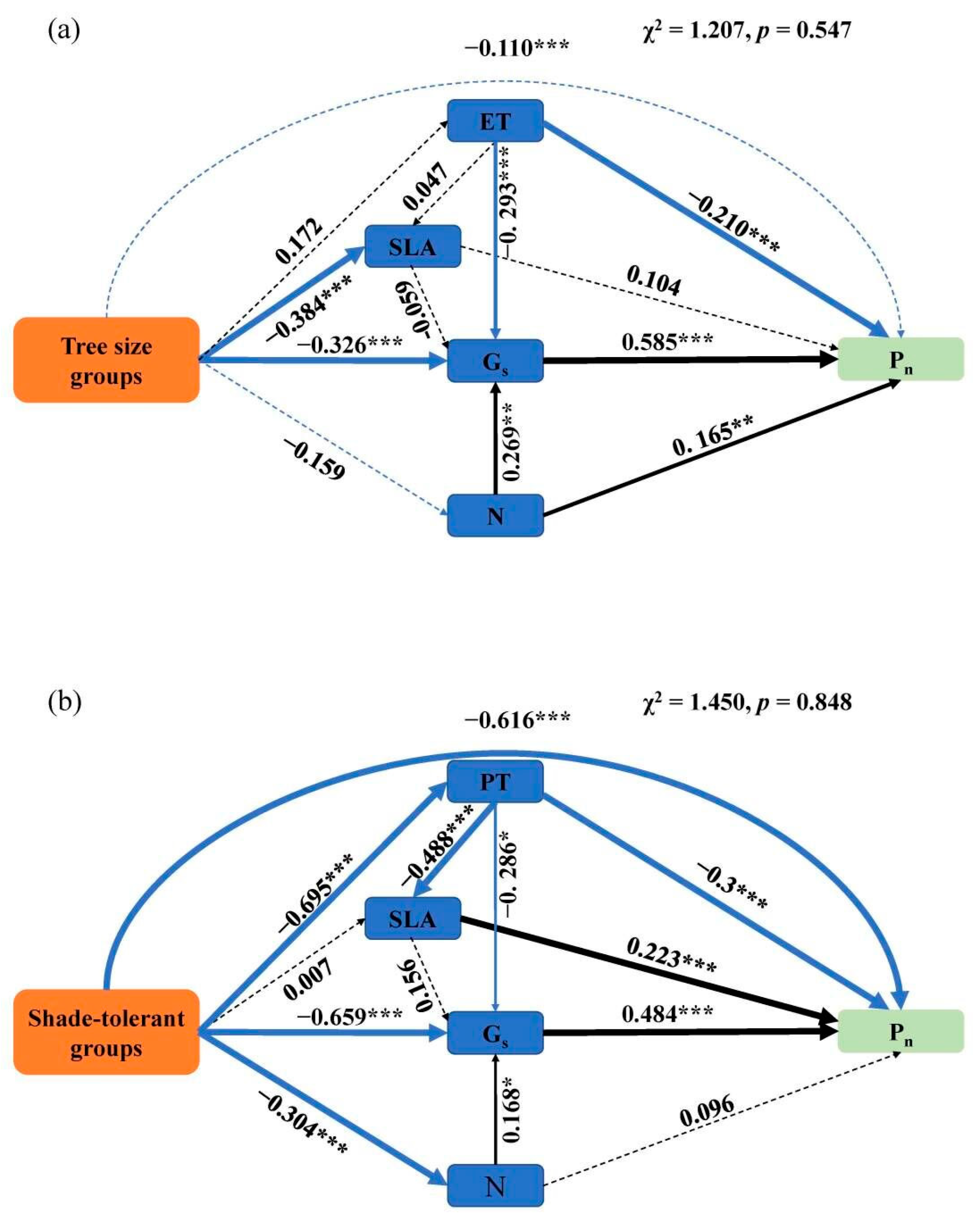
| Leaf Traits | Shade-Tolerant Groups | Leaf Traits | Shade-Tolerant Groups | ||
|---|---|---|---|---|---|
| T | p-Value | T | p-Value | ||
| Pn | 7.387 | <0.001 | PT/LT | 13.756 | <0.001 |
| SLA | −3.901 | <0.001 | ST/LT | −5.598 | <0.001 |
| Gs | 5.331 | <0.001 | C | 9.410 | <0.001 |
| ET | −4.379 | <0.001 | N | 3.504 | 0.001 |
| PT | 11.435 | <0.001 | P | 8.375 | <0.001 |
| ST | −0.036 | 0.971 | PNUE | 5.687 | <0.001 |
| PT/ST | 9.572 | <0.001 | PPUE | 3.620 | <0.001 |
| Source | Pn | SLA | Gs | |||
|---|---|---|---|---|---|---|
| F | P | F | P | F | P | |
| Species | 34.664 | <0.001 | 20.054 | <0.001 | 15.391 | <0.001 |
| Tree size | 87.059 | <0.001 | 48.959 | <0.001 | 49.800 | <0.001 |
| Species × Tree size | 6.868 | <0.001 | 7.844 | <0.001 | 7.454 | <0.001 |
| ET | PT | ST | ||||
| F | P | F | P | F | P | |
| Species | 29.440 | <0.001 | 79.129 | <0.001 | 70.469 | <0.001 |
| Tree size | 9.296 | 0.003 | 14.469 | <0.001 | 17.348 | <0.001 |
| Species × Tree size | 2.043 | 0.079 | 3.077 | 0.012 | 2.704 | 0.024 |
| PT/ST | PT/LT | ST/LT | ||||
| F | P | F | P | F | P | |
| Species | 137.328 | <0.001 | 126.407 | <0.001 | 108.730 | <0.001 |
| Tree size | 0.133 | 0.716 | 0.270 | 0.604 | 1.476 | 0.227 |
| Species × Tree size | 0.471 | 0.797 | 0.426 | 0.830 | 1.043 | 0.396 |
| C | N | P | ||||
| F | P | F | P | F | P | |
| Species | 155.950 | <0.001 | 4.792 | 0.001 | 26.268 | <0.001 |
| Tree size | 18.293 | <0.001 | 4.074 | 0.046 | 8.894 | 0.004 |
| Species × Tree size | 1.058 | 0.388 | 3.761 | 0.004 | 4.505 | 0.001 |
| PNUE | PPUE | |||||
| F | P | F | P | |||
| Species | 16.893 | <0.001 | 8.050 | <0.001 | ||
| Tree size | 46.159 | <0.001 | 40.123 | <0.001 | ||
| Species × Tree size | 3.647 | 0.004 | 1.807 | 0.118 | ||
| Species | Small Tree | Large Tree | ||
|---|---|---|---|---|
| DBH (cm) | H (m) | DBH (cm) | H (m) | |
| All species | 3.16 ± 0.12 | 4.11 ± 0.16 | 26.15 ± 1.19 | 19.18 ± 0.63 |
| Acer pictum subsp. mono | 2.74 ± 0.27 | 4.04 ± 0.32 | 24.22 ± 1.98 | 16.76 ± 1.16 |
| Acer tegmentosum | 3.1 ± 0.35 | 3.92 ± 0.15 | 14.20 ± 1.25 | 13.13 ± 1.01 |
| Ulmus laciniata | 2.58 ± 0.12 | 3.38 ± 0.21 | 26.18 ± 3.05 | 16.75 ± 0.61 |
| Betula platyphylla | 4.05 ± 0.16 | 5.40 ± 0.43 | 28.47 ± 2.25 | 21.00 ± 0.95 |
| Fraxinus mandschurica | 2.75 ± 0.27 | 3.77 ± 0.44 | 33.14 ± 2.60 | 24.19 ± 1.22 |
| Juglans mandshurica | 3.89 ± 0.37 | 4.36 ± 0.52 | 31.22 ± 2.20 | 23.62 ± 0.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.; Jin, G. Do Tree Size and Tree Shade Tolerance Affect the Photosynthetic Capacity of Broad-Leaved Tree Species? Plants 2023, 12, 523. https://doi.org/10.3390/plants12030523
Song Y, Jin G. Do Tree Size and Tree Shade Tolerance Affect the Photosynthetic Capacity of Broad-Leaved Tree Species? Plants. 2023; 12(3):523. https://doi.org/10.3390/plants12030523
Chicago/Turabian StyleSong, Yuhan, and Guangze Jin. 2023. "Do Tree Size and Tree Shade Tolerance Affect the Photosynthetic Capacity of Broad-Leaved Tree Species?" Plants 12, no. 3: 523. https://doi.org/10.3390/plants12030523
APA StyleSong, Y., & Jin, G. (2023). Do Tree Size and Tree Shade Tolerance Affect the Photosynthetic Capacity of Broad-Leaved Tree Species? Plants, 12(3), 523. https://doi.org/10.3390/plants12030523






