Enhancement of Yield, Phytochemical Content and Biological Activity of a Leafy Vegetable (Beta vulgaris L. var. cycla) by Using Organic Amendments as an Alternative to Chemical Fertilizer
Abstract
:1. Introduction
2. Results
2.1. Yield, Water Consumption and Water-Use Efficiency
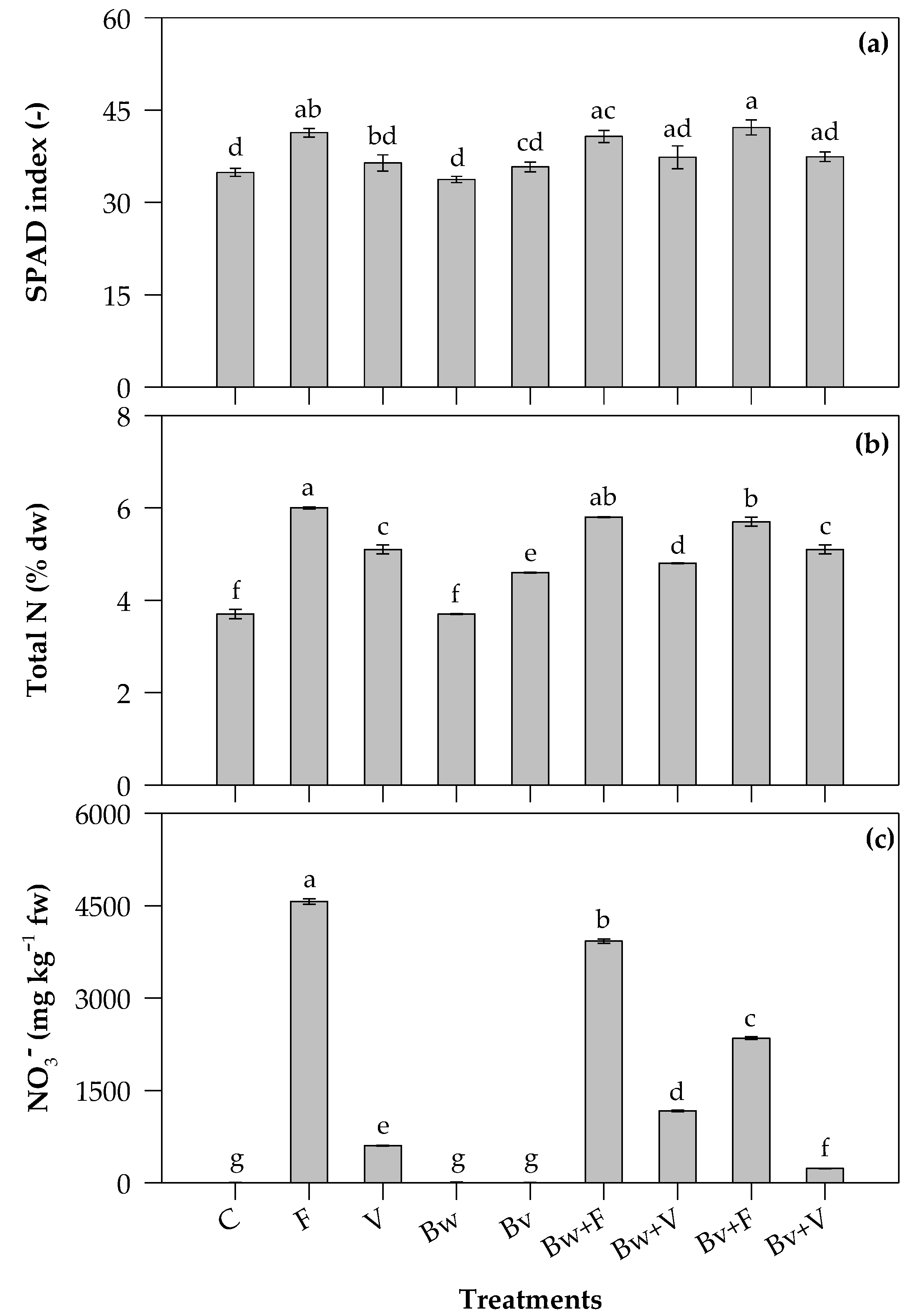
2.2. SPAD Index, Total N and NO3− Content of Leaves
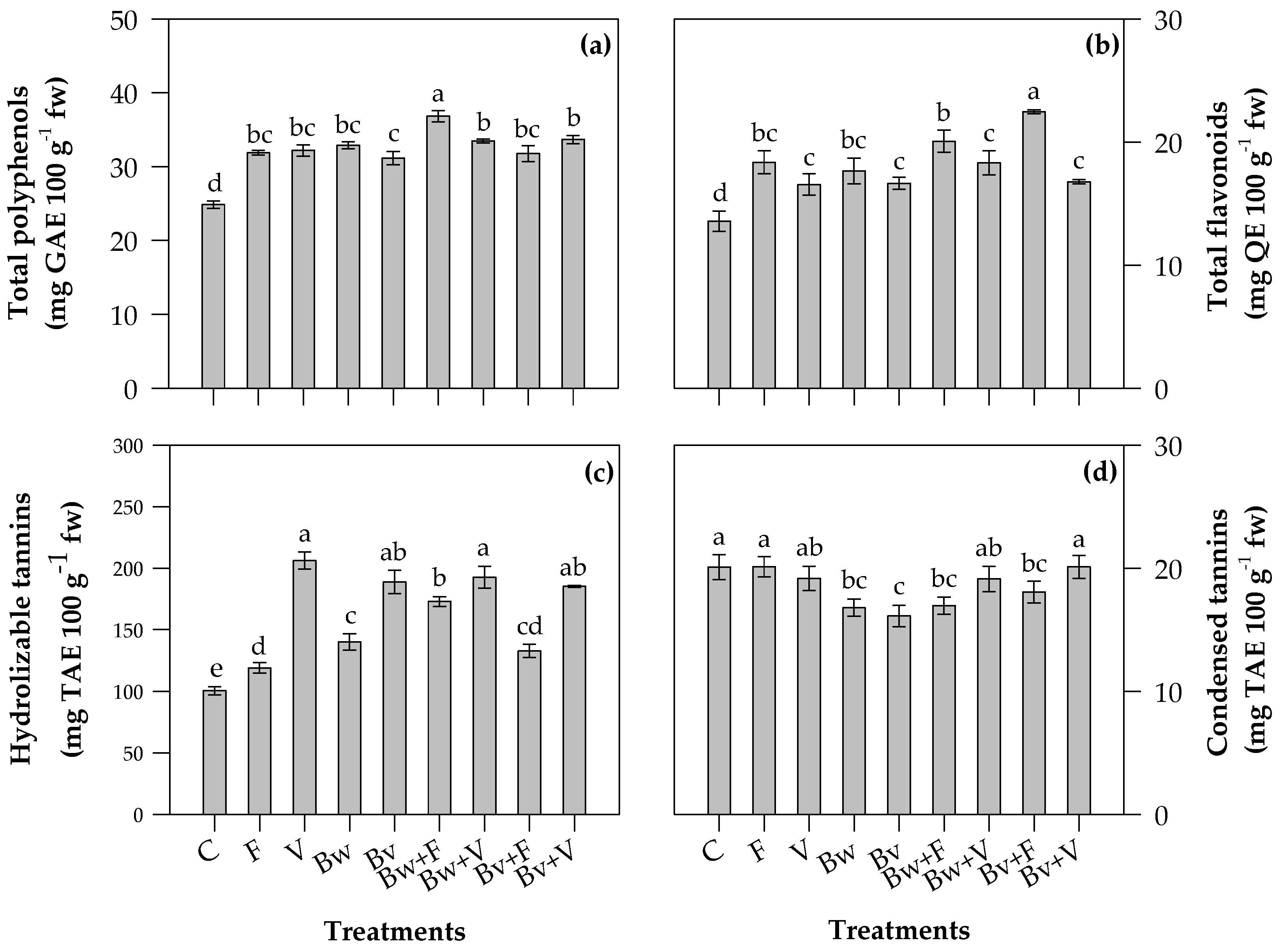
2.3. Phytochemical Contents of Leaves
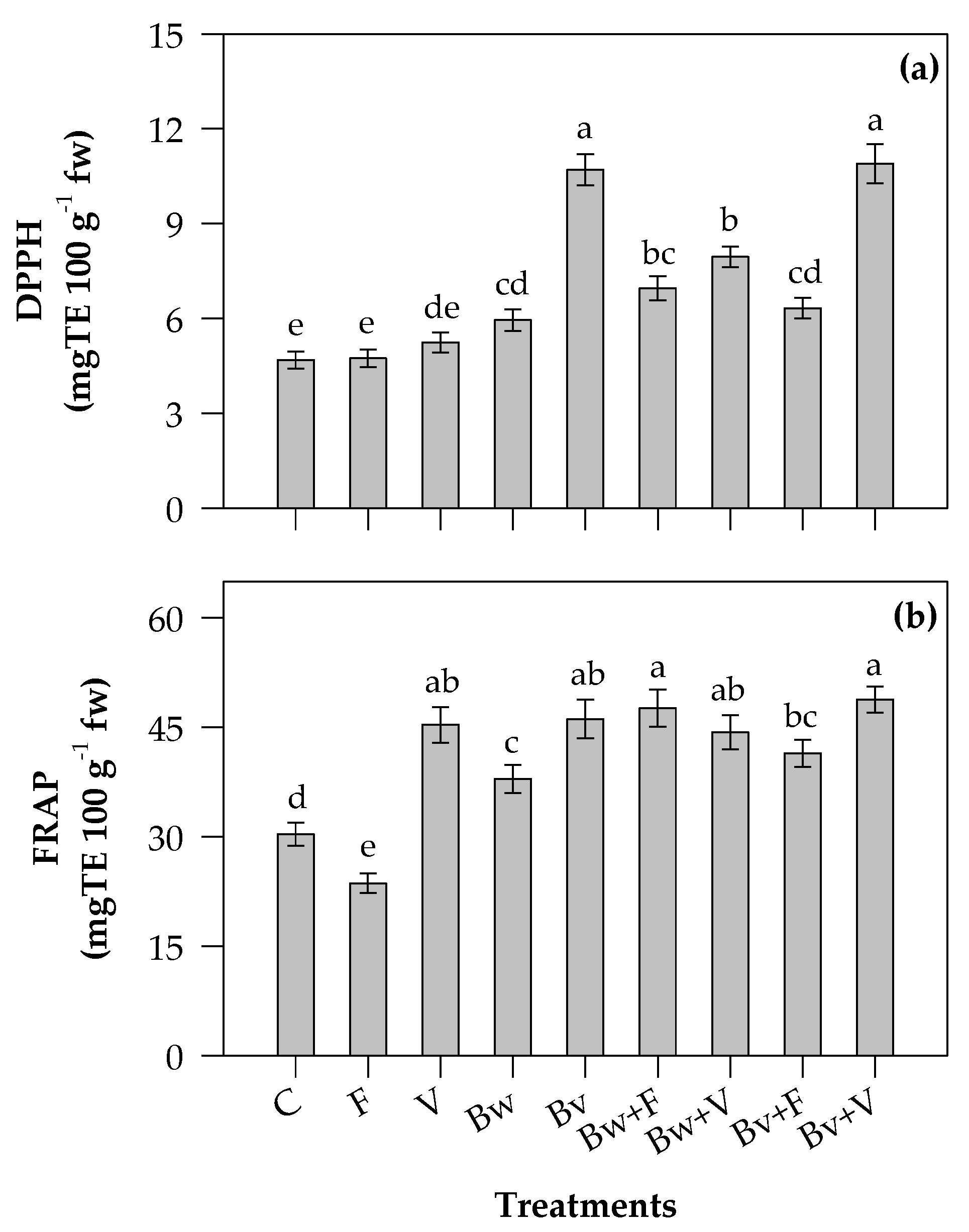
2.4. Antioxidant Activity
3. Discussion
3.1. Swiss Chard Yield
3.2. SPAD Index, Total N and NO3− Content of Leaves
3.3. Phytochemical Contents and Antioxidant Properties of Leaves
4. Materials and Methods
4.1. Experimental Site, Materials Used in the Experiment and Analyses
4.2. Experimental Layout and Setup
4.3. Plant Measurements and Analyses
4.4. Extraction Procedure of Leaf Tissues
4.5. Phytochemical Content Measurements
4.6. Antioxidant Activity Measurement
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Natesh, H.; Abbey, L.; Asiedu, S. An overview of nutritional and antinutritional factors in green leafy vegetables. Horticult. Int. J. 2017, 1, 00011. [Google Scholar] [CrossRef] [Green Version]
- Randhawa, M.A.; Khan, A.A.; Javed, M.S.; Sajid, M.W. Green leafy vegetables: A health promoting source. In Handbook of Fertility; Elsevier: Amsterdam, The Netherlands, 2015; pp. 205–220. [Google Scholar]
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L.T.; Keum, N.; Norat, T.; Greenwood, D.C.; Riboli, E.; Vatten, L.J.; Tonstad, S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality—A systematic review and dose-response meta-analysis of prospective studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef] [Green Version]
- Sacan, O.; Yanardag, R. Antioxidant and antiacetylcholinesterase activities of chard (Beta vulgaris L. var. cicla). Food Chem. Toxicol. 2010, 48, 1275–1280. [Google Scholar] [CrossRef] [PubMed]
- Ninfali, P.; Angelino, D. Nutritional and functional potential of Beta vulgaris cicla and rubra. Fitoterapia 2013, 89, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Pyo, Y.-H.; Lee, T.-C.; Logendra, L.; Rosen, R.T. Antioxidant activity and phenolic compounds of Swiss chard (Beta vulgaris subspecies cycla) extracts. Food Chem. 2004, 85, 19–26. [Google Scholar] [CrossRef]
- Fiedor, J.; Burda, K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef] [Green Version]
- Ivanović, L.; Milašević, I.; Topalović, A.; Ðurović, D.; Mugoša, B.; Knežević, M.; Vrvić, M. Nutritional and phytochemical content of Swiss chard from Montenegro, under different fertilization and irrigation treatments. Br. Food J. 2018, 121, 411–425. [Google Scholar] [CrossRef]
- Kugler, F.; Stintzing, F.C.; Carle, R. Identification of betalains from petioles of differently colored Swiss chard (Beta vulgaris L. ssp. cicla [L.] Alef. cv. Bright Lights) by high-performance liquid chromatography—Electrospray ionization mass spectrometry. J. Agric. Food Chem. 2004, 52, 2975–2981. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Ley, C.M.; Osorio-Revilla, G.; Hernández-Martínez, D.M.; Ramos-Monroy, O.A.; Gallardo-Velázquez, T. Anti-inflammatory activity of betalains: A comprehensive review. Hum. Nutr. Metab. 2021, 25, 200126. [Google Scholar] [CrossRef]
- Dias, J.S. Major classes of phytonutriceuticals in vegetables and health benefits: A review. J. Nutr. Ther. 2012, 1, 31–62. [Google Scholar] [CrossRef]
- Bian, Z.H.; Yang, Q.C.; Liu, W.K. Effects of light quality on the accumulation of phytochemicals in vegetables produced in controlled environments: A review. J. Sci. Food Agric. 2015, 95, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Boo, H.-O.; Heo, B.-G.; Gorinstein, S.; Chon, S.-U. Positive effects of temperature and growth conditions on enzymatic and antioxidant status in lettuce plants. Plant Sci. 2011, 181, 479–484. [Google Scholar] [CrossRef]
- Colonna, E.; Rouphael, Y.; Barbieri, G.; De Pascale, S. Nutritional quality of ten leafy vegetables harvested at two light intensities. Food Chem. 2016, 199, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Kader, A.A. Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biol. Technol. 2000, 20, 207–220. [Google Scholar] [CrossRef] [Green Version]
- Rouphael, Y.; Cardarelli, M.; Bassal, A.; Leonardi, C.; Giuffrida, F.; Colla, G. Vegetable quality as affected by genetic, agronomic and environmental factors. J. Food Agric. Environ. 2012, 10, 680–688. [Google Scholar]
- Snehalatha Reddy, N.; Bhatt, G. Contents of minerals in green leafy vegetables cultivated in soil fortified with different chemical fertilizers. Plant Foods Hum. Nutr. 2001, 56, 1–6. [Google Scholar] [CrossRef]
- Machado, R.; Alves-Pereira, I.; Lourenço, D.; Ferreira, R. Effect of organic compost and inorganic nitrogen fertigation on spinach growth, phytochemical accumulation and antioxidant activity. Heliyon 2020, 6, e05085. [Google Scholar] [CrossRef]
- Mampholo, B.M.; Maboko, M.M.; Soundy, P.; Sivakumar, D. Variety-specific responses of lettuce grown in a gravel-film technique closed hydroponic system to N supply on yield, morphology, phytochemicals, mineral content and safety. J. Integr. Agric. 2018, 17, 2447–2457. [Google Scholar] [CrossRef] [Green Version]
- Stefanelli, D.; Winkler, S.; Jones, R. Reduced nitrogen availability during growth improves quality in red oak lettuce leaves by minimizing nitrate content, and increasing antioxidant capacity and leaf mineral content. Agric. Sci. 2011, 2, 477. [Google Scholar] [CrossRef]
- Argyropoulou, K.; Salahas, G.; Papasavvas, A.; Hela, D. Impact of nitrogen deficiency on biomass production, morphological and biochemical characteristics of sweet basil (Ocimum basilicum L.) plants, cultivated aeroponically. Agric. Food. 2015, 3, 32–42. [Google Scholar]
- Rouphael, Y.; Kyriacou, M.C.; Petropoulos, S.A.; De Pascale, S.; Colla, G. Improving vegetable quality in controlled environments. Sci. Hortic. 2018, 234, 275–289. [Google Scholar] [CrossRef]
- Santamaria, P. Nitrate in vegetables: Toxicity, content, intake and EC regulation. J. Sci. Food Agric. 2006, 86, 10–17. [Google Scholar] [CrossRef]
- Majumdar, D. The blue baby syndrome. Resonance 2003, 8, 20–30. [Google Scholar] [CrossRef]
- Dzida, K.; Pitura, K. The influence of varied nitrogen fertilization on yield and chemical composition of Swiss chard (Beta vulgaris L. var. cicla L.). Acta Sci. Pol. Hortorum Cultus. 2008, 7, 15–24. [Google Scholar]
- Engelbrecht, G.; Ceronio, G.; Motseki, P. Effect of nitrogen levels and sources on production of Swiss Chard (Beta vulgaris var. cicla). S. Afr. J. Plant Soil. 2010, 27, 229–234. [Google Scholar] [CrossRef]
- Hailay, G.; Haymanot, A. The response of Swiss chard (Beta vulgaris L.) to nitrogen levels and intra-row spacing in Debre Berhan Central Ethiopia. J. Hortic. Postharvest Res. 2019, 2, 105–116. [Google Scholar] [CrossRef]
- Kołota, E.; Adamczewska-Sowińska, K.; Czerniak, K. Yield and nutritional value of Swiss chard grown for summer and autumn harvest. J. Agric. Sci. 2010, 2, 20–24. [Google Scholar] [CrossRef] [Green Version]
- Molitorisová, A.; Burke, C. Farm to fork strategy: Animal welfare, EU trade policy, and public participation. Appl. Econ. Perspect. Policy 2022, 1–30. [Google Scholar] [CrossRef]
- European Commission. The European green deal. Eur. Comm. 2019, 53, 24. [Google Scholar]
- Demiraj, E.; Libutti, A.; Malltezi, J.; Rroço, E.; Brahushi, F.; Monteleone, M.; Sulçe, S. Effect of organic amendments on nitrate leaching mitigation in a sandy loam soil of Shkodra district, Albania. Ital. J. Agron. 2018, 13, 93–102. [Google Scholar] [CrossRef] [Green Version]
- Gobbi, V.; Bonato, S.; Nicoletto, C.; Zanin, G. Spent mushroom substrate as organic fertilizer: Vegetable organic trials. In Proceedings of the III International Symposium on Organic Matter Management and Compost Use in Horticulture, Murcia, Spain, 20 April 2015; Volume 1146, pp. 49–56. [Google Scholar]
- Libutti, A.; Mucci, M.; Francavilla, M.; Monteleone, M. Effect of biochar amendment on nitrate retention in a silty clay loam soil. Ital. J. Agron. 2016, 11, 273–276. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Blanco, J.; Lazcano, C.; Christensen, T.H.; Muñoz, P.; Rieradevall, J.; Møller, J.; Antón, A.; Boldrin, A. Compost benefits for agriculture evaluated by life cycle assessment. A review. Agron. Sustain. Dev. 2013, 33, 721–732. [Google Scholar] [CrossRef] [Green Version]
- Nair, V.D.; Nair, P.R.; Dari, B.; Freitas, A.M.; Chatterjee, N.; Pinheiro, F.M. Biochar in the agroecosystem–climate-change–sustainability nexus. Front. Plant Sci. 2017, 8, 2051. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J. Bio-energy in the black. Front. Ecol. Environ. 2007, 5, 381–387. [Google Scholar] [CrossRef]
- Monlau, F.; Francavilla, M.; Sambusiti, C.; Antoniou, N.; Solhy, A.; Libutti, A.; Zabaniotou, A.; Barakat, A.; Monteleone, M. Toward a functional integration of anaerobic digestion and pyrolysis for a sustainable resource management. Comparison between solid-digestate and its derived pyrochar as soil amendment. Appl. Energy 2016, 169, 652–662. [Google Scholar] [CrossRef]
- Zabaniotou, A.; Rovas, D.; Delivand, M.; Francavilla, M.; Libutti, A.; Cammerino, A.; Monteleone, M. Conceptual vision of bioenergy sector development in Mediterranean regions based on decentralized thermochemical systems. Sustain. Energy Technol. Assess. 2017, 23, 33–47. [Google Scholar] [CrossRef]
- Zabaniotou, A.; Rovas, D.; Libutti, A.; Monteleone, M. Boosting circular economy and closing the loop in agriculture: Case study of a small-scale pyrolysis–biochar based system integrated in an olive farm in symbiosis with an olive mill. Environ. Dev. 2015, 14, 22–36. [Google Scholar] [CrossRef]
- Available online: https://webofscience.com (accessed on 22 September 2022).
- Abbey, L.; Young, C.; Teitel-Payne, R.; Howe, K. Evaluation of proportions of vermicompost and coir in a medium for container-grown Swiss chard. Int. J. Veg. Sci. 2012, 18, 109–120. [Google Scholar] [CrossRef]
- Paredes, C.; Cegarra, J.; Bernal, M.; Roig, A. Influence of olive mill wastewater in composting and impact of the compost on a Swiss chard crop and soil properties. Environ. Int. 2005, 31, 305–312. [Google Scholar] [CrossRef]
- Smith, D.C.; Beharee, V.; Hughes, J.C. The effects of composts produced by a simple composting procedure on the yields of Swiss chard (Beta vulgaris L. var. Flavescens) and common bean (Phaseolus vulgaris L. var. Nanus). Sci. Hortic. 2001, 91, 393–406. [Google Scholar] [CrossRef]
- Teshome, Z.T. Effects of banana peel compost rates on Swiss chard growth performance and yield in Shirka district, Oromia, Ethiopia. Heliyon 2022, 8, e10097. [Google Scholar] [CrossRef] [PubMed]
- Libutti, A.; Trotta, V.; Rivelli, A.R. Biochar, vermicompost, and compost as soil organic amendments: Influence on Growth Parameters, Nitrate and Chlorophyll Content of Swiss Chard (Beta vulgaris L. var. cycla). Agronomy 2020, 10, 346. [Google Scholar] [CrossRef] [Green Version]
- Libutti, A.; Rivelli, A.R. Quanti-qualitative response of swiss chard (Beta vulgaris L. var. cycla) to soil amendment with biochar-compost mixtures. Agronomy 2021, 11, 307. [Google Scholar] [CrossRef]
- Rivelli, A.R.; Libutti, A. Effect of Biochar and Inorganic or Organic Fertilizer Co-Application on Soil Properties, Plant Growth and Nutrient Content in Swiss Chard. Agronomy 2022, 12, 2089. [Google Scholar] [CrossRef]
- Sitienei, K.; Opala, P.; Bore, J.; Ochanda, S.; Rioba, N. Effects of Vermicompost, Tithonia Green Manure and Urea on Quality of Swiss Chard (Beta Vulgaris L. Var. cicla L.) in Kenya. Sustain. Agric. Res. 2021, 9, 55–66. [Google Scholar] [CrossRef]
- Joshi, R.; Singh, J.; Vig, A.P. Vermicompost as an effective organic fertilizer and biocontrol agent: Effect on growth, yield and quality of plants. Rev. Environ. Sci. Biotechnol. 2015, 14, 137–159. [Google Scholar] [CrossRef]
- Lazcano, C.; Domínguez, J. The use of vermicompost in sustainable agriculture: Impact on plant growth and soil fertility. In Soil Nutrients; Nova Science Publishers, Inc.: New York, NY, USA, 2011; Volume 10, p. 187. [Google Scholar]
- Xu, C.; Mou, B. Vermicompost affects soil properties and spinach growth, physiology, and nutritional value. HortScience 2016, 51, 847–855. [Google Scholar] [CrossRef] [Green Version]
- Adiloğlu, S.; Eryılmaz Açıkgöz, F.; Solmaz, Y.; Çaktü, E.; Adiloğlu, A.; Science, S. Effect of vermicompost on the growth and yield of lettuce plant (Lactuca sativa L. var. crispa). Int. J. Plant Sci. 2018, 21, 1–5. [Google Scholar] [CrossRef]
- Yang, L.; Zhao, F.; Chang, Q.; Li, T.; Li, F. Effects of vermicomposts on tomato yield and quality and soil fertility in greenhouse under different soil water regimes. Agric. Water Manag. 2015, 160, 98–105. [Google Scholar] [CrossRef]
- Lim, S.L.; Wu, T.Y.; Lim, P.N.; Shak, K.P.Y. The use of vermicompost in organic farming: Overview, effects on soil and economics. J. Sci. Food Agric. 2015, 95, 1143–1156. [Google Scholar] [CrossRef]
- Coulibaly, S.S.; Touré, M.; Kouamé, A.E.; Kambou, I.C.; Soro, S.Y.; Yéo, K.I.; Koné, S.; Zoro, B.I. Vermicompost as an Alternative to Inorganic Fertilizer to Improve Okra Productivity in Côte d’Ivoire. Open J. Soil Sci. 2021, 11, 1. [Google Scholar] [CrossRef]
- Kashem, M.A.; Sarker, A.; Hossain, I.; Islam, M.S. Comparison of the effect of vermicompost and inorganic fertilizers on vegetative growth and fruit production of tomato (Solanum lycopersicum L.). Open J. Soil Sci. 2015, 5, 53. [Google Scholar] [CrossRef] [Green Version]
- Zheng, H.; Wang, Z.; Deng, X.; Zhao, J.; Luo, Y.; Novak, J.; Herbert, S.; Xing, B. Characteristics and nutrient values of biochars produced from giant reed at different temperatures. Bioresour. Technol. 2013, 130, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Liu, Y.; Liu, S.; Li, Z.; Tan, X.; Huang, X.; Zeng, G.; Zhou, L.; Zheng, B. Biochar to improve soil fertility. A review. Agron. Sustain. Dev. 2016, 36, 36. [Google Scholar] [CrossRef]
- Sigua, G.; Novak, J.; Watts, D.; Johnson, M.; Spokas, K. Efficacies of designer biochars in improving biomass and nutrient uptake of winter wheat grown in a hard setting subsoil layer. Chemosphere 2016, 142, 176–183. [Google Scholar] [CrossRef]
- Olszyk, D.; Johnson, M.; Shiroyama, T.; Novak, J.; Cantrell, K.; Watts, D. Effects of Biochar Feedstock and Pyrolysis Temperature on Growth of Corn, Soybean, Lettuce and Carrot; Society for Environmental Toxicology Chemistry, US Environmental Protection Agency: Vancouver, BC, Canada, 2014. [Google Scholar]
- Spokas, K.A.; Cantrell, K.B.; Novak, J.M.; Archer, D.W.; Ippolito, J.A.; Collins, H.P.; Boateng, A.A.; Lima, I.M.; Lamb, M.C.; McAloon, A.J. Biochar: A synthesis of its agronomic impact beyond carbon sequestration. J. Environ. Qual. 2012, 41, 973–989. [Google Scholar] [CrossRef] [Green Version]
- Butnan, S.; Deenik, J.L.; Toomsan, B.; Antal, M.J.; Vityakon, P. Biochar characteristics and application rates affecting corn growth and properties of soils contrasting in texture and mineralogy. Geoderma 2015, 237, 105–116. [Google Scholar] [CrossRef]
- Libutti, A.; Demiraj, E.; Sulce, S.; Monteleone, M. Risk of nitrogen losses from cultivated soils: Effect of biochar amendment. Albanian J. Agric. Sci. 2018, 17, 318–330. [Google Scholar]
- Joseph, S.; Lehmann, J. Biochar for Environmental Management: Science and Technology; Earthscan: London, UK, 2009. [Google Scholar]
- Huang, L.; Gu, M.; Yu, P.; Zhou, C.; Liu, X. Biochar and vermicompost amendments affect substrate properties and plant growth of basil and tomato. Agronomy 2020, 10, 224. [Google Scholar] [CrossRef] [Green Version]
- Álvarez, J.M.; Pasian, C.; Lal, R.; López Núñez, R.; Fernández Martínez, M. Vermicompost and biochar as substitutes of growing media in ornamental-plant production. J. Appl. Hortic. 2017, 19, 205–214. [Google Scholar] [CrossRef]
- Netto, A.T.; Campostrini, E.; de Oliveira, J.G.; Bressan-Smith, R.E. Photosynthetic pigments, nitrogen, chlorophyll a fluorescence and SPAD-502 readings in coffee leaves. Sci. Hortic. 2005, 104, 199–209. [Google Scholar] [CrossRef]
- Castellanos, J.; Uvalle-Bueno, J.; Aguilar-Santelises, A. Manual de Interpretación de Análisis de Suelos y Aguas Agrícolas; Instituto de Capacitación Para la Productividad Agrícola: Chapingo, Mexico, 2000. [Google Scholar]
- Benford, D.; De Boer, J.; Carere, A.; Di Domenico, A.; Johansson, N.; Schrenk, D.; Schoeters, G.; De Voogt, P.; Dellatte, E. Opinion of the scientific panel on contaminants in the food chain on perfluorooctane sulfonate (PFOS), perfluorooctanoic acid (PFOA) and their salts. EFSA J. 2008, 653, 1–131. [Google Scholar]
- Parks, S.; Huett, D.; Campbell, L.; Spohr, L. Nitrate and nitrite in Australian leafy vegetables. Aust. J. Agric. Res. 2008, 59, 632–638. [Google Scholar] [CrossRef]
- Haider, G.; Steffens, D.; Moser, G.; Müller, C.; Kammann, C.I. Biochar reduced nitrate leaching and improved soil moisture content without yield improvements in a four-year field study. Agric. Ecosyst. Environ. 2017, 237, 80–94. [Google Scholar] [CrossRef]
- Libutti, A.; Cammerino, A.R.B.; Francavilla, M.; Monteleone, M. Soil amendment with biochar affects water drainage and nutrient losses by leaching: Experimental evidence under field-grown conditions. Agronomy 2019, 9, 758. [Google Scholar] [CrossRef] [Green Version]
- Chintala, R.; Mollinedo, J.; Schumacher, T.E.; Papiernik, S.K.; Malo, D.D.; Clay, D.E.; Kumar, S.; Gulbrandson, D.W. Nitrate sorption and desorption in biochars from fast pyrolysis. Mesoporous Mater. 2013, 179, 250–257. [Google Scholar] [CrossRef]
- Güereña, D.; Lehmann, J.; Hanley, K.; Enders, A.; Hyland, C.; Riha, S. Nitrogen dynamics following field application of biochar in a temperate North American maize-based production system. Plant Soil. 2013, 365, 239–254. [Google Scholar] [CrossRef]
- Singh Mavi, M.; Singh, G.; Singh, B.P.; Singh Sekhon, B.; Choudhary, O.P.; Sagi, S.; Berry, R. Interactive effects of rice-residue biochar and N-fertilizer on soil functions and crop biomass in contrasting soils. J. Soil Sci. Plant Nutr. 2018, 18, 41–59. [Google Scholar] [CrossRef] [Green Version]
- Herencia, J.F.; Ruiz-Porras, J.; Melero, S.; Garcia-Galavis, P.; Morillo, E.; Maqueda, C. Comparison between organic and mineral fertilization for soil fertility levels, crop macronutrient concentrations, and yield. Agron. J. 2007, 99, 973–983. [Google Scholar] [CrossRef]
- Matallana Gonzalez, M.; Martinez-Tome, M.; Torija Isasa, M. Nitrate and nitrite content in organically cultivated vegetables. Food Addit. Contam. Part B 2010, 3, 19–29. [Google Scholar] [CrossRef]
- Becker, C.; Urlić, B.; Jukić Špika, M.; Kläring, H.-P.; Krumbein, A.; Baldermann, S.; Goreta Ban, S.; Perica, S.; Schwarz, D. Nitrogen limited red and green leaf lettuce accumulate flavonoid glycosides, caffeic acid derivatives, and sucrose while losing chlorophylls, β-carotene and xanthophylls. PLoS ONE 2015, 10, e0142867. [Google Scholar] [CrossRef] [PubMed]
- Larbat, R.; Olsen, K.M.; Slimestad, R.; Løvdal, T.; Bénard, C.; Verheul, M.; Bourgaud, F.; Robin, C.; Lillo, C. Influence of repeated short-term nitrogen limitations on leaf phenolics metabolism in tomato. Phytochemistry 2012, 77, 119–128. [Google Scholar] [CrossRef]
- Wang, D.; Shi, Q.; Wang, X.; Wei, M.; Hu, J.; Liu, J.; Yang, F. Influence of cow manure vermicompost on the growth, metabolite contents, and antioxidant activities of Chinese cabbage (Brassica campestris ssp. chinensis). Biol. Fertil. Soils 2010, 46, 689–696. [Google Scholar] [CrossRef]
- Paolocci, F.; Bovone, T.; Tosti, N.; Arcioni, S.; Damiani, F. Light and an exogenous transcription factor qualitatively and quantitatively affect the biosynthetic pathway of condensed tannins in Lotus corniculatus leaves. J. Exp. Bot. 2005, 56, 1093–1103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harding, S.A.; Xue, L.-J.; Du, L.; Nyamdari, B.; Lindroth, R.L.; Sykes, R.; Davis, M.F.; Tsai, C.-J. Condensed tannin biosynthesis and polymerization synergistically condition carbon use, defense, sink strength and growth in Populus. Tree Physiol. 2014, 34, 1240–1251. [Google Scholar] [CrossRef] [Green Version]
- Fabbrini, M.; D’Amico, F.; Barone, M.; Conti, G.; Mengoli, M.; Brigidi, P.; Turroni, S. Polyphenol and tannin nutraceuticals and their metabolites: How the human gut microbiota influences their properties. Biomolecules 2022, 12, 875. [Google Scholar] [CrossRef]
- Italian Official Gazette. Ministerial Decree 248 (1999): Approval of “Official Methods of Chemical Soil Analysis”. Ordinary Supplement 185 to the Official Journal of the Italian Republic 248, 21 October 1999, Italy. Available online: www.gazzettaufficiale.it/eli/gu/1999/10/21/248/so/185/sg/pdf. (accessed on 2 May 2022).
- EBC. Comparison of European Biochar Certificate Version 4. 8 and IBI Biochar Standards Version 2.0. 2012. Available online: www.european-biochar.org/en/home (accessed on 24 October 2022).
- International Biochar Initiative (IBI). Standardized Product Definition and Product Testing Guidelines for Biochar That Is Used in Soil; IBI-STD-2.0; IBI: Toronto, ON, Canada, 2014. [Google Scholar]
- Schimmelpfennig, S.; Glaser, B. One step forward toward characterization: Some important material properties to distinguish biochars. J. Environ. Qual. 2012, 41, 1001–1013. [Google Scholar] [CrossRef] [Green Version]
- Lamorte, D.; Faraone, I.; Laurenzana, I.; Milella, L.; Trino, S.; De Luca, L.; Del Vecchio, L.; Armentano, M.F.; Sinisgalli, C.; Chiummiento, L. Future in the Past: Azorella glabra Wedd. as a source of new natural compounds with antiproliferative and cytotoxic activity on multiple myeloma cells. Int. J. Mol. Sci. 2018, 19, 3348. [Google Scholar] [CrossRef] [Green Version]
- Bruno, M.R.; Russo, D.; Cetera, P.; Faraone, I.; Lo Giudice, V.; Milella, L.; Todaro, L.; Sinisgalli, C.; Fritsch, C.; Dumarçay, S.; et al. Chemical analysis and antioxidant properties of orange-tree (Citrus sinensis L.) biomass extracts obtained via different extraction techniques. Biofuel Bioprod. Biorefin. 2020, 14, 509–520. [Google Scholar] [CrossRef]
- Broadhurst, R.B.; Jones, W.T. Analysis of condensed tannins using acidified vanillin. J. Sci. Food Agric. 1978, 29, 788–794. [Google Scholar] [CrossRef]
- Hartzfeld, P.W.; Forkner, R.; Hunter, M.D.; Hagerman, A.E. Determination of hydrolyzable tannins (gallotannins and ellagitannins) after reaction with potassium iodate. J. Agric. Food Chem. 2002, 50, 1785–1790. [Google Scholar] [CrossRef] [PubMed]
- Castronuovo, D.; Russo, D.; Libonati, R.; Faraone, I.; Candido, V.; Picuno, P.; Andrade, P.; Valentao, P.; Milella, L. Influence of shading treatment on yield, morphological traits and phenolic profile of sweet basil (Ocimum basilicum L.). Sci. Hortic. 2019, 254, 91–98. [Google Scholar] [CrossRef]
- Russo, D.; Faraone, I.; Labanca, F.; Sinisgalli, C.; Bartolo, M.; Andrade, P.B.; Valentao, P.; Milella, L. Comparison of different green-extraction techniques and determination of the phytochemical profile and antioxidant activity of Echinacea angustifolia L. extracts. Phytochem. Anal. 2019, 30, 547–555. [Google Scholar] [CrossRef] [PubMed]

| Treatment | H (cm) | LA (m2 m−2) | DM (%) | Yield (g fw m−2) | Wc (L m−2) | WUE (g fw L−1) |
|---|---|---|---|---|---|---|
| C | 9.7 ± 0.3 c | 2.4 ± 0.1 b | 7.7 ± 0.6 | 1066 ± 107.3 b | 151 ± 0.6 ab | 7.1 ± 0.7 c |
| F | 15.1 ± 1.4 a | 5.5 ± 0.9 a | 7.9 ± 0.3 | 2798 ± 38.7 a | 163 ± 8.2 ab | 16.9 ± 1.9 a |
| V | 13.6 ± 0.4 ab | 5.2 ± 0.1 a | 6.9 ± 0.4 | 2618 ± 35.3 a | 159 ± 4.2 ab | 16.6 ± 0.5 a |
| Bw | 9.8 ± 0.3 c | 2.4 ± 0.0 b | 7.8 ± 0.3 | 1056 ± 39.5 b | 140 ± 1.5 b | 7.5 ± 0.3 bc |
| Bv | 10.6 ± 0.3 bc | 2.6 ± 0.1 b | 7.2 ± 0.3 | 1184 ± 65.7 b | 139 ± 5.2 b | 8.6 ± 0.7 bc |
| Bw + F | 13.2 ± 1.1 ac | 4.3 ± 0.6 ab | 8.0 ± 0.3 | 2070 ± 337.1 ab | 163 ± 6.9 ab | 12.5 ± 1.7 ab |
| Bw + V | 13.2 ± 1.4 ac | 3.8 ± 0.6 ab | 8.1 ± 0.4 | 1898 ± 372.4 ab | 150 ± 9.1 ab | 12.4 ± 1.7 ac |
| Bv + F | 14.3 ± 0.5 a | 5.4 ± 0.6 a | 7.1 ± 0.2 | 2676 ± 369.5 a | 173 ± 9.3 a | 15.3 ± 1.4 a |
| Bv + V | 14.4 ± 0.4 a | 4.5 ± 0.0 a | 7.1 ± 0.3 | 2461 ± 32.8 a | 165 ± 1.6 ab | 14.9 ± 0.3 a |
| Significance | *** | *** | ns | *** | ** | *** |
| Property | Soil | Vermicompost | Wood Chip Biochar | Vine Pruning Biochar |
|---|---|---|---|---|
| pH (-) | 7.6 ± 0.1 | 7.6 ± 0.1 | 8.9 ± 0.1 | 10.6 ± 0.1 |
| EC ( mS m−1) | 0.6 ± 0.1 | 265.0 ± 0.0 | 52.0 ± 0.0 | 249.0 ± 0.0 |
| Moisture (% dw) | 6.0 ± 0.1 | 4.0 ± 0.2 | 5.6 ± 0.1 | 15.3 ± 0.3 |
| Volatile solids (% dw) | - | 27.5 ± 0.6 | 42.3 ± 0.4 | 15.3 ± 0.3 |
| Ash (% dw) | - | 72.2 ± 0.6 | 4.4 ± 0.2 | 9.9 ± 0.0 |
| Fixed carbon (% dw) | - | 0.2 ± 0.0 | 53.3 ± 0.2 | 74.8 ± 0.3 |
| C (% dw) | 0.8 ± 0.0 | 11.3 ± 0.0 | 68.3 ± 0.1 | 67.7 ± 0.9 |
| H (% dw) | - | 1.5 ± 0.1 | 4.0 ± 0.0 | 2.1 ± 0.0 |
| N (% dw) | 0.2 ± 0.0 | 1.5 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 |
| S (% dw) | - | 0.3 ± 0.0 | 0.03 ± 0.0 | 0.2 ± 0.0 |
| Corg (% dw) | 0.6 ± 0.0 | 7.8 ± 0.1 | 66.3 ± 0.1 | 67.0 ± 0.9 |
| O (% dw) | - | 5.2 ± 0.2 | 22.3 ± 0.3 | 17.9 ± 1.5 |
| H/Corg (-) | - | - | 0.7 ± 0.0 | 0.4 ± 0.0 |
| O/Corg (-) | - | - | 0.4 ± 0.0 | 0.2 ± 0.0 |
| C/N (-) | 3.9 ± 0.1 | 5.0 ± 0.2 | 67.2 ± 2.0 | 66.2 ± 0.1 |
| NO3− (mg kg−1) | 49.1 ± 1.9 | 11574 ± 445 | <0.1 | <0.1 |
| NH4+ (mg kg−1) | <0.1 | 27.7 ± 0.8 | <0.1 | <0.1 |
| Treatment | Extraction Yield (%) |
|---|---|
| C | 5.10 |
| F | 5.79 |
| V | 5.77 |
| Bw | 5.91 |
| Bv | 5.69 |
| Bw + F | 6.67 |
| Bw + V | 6.11 |
| Bv + F | 5.51 |
| Bv + V | 5.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Libutti, A.; Russo, D.; Lela, L.; Ponticelli, M.; Milella, L.; Rivelli, A.R. Enhancement of Yield, Phytochemical Content and Biological Activity of a Leafy Vegetable (Beta vulgaris L. var. cycla) by Using Organic Amendments as an Alternative to Chemical Fertilizer. Plants 2023, 12, 569. https://doi.org/10.3390/plants12030569
Libutti A, Russo D, Lela L, Ponticelli M, Milella L, Rivelli AR. Enhancement of Yield, Phytochemical Content and Biological Activity of a Leafy Vegetable (Beta vulgaris L. var. cycla) by Using Organic Amendments as an Alternative to Chemical Fertilizer. Plants. 2023; 12(3):569. https://doi.org/10.3390/plants12030569
Chicago/Turabian StyleLibutti, Angela, Daniela Russo, Ludovica Lela, Maria Ponticelli, Luigi Milella, and Anna Rita Rivelli. 2023. "Enhancement of Yield, Phytochemical Content and Biological Activity of a Leafy Vegetable (Beta vulgaris L. var. cycla) by Using Organic Amendments as an Alternative to Chemical Fertilizer" Plants 12, no. 3: 569. https://doi.org/10.3390/plants12030569
APA StyleLibutti, A., Russo, D., Lela, L., Ponticelli, M., Milella, L., & Rivelli, A. R. (2023). Enhancement of Yield, Phytochemical Content and Biological Activity of a Leafy Vegetable (Beta vulgaris L. var. cycla) by Using Organic Amendments as an Alternative to Chemical Fertilizer. Plants, 12(3), 569. https://doi.org/10.3390/plants12030569







