From Genes to Molecules, Secondary Metabolism in Botrytis cinerea: New Insights into Anamorphic and Teleomorphic Stages
Abstract
:1. Introduction
2. Botrytis cinerea Pers. Fr. (The Anamorph Stage)
2.1. From Genes to Molecules
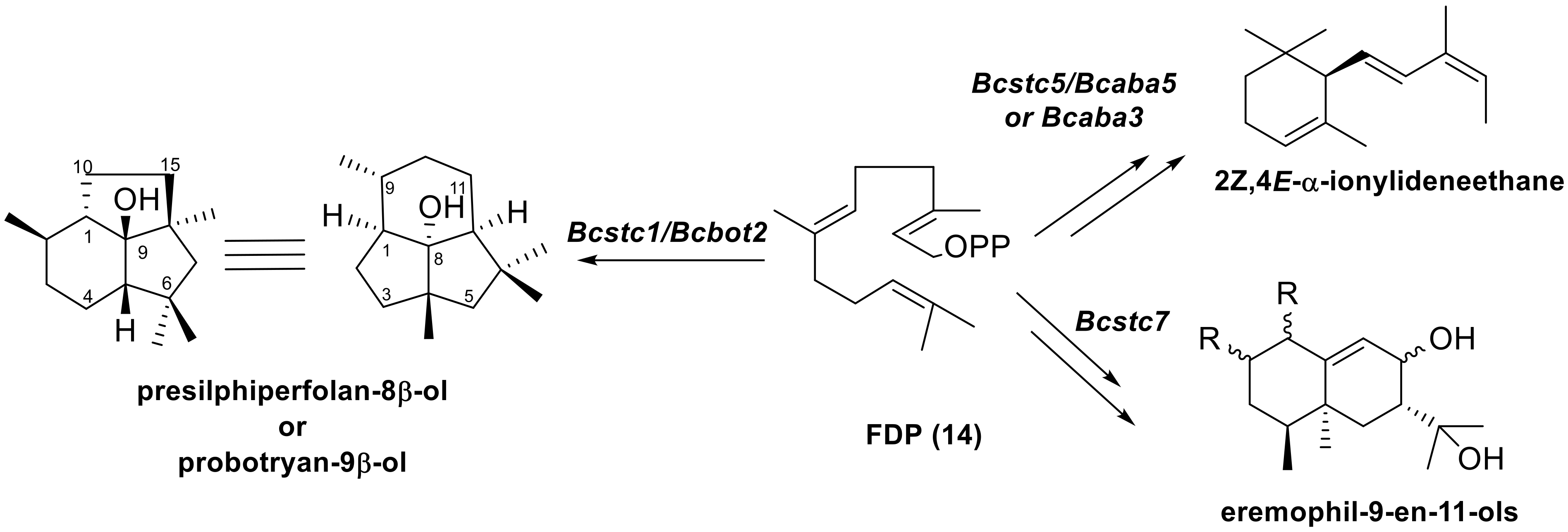
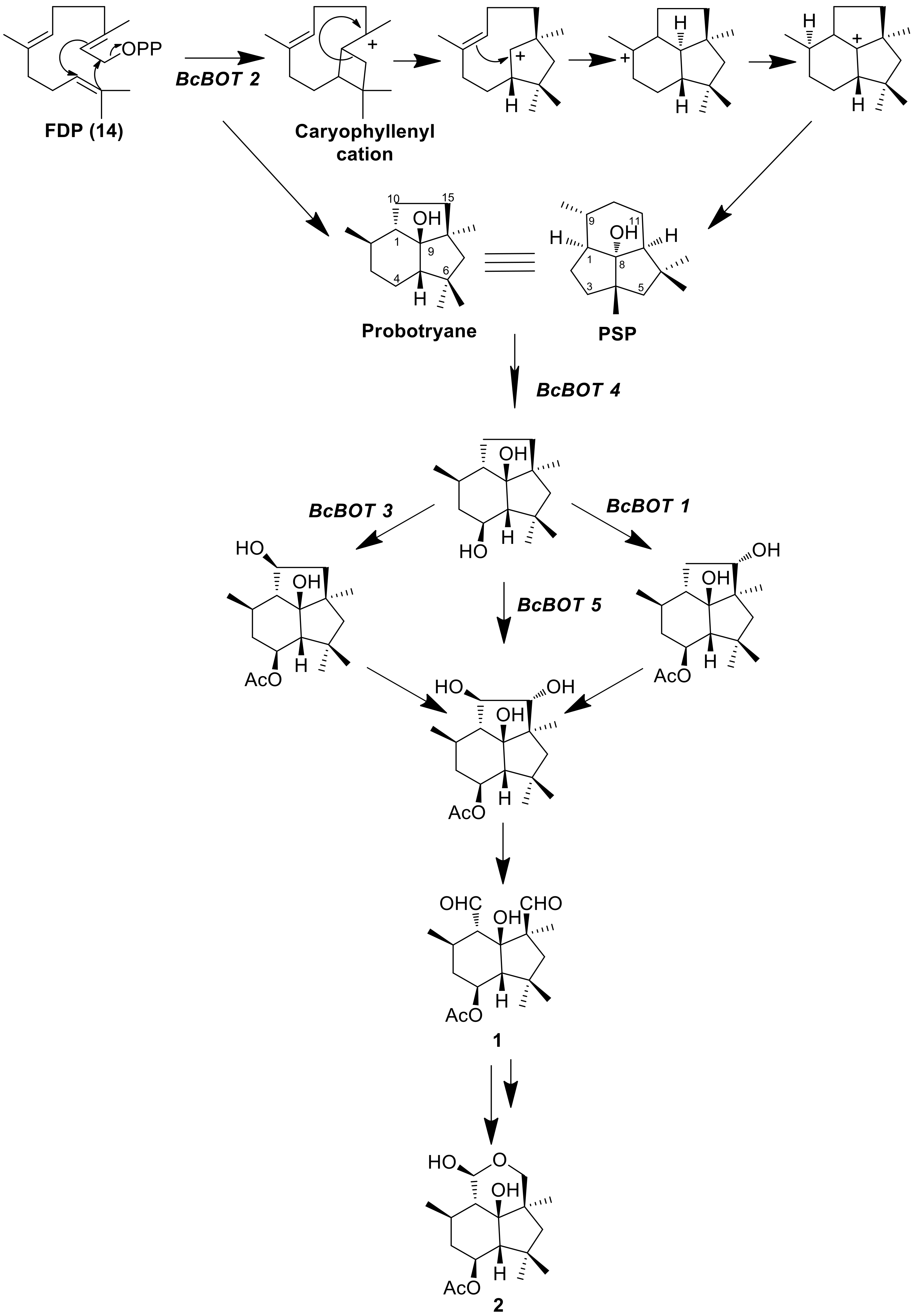
2.1.1. Botrydial Gene Cluster
2.1.2. ABA Gene Cluster
2.1.3. Eremophilenol Genes
2.1.4. Retinal and Carotenoid Gene Cluster
2.1.5. Botcinic Acid and Botcinins Gene Cluster
2.1.6. Melanin Gene Cluster
2.1.7. Pyrones, Resorcylic Acids and Resorcinols
2.1.8. Siderophore Genes
| Gene Name(s) | Gene ID in NCBI | Gene ID in EnsemblFungi | Metabolite When Isolated or Predicted |
|---|---|---|---|
| STCs (sesquiterpene cyclases) | |||
| Bcstc1/Bcbot2 | BC1G_16381 | Bcin12g06390 | Botrydial [33] |
| Bcstc2 | BC1G_09560 | Bcin08g02350 | Unknown sesquiterpenes |
| Bcstc3 | BC1G_06357 | Bcin13g05830 | |
| Bcstc4 | BC1G_14308 | Bcin04g03550 | |
| Bcstc5 | BC1G_10537 | Bcin01g03520 | Abscisic acid [34] |
| Bcstc7 | BC1G_12849 | Bcin11g06510 | Eremophil-9-en-11-ols [32] |
| DTCs (diterpene cyclases) | |||
| Bcdtc1 | BC1G_13295 | Bcin01g04920 | Unknown diterpenes |
| Bcdtc2 | BC1G_06148 | ||
| Bcdtc3 | BC1G_06751 | Bcin08g03560 | |
| Bcpax1 | BC1G_01823 | Bcin05g05670 | Unknown indole-diterpene |
| Bcphs1 | BC1G_13908 | Bcin01g04560 | Retinal [50] |
| PKSs (polyketide synthases) | |||
| Bcpks6/Bcboa6 | BC1G_16087 | Bcin01g00060 | Botcinic acid [53,60] |
| Bcpks9/Bcboa9 | BC1G_15839 | Bcin01g00090 | |
| Bcpks12 | BC1G_06876 | Bcin02g08770 | Melanin [55] |
| Bcpks13 | BC1G_14497 | Bcin03g08050 | |
| Bcpks1 | BC1G_13366 | Bcin14g00600 | Unknown polyketides |
| Bcpks2 | BC1G_02586 | Bcin02g01680 | |
| Bcpks4 | BC1G_04786 | Bcin11g02700 | |
| Bcpks8 | BC1G_02704 | Bcin07g02920 | |
| Bcpks10 | BC1G_04310 | Bcin13g01510 | |
| Bcpks11 | BC1G_09042 | Bcin14g01290 | |
| Bcpks14 | BC1G_08227 | Bcin16g01830 | |
| Bcpks15 | BC1G_01752 | Bcin05g06220 | |
| Bcpks16 | BC1G_10687 | Bcin16g05040 | |
| Bcpks17 | BC1G_01953 | Bcin03g02010 | |
| Bcpks18 | BC1G_06884 | Bcin02g08830 | |
| Bcpks19 | BC1G_16074 | Bcin08g00290 | |
| Bcpks20 | BC1G_07030 | Bcin04g00640 | |
| Bcpks21 | BC1G_13114 | Bcin05g08400 | |
| Bcchs1/Bcpks | BC1G_06032 | Bcin13g02130 | Pyrones, resorcylic acids and resorcinols [58] |
| Gene Name(s) | Gene ID in NCBI | Gene ID in EnsemblFungi | Metabolite When Isolated or Predicted |
| PKS-NRPS hybrids | |||
| Bcpks3 | BC1G_00695 | Bcin03g04360 | Unknown amino-acid containing- polyketides (PKS-NRPS hybrids) |
| Bcpks5 | BC1G_15479 | Bcin01g11550 | |
| Bcpks7 | BC1G_15702 | Bcin10g00040 | |
| NRPSs (non-ribosomal peptide synthetases) | |||
| Bcnrps2 | BC1G_03511 | Bcin12g00690 | Ferrichrome siderophores [59] |
| Bcnrps3 | BC1G_10928 | Bcin16g03570 | |
| Bcnrps7 | BC1G_15494 | Bcin01g11450 | |
| Bcnrps6 | BC1G_10567 | Bcin01g03730 | Coprogene siderophore [59] |
| Bcnrps1 | BC1G_07441 | Bcin12g04980 | Unknown peptides |
| Bcnrps4 | BC1G_02495 | Bcin02g02380 | |
| Bcnrps5 | BC1G_10622 | Bcin04g01390 | |
| Bcnrps8 | BC1G_04782 | Bcin11g02650 | |
| Bcnrps9 | BC1G_09041 | Bcin14g01300 | |
| DMATS (DiMethylAllylTryptophan Synthases) | |||
| Bcdmats1 | BC1G_08209 | Bcin16g01940 | Unknown alkaloids |
| Bcdmats2 | BC1G_15920 | Bcin14g04900 | |
2.2. New Insights in the Secondary Metabolism of B. cinerea, January 2015 to October 2022
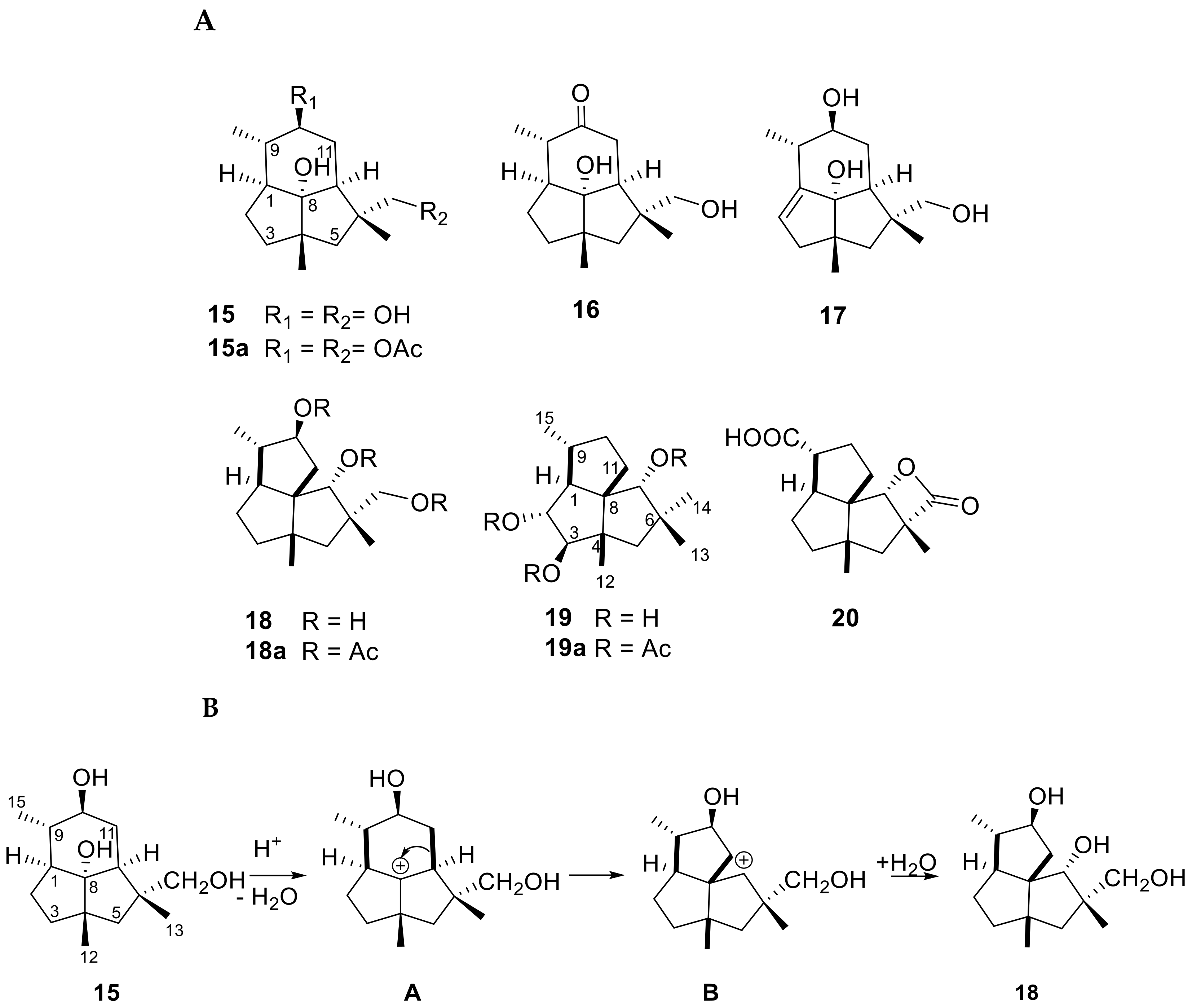
2.2.1. Botrydial Biosynthetic Gene Cluster Studies
2.2.2. Botrydial Applications
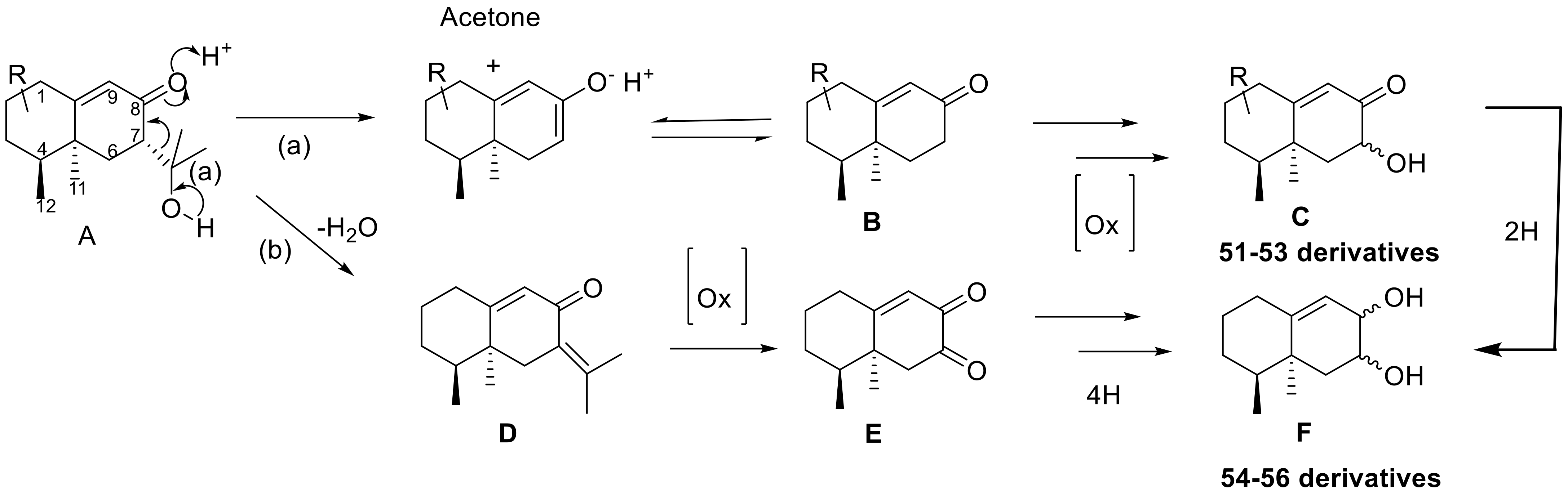
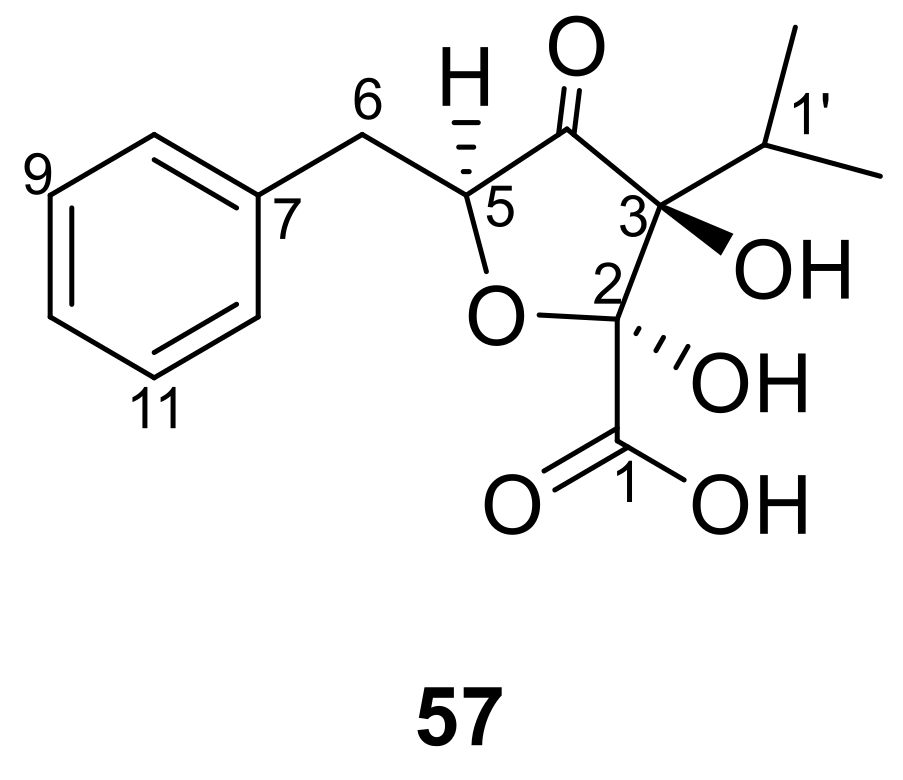
2.2.3. New Polyketides from B. cinerea
2.2.4. Abscisic Acid Biosynthetic Gene Cluster Studies
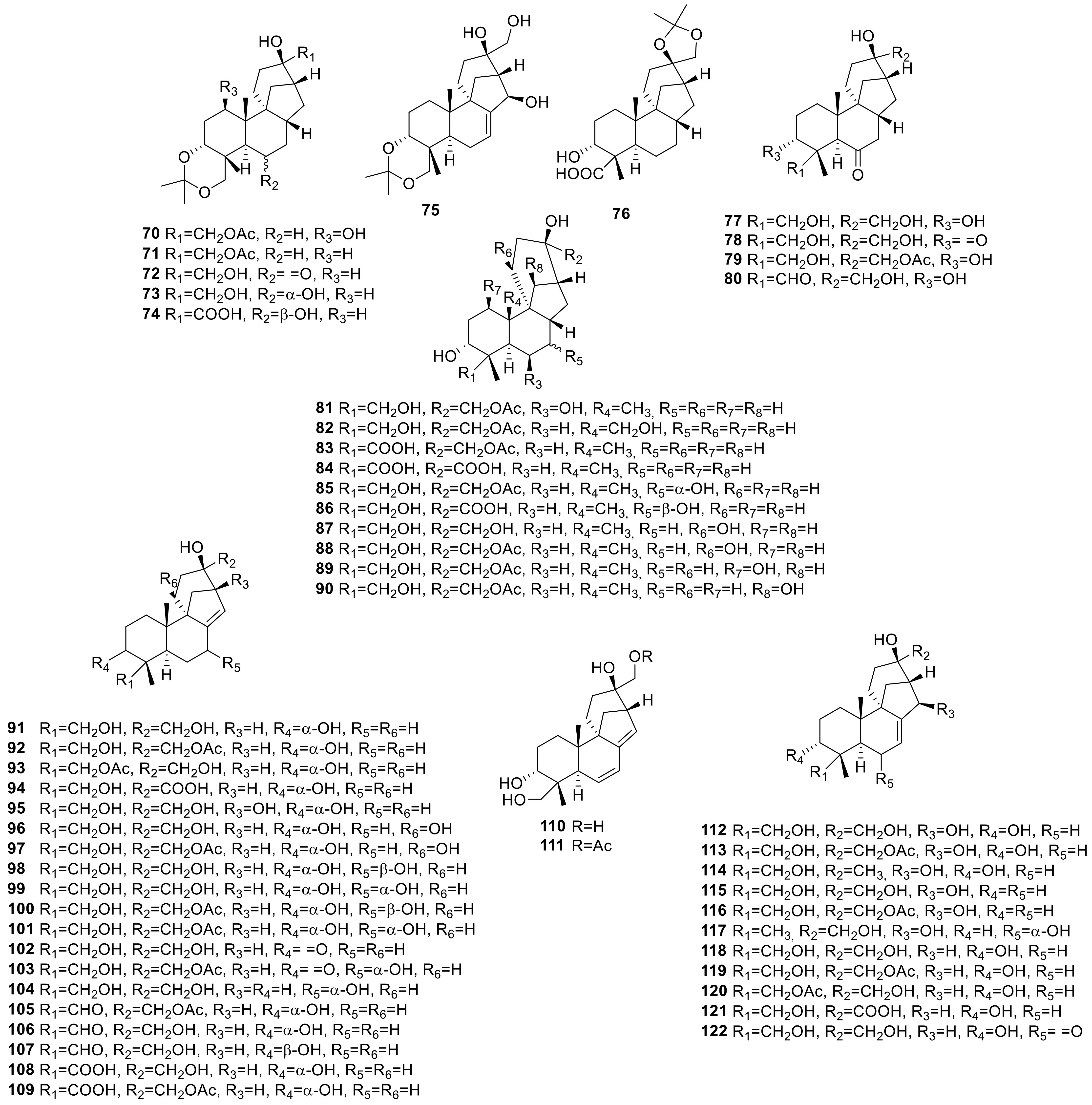
2.2.5. Eremophilenes Isolated from B. cinerea
2.2.6. Other Metabolites Isolated from B. cinerea
3. Botryotinia fuckeliana (de Bary) Wetzel (The Teleomorph Stage)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Agrios, G. Plant Pathology, 5th ed.; Academic Press: Cambridge, MA, USA, 2004; 922p. [Google Scholar] [CrossRef]
- Coley-Smith, J.R.; Verhoeff, K.; Jarvis, W.R. The Biology of Botrytis; Academic Press: London, UK, 1980. [Google Scholar]
- Mansfield, J.W. Mechanisms of Resistance to Botrytis. In The Biology of Botrytis; Coley-Smith, J.R., Verhoeff, K., Jarvis, W.R., Eds.; Academic Press: London, UK, 1980; pp. 181–218. [Google Scholar]
- Micheli, P.A. Nova Plantarum Genera Juxta Tournefortii Methodum Disposita; Typis Bernardi Paperinii: Florence, Italy, 1729. [Google Scholar] [CrossRef]
- Persoon, C.H. Synopsis Methodica Fungarum; Henricus Dieterich: Göttingen, Germany, 1801. [Google Scholar]
- Walker, A.S. Diversity within and between Species of Botrytis. In Botrytis—The Fungus, the Pathogen and Its Management in Agricultural Systems; Fillinger, S., Elad, Y., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 91–125. [Google Scholar] [CrossRef]
- Elad, Y.; Pertot, I.; Cotes Prado, A.M.; Stewart, A. Plant Hosts of Botrytis spp. In Botrytis—The Fungus, the Pathogen and Its Management in Agricultural Systems; Fillinger, S., Elad, Y., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 413–486. [Google Scholar] [CrossRef]
- Elad, Y.; Vivier, M.; Fillinger, S. Ugly. In Botrytis—The Fungus, the Pathogen and Its Management in Agricultural Systems; Fillinger, S., Elad, Y., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–15. [Google Scholar] [CrossRef]
- Fillinger, S.; Elad, Y. Botrytis—The Fungus, the Pathogen and Its Management in Agricultural Systems; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–486. [Google Scholar] [CrossRef]
- Wingfield, M.J.; De Beer, Z.W.; Slippers, B.; Wingfield, B.D.; Groenewald, J.Z.; Lombard, L.; Crous, P.W. One Fungus, One Name Promotes Progressive Plant Pathology. Mol. Plant Pathol. 2012, 13, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Johnston, P.R.; Seifert, K.A.; Stone, J.K.; Rossman, A.Y.; Marvanová, L. Recommendations on Generic Names Competing for Use in Leotiomycetes (Ascomycota). IMA Fungus 2014, 5, 91–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 Fungal Pathogens in Molecular Plant Pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, F.R.; Gárriz, A.; Marina, M.; Romero, F.M.; Gonzalez, M.E.; Collado, I.G.; Pieckenstain, F.L. The Sesquiterpene Botrydial Produced by Botrytis cinerea Induces the Hypersensitive Response on Plant Tissues and Its Action Is Modulated by Salicylic Acid and Jasmonic Acid Signaling. Mol. Plant-Microbe Interact. 2011, 24, 888–896. [Google Scholar] [CrossRef] [Green Version]
- Collado, I.G.; Viaud, M. Secondary Metabolism in Botrytis cinerea: Combining Genomic and Metabolomic Approaches. In Botrytis—The Fungus, the Pathogen and Its Management in Agricultural Systems; Springer International Publishing: Cham, Switzerland, 2016; pp. 291–313. [Google Scholar] [CrossRef]
- Colmenares, A.J.; Aleu, J.; Durán-Patrón, R.; Collado, I.G.; Hernández-Galán, R. The Putative Role of Botrydial and Related Metabolites in the Infection Mechanism of Botrytis cinerea. J. Chem. Ecol. 2002, 28, 997–1005. [Google Scholar] [CrossRef]
- Tani, H.; Koshino, H.; Sakuno, E.; Nakajima, H. Botcinins A, B, C, and D, Metabolites Produced by Botrytis cinerea, and Their Antifungal Activity against Magnaporthe grisea, a Pathogen of Rice Blast Disease. J. Nat. Prod. 2005, 68, 1768–1772. [Google Scholar] [CrossRef]
- Tani, H.; Koshino, H.; Sakuno, E.; Cutler, H.G.; Nakajima, H. Botcinins E and F and Botcinolide from Botrytis cinerea and Structural Revision of Botcinolides. J. Nat. Prod. 2006, 69, 722–725. [Google Scholar] [CrossRef]
- Deighton, N.; Muckenschnabel, I.; Colmenares, A.J.; Collado, I.G.; Williamson, B. Botrydial Is Produced in Plant Tissues Infected by Botrytis cinerea. Phytochemistry 2001, 57, 689–692. [Google Scholar] [CrossRef]
- Cutler, H.G.; Parker, S.R.; Ross, S.A.; Crumley, F.G.; Schreiner, P.R. Homobotcinolide: A Biologically Active Natural Homolog of Botcinolide from Botrytis cinerea. Biosci. Biotechnol. Biochem. 1996, 60, 656–658. [Google Scholar] [CrossRef]
- Marumo, S.; Katayama, M.; Komori, E.; Ozaki, Y.; Natsume, M.; Kondo, S. Microbial Production of Abscisic Acid by Botrytis cinerea. Agric. Biol. Chem. 1982, 46, 1967–1968. [Google Scholar] [CrossRef]
- Moraga, J.; Pinedo, C.; Durán-Patrón, R.; Collado, I.G.; Hernández-Galán, R. Botrylactone: New Interest in an Old Molecule—Review of Its Absolute Configuration and Related Compounds. Tetrahedron 2011, 67, 417–420. [Google Scholar] [CrossRef] [Green Version]
- Botubol, J.M.; Durán-Pena, M.J.; Macías-Sánchez, A.J.; Hanson, J.R.; Collado, I.G.; Hernández-Galán, R. The Asymmetric Total Synthesis of Cinbotolide: A Revision of the Original Structure. J. Org. Chem. 2014, 79, 11349–11358. [Google Scholar] [CrossRef] [PubMed]
- Konetschny-Rapp, S.; Jung, G.; Huschka, H.G.; Winkelmann, G. Isolation and Identification of the Principal Siderophore of the Plant Pathogenic Fungus Botrytis cinerea. Biol. Met. 1988, 1, 90–98. [Google Scholar] [CrossRef]
- Colmenares, A.J.; Durán-Patrón, R.M.; Hernández-Galán, R.; Collado, I.G. Four New Lactones from Botrytis cinerea. J. Nat. Prod. 2002, 65, 1724–1726. [Google Scholar] [CrossRef]
- Cutler, H.G.; Springer, J.P.; Arrendale, R.F.; Arison, B.H.; Cole, P.D.; Roberts, R.G. Cinereain: A Novel Metabolite with Plant Growth Regulating Properties from Botrytis cinerea. Agric. Biol. Chem. 1988, 52, 1725–1733. [Google Scholar] [CrossRef]
- Siewers, V.; Smedsgaard, J.; Tudzynski, P. The P450 Monooxygenase BcABA1 Is Essential for Abscisic Acid Biosynthesis in Botrytis cinerea. Appl. Environ. Microbiol. 2004, 70, 3868–3876. [Google Scholar] [CrossRef] [Green Version]
- Siewers, V.; Viaud, M.; Jimenez-Teja, D.; Collado, I.G.; Gronover, C.S.; Pradier, J.-M.-M.; Tudzynski, B.; Tudzynski, P. Functional Analysis of the Cytochrome P450 Monooxygenase Gene Bcbot1 of Botrytis cinerea Indicates That Botrydial Is a Strain-Specific Virulence Factor. Mol. Plant-Microbe Interact. 2005, 18, 602–612. [Google Scholar] [CrossRef] [Green Version]
- Siewers, V.; Kokkelink, L.; Smedsgaard, J.; Tudzynski, P. Identification of an Abscisic Acid Gene Cluster in the Grey Mold Botrytis cinerea. Appl. Environ. Microbiol. 2006, 72, 4619–4626. [Google Scholar] [CrossRef] [Green Version]
- Amselem, J.; Cuomo, C.A.; van Kan, J.A.L.; Viaud, M.; Benito, E.P.; Couloux, A.; Coutinho, P.M.; de Vries, R.P.; Dyer, P.S.; Fillinger, S.; et al. Genomic Analysis of the Necrotrophic Fungal Pathogens Sclerotinia sclerotiorum and Botrytis cinerea. PLoS Genet. 2011, 7, e1002230. [Google Scholar] [CrossRef] [Green Version]
- Staats, M.; van Kan, J.A.L. Genome Update of Botrytis cinerea Strains B05.10 and T4. Eukaryot. Cell 2012, 11, 1413–1414. [Google Scholar] [CrossRef] [Green Version]
- Howe, K.L.; Contreras-Moreira, B.; De Silva, N.; Maslen, G.; Akanni, W.; Allen, J.; Alvarez-Jarreta, J.; Barba, M.; Bolser, D.M.; Cambell, L.; et al. Ensembl Genomes 2020—Enabling Non-Vertebrate Genomic Research. Nucleic Acids Res. 2020, 48, D689–D695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suárez, I.; González-Rodríguez, V.E.; Viaud, M.; Garrido, C.; Collado, I.G. Identification of the Sesquiterpene Cyclase Involved in the Biosynthesis of (+)-4-epi-eremophil-9-en-11-ol Derivatives Isolated from Botrytis cinerea. ACS Chem. Biol. 2020, 15, 2775–2782. [Google Scholar] [CrossRef] [PubMed]
- Pinedo, C.; Wang, C.M.; Pradier, J.M.; Dalmais, B.; Choquer, M.; Le Pêcheur, P.; Morgant, G.; Collado, I.G.; Cane, D.E.; Viaud, M. Sesquiterpene Synthase from the Botrydial Biosynthetic Gene Cluster of the Phytopathogen Botrytis cinerea. ACS Chem. Biol. 2008, 3, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo-Bueno, I.; González-Rodríguez, V.E.; Simon, A.; Dalmais, B.; Pradier, J.M.; Le Pêcheur, P.; Mercier, A.; Walker, A.S.; Garrido, C.; Collado, I.G.; et al. Biosynthesis of Abscisic Acid in Fungi: Identification of a Sesquiterpene Cyclase as the Key Enzyme in Botrytis cinerea. Environ. Microbiol. 2018, 20, 2469–2482. [Google Scholar] [CrossRef] [PubMed]
- Takino, J.; Kozaki, T.; Sato, Y.; Liu, C.; Ozaki, T.; Minami, A.; Oikawa, H. Unveiling Biosynthesis of the Phytohormone Abscisic Acid in Fungi: Unprecedented Mechanism of Core Scaffold Formation Catalyzed by an Unusual Sesquiterpene Synthase. J. Am. Chem. Soc. 2018, 140, 12392–12395. [Google Scholar] [CrossRef]
- Moraga, J.; Dalmais, B.; Izquierdo-Bueno, I.; Aleu, J.; Hanson, J.R.; Hernández-Galán, R.; Viaud, M.; Collado, I.G. Genetic and Molecular Basis of Botrydial Biosynthesis: Connecting Cytochrome P450-Encoding Genes to Biosynthetic Intermediates. ACS Chem. Biol. 2016, 11, 2838–2846. [Google Scholar] [CrossRef] [Green Version]
- Porquier, A.; Morgant, G.; Moraga, J.; Dalmais, B.; Luyten, I.; Simon, A.; Pradier, J.M.; Amselem, J.; Collado, I.G.; Viaud, M. The Botrydial Biosynthetic Gene Cluster of Botrytis cinerea Displays a Bipartite Genomic Structure and Is Positively Regulated by the Putative Zn(II)2Cys6 Transcription Factor BcBot6. Fungal Genet. Biol. 2016, 96, 33–46. [Google Scholar] [CrossRef]
- Elad, Y.; Williamson, B.; Tudzynski, P.; Delen, N. Botrytis: Biology, Pathology and Control; Springer: Dordrecht, The Netherlands, 2004; pp. 1–17. [Google Scholar] [CrossRef]
- Tudzynski, B.; Sharon, A. Biosynthesis, Biological Role and Application of Fungal Phytohormones. Ind. Appl. 2002, 10, 183–211. [Google Scholar] [CrossRef]
- Dörffling, K.; Petersen, W.; Sprecher, E.; Urbasch, I.; Hanssen, H.P. Abscisic Acid in Phytopathogenic Fungi of the Genera Botrytis, Ceratocystis, Fusarium, and Rhizoctonia. Z. Naturforsch. Sect. C J. Biosci. 1984, 39, 683–684. [Google Scholar] [CrossRef]
- Nambara, E.; Marion-Poll, A. Abscisic Acid Biosynthesis and Catabolism. Annu. Rev. Plant Biol. 2005, 56, 165–185. [Google Scholar] [CrossRef] [Green Version]
- Gong, T.; Shu, D.; Zhao, M.; Zhong, J.; Deng, H.Y.; Tan, H. Isolation of Genes Related to Abscisic Acid Production in Botrytis cinerea TB-3-H8 by CDNA-AFLP. J. Basic Microbiol. 2014, 54, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Otto, M.; Teixeira, P.G.; Vizcaino, M.I.; David, F.; Siewers, V. Integration of a Multi-Step Heterologous Pathway in Saccharomyces cerevisiae for the Production of Abscisic Acid. Microb. Cell Fact. 2019, 18, 205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, Z.; Shu, D.; Sun, Q.; Chen, D.B.; Li, Z.M.; Luo, D.; Yang, J.; Tan, H. The BcLAE1 Is Involved in the Regulation of ABA Biosynthesis in Botrytis cinerea TB-31. Front. Microbiol. 2022, 13, 969499. [Google Scholar] [CrossRef] [PubMed]
- Pinedo, C.; Moraga, J.; Barúa, J.; González-Rodríguez, V.E.; Aleu, J.; Durán-Patrón, R.; Macías-Sánchez, A.J.; Hanson, J.R.; Viaud, M.; Hernández-Galán, R.; et al. Chemically Induced Cryptic Sesquiterpenoids and Expression of Sesquiterpene Cyclases in Botrytis cinerea Revealed New Sporogenic (+)-4-epieremophil-9-en-11-ols. ACS Chem. Biol. 2016, 11, 1391–1400. [Google Scholar] [CrossRef]
- Suárez, I.; da Silva Lima, G.; Conti, R.; Pinedo, C.; Moraga, J.; Barúa, J.; de Oliveira, A.L.L.; Aleu, J.; Durán-Patrón, R.; Macías-Sánchez, A.J.; et al. Structural and Biosynthetic Studies on Eremophilenols Related to the Phytoalexin Capsidiol, Produced by Botrytis cinerea. Phytochemistry 2018, 154, 10–18. [Google Scholar] [CrossRef]
- Christianson, D.W. Structural Biology and Chemistry of the Terpenoid Cyclases. Chem. Rev. 2006, 106, 3412–3442. [Google Scholar] [CrossRef] [PubMed]
- Quin, M.B.; Flynn, C.M.; Schmidt-Dannert, C. Traversing the Fungal Terpenome. Nat. Prod. Rep. 2014, 31, 1449–1473. [Google Scholar] [CrossRef] [Green Version]
- Saikia, S.; Nicholson, M.J.; Young, C.; Parker, E.J.; Scott, B. The Genetic Basis for Indole-Diterpene Chemical Diversity in Filamentous Fungi. Mycol. Res. 2008, 112, 184–199. [Google Scholar] [CrossRef]
- Schumacher, J.; Simon, A.; Cohrs, K.C.; Viaud, M.; Tudzynski, P. The Transcription Factor BcLTF1 Regulates Virulence and Light Responses in the Necrotrophic Plant Pathogen Botrytis cinerea. PLoS Genet. 2014, 10, e1004040. [Google Scholar] [CrossRef] [Green Version]
- Schumacher, J. How Light Affects the Life of Botrytis. Fungal Genet. Biol. 2017, 106, 26–41. [Google Scholar] [CrossRef]
- Kroken, S.; Glass, N.L.; Taylor, J.W.; Yoder, O.C.; Turgeon, B.G. Phylogenomic Analysis of Type I Polyketide Synthase Genes in Pathogenic and Saprobic Ascomycetes. Proc. Natl. Acad. Sci. USA 2003, 100, 15670–15675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalmais, B.; Schumacher, J.; Moraga, J.; Le Pêcheur, P.; Tudzynski, B.; Collado, I.G.; Viaud, M. The Botrytis cinerea Phytotoxin Botcinic Acid Requires Two Polyketide Synthases for Production and Has a Redundant Role in Virulence with Botrydial. Mol. Plant Pathol. 2011, 12, 564–579. [Google Scholar] [CrossRef] [PubMed]
- Porquier, A.; Moraga, J.; Morgant, G.; Dalmais, B.; Simon, A.; Sghyer, H.; Collado, I.G.; Viaud, M. Botcinic Acid Biosynthesis in Botrytis cinerea Relies on a Subtelomeric Gene Cluster Surrounded by Relics of Transposons and Is Regulated by the Zn2Cys6 Transcription Factor BcBoa13. Curr. Genet. 2019, 65, 965–980. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, J. DHN Melanin Biosynthesis in the Plant Pathogenic Fungus Botrytis cinerea Is Based on Two Developmentally Regulated Key Enzyme (PKS)-Encoding Genes. Mol. Microbiol. 2016, 99, 729–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; He, Y.; Zhu, P.; Chen, L.; Wang, Y.; Ni, B.; Xu, L. Loss of Bcbrn1 and Bcpks13 in Botrytis cinerea Not Only Blocks Melanization but Also Increases Vegetative Growth and Virulence. Mol. Plant-Microbe Interact. 2015, 28, 1091–1101. [Google Scholar] [CrossRef] [Green Version]
- Cheung, N.; Tian, L.; Liu, X.; Li, X. The Destructive Fungal Pathogen Botrytis cinerea—Insights from Genes Studied with Mutant Analysis. Pathogens 2020, 9, 923. [Google Scholar] [CrossRef]
- Jeya, M.; Kim, T.S.; Tiwari, M.K.; Li, J.; Zhao, H.; Lee, J.K. The Botrytis cinerea Type III Polyketide Synthase Shows Unprecedented High Catalytic Efficiency toward Long Chain Acyl-CoAs. Mol. Biosyst. 2012, 8, 2864–2867. [Google Scholar] [CrossRef]
- Bushley, K.E.; Turgeon, B.G. Phylogenomics Reveals Subfamilies of Fungal Nonribosomal Peptide Synthetases and Their Evolutionary Relationships. BMC Evol. Biol. 2010, 10, 26. [Google Scholar] [CrossRef] [Green Version]
- Massaroli, M.; Moraga, J.; Bastos Borges, K.; Ramírez-Fernández, J.; Viaud, M.; González Collado, I.; Durán-Patrón, R.; Hernández-Galán, R. A Shared Biosynthetic Pathway for Botcinins and Botrylactones Revealed through Gene Deletions. ChemBioChem 2013, 14, 132–136. [Google Scholar] [CrossRef]
- Collado, I.G.; Sánchez, A.J.M.; Hanson, J.R. Fungal Terpene Metabolites: Biosynthetic Relationships and the Control of the Phytopathogenic Fungus Botrytis cinerea. Nat. Prod. Rep. 2007, 24, 674–686. [Google Scholar] [CrossRef]
- Collado, I.G.; Aleu, J.; Hernandez-Galan, R.; Duran-Patron, R. Botrytis Species: An Intriguing Source of Metabolites with a Wide Range of Biological Activities. Structure, Chemistry and Bioactivity of Metabolites Isolated from Botrytis Species. Curr. Org. Chem. 2000, 4, 1261–1286. [Google Scholar] [CrossRef] [Green Version]
- Bradshaw, A.P.W.; Hanson, J.R.; Nyfeler, R. Studies in Terpenoid Biosynthesis, Part 24. The Formation of the Carbon Skeleton of the Sesquiterpenoid Dihydrobotrydial. J. Chem. Soc. Perkin Trans. 1 1981, 1469–1472. [Google Scholar] [CrossRef]
- Bradshaw, A.P.W.; Hanson, J.R.; Nyfeler, R.; Sadler, I.H. Studies in Terpenoid Biosynthesis. Part 25. The Fate of the Mevalonoid Hydrogen Atoms in the Biosynthesis of the Sesquiterpenoid Dihydrobotrydial. J. Chem. Soc. Perkin Trans. 1 1982, 2187–2192. [Google Scholar] [CrossRef]
- Durán-Patrón, R.; Colmenares, A.J.; Hernández-Galán, R.; Collado, I.G. Some Key Metabolic Intermediates in the Biosynthesis of Botrydial and Related Compounds. Tetrahedron 2001, 57, 1929–1933. [Google Scholar] [CrossRef]
- Wang, C.M.; Hopson, R.; Lin, X.; Cane, D.E. Biosynthesis of the Sesquiterpene Botrydial in Botrytis cinerea. Mechanism and Stereochemistry of the Enzymatic Formation of Presilphiperfolan-8β-ol. J. Am. Chem. Soc. 2009, 131, 8360–8361. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, G.F.; Moraga, J.; Takahashi, J.A.; Viaud, M.; Hanson, J.R.; Galán, R.H.; Collado, I.G. The Formation of Sesquiterpenoid Presilphiperfolane and Cameroonane Metabolites in the Bcbot4 Null Mutant of Botrytis cinerea. Org. Biomol. Chem. 2017, 15, 5357–5363. [Google Scholar] [CrossRef] [Green Version]
- Collado, I.G.; Hanson, J.R.; Sanchez, A.J.M. The Botryane Sesquiterpenoid Metabolism of the Fungus Botrytis cinerea. J. Chem. Res. 2017, 41, 435–440. [Google Scholar] [CrossRef]
- D’Ambrosio, J.M.; Gonorazky, G.; Sueldo, D.J.; Moraga, J.; Di Palma, A.A.; Lamattina, L.; Collado, I.G.; Laxalt, A.M. The Sesquiterpene Botrydial from Botrytis cinerea Induces Phosphatidic Acid Production in Tomato Cell Suspensions. Planta 2018, 247, 1001–1009. [Google Scholar] [CrossRef]
- Vignatti, P.; Gonzalez, M.E.; Jofré, E.C.; Bolívar-Anillo, H.J.; Moraga, J.; Viaud, M.; Collado, I.G.; Pieckenstain, F.L. Botrydial Confers Botrytis cinerea the Ability to Antagonize Soil and Phyllospheric Bacteria. Fungal Biol. 2020, 124, 54–64. [Google Scholar] [CrossRef]
- Moraga, J.; Izquierdo-Bueno Reina, I.; Pinedo, C.; Hernández-Galán, R.; Viaud, M.; Collado, I.G. Impairment of Botrydial Production in Botrytis cinerea Allows the Isolation of Undescribed Polyketides and Reveals New Insights into the Botcinins Biosynthetic Pathway. Phytochemistry 2021, 183, 112627. [Google Scholar] [CrossRef]
- Inomata, M.; Hirai, N.; Yoshida, R.; Ohigashi, H. The Biosynthetic Pathway to Abscisic Acid via Ionylideneethane in the Fungus Botrytis cinerea. Phytochemistry 2004, 65, 2667–2678. [Google Scholar] [CrossRef] [PubMed]
- Mauch-Mani, B.; Mauch, F. The Role of Abscisic Acid in Plant–Pathogen Interactions. Curr. Opin. Plant Biol. 2005, 8, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Asselbergh, B.; Curvers, K.; França, S.C.; Audenaert, K.; Vuylsteke, M.; Van Breusegem, F.; Höfte, M. Resistance to Botrytis cinerea in Sitiens, an Abscisic Acid-Deficient Tomato Mutant, Involves Timely Production of Hydrogen Peroxide and Cell Wall Modifications in the Epidermis. Plant Physiol. 2007, 144, 1863–1877. [Google Scholar] [CrossRef] [Green Version]
- Paddon, C.J.; Westfall, P.J.; Pitera, D.J.; Benjamin, K.; Fisher, K.; McPhee, D.; Leavell, M.D.; Tai, A.; Main, A.; Eng, D.; et al. High-Level Semi-Synthetic Production of the Potent Antimalarial Artemisinin. Nature 2013, 496, 528–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takino, J.; Kozaki, T.; Ozaki, T.; Liu, C.; Minami, A.; Oikawa, H. Elucidation of Biosynthetic Pathway of a Plant Hormone Abscisic Acid in Phytopathogenic Fungi. Biosci. Biotechnol. Biochem. 2019, 83, 1642–1649. [Google Scholar] [CrossRef]
- Suárez, I.; Pinedo, C.; Aleu, J.; Durán-Patrón, R.; Macías-Sánchez, A.J.; Hernández-Galán, R.; Collado, I.G. The Complemented Mutant ComplΔBcstc7niaD, in the STC7 of Botrytis cinerea Led to the Characterization of 11,12,13-tri-nor-Eremophilenols Derivatives. Phytochemistry 2022, 193, 113003. [Google Scholar] [CrossRef]
- Coca-Ruíz, V.; Suárez, I.; Aleu, J.; Collado, I.G. Structures, Occurrences and Biosynthesis of 11,12,13-tri- nor-Sesquiterpenes, an Intriguing Class of Bioactive Metabolites. Plants 2022, 11, 769. [Google Scholar] [CrossRef]
- Pinto, A.A.; Barúa, J.E.; Almeida, M.O.; Viaud, M.; Zorrilla, D.; Collado, I.G.; Macías-Sánchez, A.J.; Durán-Patrón, R. Structural and Biosynthetic Studies of Botrycinereic Acid, a New Cryptic Metabolite from the Fungus Botrytis cinerea. Bioorg. Chem. 2022, 127, 105979. [Google Scholar] [CrossRef]
- Leisen, T.; Werner, J.; Pattar, P.; Safari, N.; Ymeri, E.; Sommer, F.; Schroda, M.; Suárez, I.; Collado, I.G.; Scheuring, D.; et al. Multiple Knockout Mutants Reveal a High Redundancy of Phytotoxic Compounds Contributing to Necrotrophic Pathogenesis of Botrytis cinerea. PLoS Pathog. 2022, 18, e1010367. [Google Scholar] [CrossRef]
- Ronzon, Q.; Zhang, W.; Charote, T.; Casaretto, N.; Frison, G.; Nay, B. Total Synthesis of (+)-Cinereain and (−)-Janoxepin through a Fragment Coupling/Retro-Claisen Rearrangement Cascade. Angew. Chemie Int. Ed. 2022, 61, e202212855. [Google Scholar] [CrossRef]
- Kim, M.Y.; Sohn, J.H.; Jang, J.H.; Ahn, J.S.; Oh, H. Two New Botcinin Derivatives Encountered in the Studies of Secondary Metabolites from the Marine-Derived Fungus Botryotinia sp. SF-5275. J. Antibiot. 2012, 65, 161–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, T.; Wang, G.; Zeng, D.; Chen, H. Cytotoxic Metabolites from Botryotinia fuckeliana A-S-3: An Endophytic Fungus from Ajuga decumbens. Phytochem. Lett. 2015, 13, 206–211. [Google Scholar] [CrossRef]
- Niu, S.; Peng, G.; Xia, J.M.; Xie, C.L.; Li, Z.; Yang, X.W. A New Pimarane Diterpenoid from the Botryotinia fuckeliana Fungus Isolated from Deep-Sea Water. Chem. Biodivers. 2019, 16, e1900519. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Xia, J.M.; Li, Z.; Yang, L.H.; Yi, Z.W.; Xie, C.L.; Peng, G.; Luo, Z.H.; Shao, Z.; Yang, X.W. Aphidicolin Chemistry of the Deep-Sea-Derived Fungus Botryotinia fuckeliana MCCC 3A00494. J. Nat. Prod. 2019, 82, 2307–2331. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Xie, C.L.; Xia, J.M.; Liu, Q.M.; Peng, G.; Liu, G.M.; Yang, X.W. Botryotins A-H, Tetracyclic Diterpenoids Representing Three Carbon Skeletons from a Deep-Sea-Derived Botryotinia fuckeliana. Org. Lett. 2020, 22, 580–583. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.X.; Zhi, D.J.; Ren, H.; Li, Z.Y.; Hu, Q.L.; Liu, Y.H.; Zhang, Z.X.; Fei, D.Q. A New Succinate Derivative from Ajuga decumbens. Nat. Prod. Commun. 2016, 11, 497–498. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Luo, M.; Liu, F.; Wang, D.; Pang, X.; Zhao, T.; Xu, L.; Wu, X.; Xia, M.; Yang, X. Cytochalasan and Tyrosine-Derived Alkaloids from the Marine Sediment-Derived Fungus Westerdykella dispersa and Their Bioactivities. Sci. Rep. 2017, 7, 11956. [Google Scholar] [CrossRef] [Green Version]
- Santos, G.B.; Almeida, M.O.; Cardoso, I.A.; Manfrim, V.; Chagas, F.O.; Toledo, J.S.; Pinzan, C.C.; De Araujo, A.S.; Cruz, A.K.; Pupo, M.T.; et al. The Semisynthetic Landscape of Aphidicolin: Inspiration towards Leishmanicidal Compounds. J. Braz. Chem. Soc. 2014, 25, 1885–1899. [Google Scholar] [CrossRef]
- Baranovskiy, A.G.; Babayeva, N.D.; Suwa, Y.; Gu, J.; Pavlov, Y.I.; Tahirov, T.H. Structural Basis for Inhibition of DNA Replication by Aphidicolin. Nucleic Acids Res. 2014, 42, 14013–14021. [Google Scholar] [CrossRef] [Green Version]
- Reveglia, P.; Cimmino, A.; Masi, M.; Nocera, P.; Berova, N.; Ellestad, G.; Evidente, A. Pimarane Diterpenes: Natural Source, Stereochemical Configuration, and Biological Activity. Chirality 2018, 30, 1115–1134. [Google Scholar] [CrossRef]
- Li, D.; Xu, Y.; Shao, C.L.; Yang, R.Y.; Zheng, C.J.; Chen, Y.Y.; Fu, X.M.; Qian, P.Y.; She, Z.G.; De Voogd, N.J.; et al. Antibacterial Bisabolane-Type Sesquiterpenoids from the Sponge-Derived Fungus Aspergillus sp. Mar. Drugs 2012, 10, 234–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, L.R.; Li, D.Y.; Li, Z.L.; Hua, H.M.; Wang, P.L.; Wu, X. A New Cyclonerol Derivative from a Marine-Derived Fungus Ascotricha sp. ZJ-M-5. Nat. Prod. Res. 2013, 27, 847–850. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.M.; Ju, G.L.; Xiao, L.; Zhang, X.F.; Du, F.Y. Cyclodepsipeptides and Sesquiterpenes from Marine-Derived Fungus Trichothecium roseum and Their Biological Functions. Mar. Drugs 2018, 16, 519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Y.P.; Fang, S.T.; Miao, F.P.; Yin, X.L.; Ji, N.Y. Diterpenes and Sesquiterpenes from the Marine Algicolous Fungus Trichoderma harzianum X-5. J. Nat. Prod. 2018, 81, 2553–2559. [Google Scholar] [CrossRef] [PubMed]
- Prasad, C. Bioactive Cyclic Dipeptides. Peptides 1995, 16, 151–164. [Google Scholar] [CrossRef]
- Ying, J.; Lin, R.; Xu, P.; Wu, Y.; Liu, Y.; Zhao, Y. Prebiotic Formation of Cyclic Dipeptides under Potentially Early Earth Conditions. Sci. Rep. 2018, 8, 936. [Google Scholar] [CrossRef] [Green Version]
- Challis, G.L. Mining Microbial Genomes for New Natural Products and Biosynthetic Pathways. Microbiology 2008, 154, 1555–1569. [Google Scholar] [CrossRef]
- Macheleidt, J.; Mattern, D.J.; Fischer, J.; Netzker, T.; Weber, J.; Schroeckh, V.; Valiante, V.; Brakhage, A.A. Regulation and Role of Fungal Secondary Metabolites. Annu. Rev. Genet. 2016, 50, 371–392. [Google Scholar] [CrossRef]
- Scherlach, K.; Hertweck, C. Triggering Cryptic Natural Product Biosynthesis in Microorganisms. Org. Biomol. Chem. 2009, 7, 1753. [Google Scholar] [CrossRef]















Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva Ripardo-Filho, H.; Coca Ruíz, V.; Suárez, I.; Moraga, J.; Aleu, J.; Collado, I.G. From Genes to Molecules, Secondary Metabolism in Botrytis cinerea: New Insights into Anamorphic and Teleomorphic Stages. Plants 2023, 12, 553. https://doi.org/10.3390/plants12030553
da Silva Ripardo-Filho H, Coca Ruíz V, Suárez I, Moraga J, Aleu J, Collado IG. From Genes to Molecules, Secondary Metabolism in Botrytis cinerea: New Insights into Anamorphic and Teleomorphic Stages. Plants. 2023; 12(3):553. https://doi.org/10.3390/plants12030553
Chicago/Turabian Styleda Silva Ripardo-Filho, Haroldo, Víctor Coca Ruíz, Ivonne Suárez, Javier Moraga, Josefina Aleu, and Isidro G. Collado. 2023. "From Genes to Molecules, Secondary Metabolism in Botrytis cinerea: New Insights into Anamorphic and Teleomorphic Stages" Plants 12, no. 3: 553. https://doi.org/10.3390/plants12030553
APA Styleda Silva Ripardo-Filho, H., Coca Ruíz, V., Suárez, I., Moraga, J., Aleu, J., & Collado, I. G. (2023). From Genes to Molecules, Secondary Metabolism in Botrytis cinerea: New Insights into Anamorphic and Teleomorphic Stages. Plants, 12(3), 553. https://doi.org/10.3390/plants12030553









