Phytochemical Characterization and In Vitro Anti-Inflammatory Evaluation in RAW 264.7 Cells of Jatropha cordata Bark Extracts
Abstract
1. Introduction
2. Results and Discussion
2.1. Yields
2.2. Qualitative Analysis of Phytochemical Compounds
2.3. GC–MS Analysis
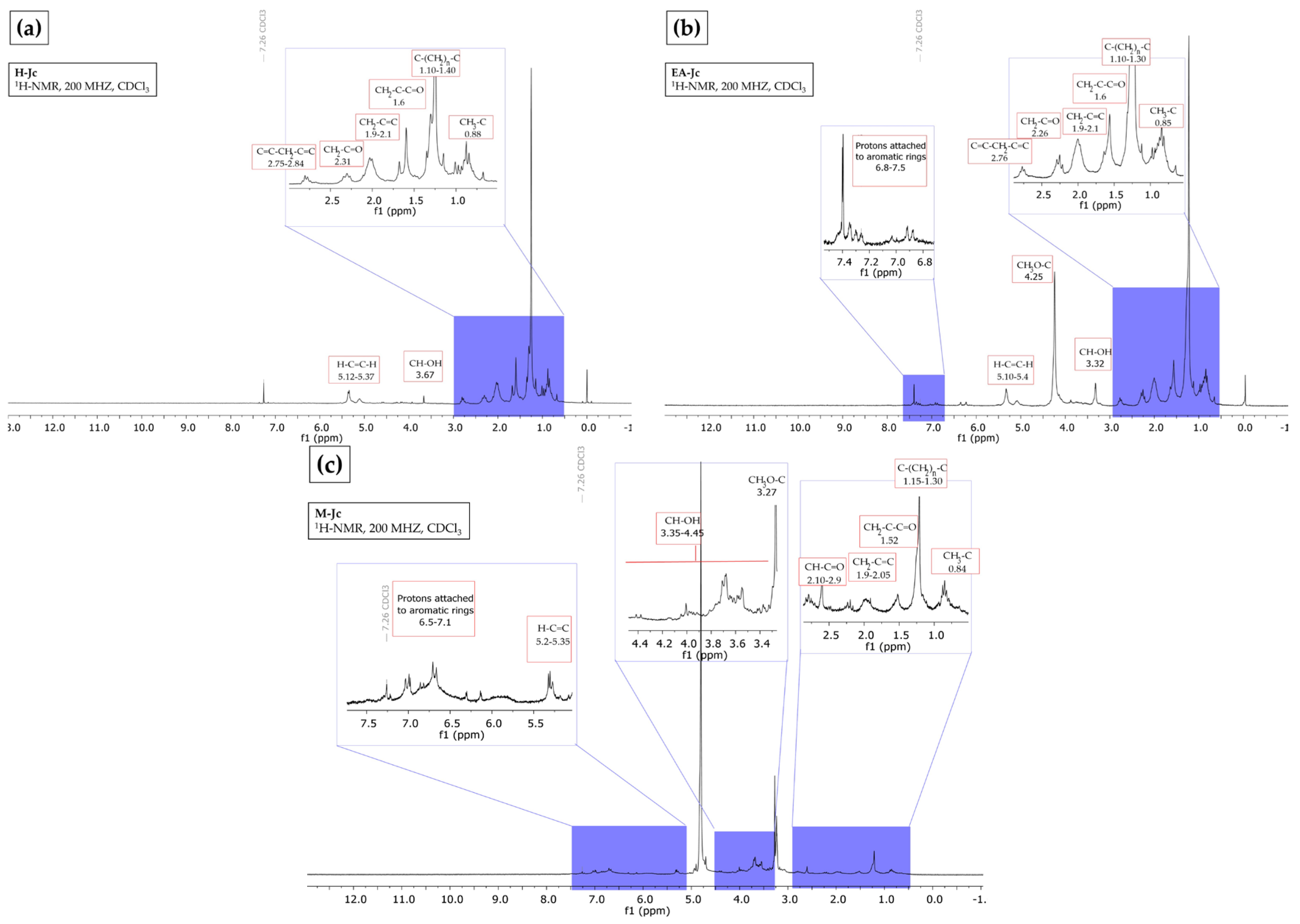
2.4. Nuclear Magnetic Resonance
2.5. In Vitro Anti-Inflammatory Activity
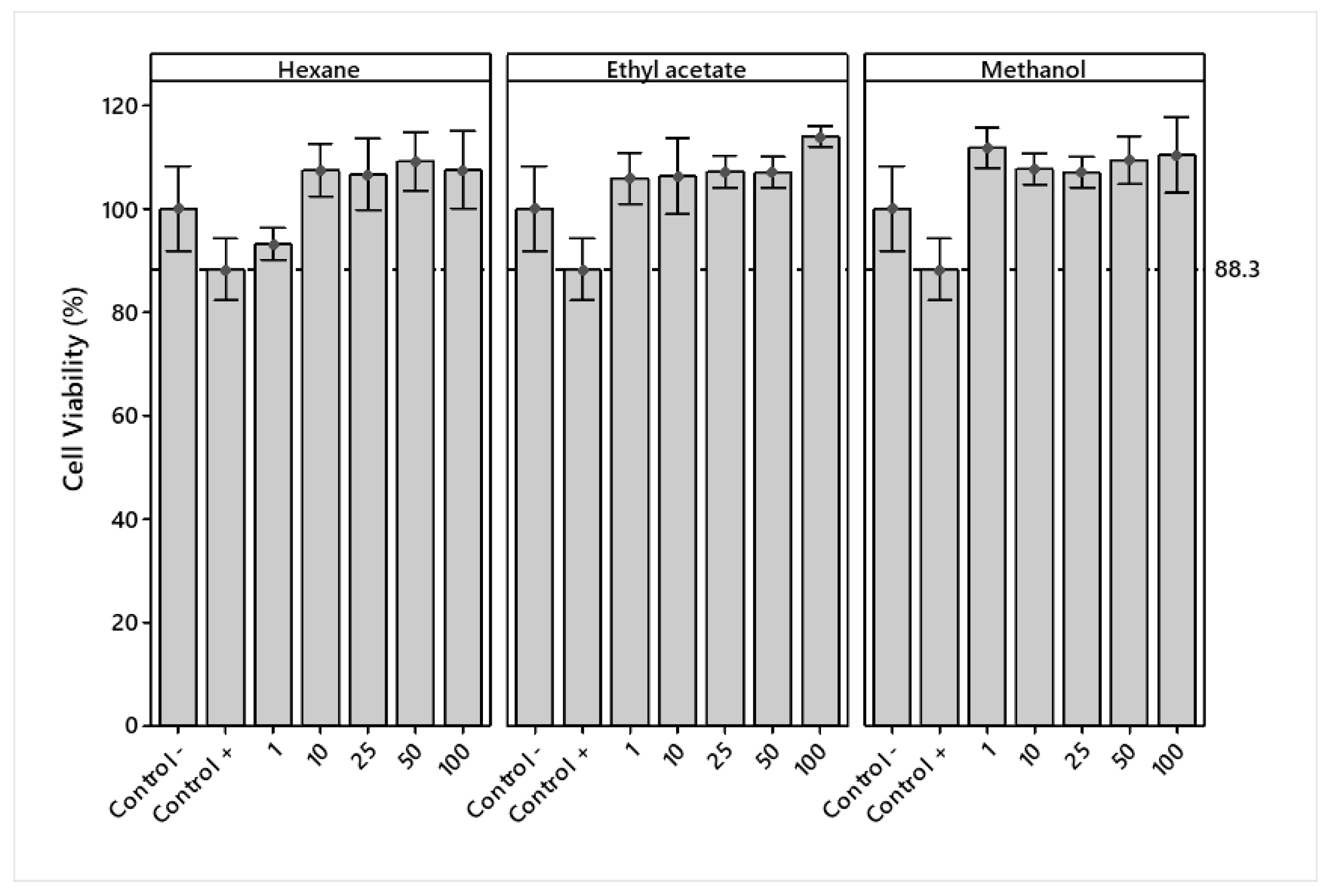
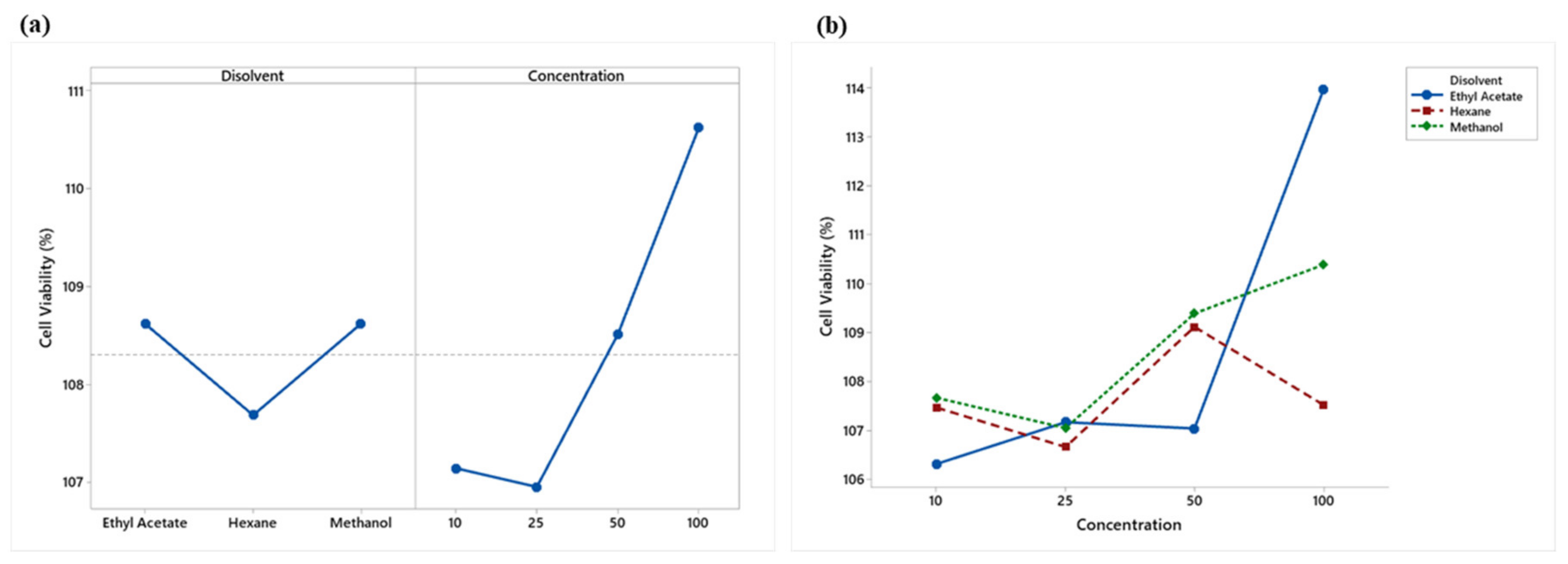
2.5.1. Cell Viability
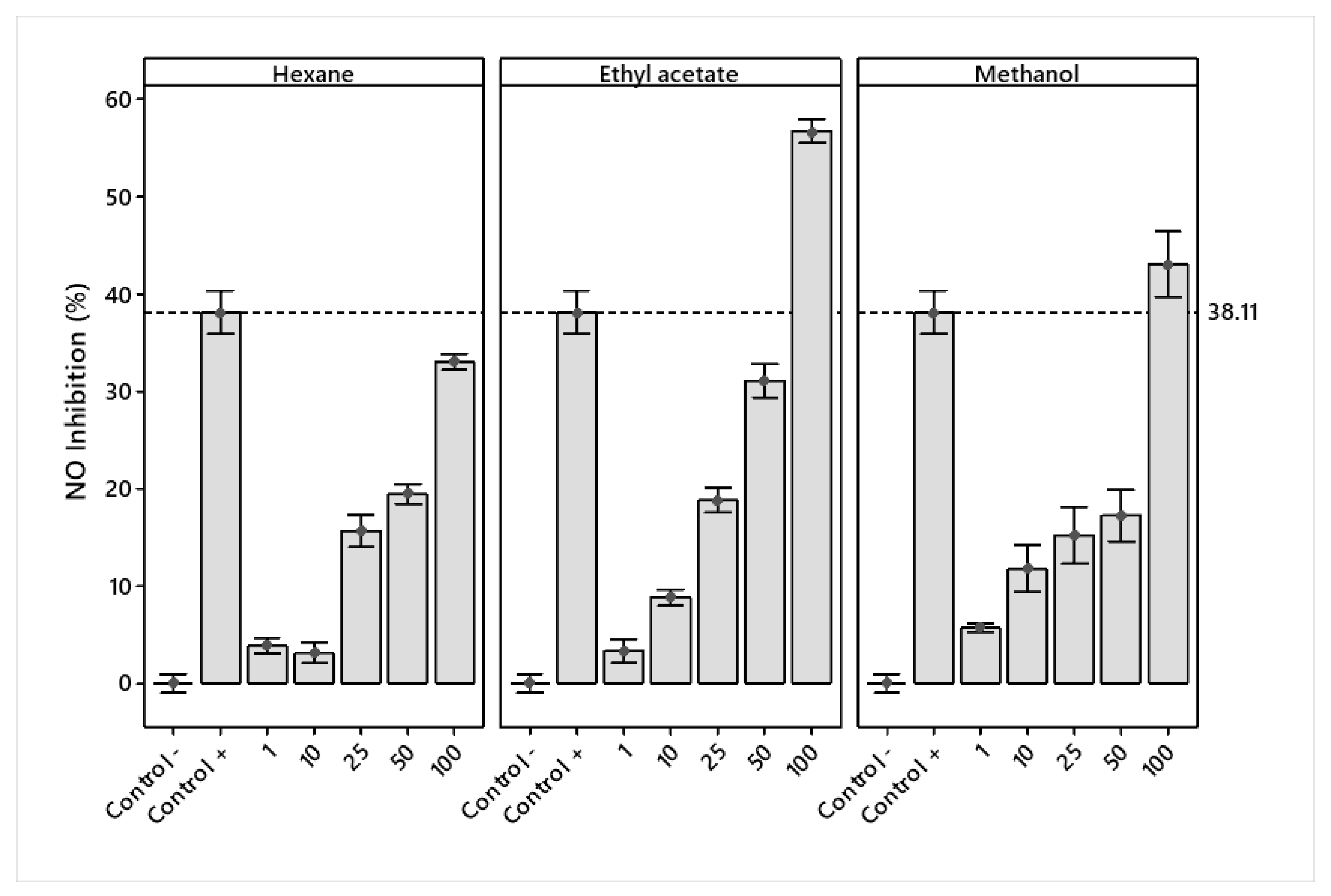
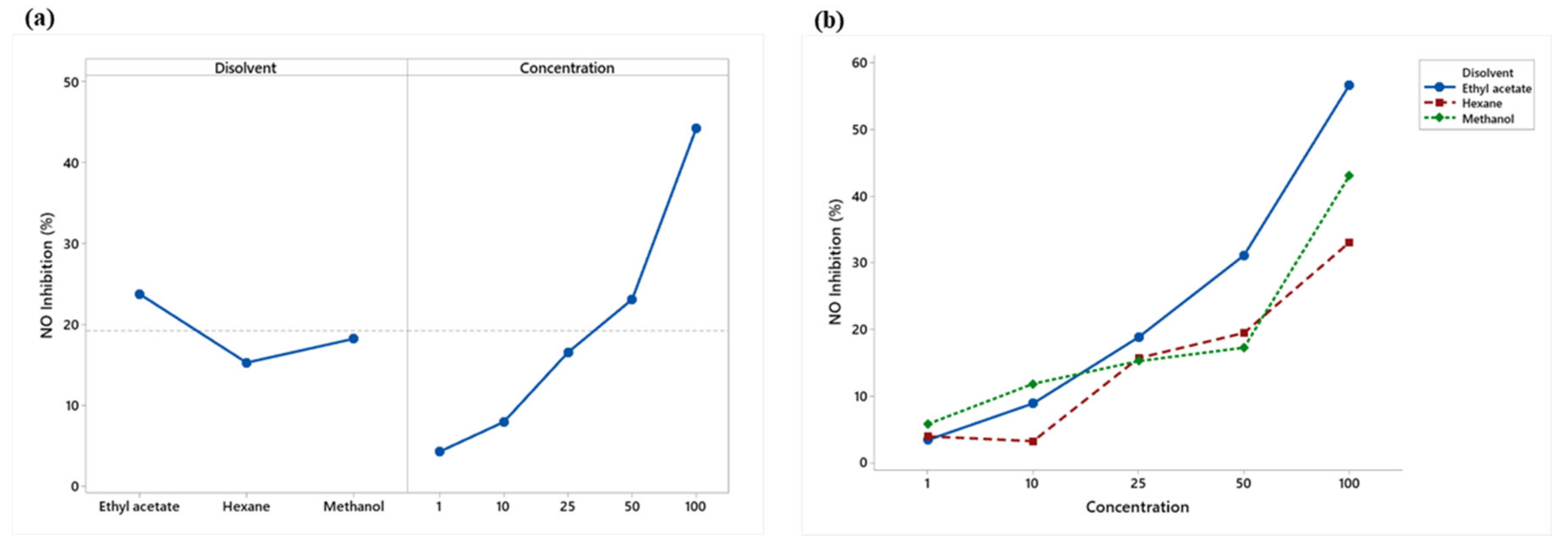
2.5.2. Nitric Oxide Production (NO)
3. Materials and Methods
3.1. Collection of Plant Material
3.2. Extraction
3.3. Qualitative Phytochemical Analysis
3.4. GC–MS Chromatographic Analysis
3.5. Nuclear Magnetic Resonance
3.6. In Vitro Anti-Inflammatory Activity
3.6.1. Cell Culture and Viability Assay
3.6.2. Nitric Oxide (NO) Production
- (1)
- A calibration curve was determined using the concentrations 0, 1, 5, 10, 10, 20, 40, 60, 60, 100 µg/mL of NaNO2.where a = absorbance and concentration of sodium nitrate.
- (2)
- The corrected absorbance, (ac), was calculated for each crude extract, at concentrations of 0, 1, 10, 25, 50, and 100 µg/mL by the difference:where aS = absorbance of the sample and = average absorbance at zero concentration of NaNO2 curve.
- (3)
- The concentration of NaNO2 (µM) present in each of the extracts was determined by the equation:where = micromolar concentration of sodium nitrate.
- (4)
- The percentage of NaNO2 in each extract was obtained by the following equation:
- (5)
- Finally, the percentage inhibition of nitric oxide (%INO) was calculated by the following equation:
3.7. Statistical Analysis
4. Conclusions and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baz, F.E.; Aly, H.; Abd-Alla, H.I.; Saad, S.A. Bioactive flavonoid glycosides and anti-diabetic activity of Jatropha curcas on Streptozotocin-induced diabetic rats. Int. J. Pharm. Sci. Rev. Res. 2014, 29, 143–156. [Google Scholar]
- Hadi, V.; Hotard, M.; Ling, T.; Salinas, Y.G.; Palacios, G.; Connelly, M.; Rivas, F. Evaluation of Jatropha isabelli natural products and their synthetic analogs as potential antimalarial therapeutic agents. Eur. J. Med. Chem. 2013, 65, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Sutthivaiyakit, S.; Mongkolvisut, W.; Prabpai, S.; Kongsaeree, P. Diterpenes, Sesquiterpenes, and a Sesquiterpene-Coumarin Conjugate from Jatropha integerrima. J. Nat. Prod. 2009, 72, 2024–2027. [Google Scholar] [CrossRef] [PubMed]
- Falodun, A.; Imieje, V.; Erharuyi, O.; Joy, A.; Langer, P.; Jacob, M.; Khan, S.; Abaldry, M.; Hamann, M. Isolation of antileishmanial, antimalarial and antimicrobial metabolites from Jatropha multifida. Asian Pac. J. Trop. Biomed. 2014, 4, 374–378. [Google Scholar] [CrossRef]
- Filho, A.A.P.; França, C.R.C.; Oliveira, D.D.S.; Mendes, R.; Gonçalves, J.D.R.S.; Rosa, I.G. Evaluation of the molluscicidal potential of hydroalcoholic extracts of Jatropha gossypiifolia Linnaeus, 1753 on Biomphalaria glabrata (Say, 1818). Rev. Inst. Med. Trop. Sao Paulo 2014, 56, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Erharuyi, O.; Engel-Lutz, N.; Ahomafor, J.; Imieje, V.; Falodun, A.; Nebe, B.; Langer, P. Anticancer activity of five forest crops used in African folklore: Antiproliferative and pro-apoptotic effects. Nat. Prod. Res. 2014, 28, 740–745. [Google Scholar] [CrossRef]
- Pertino, M.; Schmeda-Hirschmann, G.; Rodríguez, J.A.; Theoduloz, C. Gastroprotective effect and cytotoxicity of terpenes from the Paraguayan crude drug “yagua rova” (Jatropha isabelli). J. Ethnopharmacol. 2007, 111, 553–559. [Google Scholar] [CrossRef]
- Ambriz-Pérez, D.L.; Bang, W.Y.; Nair, V.; Angulo-Escalante, M.A.; Cisneros-Zevallos, L.; Heredia, J.B. Protective Role of Flavonoids and Lipophilic Compounds from Jatropha platyphylla on the Suppression of Lipopolysaccharide (LPS)-Induced Inflammation in Macrophage Cells. J. Agric. Food Chem. 2016, 64, 1899–1909. [Google Scholar] [CrossRef]
- Suzuki, T.; Eto, K.; Kubota, Y.; Katayama, T.; Pankasemsuk, T. Antioxidative catechol lignans/neolignans isolated from defatted kernel of Jatropha curcas. J. Wood Sci. 2016, 62, 339–348. [Google Scholar] [CrossRef]
- Batista, P.H.J.; Andrade, J.R.M.; Matos, T.S.; Sousa, T.S.; Pinto, F.C.L.; Silveira, E.R.; Loiola, M.I.B.; Pessoa, O.D.L. Terpenoids and coumarins from Jatropha ribifolia (Pohl) Baill. Chem. Nova 2014, 37, 1010–1014. [Google Scholar]
- dos Santos, A.F.; Fonseca, S.A.; César, F.A.; Albuquerque, M.C.P.D.A.; Santana, J.V.; Santana, A.E.G. A penta-substituted pyridine alkaloid from the rhizome of Jatropha elliptica (Pohl) Muell. Arg. is active against Schistosoma mansoni and Biomphalaria glabrata. Parasitol. Res. 2014, 113, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, J.K.; Froeder, A.L.F.; Janovik, V.; Venturini, T.P.; Pereira, R.P.; Boligon, A.A.; de Brum, T.F.; Alves, S.H.; da Rocha, J.B.T.; Athayde, M.L. Antioxidant capacity, antimicrobial activity and triterpenes isolated from Jatropha isabellei Müll Arg. Nat. Prod. Res. 2013, 27, 1049–1059. [Google Scholar] [CrossRef]
- Popham, R.A. Developmental anatomy of seedling of Jatropha cordata. Ohio J. Sci. 1974, 47, 1–20. [Google Scholar]
- Gámez-Meza, N.; Alday-Lara, P.P.; Makkar, H.P.; Becker, K.; A Medina-Juárez, L. Chemical characterisation of kernels, kernel meals and oils from Jatropha cordata and Jatropha cardiophylla seeds. J. Sci. Food Agric. 2013, 93, 1706–1710. [Google Scholar] [CrossRef] [PubMed]
- Vega-Ruiz, Y.; Hayano-Kanashiro, C.; Gámez-Meza, N.; Medina-Juárez, L. Determination of Chemical Constituents and Antioxidant Activities of Leaves and Stems from Jatropha cinerea (Ortega) Müll. Arg and Jatropha cordata (Ortega) Müll. Arg. Plants 2021, 10, 212. [Google Scholar] [CrossRef]
- Sharma, A.K.; Gangwar, M.; Kumar, D.; Nath, G.; Kumar-Sinha, A.S.; Tripathi, Y.B. Phytochemical characterization, anti-microbial activity and reducing potential of seed oil, latex, machine oil and presscake of Jatropha curcas. Avicenna J. Phytomed. 2016, 6, 366–375. [Google Scholar]
- Nazeema, T.H.; Girija, S. Characterization of the active antiproliferative principles of J. curcas and J. gossypyfolia on HeLa Cell lines. Int. J. Pharm. Pharm. Sci. 2013, 5, 347–355. [Google Scholar]
- Serrano-Gallardo, L.B.; Castillo-Maldonado, I.; Borjón-Ríos, C.-G.; Rivera-Guillén, M.A.; Morán-Martínez, J.; Téllez-López, M.A.; García-Salcedo, J.J.; Pedroza-Escobar, D.; Vega-Menchaca, M.C. Antimicrobial activity and toxicity of plants from northern Mexico. Indian J. Tradit. Knowl. 2017, 16, 203–207. [Google Scholar]
- Halket, J.M.; Waterman, D.; Przyborowska, A.M.; Patel, R.K.P.; Fraser, P.D.; Bramley, P.M. Chemical derivatization and mass spectral libraries in metabolic profiling by GC/MS and LC/MS/MS. J. Exp. Bot. 2005, 56, 219–243. [Google Scholar] [CrossRef]
- Méndez-López, L.F.; Garza-González, E.; Ríos, M.Y.; Ramírez-Cisneros, M.; Alvarez, L.; González-Maya, L.; Sánchez-Carranza, J.N.; Camacho-Corona, M.D.R. Metabolic Profile and Evaluation of Biological Activities of Extracts from the Stems of Cissus trifoliata. Int. J. Mol. Sci. 2020, 21, 930. [Google Scholar] [CrossRef]
- Ogbobe, O.; Akano, V. The physico-chemical properties of the seed and seed oil of Jatropha gossipifolia. Plant Foods Hum. Nutr. 1993, 43, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Hosamani, K.; Katagi, K. Characterization and structure elucidation of 12-hydroxyoctadec-cis-9-enoic acid in Jatropha gossypifolia and Hevea brasiliensis seed oils: A rich source of hydroxy fatty acid. Chem. Phys. Lipids 2008, 152, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, N.; Kumar, P.; Mishra, S.; Verma, S.; Malik, A.; Sharma, S. Insecticidal activity of Jatropha curcas extracts against housefly, Musca domestica. Environ. Sci. Pollut. Res. 2015, 22, 14793–14800. [Google Scholar] [CrossRef]
- Othman, A.R.; Abdullah, N.; Ahmad, S.; Ismail, I.S.; Zakaria, M.P. Elucidation of in-vitro anti-inflammatory bioactive compounds isolated from Jatropha curcas L. plant root. BMC Complement. Altern. Med. 2015, 15, 11. [Google Scholar] [CrossRef] [PubMed]
- Thi, H.T.; Le, B.A.; Le, H.N.T.; Okitsu, K.; Imamura, K.; Takenaka, N.; Van Luu, B.V.; Maeda, Y. Screening of fatty acids, saccharides, and phytochemicals in Jatropha curcas seed kernel as their trimethylsilyl derivatives using gas chromatography/mass spectrometry. J. Chromatogr. B 2018, 1102–1103, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Ahmad, S.H.; Mohamed, M.T.M.; Ab Rahman, M.Z. Antimicrobial Compounds from Leaf Extracts of Jatropha curcas, Psidium guajava, and Andrographis paniculata. Sci. World J. 2014, 2014, 635240. [Google Scholar] [CrossRef] [PubMed]
- Alhaj, W.T.; Moharram, B.A.; Al-Maqtari, T.; Al-Mahbashi, H.M.; Al-Doaiss, A.A. The Potential of Jatropha variegata Fruits as a Natural Contraceptive: Antifertility Activity and Phytochemical Analysis. Evid.-Based Complement. Altern. Med. 2022, 2022, 1365526. [Google Scholar] [CrossRef] [PubMed]
- Ravindranath, N.; Reddy, M.R.; Ramesh, C.; Ramu, R.; Prabhakar, A.; Jagadeesh, B.; Das, B. New Lathyrane and Podocarpane Diterpenoids from Jatropha curcas. Chem. Pharm. Bull. 2004, 52, 608–611. [Google Scholar] [CrossRef]
- Luo, S.-Y.; Pu, R.; Tang, Y.-Q.; Fan, R.-Z.; Yin, S.; Tang, G.-H. Euphane- and 19(10→9)abeo-euphane-type triterpenoids from Jatropha gossypiifolia. Fitoterapia 2020, 143, 104582. [Google Scholar] [CrossRef]
- Oskoueian, E.; Oskoueian, A.; Shakeri, M.; Jahromi, M.F. Benefits and Challenges of Jatropha Meal as Novel Biofeed for Animal Production. Vet. Sci. 2021, 8, 179. [Google Scholar] [CrossRef]
- Rogero, M.M.; Calder, P.C. Obesity, Inflammation, Toll-Like Receptor 4 and Fatty Acids. Nutrients 2018, 10, 432. [Google Scholar] [CrossRef] [PubMed]
- Li, A.C.; Glass, C.K. PPAR- and LXR-dependent pathways controlling lipid metabolism and the development of atherosclerosis. J. Lipid Res. 2004, 45, 2161–2173. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.I.; Naina Mohamed, I. Interdependence of Anti-Inflammatory and Antioxidant Properties of Squalene-Implication for Cardiovascular Health. Life 2021, 11, 103. [Google Scholar] [CrossRef]
- Lou-Bonafonte, J.M.; Martínez-Beamonte, R.; Sanclemente, T.; Surra, J.C.; Herrera-Marcos, L.V.; Sanchez-Marco, J.; Arnal, C.; Osada, J. Current Insights into the Biological Action of Squalene. Mol. Nutr. Food Res. 2018, 62, e1800136. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, L.; Hou, W.; Wu, J. β-Sitosterol Alleviates Inflammatory Response via Inhibiting the Activation of ERK/p38 and NF-κB Pathways in LPS-Exposed BV2 Cells. BioMed Res. Int. 2020, 2020, 7532306. [Google Scholar] [CrossRef] [PubMed]
- Reiter, E.; Jiang, Q.; Christen, S. Anti-inflammatory properties of α- and γ-tocopherol. Mol. Asp. Med. 2007, 28, 668–691. [Google Scholar] [CrossRef] [PubMed]
- Ranard, K.M.; Kuchan, M.J.; Erdman, J.W., Jr. α-Tocopherol, but Not γ-Tocopherol, Attenuates the Expression of Selective Tumor Necrosis Factor-Alpha-Induced Genes in Primary Human Aortic Cell Lines. Lipids 2019, 54, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Brejchova, K.; Balas, L.; Paluchova, V.; Brezinova, M.; Durand, T.; Kuda, O. Understanding FAHFAs: From structure to metabolic regulation. Prog. Lipid Res. 2020, 79, 101053. [Google Scholar] [CrossRef]
- Harborne, J.B. Phytochemical Methods A Guide to Modern Techniques of Plant Analysis, 3rd ed.; Springer Science & Busines Media: Dordrecht, The Netherlands, 1998. [Google Scholar]
- Dasgupta, D.; Rahman, M. Phytochemical Screening and Pharmacological Activities with Respect To Analgesic and Anti-Inflammatory Activity of Methanolic Extract of Jatropha curcas Fruit. Int. J. Pharm. Sci. Res. 2019, 10, 5211–5215. [Google Scholar] [CrossRef]
- Romero-Estrada, A.; Maldonado-Magaña, A.; González-Christen, J.; Bahena, S.M.; Garduño-Ramírez, M.L.; Rodríguez-López, V.; Alvarez, L. Anti-inflammatory and antioxidative effects of six pentacyclic triterpenes isolated from the Mexican copal resin of Bursera copallifera. BMC Complement. Altern. Med. 2016, 16, 422. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Lee, K.; Lee, M.; Ham, I.; Choi, H.-Y. Inhibition of lipopolysaccharide-induced nitric oxide and prostaglandin E2 production by chloroform fraction of Cudrania tricuspidata in RAW 264.7 macrophages. BMC Complement. Altern. Med. 2012, 12, 250. [Google Scholar] [CrossRef] [PubMed]
| Phytochemical constituents | H-Jc | EA-Jc | M-Jc |
|---|---|---|---|
| Tannins | - | ++ | +++ |
| Alkaloids | - | + | + |
| Saponins | - | + | + |
| Flavonoids | - | + | ++ |
| Triterpenes or steroids | +++ | ++ | + |
| Phytochemical Group | Identified Compound | Chemical Formula | Molecular Weight | RT (min) | Abundance (%) | |||
|---|---|---|---|---|---|---|---|---|
| H-Jc | EA-Jc | M-Jc | ||||||
| Aromatic Aldehyde | Benzaldehyde | C7H6O | 106 | 6.26 | ND | 45.34 | 54.46 | |
| Benzaldehyde dimethyl acetal | C9H12O2 | 152 | 8.35 | ND | ND | 1.53 | ||
| Alcohol | Benzyl alcohol | C7H8O | 108 | 7.58 | ND | ND | 1.91 | |
| Terpenoids | 3,7,11,15-tetramethyl-2-hexadecen-1-ol (phytol) | C20H40O | 296 | 17.69 | 0.49 | 1.19 | ND | |
| Squalene | C30H50 | 410 | 28.98 | 1.72 | 1.00 | ND | ||
| Stigmasta-5,22-dien-3-ol | C29H48O | 412 | 34.80 | 4.33 | 2.52 | ND | ||
| γ-sitosterol | C29H50O | 414 | 35.87 | 9.53 | 8.63 | ND | ||
| Fatty acid | Saturated | n-hexadecanoic acid (palmitic acid) | C16H32O2 | 256 | 19.08 | 9.80 | 13.22 | 0.48 |
| Unsaturated | 9,12-octadecadienoic acid | C18H32O2 | 280 | 20.81 | 19.86 | 20.08 | ND | |
| Saturated | octadecanoic acid | C18H36O2 | 284 | 20.93 | 2.23 | 2.86 | ND | |
| Fatty ester | Saturated | Hexadecanoic acid, methyl ester (palmitic acid methyl ester) | C17H34O2 | 270 | 18.57 | 6.58 | 0.71 | 0.71 |
| Unsaturated | 9,12-octadecadienoic acid, methyl ester (linoleic acid methyl ester) | C19H34O2 | 294 | 20.21 | 6.04 | 0.46 | 0.52 | |
| Unsaturated | 6,9,12-octadecatrienoic acid, methyl ester | C19H32O2 | 292 | 20.27 | ND | 1.04 | 1.29 | |
| Unsaturated | 9,12,15-octadecatrienoic acid, methyl ester | C19H32O2 | 292 | 20.29 | 13.91 | ND | ND | |
| Saturated | octadecanoic acid, methyl ester | C19H38O2 | 298 | 20.48 | 1.39 | ND | ND | |
| Saturated | Eicosanoic acid, methyl ester | C21H42O2 | 326 | 22.24 | 0.39 | ND | ND | |
| Saturated | Docosanoic acid, methyl ester | C23H46O2 | 354 | 24.34 | 1.06 | ND | ND | |
| Saturated | Tetracosanoic acid, methyl ester | C25H50O2 | 382 | 27.50 | 1.15 | ND | ND | |
| Saturated | Hexacosanoic acid, methyl ester | C27H54O2 | 410 | 30.26 | 0.74 | ND | ND | |
| Saturated | Octacosanoic acid, methyl ester | C29H58O2 | 438 | 32.68 | 1.17 | ND | ND | |
| Alkane | Nonacosane | C29H60 | 408 | 29.85 | 2.10 | ND | ND | |
| Heptacosane | C27H56 | 380 | 32.19 | 1.30 | ND | ND | ||
| Vitamin E | α-tocopherol | C29H50O2 | 430 | 32.79 | 4.17 | 2.96 | ND | |
| Grand Total | 87.96 | 100.0 | 60.9 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Nevárez, Y.B.; Angulo-Escalante, M.A.; Montes-Avila, J.; Guerrero-Alonso, A.; Christen, J.G.; Hurtado-Díaz, I.; Heredia, J.B.; Quintana-Obregón, E.A.; Alvarez, L. Phytochemical Characterization and In Vitro Anti-Inflammatory Evaluation in RAW 264.7 Cells of Jatropha cordata Bark Extracts. Plants 2023, 12, 560. https://doi.org/10.3390/plants12030560
Jiménez-Nevárez YB, Angulo-Escalante MA, Montes-Avila J, Guerrero-Alonso A, Christen JG, Hurtado-Díaz I, Heredia JB, Quintana-Obregón EA, Alvarez L. Phytochemical Characterization and In Vitro Anti-Inflammatory Evaluation in RAW 264.7 Cells of Jatropha cordata Bark Extracts. Plants. 2023; 12(3):560. https://doi.org/10.3390/plants12030560
Chicago/Turabian StyleJiménez-Nevárez, Yazmín B., Miguel Angel Angulo-Escalante, Julio Montes-Avila, Araceli Guerrero-Alonso, Judith González Christen, Israel Hurtado-Díaz, J. Basilio Heredia, Eber Addí Quintana-Obregón, and Laura Alvarez. 2023. "Phytochemical Characterization and In Vitro Anti-Inflammatory Evaluation in RAW 264.7 Cells of Jatropha cordata Bark Extracts" Plants 12, no. 3: 560. https://doi.org/10.3390/plants12030560
APA StyleJiménez-Nevárez, Y. B., Angulo-Escalante, M. A., Montes-Avila, J., Guerrero-Alonso, A., Christen, J. G., Hurtado-Díaz, I., Heredia, J. B., Quintana-Obregón, E. A., & Alvarez, L. (2023). Phytochemical Characterization and In Vitro Anti-Inflammatory Evaluation in RAW 264.7 Cells of Jatropha cordata Bark Extracts. Plants, 12(3), 560. https://doi.org/10.3390/plants12030560









