Genetic Improvement of Camelina sativa (L.) Crantz: Opportunities and Challenges
Abstract
:1. Introduction
2. Cultivation
3. Uses and Potential
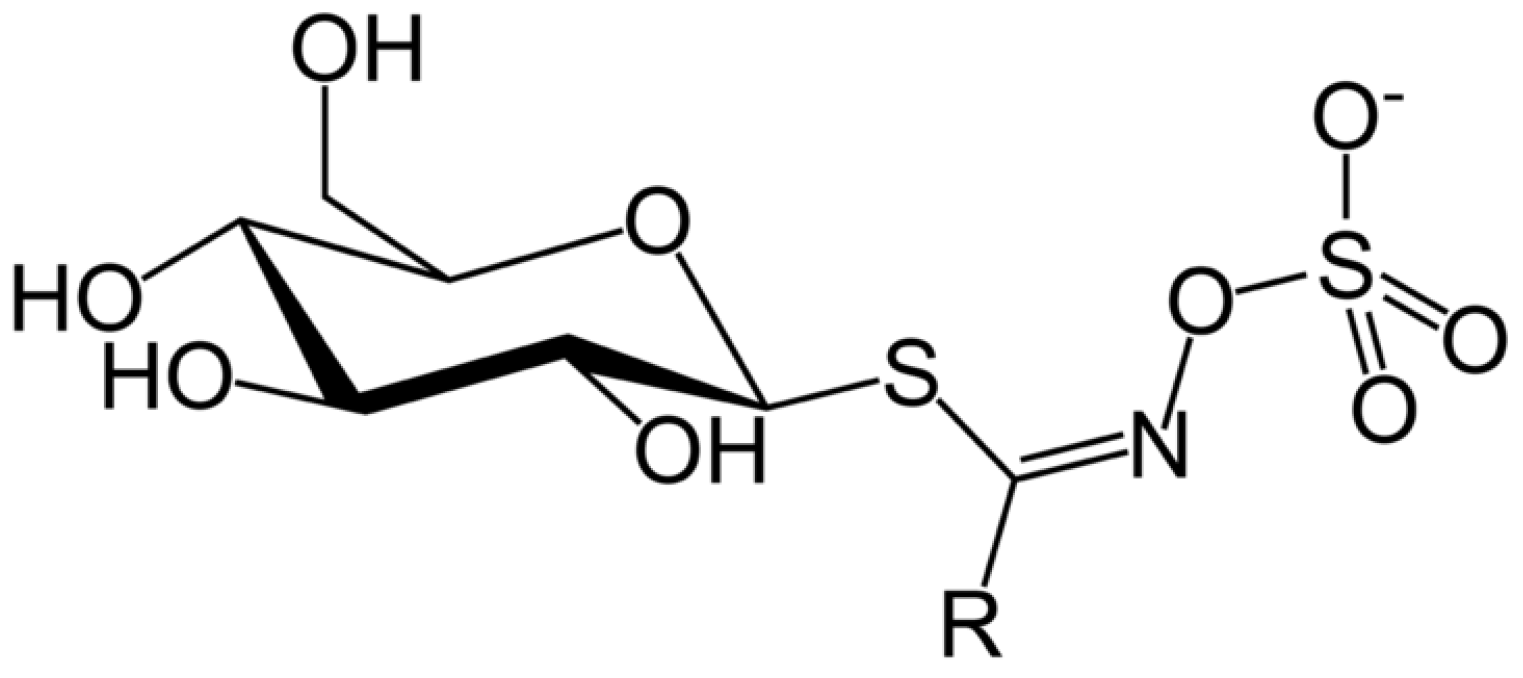
4. Antinutritional Compounds: Glucosinolates
5. Genetic Resources and Varieties Constitution
6. Biotechnological Approach
6.1. GMO Technology
6.2. GE Technology
7. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zubr, J. Oil-Seed Crop: Camelina Sativa. Ind. Crops Prod. 1997, 6, 113–119. [Google Scholar] [CrossRef]
- Vollmann, J.; Moritz, T.; Kargl, C.; Baumgartner, S.; Wagentristl, H. Agronomic Evaluation of Camelina Genotypes Selected for Seed Quality Characteristics. Ind. Crops Prod. 2007, 26, 270–277. [Google Scholar] [CrossRef]
- Berti, M.; Gesch, R.; Eynck, C.; Anderson, J.; Cermak, S. Camelina Uses, Genetics, Genomics, Production, and Management. Ind. Crops Prod. 2016, 94, 690–710. [Google Scholar] [CrossRef]
- Francis, A.; Warwick, S.I. The Biology of Canadian Weeds. 142. Camelina Alyssum (Mill.) Thell.; C. Microcarpa Andrz. Ex DC.; C. Sativa (L.) Crantz. Can. J. Plant Sci. 2009, 89, 791–810. [Google Scholar] [CrossRef]
- Sainger, M.; Jaiwal, A.; Sainger, P.A.; Chaudhary, D.; Jaiwal, R.; Jaiwal, P.K. Advances in Genetic Improvement of Camelina sativa for Biofuel and Industrial Bio-Products. Renew. Sustain. Energy Rev. 2017, 68, 623–637. [Google Scholar] [CrossRef]
- Ghamkhar, K.; Croser, J.; Aryamanesh, N.; Campbell, M.; Kon’kova, N.; Francis, C. Camelina (Camelina sativa (L.) Crantz) as an Alternative Oilseed: Molecular and Ecogeographic Analyses. Genome 2010, 53, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Falk, K.C. Camelina (Camelina Sativa). Biofuel Crops Prod. Physiol. Genet. 2013, 369–391. [Google Scholar] [CrossRef] [Green Version]
- Larsson, M. Cultivation and Processing of Linum Usitatissimum and Camelina sativa in Southern Scandinavia during the Roman Iron Age. Veg. Hist. Archaeobotany 2013, 22, 509–520. [Google Scholar] [CrossRef]
- Masella, P.; Galasso, I. A Comparative Cradle-to-Gate Life Cycle Study of Bio-Energy Feedstock from Camelina Sativa, an Italian Case Study. Sustainability 2020, 12, 9590. [Google Scholar] [CrossRef]
- Gao, Y.; Jiang, C.; Zhang, Y.; Liu, L.; Wang, Y.; Kim, D.-S.; Yu, J.; Diao, J.; Wu, N.; Chen, M.; et al. Agronomic Performance of Camelina Genotypes Selected for Seed Yield and Quality Characteristics in Eastern China. Ind. Crops Prod. 2022, 184, 115077. [Google Scholar] [CrossRef]
- Masella, P.; Martinelli, T.; Galasso, I. Agronomic Evaluation and Phenotypic Plasticity of Camelina sativa Growing in Lombardia, Italy. Crop Pasture Sci. 2014, 65, 453–460. [Google Scholar] [CrossRef]
- Hergert, G.W.; Margheim, J.F.; Pavlista, A.D.; Martin, D.L.; Supalla, R.J.; Isbell, T.A. Yield, Irrigation Response, and Water Productivity of Deficit to Fully Irrigated Spring Canola. Agric. Water Manag. 2016, 168, 96–103. [Google Scholar] [CrossRef] [Green Version]
- Matteo, R.; D’Avino, L.; Ramirez-Cando, L.J.; Pagnotta, E.; Angelini, L.G.; Spugnoli, P.; Tavarini, S.; Ugolini, L.; Foschi, L.; Lazzeri, L. Camelina (Camelina sativa L. Crantz) under Low-Input Management Systems in Northern Italy: Yields, Chemical Characterization and Environmental Sustainability. Ital. J. Agron. 2020, 15, 132–143. [Google Scholar] [CrossRef]
- Orczewska-Dudek, S.; Pietras, M.; Puchała, M.; Nowak, J. Oil and Camelina Cake as Sources of Polyunsaturated Fatty Acids in the Diets of Laying Hens: Effect on Hen Performance, Fatty Acid Profile of Yolk Lipids, and Egg Sensory Quality. Ann. Anim. Sci. 2020, 20, 1365–1377. [Google Scholar] [CrossRef]
- Martinelli, T.; Galasso, I. Phenological Growth Stages of Camelina sativa According to the Extended BBCH Scale. Ann. Appl. Biol. 2011, 158, 87–94. [Google Scholar] [CrossRef]
- King, K.; Li, H.; Kang, J.; Lu, C. Mapping Quantitative Trait Loci for Seed Traits in Camelina Sativa. Theor. Appl. Genet. 2019, 132, 2567–2577. [Google Scholar] [CrossRef]
- Angelini, L.G.; Abou Chehade, L.; Foschi, L.; Tavarini, S. Performance and Potentiality of Camelina (Camelina sativa L. Crantz) Genotypes in Response to Sowing Date under Mediterranean Environment. Agronomy 2020, 10, 1929. [Google Scholar] [CrossRef]
- Russo, R.; Reggiani, R. Antinutritive Compounds in Twelve <I>Camelina sativa </I>Genotypes. Am. J. Plant Sci. 2012, 03, 1408–1412. [Google Scholar] [CrossRef] [Green Version]
- Neupane, D.; Lohaus, R.H.; Solomon, J.K.Q.; Cushman, J.C. Realizing the Potential of Camelina sativa as a Bioenergy Crop for a Changing Global Climate. Plants 2022, 11, 772. [Google Scholar] [CrossRef]
- Abbass, K.; Qasim, M.Z.; Song, H.; Murshed, M.; Mahmood, H.; Younis, I. A Review of the Global Climate Change Impacts, Adaptation, and Sustainable Mitigation Measures. Environ. Sci. Pollut. Res. 2022, 29, 42539–42559. [Google Scholar] [CrossRef]
- Rostami Ahmadvandi, H.; Faghihi, A. Adapted Oilseed Crops with the Ability to Grow Economically in Dryland Conditions in Iran. Agrotech. Ind. Crops 2021, 1, 122–128. [Google Scholar] [CrossRef]
- Zanetti, F.; Eynck, C.; Christou, M.; Krzyżaniak, M.; Righini, D.; Alexopoulou, E.; Stolarski, M.J.; Van Loo, E.N.; Puttick, D.; Monti, A. Agronomic Performance and Seed Quality Attributes of Camelina (Camelina sativa L. Crantz) in Multi-Environment Trials across Europe and Canada. Ind. Crops Prod. 2017, 107, 602–608. [Google Scholar] [CrossRef] [Green Version]
- Gore, M.; Kurt, O. Evaluation of Camelina Genotypes Grown in Winter at Different Sowing Times in Northern Turkey Ecological Conditions in Terms of Yield and Oil Ratio. Agrotech. Ind. Crops 2022, 31, 1397–1404. [Google Scholar] [CrossRef]
- Blackshaw, R.; Johnson, E.; Gan, Y.; May, W.; McAndrew, D.; Barthet, V.; McDonald, T.; Wispinski, D. Alternative Oilseed Crops for Biodiesel Feedstock on the Canadian Prairies. Can. J. Plant Sci. 2011, 91, 889–896. [Google Scholar] [CrossRef]
- Berti, M.; Gesch, R.; Johnson, B.; Ji, Y.; Seames, W.; Aponte, A. Double- and Relay-Cropping of Energy Crops in the Northern Great Plains, USA. Ind. Crops Prod. 2015, 75, 26–34. [Google Scholar] [CrossRef]
- Estakhr, A.; Ranjbar, G. The Preliminary Study of Camelina Compatibility as a New Oil Crop in the Temperate Region of Fars Province. Agrotech. Ind. Crops 2021, 1, 77–84. [Google Scholar] [CrossRef]
- Zubr, J. Qualitative Variation of Camelina sativa Seed from Different Locations. Ind. Crops Prod. 2003, 17, 161–169. [Google Scholar] [CrossRef]
- Sydor, M.; Kurasiak-Popowska, D.; Stuper-Szablewska, K.; Rogoziński, T. Camelina Sativa. Status Quo and Future Perspectives. Ind. Crops Prod. 2022, 187, 115531. [Google Scholar] [CrossRef]
- Tejera, N.; Vauzour, D.; Betancor, M.B.; Sayanova, O.; Usher, S.; Cochard, M.; Rigby, N.; Ruiz-Lopez, N.; Menoyo, D.; Tocher, D.R.; et al. A Transgenic Camelina sativa Seed Oil Effectively Replaces Fish Oil as a Dietary Source of Eicosapentaenoic Acid in Mice. J. Nutr. 2016, 146, 227–235. [Google Scholar] [CrossRef] [Green Version]
- West, A.L.; Miles, E.A.; Lillycrop, K.A.; Napier, J.A.; Calder, P.C.; Burdge, G.C. Genetically Modified Plants Are an Alternative to Oily Fish for Providing N-3 Polyunsaturated Fatty Acids in the Human Diet: A Summary of the Findings of a Biotechnology and Biological Sciences Research Council Funded Project. Nutr. Bull. 2021, 46, 60–68. [Google Scholar] [CrossRef]
- Dharavath, R.N.; Singh, S.; Chaturvedi, S.; Luqman, S. Camelina sativa (L.) Crantz A Mercantile Crop with Speckled Pharmacological Activities. Ann. Phytomedicine Int. J. 2016, 5, 6–26. [Google Scholar] [CrossRef]
- Mondor, M.; Hernández-Álvarez, A.J. Camelina sativa Composition, Attributes, and Applications: A Review. Eur. J. Lipid Sci. Technol. 2022, 124, 2100035. [Google Scholar] [CrossRef]
- Musazadeh, V.; Dehghan, P.; Azadmard-Damirchi, S. Effectiveness of Co-Administration of Camelina Oil and Caloric Restriction on Cardiometabolic Risk Factors, Liver Function and Mental Health in Patients with Non-Alcoholic Fatty Liver Disease: A Blinded Randomized Controlled Trial Protocol. J. Nutr. Food Secur. 2022, 9, 379–387. [Google Scholar] [CrossRef]
- Jaśkiewicz, T.; Sagan, A.; Puzio, I. Effect of the Camelina sativa Oil on the Performance, Essential Fatty Acid Level in Tissues and Fat-Soluble Vitamins Content in the Livers of Broiler Chickens. Livest. Sci. 2014, 165, 74–79. [Google Scholar] [CrossRef]
- Orczewska-Dudek, S.; Pietras, M. The Effect of Dietary Camelina sativa Oil or Cake in the Diets of Broiler Chickens on Growth Performance, Fatty Acid Profile, and Sensory Quality of Meat. Animals 2019, 9, 734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Marzo, D.; Laudadio, V.; Khan, R.U.; Tufarelli, V.; Maiorano, G. Feeding of Camelina sativa Seeds to Light-Type Gentile Di Puglia Lambs: Effect on Productive Performance and Muscle Fatty Acid Composition. Anim. Biotechnol. 2022, 1–7. [Google Scholar] [CrossRef]
- Zlepkin, V.A.; Salomatin, V.V.; Ryadnov, A.A.; Zlepkina, N.A.; Mishurova, M.N.; Ryadnova, T.A.; Kurskaya, Y.A. Vegetable Oil Various Types Together with Enzyme Preparation Influence on Broiler Chickens’ Meat Productivity and Quality. IOP Conf. Ser. Earth Environ. Sci. 2022, 965, 012035. [Google Scholar] [CrossRef]
- Ciurescu, G.; Idriceanu, L.; Gheorghe, A.; Ropotă, M.; Drăghici, R. Meat Quality in Broiler Chickens Fed on Cowpea (Vigna Unguiculata [L.] Walp) Seeds. Sci. Rep. 2022, 12, 9685. [Google Scholar] [CrossRef]
- Lolli, S.; Grilli, G.; Ferrari, L.; Battelli, G.; Pozzo, S.; Galasso, I.; Russo, R.; Brasca, M.; Reggiani, R.; Ferrante, V. Effect of Different Percentage of Camelina sativa Cake in Laying Hens Diet: Performance, Welfare, and Eggshell Quality. Animals 2020, 10, 1396. [Google Scholar] [CrossRef]
- Peiretti, P.G.; Mussa, P.P.; Prola, L.; Meineri, G. Use of Different Levels of False Flax (Camelina sativa L.) Seed in Diets for Fattening Rabbits. Livest. Sci. 2007, 107, 192–198. [Google Scholar] [CrossRef]
- Colombini, S.; Broderick, G.A.; Galasso, I.; Martinelli, T.; Rapetti, L.; Russo, R.; Reggiani, R. Evaluation of Camelina sativa (L.) Crantz Meal as an Alternative Protein Source in Ruminant Rations. J. Sci. Food Agric. 2014, 94, 736–743. [Google Scholar] [CrossRef]
- Colonna, M.A.; Giannico, F.; Tufarelli, V.; Laudadio, V.; Selvaggi, M.; De Mastro, G.; Tedone, L. Dietary Supplementation with Camelina sativa (L. Crantz) Forage in Autochthonous Ionica Goats: Effects on Milk and Caciotta Cheese Chemical, Fatty Acid Composition and Sensory Properties. Animals 2021, 11, 1589. [Google Scholar] [CrossRef] [PubMed]
- Mierlita, D.; Daraban, S.; Lup, F.; Chereji, A. The Effect of Grazing Management and Camelina Seed Supplementation in the Diet on Milk Performance and Milk Fatty Acid Composition of Dairy Ewes. J. Food Agric. Environ. 2011, 9, 368–373. [Google Scholar]
- Tedone, L.; Giannico, F.; Tufarelli, V.; Laudadio, V.; Selvaggi, M.; De Mastro, G.; Colonna, M.A. Camelina sativa (L. Crantz) Fresh Forage Productive Performance and Quality at Different Vegetative Stages: Effects of Dietary Supplementation in Ionica Goats on Milk Quality. Agriculture 2022, 12, 91. [Google Scholar] [CrossRef]
- Taranu, I.; Gras, M.; Pistol, G.C.; Motiu, M.; Marin, D.E.; Lefter, N.; Ropota, M.; Habeanu, M. ω-3 PUFA Rich Camelina Oil By-Products Improve the Systemic Metabolism and Spleen Cell Functions in Fattening Pigs. PLOS ONE 2014, 9, e110186. [Google Scholar] [CrossRef] [PubMed]
- Juodka, R.; Nainienė, R.; Juškienė, V.; Juška, R.; Leikus, R.; Kadžienė, G.; Stankevičienė, D. Camelina (Camelina sativa (L.) Crantz) as Feedstuffs in Meat Type Poultry Diet: A Source of Protein and n-3 Fatty Acids. Animals 2022, 12, 295. [Google Scholar] [CrossRef]
- Riaz, R.; Ahmed, I.; Sizmaz, O.; Ahsan, U. Use of Camelina sativa and By-Products in Diets for Dairy Cows: A Review. Animals 2022, 12, 1082. [Google Scholar] [CrossRef]
- Hixson, S.M.; Parrish, C.C.; Anderson, D.M. Full Substitution of Fish Oil with Camelina (Camelina Sativa) Oil, with Partial Substitution of Fish Meal with Camelina Meal, in Diets for Farmed Atlantic Salmon (Salmo Salar) and Its Effect on Tissue Lipids and Sensory Quality. Food Chem. 2014, 157, 51–61. [Google Scholar] [CrossRef]
- Ruiz-Lopez, N.; Haslam, R.P.; Napier, J.A.; Sayanova, O. Successful High-level Accumulation of Fish Oil Omega-3 Long-chain Polyunsaturated Fatty Acids in a Transgenic Oilseed Crop. Plant J. 2014, 77, 198–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, X.; Hixson, S.M.; Hori, T.S.; Booman, M.; Parrish, C.C.; Anderson, D.M.; Rise, M.L. Atlantic Salmon (Salmo Salar) Liver Transcriptome Response to Diets Containing Camelina sativa Products. Comp. Biochem. Physiol. Part D Genom. Proteom. 2015, 14, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Betancor, M.B.; Sprague, M.; Sayanova, O.; Usher, S.; Campbell, P.J.; Napier, J.A.; Caballero, M.J.; Tocher, D.R. Evaluation of a High-EPA Oil from Transgenic Camelina sativa in Feeds for Atlantic Salmon (Salmo Salar L.): Effects on Tissue Fatty Acid Composition, Histology and Gene Expression. Aquaculture 2015, 444, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Tocher, D.R. Omega-3 Long-Chain Polyunsaturated Fatty Acids and Aquaculture in Perspective. Aquaculture 2015, 449, 94–107. [Google Scholar] [CrossRef]
- Betancor, M.B.; Sprague, M.; Montero, D.; Usher, S.; Sayanova, O.; Campbell, P.J.; Napier, J.A.; Caballero, M.J.; Izquierdo, M.; Tocher, D.R. Replacement of Marine Fish Oil with de Novo Omega-3 Oils from Transgenic Camelina sativa in Feeds for Gilthead Sea Bream (Sparus Aurata L.). Lipids 2016, 51, 1171–1191. [Google Scholar] [CrossRef]
- Wei, M.; Anderson, D.M.; Zhang, Z.; Colombo, S.M. High-Oil Residue Camelina Meal, a Viable Source of Protein at Low Levels in Diets for Juvenile Salmonids. Aquac. Nutr. 2020, 26, 558–567. [Google Scholar] [CrossRef]
- Ruyter, B.; Bou, M.; Berge, G.M.; Mørkøre, T.; Sissener, N.H.; Sanden, M.; Lutfi, E.; Romarheim, O.-H.; Krasnov, A.; Østbye, T.-K.K. A Dose-Response Study with Omega-3 Rich Canola Oil as a Novel Source of Docosahexaenoic Acid (DHA) in Feed for Atlantic Salmon (Salmo Salar) in Seawater; Effects on Performance, Tissue Fatty Acid Composition, and Fillet Quality. Aquaculture 2022, 561, 738733. [Google Scholar] [CrossRef]
- Toyes-Vargas, E.A.; Magallón-Barajas, F.J.; Parrish, C.C. Lipid Variations in Tilapia (Var. GIFT Oreochromis Sp.) Tissues Due to Dietary Replacement of Fish Oil with Camelina Oil (Camelina Sativa). Aquac. Res. 2022, 53, 2819–2832. [Google Scholar] [CrossRef]
- Balanuca, B.; Stan, R.; Hanganu, A.; Lungu, A.; Iovu, H. Design of New Camelina Oil-Based Hydrophilic Monomers for Novel Polymeric Materials. J. Am. Oil Chem. Soc. 2015, 92, 881–891. [Google Scholar] [CrossRef]
- Li, N.; Qi, G.; Sun, X.S.; Xu, F.; Wang, D. Adhesion Properties of Camelina Protein Fractions Isolated with Different Methods. Ind. Crops Prod. 2015, 69, 263–272. [Google Scholar] [CrossRef]
- Li, Y.; Sun, X.S. Camelina Oil Derivatives and Adhesion Properties. Ind. Crops Prod. 2015, 73, 73–80. [Google Scholar] [CrossRef]
- Kim, N.; Li, Y.; Sun, X.S. Epoxidation of Camelina sativa Oil and Peel Adhesion Properties. Ind. Crops Prod. 2015, 64, 1–8. [Google Scholar] [CrossRef]
- Nosal, H.; Nowicki, J.; Warzała, M.; Nowakowska-Bogdan, E.; Zarębska, M. Synthesis and Characterization of Alkyd Resins Based on Camelina sativa Oil and Polyglycerol. Prog. Org. Coat. 2015, 86, 59–70. [Google Scholar] [CrossRef]
- Piravi-vanak, Z.; Azadmard-Damirchi, S.; Kahrizi, D.; Mooraki, N.; Ercisli, S.; Savage, G.P.; Rostami Ahmadvandi, H.; Martinez, F. Physicochemical Properties of Oil Extracted from Camelina (Camelina Sativa) Seeds as a New Source of Vegetable Oil in Different Regions of Iran. J. Mol. Liq. 2022, 345, 117043. [Google Scholar] [CrossRef]
- Mališová, M.; Horňáček, M.; Hudec, P.; Mikulec, J.; Slezáčková, M.; Hájeková, E. Preparation and Characterization of K-Loaded Mg/Al Mixed Oxides Obtained from Hydrotalcites for Transesterification of Camelina sativa Oil. Chem. Pap. 2022, 76, 7585–7596. [Google Scholar] [CrossRef]
- Arshad, M.K.; Mohanty, A.; Acker, R.V.; Riddle, R.; Todd, J.; Khalil, H.; Misra, M. Valorization of Camelina Oil to Biobased Materials and Biofuels for New Industrial Uses: A Review. RSC Adv. 2022, 12, 27230–27245. [Google Scholar] [CrossRef]
- Shonnard, D.R.; Williams, L.; Kalnes, T.N. Camelina-Derived Jet Fuel and Diesel: Sustainable Advanced Biofuels. Environ. Prog. Sustain. Energy 2010, 29, 382–392. [Google Scholar] [CrossRef]
- Campbell, M.C.; Rossi, A.F.; Erskine, W. Camelina (Camelina sativa (L.) Crantz): Agronomic Potential in Mediterranean Environments and Diversity for Biofuel and Food Uses. Crop Pasture Sci. 2013, 64, 388. [Google Scholar] [CrossRef]
- Gesch, R.W.; Archer, D.W. Double-Cropping with Winter Camelina in the Northern Corn Belt to Produce Fuel and Food. Ind. Crops Prod. 2013, 44, 718–725. [Google Scholar] [CrossRef]
- Li, X.; Mupondwa, E. Life Cycle Assessment of Camelina Oil Derived Biodiesel and Jet Fuel in the Canadian Prairies. Sci. Total Environ. 2014, 481, 17–26. [Google Scholar] [CrossRef]
- Obour, A.K.; Sintim, H.Y.; Obeng, E.; Jeliazkov, D.V. Oilseed Camelina (Camelina sativa L. Crantz): Production Systems, Prospects and Challenges in the USA Great Plains. Adv. Plants Agric. Res. 2015, 2, 68–76. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Caldwell, C.; Corscadden, K.; He, Q.S.; Li, J. An Evaluation of Biodiesel Production from Camelina sativa Grown in Nova Scotia. Ind. Crops Prod. 2016, 81, 162–168. [Google Scholar] [CrossRef]
- Bacenetti, J.; Restuccia, A.; Schillaci, G.; Failla, S. Biodiesel Production from Unconventional Oilseed Crops (Linum Usitatissimum L. and Camelina sativa L.) in Mediterranean Conditions: Environmental Sustainability Assessment. Renew. Energy 2017, 112, 444–456. [Google Scholar] [CrossRef]
- Ahmad, M.; Waraich, E.A.; Hafeez, M.B.; Zulfiqar, U.; Ahmad, Z.; Iqbal, M.A.; Raza, A.; Slam, M.S.; Rehman, A.; Younis, U.; et al. Changing Climate Scenario: Perspectives of Camelina sativa as Low-Input Biofuel and Oilseed Crop. In Global Agricultural Production: Resilience to Climate Change; Ahmed, M., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 197–236. ISBN 978-3-031-14973-3. [Google Scholar]
- Tanwar, B.; Goyal, A. Oilseeds: Health Attributes and Food Applications; Springer: Singapore, 2021; ISBN 9789811541933. [Google Scholar]
- Karvonen, H.M.; Aro, A.; Tapola, N.S.; Salminen, I.; Uusitupa, M.I.J.; Sarkkinen, E.S. Effect of [Alpha]-Linolenic Acid[Ndash ]Rich Camelina sativa Oil on Serum Fatty Acid Composition and Serum Lipids in Hypercholesterolemic Subjects. Metab.-Clin. Exp. 2002, 51, 1253–1260. [Google Scholar] [CrossRef] [PubMed]
- Manninen, S.; Lankinen, M.; Erkkilä, A.; Nguyen, S.D.; Ruuth, M.; de Mello, V.; Öörni, K.; Schwab, U. The Effect of Intakes of Fish and Camelina sativa Oil on Atherogenic and Anti-Atherogenic Functions of LDL and HDL Particles: A Randomized Controlled Trial. Atherosclerosis 2019, 281, 56–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, F.; El Habbasha, E.S. Chemical Composition, Medicinal Impacts and Cultivation of Camelina (Camelina Sativa): Review. Int.J. PharmTech Res. 2015, 8, 114–122. [Google Scholar]
- Ratusz, K.; Symoniuk, E.; Wroniak, M.; Rudzińska, M. Bioactive Compounds, Nutritional Quality and Oxidative Stability of Cold-Pressed Camelina (Camelina sativa L.) Oils. Appl. Sci. 2018, 8, 2606. [Google Scholar] [CrossRef] [Green Version]
- Christodoulou, C.; Mavrommatis, A.; Mitsiopoulou, C.; Symeon, G.; Dotas, V.; Sotirakoglou, K.; Kotsampasi, B.; Tsiplakou, E. Assessing the Optimum Level of Supplementation with Camelina Seeds in Ewes’ Diets to Improve Milk Quality. Foods Basel Switz. 2021, 10, 2076. [Google Scholar] [CrossRef]
- Nain, S.; Oryschak, M.A.; Betti, M.; Beltranena, E. Camelina sativa Cake for Broilers: Effects of Increasing Dietary Inclusion from 0 to 24% on Tissue Fatty Acid Proportions at 14, 28, and 42 d of Age. Poult. Sci. 2015, 94, 1247–1258. [Google Scholar] [CrossRef]
- Russo, R.; Galasso, I.; Reggiani, R. Variability in Glucosinolate Content among Camelina Species. Am. J. Plant Sci. 2014, 05, 294–298. [Google Scholar] [CrossRef] [Green Version]
- Lokesh, K.; Sethi, V.; Nikolaidis, T.; Goodger, E.; Nalianda, D. Life Cycle Greenhouse Gas Analysis of Biojet Fuels with a Technical Investigation into Their Impact on Jet Engine Performance. Biomass Bioenergy 2015, 77, 26–44. [Google Scholar] [CrossRef] [Green Version]
- Fröhlich, A.; Rice, B. Evaluation of Camelina sativa Oil as a Feedstock for Biodiesel Production. Ind. Crops Prod. 2005, 21, 25–31. [Google Scholar] [CrossRef]
- Ciubota-Rosie, C.; Ruiz, J.R.; Ramos, M.J.; Pérez, Á. Biodiesel from Camelina Sativa: A Comprehensive Characterisation. Fuel 2013, 105, 572–577. [Google Scholar] [CrossRef]
- Zubr, J. Dietary Fatty Acids and Amino Acids of Camelina sativa Seed. J. Food Qual. 2003, 26, 451–462. [Google Scholar] [CrossRef]
- Matthaus, B.; Zubr, J. Variability of Specific Components in Camelina sativa Oilseed Cakes. Ind. Crops Prod. 2000, 12, 9–18. [Google Scholar] [CrossRef]
- Nour-Eldin, H.H.; Madsen, S.R.; Engelen, S.; Jørgensen, M.E.; Olsen, C.E.; Andersen, J.S.; Seynnaeve, D.; Verhoye, T.; Fulawka, R.; Denolf, P.; et al. Reduction of Antinutritional Glucosinolates in Brassica Oilseeds by Mutation of Genes Encoding Transporters. Nat. Biotechnol. 2017, 35, 377–382. [Google Scholar] [CrossRef]
- Regolamento (UE) 2017/1017 della Commissione, del 15 Giugno 2017, che Modifica il Regolamento (UE) n. 68/2013 Concernente il Catalogo delle Materie prime per Mangimi (Testo Rilevante ai fini del SEE.). Available online: http://data.europa.eu/eli/reg/2017/1017/oj (accessed on 23 January 2023).
- Amyot, L.; McDowell, T.; Martin, S.L.; Renaud, J.; Gruber, M.Y.; Hannoufa, A. Assessment of Antinutritional Compounds and Chemotaxonomic Relationships between Camelina sativa and Its Wild Relatives. J. Agric. Food Chem. 2019, 67, 796–806. [Google Scholar] [CrossRef]
- Halkier, B.A.; Gershenzon, J. Biology and Biochemistry of Glucosinolates. Annu. Rev. Plant Biol. 2006, 57, 303–333. [Google Scholar] [CrossRef] [Green Version]
- Schuster, A.; Friedt, W. Glucosinolate Content and Composition as Parameters of Quality of Camelina Seed. Ind. Crops Prod. 1998, 7, 297–302. [Google Scholar] [CrossRef]
- Shakour, Z.T.; Shehab, N.G.; Gomaa, A.S.; Wessjohann, L.A.; Farag, M.A. Metabolic and Biotransformation Effects on Dietary Glucosinolates, Their Bioavailability, Catabolism and Biological Effects in Different Organisms. Biotechnol. Adv. 2022, 54, 107784. [Google Scholar] [CrossRef]
- De Groef, B.; Decallonne, B.R.; Van der Geyten, S.; Darras, V.M.; Bouillon, R. Perchlorate versus Other Environmental Sodium/Iodide Symporter Inhibitors: Potential Thyroid-Related Health Effects. Eur. J. Endocrinol. 2006, 155, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Wittstock, U.; Halkier, B.A. Glucosinolate Research in the Arabidopsis Era. Trends Plant Sci. 2002, 7, 263–270. [Google Scholar] [CrossRef]
- Diebold, R.; Schuster, J.; Däschner, K.; Binder, S. The Branched-Chain Amino Acid Transaminase Gene Family in Arabidopsis Encodes Plastid and Mitochondrial Proteins. Plant Physiol. 2002, 129, 540–550. [Google Scholar] [CrossRef] [Green Version]
- Schuster, J.; Knill, T.; Reichelt, M.; Gershenzon, J.; Binder, S. Branched-Chain Aminotransferase4 Is Part of the Chain Elongation Pathway in the Biosynthesis of Methionine-Derived Glucosinolates in Arabidopsis. Plant Cell 2006, 18, 2664–2679. [Google Scholar] [CrossRef] [Green Version]
- Falk, K.L.; Vogel, C.; Textor, S.; Bartram, S.; Hick, A.; Pickett, J.A.; Gershenzon, J. Glucosinolate Biosynthesis: Demonstration and Characterization of the Condensing Enzyme of the Chain Elongation Cycle in Eruca Sativa. Phytochemistry 2004, 65, 1073–1084. [Google Scholar] [CrossRef]
- Textor, S.; de Kraker, J.-W.; Hause, B.; Gershenzon, J.; Tokuhisa, J.G. MAM3 Catalyzes the Formation of All Aliphatic Glucosinolate Chain Lengths in Arabidopsis. Plant Physiol. 2007, 144, 60–71. [Google Scholar] [CrossRef] [Green Version]
- Sawada, Y.; Kuwahara, A.; Nagano, M.; Narisawa, T.; Sakata, A.; Saito, K.; Hirai, M.Y. Omics-Based Approaches to Methionine Side Chain Elongation in Arabidopsis: Characterization of the Genes Encoding Methylthioalkylmalate Isomerase and Methylthioalkylmalate Dehydrogenase. Plant Cell Physiol. 2009, 50, 1181–1190. [Google Scholar] [CrossRef] [Green Version]
- Knill, T.; Reichelt, M.; Paetz, C.; Gershenzon, J.; Binder, S. Arabidopsis Thaliana Encodes a Bacterial-Type Heterodimeric Isopropylmalate Isomerase Involved in Both Leu Biosynthesis and the Met Chain Elongation Pathway of Glucosinolate Formation. Plant Mol. Biol. 2009, 71, 227–239. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Mawhinney, T.P.; Preuss, M.L.; Schroeder, A.C.; Chen, B.; Abraham, L.; Jez, J.M.; Chen, S. A Redox-Active Isopropylmalate Dehydrogenase Functions in the Biosynthesis of Glucosinolates and Leucine in Arabidopsis. Plant J. Cell Mol. Biol. 2009, 60, 679–690. [Google Scholar] [CrossRef]
- Gigolashvili, T.; Yatusevich, R.; Rollwitz, I.; Humphry, M.; Gershenzon, J.; Flügge, U.-I. The Plastidic Bile Acid Transporter 5 Is Required for the Biosynthesis of Methionine-Derived Glucosinolates in Arabidopsis Thaliana. Plant Cell 2009, 21, 1813–1829. [Google Scholar] [CrossRef] [Green Version]
- Textor, S.; Bartram, S.; Kroymann, J.; Falk, K.L.; Hick, A.; Pickett, J.A.; Gershenzon, J. Biosynthesis of Methionine-Derived Glucosinolates in Arabidopsis Thaliana: Recombinant Expression and Characterization of Methylthioalkylmalate Synthase, the Condensing Enzyme of the Chain-Elongation Cycle. Planta 2004, 218, 1026–1035. [Google Scholar] [CrossRef]
- de Kraker, J.-W.; Gershenzon, J. From Amino Acid to Glucosinolate Biosynthesis: Protein Sequence Changes in the Evolution of Methylthioalkylmalate Synthase in Arabidopsis. Plant Cell 2011, 23, 38–53. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Lee, S.G.; Augustine, R.; Reichelt, M.; Vassão, D.G.; Palavalli, M.H.; Allen, A.; Gershenzon, J.; Jez, J.M.; Bisht, N.C. Molecular Basis of the Evolution of Methylthioalkylmalate Synthase and the Diversity of Methionine-Derived Glucosinolates. Plant Cell 2019, 31, 1633–1647. [Google Scholar] [CrossRef]
- He, Y.; Chen, B.; Pang, Q.; Strul, J.M.; Chen, S. Functional Specification of Arabidopsis Isopropylmalate Isomerases in Glucosinolate and Leucine Biosynthesis. Plant Cell Physiol. 2010, 51, 1480–1487. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Chen, L.; Zhou, Y.; Mawhinney, T.P.; Chen, B.; Kang, B.-H.; Hauser, B.A.; Chen, S. Functional Characterization of Arabidopsis Thaliana Isopropylmalate Dehydrogenases Reveals Their Important Roles in Gametophyte Development. New Phytol. 2011, 189, 160–175. [Google Scholar] [CrossRef]
- He, Y.; Galant, A.; Pang, Q.; Strul, J.M.; Balogun, S.F.; Jez, J.M.; Chen, S. Structural and Functional Evolution of Isopropylmalate Dehydrogenases in the Leucine and Glucosinolate Pathways of Arabidopsis Thaliana. J. Biol. Chem. 2011, 286, 28794–28801. [Google Scholar] [CrossRef] [Green Version]
- Knill, T.; Schuster, J.; Reichelt, M.; Gershenzon, J.; Binder, S. Arabidopsis Branched-Chain Aminotransferase 3 Functions in Both Amino Acid and Glucosinolate Biosynthesis. Plant Physiol. 2008, 146, 1028–1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lächler, K.; Imhof, J.; Reichelt, M.; Gershenzon, J.; Binder, S. The Cytosolic Branched-Chain Aminotransferases of Arabidopsis Thaliana Influence Methionine Supply, Salvage and Glucosinolate Metabolism. Plant Mol. Biol. 2015, 88, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Hull, A.K.; Vij, R.; Celenza, J.L. Arabidopsis Cytochrome P450s That Catalyze the First Step of Tryptophan-Dependent Indole-3-Acetic Acid Biosynthesis. Proc. Natl. Acad. Sci. USA 2000, 97, 2379–2384. [Google Scholar] [CrossRef] [Green Version]
- Wittstock, U.; Halkier, B.A. Cytochrome P450 CYP79A2 from Arabidopsis Thaliana L. Catalyzes the Conversion of L-Phenylalanine to Phenylacetaldoxime in the Biosynthesis of Benzylglucosinolate. J. Biol. Chem. 2000, 275, 14659–14666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikkelsen, M.D.; Hansen, C.H.; Wittstock, U.; Halkier, B.A. Cytochrome P450 CYP79B2 from Arabidopsis Catalyzes the Conversion of Tryptophan to Indole-3-Acetaldoxime, a Precursor of Indole Glucosinolates and Indole-3-Acetic Acid. J. Biol. Chem. 2000, 275, 33712–33717. [Google Scholar] [CrossRef] [Green Version]
- Hansen, C.H.; Wittstock, U.; Olsen, C.E.; Hick, A.J.; Pickett, J.A.; Halkier, B.A. Cytochrome P450 CYP79F1 from Arabidopsis Catalyzes the Conversion of Dihomomethionine and Trihomomethionine to the Corresponding Aldoximes in the Biosynthesis of Aliphatic Glucosinolates. J. Biol. Chem. 2001, 276, 11078–11085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Glawischnig, E.; Jørgensen, K.; Naur, P.; Jørgensen, B.; Olsen, C.-E.; Hansen, C.H.; Rasmussen, H.; Pickett, J.A.; Halkier, B.A. CYP79F1 and CYP79F2 Have Distinct Functions in the Biosynthesis of Aliphatic Glucosinolates in Arabidopsis. Plant J. Cell Mol. Biol. 2003, 33, 923–937. [Google Scholar] [CrossRef] [Green Version]
- Glawischnig, E. The Role of Cytochrome P450 Enzymes in the Biosynthesis of Camalexin. Biochem. Soc. Trans. 2006, 34, 1206–1208. [Google Scholar] [CrossRef] [Green Version]
- Czerniawski, P.; Piasecka, A.; Bednarek, P. Evolutionary Changes in the Glucosinolate Biosynthetic Capacity in Species Representing Capsella, Camelina and Neslia Genera. Phytochemistry 2021, 181, 112571. [Google Scholar] [CrossRef]
- Hopkins, R.J.; van Dam, N.M.; van Loon, J.J.A. Role of Glucosinolates in Insect-Plant Relationships and Multitrophic Interactions. Annu. Rev. Entomol. 2009, 54, 57–83. [Google Scholar] [CrossRef]
- Sønderby, I.E.; Geu-Flores, F.; Halkier, B.A. Biosynthesis of Glucosinolates--Gene Discovery and Beyond. Trends Plant Sci. 2010, 15, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Ouassou, M.; Mukhaimar, M.; El Amrani, A.; Kroymann, J.; Chauveau, O. Biosynthesis of indole glucosinolates and ecological role of secondary modification pathways. C. R. Biol. 2019, 342, 58–80. [Google Scholar] [CrossRef] [PubMed]
- Petersen, B.L.; Chen, S.; Hansen, C.H.; Olsen, C.E.; Halkier, B.A. Composition and Content of Glucosinolates in Developing Arabidopsis Thaliana. Planta 2002, 214, 562–571. [Google Scholar] [CrossRef]
- Brown, P.D.; Tokuhisa, J.G.; Reichelt, M.; Gershenzon, J. Variation of Glucosinolate Accumulation among Different Organs and Developmental Stages of Arabidopsis Thaliana. Phytochemistry 2003, 62, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Quéro, A.; Molinié, R.; Mathiron, D.; Thiombiano, B.; Fontaine, J.-X.; Brancourt, D.; Van Wuytswinkel, O.; Petit, E.; Demailly, H.; Mongelard, G.; et al. Metabolite Profiling of Developing Camelina sativa Seeds. Metabolomics 2016, 12, 186. [Google Scholar] [CrossRef]
- Tavarini, S.; De Leo, M.; Matteo, R.; Lazzeri, L.; Braca, A.; Angelini, L.G. Flaxseed and Camelina Meals as Potential Sources of Health-Beneficial Compounds. Plants Basel Switz. 2021, 10, 156. [Google Scholar] [CrossRef] [PubMed]
- Sawada, Y.; Toyooka, K.; Kuwahara, A.; Sakata, A.; Nagano, M.; Saito, K.; Hirai, M.Y. Arabidopsis Bile Acid:Sodium Symporter Family Protein 5 Is Involved in Methionine-Derived Glucosinolate Biosynthesis. Plant Cell Physiol. 2009, 50, 1579–1586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, L.; Halkier, B.A. Biosynthesis of Glucosinolates in the Developing Silique Walls and Seeds of Sinapis Alba. Phytochemistry 1998, 48, 1145–1150. [Google Scholar] [CrossRef]
- Chen, S.; Petersen, B.L.; Olsen, C.E.; Schulz, A.; Halkier, B.A. Long-Distance Phloem Transport of Glucosinolates in Arabidopsis. Plant Physiol. 2001, 127, 194–201. [Google Scholar] [CrossRef] [Green Version]
- Ellerbrock, B.L.; Kim, J.H.; Jander, G. Contribution of Glucosinolate Transport to Arabidopsis Defense Responses. Plant Signal. Behav. 2007, 2, 282–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nour-Eldin, H.H.; Andersen, T.G.; Burow, M.; Madsen, S.R.; Jørgensen, M.E.; Olsen, C.E.; Dreyer, I.; Hedrich, R.; Geiger, D.; Halkier, B.A. NRT/PTR Transporters Are Essential for Translocation of Glucosinolate Defence Compounds to Seeds. Nature 2012, 488, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Léran, S.; Varala, K.; Boyer, J.-C.; Chiurazzi, M.; Crawford, N.; Daniel-Vedele, F.; David, L.; Dickstein, R.; Fernandez, E.; Forde, B.; et al. A Unified Nomenclature of NITRATE TRANSPORTER 1/PEPTIDE TRANSPORTER Family Members in Plants. Trends Plant Sci. 2014, 19, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Nour-Eldin, H.H.; Halkier, B.A. The Emerging Field of Transport Engineering of Plant Specialized Metabolites. Curr. Opin. Biotechnol. 2013, 24, 263–270. [Google Scholar] [CrossRef]
- Xu, D.; Hunziker, P.; Koroleva, O.; Blennow, A.; Crocoll, C.; Schulz, A.; Nour-Eldin, H.H.; Halkier, B.A. GTR-Mediated Radial Import Directs Accumulation of Defensive Glucosinolates to Sulfur-Rich Cells in the Phloem Cap of Arabidopsis Inflorescence Stem. Mol. Plant 2019, 12, 1474–1484. [Google Scholar] [CrossRef] [PubMed]
- Nambiar, D.M.; Kumari, J.; Augustine, R.; Kumar, P.; Bajpai, P.K.; Bisht, N.C. GTR1 and GTR2 Transporters Differentially Regulate Tissue-Specific Glucosinolate Contents and Defence Responses in the Oilseed Crop Brassica Juncea. Plant Cell Environ. 2021, 44, 2729–2743. [Google Scholar] [CrossRef]
- Hölzl, G.; Rezaeva, B.R.; Kumlehn, J.; Dörmann, P. Ablation of Glucosinolate Accumulation in the Oil Crop Camelina sativa by Targeted Mutagenesis of Genes Encoding the Transporters GTR1 and GTR2 and Regulators of Biosynthesis MYB28 and MYB29. Plant Biotechnol. J. 2023, 21, 189–201. [Google Scholar] [CrossRef]
- Noret, N.; Meerts, P.; Tolrà, R.; Poschenrieder, C.; Barceló, J.; Escarre, J. Palatability of Thlaspi Caerulescens for Snails: Influence of Zinc and Glucosinolates. New Phytol. 2005, 165, 763–772. [Google Scholar] [CrossRef]
- Alexandre, E.M.C.; Moreira, S.A.; Pinto, C.A.; Pintado, M.; Saraiva, J.A. Analysis of Glucosinolates Content in Food Products. In Glucosinolates: Properties, Recovery, and Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 213–250. ISBN 978-0-12-816493-8. [Google Scholar]
- Baenas, N.; Cartea, M.E.; Moreno, D.A.; Tortosa, M.; Francisco, M. Processing and Cooking Effects on Glucosinolates and Their Derivatives. In Glucosinolates: Properties, Recovery, and Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 181–212. ISBN 978-0-12-816493-8. [Google Scholar]
- Galanakis, C.M. Recovery Techniques, Stability, and Applications of Glucosinolates. In Glucosinolates: Properties, Recovery, and Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 251–280. ISBN 978-0-12-816493-8. [Google Scholar]
- European Parliament. Available online: https://www.europarl.europa.eu/portal (accessed on 26 November 2022).
- Gugel, R.K.; Falk, K. Agronomic and Seed Quality Evaluation of Camelina sativa in Western Canada. Can. J. Plant Sci. 2006, 86, 1047–1058. [Google Scholar] [CrossRef]
- Manca, A.; Pecchia, P.; Mapelli, S.; Masella, P.; Galasso, I. Evaluation of Genetic Diversity in a Camelina sativa (L.) Crantz Collection Using Microsatellite Markers and Biochemical Traits. Genet. Resour. Crop Evol. 2012, 60, 1223–1236. [Google Scholar] [CrossRef]
- Singh, R.; Bollina, V.; Higgins, E.E.; Clarke, W.E.; Eynck, C.; Sidebottom, C.; Gugel, R.; Snowdon, R.; Parkin, I.A.P. Single-Nucleotide Polymorphism Identification and Genotyping in Camelina Sativa. Mol. Breed. New Strateg. Plant Improv. 2015, 35, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galasso, I.; Manca, A.; Braglia, L.; Ponzoni, E.; Breviario, D. Genomic Fingerprinting of Camelina Species Using CTBP as Molecular Marker. Am. J. Plant Sci. 2015, 6, 1184–1200. [Google Scholar] [CrossRef] [Green Version]
- Faure, J.-D.; Tepfer, M. Camelina, a Swiss Knife for Plant Lipid Biotechnology. OCL 2016, 23, D503. [Google Scholar] [CrossRef]
- Luo, Z.; Brock, J.; Dyer, J.M.; Kutchan, T.; Schachtman, D.; Augustin, M.; Ge, Y.; Fahlgren, N.; Abdel-Haleem, H. Genetic Diversity and Population Structure of a Camelina sativa Spring Panel. Front. Plant Sci. 2019, 10, 184. [Google Scholar] [CrossRef] [Green Version]
- Kagale, S.; Koh, C.; Nixon, J.; Bollina, V.; Clarke, W.E.; Tuteja, R.; Spillane, C.; Robinson, S.J.; Links, M.G.; Clarke, C.; et al. The Emerging Biofuel Crop Camelina sativa Retains a Highly Undifferentiated Hexaploid Genome Structure. Nat. Commun. 2014, 5, 3706. [Google Scholar] [CrossRef] [Green Version]
- Tepfer, M.; Hurel, A.; Tellier, F.; Jenczewski, E. Evaluation of the Progeny Produced by Interspecific Hybridization between Camelina sativa and C. Microcarpa. Ann. Bot. 2020, 125, 993–1002. [Google Scholar] [CrossRef]
- Kumar, D.; Yadav, A.; Ahmad, R.; Dwivedi, U.N.; Yadav, K. CRISPR-Based Genome Editing for Nutrient Enrichment in Crops: A Promising Approach Toward Global Food Security. Front. Genet. 2022, 13, 932859. [Google Scholar] [CrossRef]
- Legislation for Plants Produced by Certain New Genomic Techniques. Available online: https://ec.europa.eu/info/law/better-regulation/have-your-say/initiatives/13119-Legislation-for-plants-produced-by-certain-new-genomic-techniques_en (accessed on 23 January 2023).
- CPVO | Community Plant Variety Office. Available online: https://cpvo.europa.eu/en (accessed on 20 November 2022).
- Welcome to Arrow Seed- WE KNOW SEED. Available online: https://arrowseed.com/ (accessed on 20 November 2022).
- GRIN. Available online: https://www.ars-grin.gov/ (accessed on 20 November 2022).
- Government of Canada, C.F.I.A. Directive 94-08 (Dir 94-08) Assessment Criteria for Determining Environmental Safety of Plants With Novel Traits. Available online: https://inspection.canada.ca/plant-varieties/plants-with-novel-traits/applicants/directive-94-08/eng/1512588596097/1512588596818 (accessed on 18 November 2022).
- Büchsenschütz-Nothdurft, A.; Schuster, A.; Friedt, W. Breeding for Modified Fatty Acid Composition via Experimental Mutagenesis in Camelina sativa (L.) Crtz. Ind. Crops Prod. 1998, 7, 291–295. [Google Scholar] [CrossRef]
- Li, H.; Hu, X.; Lovell, J.T.; Grabowski, P.P.; Mamidi, S.; Chen, C.; Amirebrahimi, M.; Kahanda, I.; Mumey, B.; Barry, K.; et al. Genetic Dissection of Natural Variation in Oilseed Traits of Camelina by Whole-Genome Resequencing and QTL Mapping. Plant Genome 2021, 14, e20110. [Google Scholar] [CrossRef] [PubMed]
- Khan, E.U.; Liu, J.-H. Plant Biotechnological Approaches for the Production and Commercialization of Transgenic Crops. Biotechnol. Biotechnol. Equip. 2009, 23, 1281–1288. [Google Scholar] [CrossRef] [Green Version]
- Mudalkar, S.; Golla, R.; Ghatty, S.; Reddy, A.R. De Novo Transcriptome Analysis of an Imminent Biofuel Crop, Camelina sativa L. Using Illumina GAIIX Sequencing Platform and Identification of SSR Markers. Plant Mol. Biol. 2014, 84, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Ozseyhan, M.E.; Li, P.; Na, G.; Li, Z.; Wang, C.; Lu, C. Improved Fatty Acid Profiles in Seeds of Camelina sativa by Artificial MicroRNA Mediated FATB Gene Suppression. Biochem. Biophys. Res. Commun. 2018, 503, 621–624. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.; Kang, J. Generation of Transgenic Plants of a Potential Oilseed Crop Camelina sativa by Agrobacterium-Mediated Transformation. Plant Cell Rep. 2008, 27, 273–278. [Google Scholar] [CrossRef]
- Sitther, V.; Tabatabai, B.; Enitan, O.; Dhekney, S. Agrobacterium-Mediated Transformation of Camelina sativa for Production of Transgenic Plants. J. Biol. Methods 2018, 5, e83. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Yu, L.; Yung, K.-F.; Leung, D.Y.; Sun, F.; Lim, B.L. Over-Expression of AtPAP2 in Camelina sativa Leads to Faster Plant Growth and Higher Seed Yield. Biotechnol. Biofuels 2012, 5, 19. [Google Scholar] [CrossRef] [Green Version]
- Roy Choudhury, S.; Riesselman, A.J.; Pandey, S. Constitutive or Seed-Specific Overexpression of Arabidopsis G-Protein γ Subunit 3 (AGG3) Results in Increased Seed and Oil Production and Improved Stress Tolerance in Camelina Sativa. Plant Biotechnol. J. 2014, 12, 49–59. [Google Scholar] [CrossRef]
- Dahee An; Mi Chung Suh Overexpression of Arabidopsis WRI1 Enhanced Seed Mass and Storage Oil Content in Camelina Sativa. Plant Biotechnol. Rep. 2015, 9, 137–148. [CrossRef]
- Dalal, J.; Lopez, H.; Vasani, N.B.; Hu, Z.; Swift, J.E.; Yalamanchili, R.; Dvora, M.; Lin, X.; Xie, D.; Qu, R.; et al. A Photorespiratory Bypass Increases Plant Growth and Seed Yield in Biofuel Crop Camelina Sativa. Biotechnol. Biofuels 2015, 8, 175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chhikara, S.; Abdullah, H.M.; Akbari, P.; Schnell, D.; Dhankher, O.P. Engineering Camelina sativa (L.) Crantz for Enhanced Oil and Seed Yields by Combining Diacylglycerol Acyltransferase1 and Glycerol-3-Phosphate Dehydrogenase Expression. Plant Biotechnol. J. 2018, 16, 1034–1045. [Google Scholar] [CrossRef] [Green Version]
- Cai, G.; Fan, C.; Liu, S.; Yang, Q.; Liu, D.; Wu, J.; Li, J.; Zhou, Y.; Guo, L.; Wang, X. Nonspecific Phospholipase C6 Increases Seed Oil Production in Oilseed Brassicaceae Plants. New Phytol. 2020, 226, 1055–1073. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.; Ito, K.; Tominaga, M. Heterologous Transformation of Camelina sativa with High-Speed Chimeric Myosin XI-2 Promotes Plant Growth and Leads to Increased Seed Yield. Plant Biotechnol. Tokyo Jpn. 2020, 37, 253–259. [Google Scholar] [CrossRef]
- Cai, G.; Wang, G.; Kim, S.-C.; Li, J.; Zhou, Y.; Wang, X. Increased Expression of Fatty Acid and ABC Transporters Enhances Seed Oil Production in Camelina. Biotechnol. Biofuels 2021, 14, 49. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Silva, J.E.; Podicheti, R.; Macrander, J.; Yang, W.; Nazarenus, T.J.; Nam, J.-W.; Jaworski, J.G.; Lu, C.; Scheffler, B.E.; et al. Camelina Seed Transcriptome: A Tool for Meal and Oil Improvement and Translational Research. Plant Biotechnol. J. 2013, 11, 759–769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Na, G.; Aryal, N.; Fatihi, A.; Kang, J.; Lu, C. Seed-Specific Suppression of ADP-Glucose Pyrophosphorylase in Camelina sativa Increases Seed Size and Weight. Biotechnol. Biofuels 2018, 11, 330. [Google Scholar] [CrossRef]
- Horn, P.J.; Silva, J.E.; Anderson, D.; Fuchs, J.; Borisjuk, L.; Nazarenus, T.J.; Shulaev, V.; Cahoon, E.B.; Chapman, K.D. Imaging Heterogeneity of Membrane and Storage Lipids in Transgenic Camelina sativa Seeds with Altered Fatty Acid Profiles. Plant J. Cell Mol. Biol. 2013, 76, 138–150. [Google Scholar] [CrossRef]
- Petrie, J.R.; Shrestha, P.; Belide, S.; Kennedy, Y.; Lester, G.; Liu, Q.; Divi, U.K.; Mulder, R.J.; Mansour, M.P.; Nichols, P.D.; et al. Metabolic Engineering Camelina sativa with Fish Oil-like Levels of DHA. PloS ONE 2014, 9, e85061. [Google Scholar] [CrossRef]
- Snapp, A.R.; Kang, J.; Qi, X.; Lu, C. A Fatty Acid Condensing Enzyme from Physaria Fendleri Increases Hydroxy Fatty Acid Accumulation in Transgenic Oilseeds of Camelina Sativa. Planta 2014, 240, 599–610. [Google Scholar] [CrossRef]
- Huai, D.; Zhang, Y.; Zhang, C.; Cahoon, E.B.; Zhou, Y. Combinatorial Effects of Fatty Acid Elongase Enzymes on Nervonic Acid Production in Camelina Sativa. PLoS ONE 2015, 10, e0131755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Rice, A.; McGlew, K.; Shaw, V.; Park, H.; Clemente, T.; Pollard, M.; Ohlrogge, J.; Durrett, T.P. Metabolic Engineering of Oilseed Crops to Produce High Levels of Novel Acetyl Glyceride Oils with Reduced Viscosity, Freezing Point and Calorific Value. Plant Biotechnol. J. 2015, 13, 858–865. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Park, J.; Kim, D.; Kim, A.; Suh, M. Functional Analysis of Diacylglycerol Acyltransferase1 Genes from Camelina sativa and Effects of CsDGAT1B Overexpression on Seed Mass and Storage Oil Content in C. Sativa. Plant Biotechnol. Rep. 2016, 10, 141–153. [Google Scholar] [CrossRef]
- Hu, Z.; Wu, Q.; Dalal, J.; Vasani, N.; Lopez, H.O.; Sederoff, H.W.; Qu, R. Accumulation of Medium-Chain, Saturated Fatty Acyl Moieties in Seed Oils of Transgenic Camelina Sativa. PLoS ONE 2017, 12, e0172296. [Google Scholar] [CrossRef] [Green Version]
- Marmon, S.; Sturtevant, D.; Herrfurth, C.; Chapman, K.; Stymne, S.; Feussner, I. Two Acyltransferases Contribute Differently to Linolenic Acid Levels in Seed Oil. Plant Physiol. 2017, 173, 2081–2095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Brost, J.; Hutcheon, C.; Guilfoil, R.; Wilson, A.K.; Leung, S.; Shewmaker, C.K.; Rooke, S.; Nguyen, T.; Kiser, J.; et al. Transformation of the Oilseed Crop Camelina sativa by Agrobacterium-Mediated Floral Dip and Simple Large-Scale Screening of Transformants. Vitro Cell. Dev. Biol.-Plant 2012, 48, 462–468. [Google Scholar] [CrossRef]
- Obour, A.K.; Obeng, E.; Mohammed, Y.A.; Ciampitti, I.A.; Durrett, T.P.; Aznar-Moreno, J.A.; Chen, C. Camelina Seed Yield and Fatty Acids as Influenced by Genotype and Environment. Agron. J. 2017, 109, 947–956. [Google Scholar] [CrossRef] [Green Version]
- Righini, D.; Zanetti, F.; Martínez-Force, E.; Mandrioli, M.; Toschi, T.G.; Monti, A. Shifting Sowing of Camelina from Spring to Autumn Enhances the Oil Quality for Bio-Based Applications in Response to Temperature and Seed Carbon Stock. Ind. Crops Prod. 2019, 137, 66–73. [Google Scholar] [CrossRef]
- Bansal, S.; Durrett, T.P. Camelina Sativa: An Ideal Platform for the Metabolic Engineering and Field Production of Industrial Lipids. Biochimie 2016, 120, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Murphy, E.J. Camelina (Camelina Sativa). In Industrial Oil Crops; Elsevier: Amsterdam, The Netherlands, 2016; pp. 207–230. ISBN 978-1-893997-98-1. [Google Scholar]
- Walsh, K.D.; Puttick, D.M.; Hills, M.J.; Yang, R.-C.; Topinka, K.C.; Hall, L.M. Short Communication: First Report of Outcrossing Rates in Camelina [Camelina sativa (L.) Crantz], a Potential Platform for Bioindustrial Oils. Can. J. Plant Sci. 2012, 92, 681–685. [Google Scholar] [CrossRef] [Green Version]
- GM Freeze | UK Field Trials. Available online: https://www.gmfreeze.org/why-freeze/uk-field-trials/ (accessed on 21 November 2022).
- Ricroch, A.; Clairand, P.; Harwood, W. Use of CRISPR Systems in Plant Genome Editing: Toward New Opportunities in Agriculture. Emerg. Top. Life Sci. 2017, 1, 169–182. [Google Scholar] [CrossRef] [Green Version]
- Jiang, W.Z.; Henry, I.M.; Lynagh, P.G.; Comai, L.; Cahoon, E.B.; Weeks, D.P. Significant Enhancement of Fatty Acid Composition in Seeds of the Allohexaploid, Camelina Sativa, Using CRISPR/Cas9 Gene Editing. Plant Biotechnol. J. 2017, 15, 648–657. [Google Scholar] [CrossRef] [Green Version]
- Morineau, C.; Bellec, Y.; Tellier, F.; Gissot, L.; Kelemen, Z.; Nogué, F.; Faure, J.-D. Selective Gene Dosage by CRISPR-Cas9 Genome Editing in Hexaploid Camelina Sativa. Plant Biotechnol. J. 2017, 15, 729–739. [Google Scholar] [CrossRef] [Green Version]
- Aznar-Moreno, J.A.; Durrett, T.P. Simultaneous Targeting of Multiple Gene Homeologs to Alter Seed Oil Production in Camelina Sativa. Plant Cell Physiol. 2017, 58, 1260–1267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozseyhan, M.E.; Kang, J.; Mu, X.; Lu, C. Mutagenesis of the FAE1 Genes Significantly Changes Fatty Acid Composition in Seeds of Camelina Sativa. Plant Physiol. Biochem. PPB 2018, 123, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Lyzenga, W.J.; Harrington, M.; Bekkaoui, D.; Wigness, M.; Hegedus, D.D.; Rozwadowski, K.L. CRISPR/Cas9 Editing of Three CRUCIFERIN C Homoeologues Alters the Seed Protein Profile in Camelina Sativa. BMC Plant Biol. 2019, 19, 292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.-R.; Jeon, I.; Yu, H.; Kim, S.-G.; Kim, H.-S.; Ahn, S.-J.; Lee, J.; Lee, S.-K.; Kim, H.U. Increasing Monounsaturated Fatty Acid Contents in Hexaploid Camelina sativa Seed Oil by FAD2 Gene Knockout Using CRISPR-Cas9. Front. Plant Sci. 2021, 12, 702930. [Google Scholar] [CrossRef] [PubMed]
- Gehringer, A.; Friedt, W.; Lühs, W.; Snowdon, R.J. Genetic Mapping of Agronomic Traits in False Flax (Camelina sativa Subsp. Sativa). Genome 2006, 49, 1555–1563. [Google Scholar] [CrossRef]
- Kang, J.; Snapp, A.R.; Lu, C. Identification of Three Genes Encoding Microsomal Oleate Desaturases (FAD2) from the Oilseed Crop Camelina Sativa. Plant Physiol. Biochem. PPB 2011, 49, 223–229. [Google Scholar] [CrossRef]
- Russo, R.; Reggiani, R. Glucosinolates and Sinapine in Camelina Meal. Food Nutr. Sci. 2017, 8, 1063–1073. [Google Scholar] [CrossRef] [Green Version]
- Cherian, G. Camelina sativa in Poultry Diets: Opportunities and Challenges. In Biofuel Co-Poducts as Livestock Feed: Opportunities and Challenges; FAO: Rome, Italy, 2012. [Google Scholar]
- Kliebenstein, D.J.; Kroymann, J.; Brown, P.; Figuth, A.; Pedersen, D.; Gershenzon, J.; Mitchell-Olds, T. Genetic Control of Natural Variation in Arabidopsis Glucosinolate Accumulation. Plant Physiol. 2001, 126, 811–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inc, Y.B. Yield10 Bioscience Announces an Update on the Camelina Line E3902 Development Program for Producing Low-Carbon Feedstock Oil for Renewable Diesel. Available online: https://www.globenewswire.com/en/news-release/2022/03/03/2396309/34378/en/Yield10-Bioscience-Announces-an-Update-on-the-Camelina-Line-E3902-Development-Program-for-Producing-Low-carbon-Feedstock-Oil-for-Renewable-Diesel.html (accessed on 20 November 2022).
- Highlights|Home|Panel for the Future of Science and Technology (STOA) | European Parliament. Available online: https://www.europarl.europa.eu/stoa/en/home/highlights (accessed on 21 November 2022).
- CPVO Essentially derived varieties. Available online: https://cpvo.europa.eu/sites/default/files/documents/articles/EDV_presentation_PlantumNL_March_2006_BK.pdf (accessed on 23 January 2023).


| Uses | Details | References | |
|---|---|---|---|
| Human nutrition | Food | [28,29,30,31,32,33] | |
| Diet supplements | |||
| Animal feed | Bird | Chicken broilers | [19,34,35,36,37,38,39] |
| Laying hens | |||
| Mammals | Cows | [40,41,42,43,44,45,46,47,47] | |
| Swine | |||
| Sheep | |||
| Rabbit | |||
| Swine | |||
| Fish | Salmon | [48,49,50,51,52,53,54,55,56] | |
| Trout | |||
| Other fish | |||
| Chemicals | Polymers | [57,58,59,60,61,62,63,64] | |
| Adhesives | |||
| Resins | |||
| Cosmetics ingredients | |||
| Fuels | Biodiesel | [1,3,5,13,64,65,66,67,68,69,70,71,72] | |
| Jet fuel | |||
| Seed Quality Traits Improved | Biotechnological Approach Used | Target/ Introduced Gene(s) | Promoter Used | Selectable Marker | Final Product/Major Results | Reference |
|---|---|---|---|---|---|---|
| Seed yield increase | single transgene overexpression | Arabidopsis purple acid phosphate (AtPAP2) | constitutive promoter not specified | BASTA (Bar gene) herbicide | 50% higher seed yields with increased seed size | [160] |
| single transgene overexpression | Arabidopsis G-protein γ subunit 3 (AGG3) | CaMV35S and seed-specific soybean glycinin | DsRed fluorescence and Bar gene | Increased seed size, number, and seed mass | [161] | |
| single transgene expression | Arabidopsis WRINKLED1 (AtWRI1) | Seed-specific SiW6 promoter | BASTA herbicide | Enhances seed oil content, seed mass and seed size | [162] | |
| transgenes cassette overexpression | E.coli chloroplast glycolate dehydrogenase (GDH), glyoxylate carboxylase (GCL), and tartronic semialdehyde reductase (TSR) | CaMV35S promoter, tobacco EntCUP4 promoter, Arabidopsis ACTIN2 promoter | seed mCherry fluorescence, phosphinothricin herbicide | enhanced CO2 use efficiency increased plant grown up to 50% | [163] | |
| transgenes cassette expression | Arabidopsis diacylglycerol acyltransferase1 (DGAT1), and a yeast cytosolic glycerol-3-phosphate dehydrogenase (GPD1) | seed specific oleosin and glycinin promoters from soybean | DsRed fluorescence and Bar gene | up to 52% increase in seed mass, and up to 13% higher seed oil content | [164] | |
| single transgene expression | nonspecific phospholipase C6 (NPC6) | not specified | hygromycin B antibiotics | increase seed oil content, seed weight, and oil yield | [165] | |
| single transgene expression | chimeric arabidopsis myosin XI-2 | Arabidopsis myosin XI-2 promoter | hygromycin B antibiotics | improve plant growth, total seed yield increase as the total seed number | [166] | |
| transgenes cassette overexpression | At lipid transporters, FAX1 (fatty acid export1), and ABCA9 (ATP-binding cassette transporter subfamily A9) | CaMV35S promoter | kanamycin antibiotic in plates | increased expression of fatty acid, and seed oil production, increased seed weight and size | [167] | |
| Seed protein content | RNAi suppression | 12S and 2Sinapin protein | seed specific soybean glycine 1 | DsRed fluorescence and Bar gene | seed storage protein (SPP) modulation | [168] |
| RNAi suppression | ADP-glucose pyrophosphorylase (AGPase) | seed-specific phaseolin promoter | DsRed fluorescence | enhanced seed protein content and seed size | [169] | |
| Seed oil modulation | RNAi suppression | camelina FAD2 and FAE1 | not specificized | DsRed fluorescence and Bar gene | increase up to 50% oleic acid | [168] |
| RNAi suppression | fatty acid desaturase 3 (FAD3) and FAE1 | soybean glycinin-1 promoter | DsRed fluorescence and Bar gene | seeds with high linoleate content (approximately 57% of total FA) | [170] | |
| transgenes cassette expression | set of genes of Δ6-desaturase pathway | Different seed-specific promoters, such as Arabidopsis FAE1 promoter, flax Cnl1 and Cnl2, and Brassica napus napin promoter | BASTA herbicide | >12% of DHA, high ω3/ω6 ratio | [170,171] | |
| transgenes cassette expression | microalgal and yeast set of genes for EPA synthesis | Different seed-specific promoters, such as Vicia faba USP, and sucrose binding protein promoter; napin promoter, flax seed specific conlinin 1 (Cnl1) | DsRed fluorescence protein | EPA and DHA content levels in camelina equivalent to those in fish oils | [49] | |
| transgenes cassette expression | Ricinus communis fatty acid hydroxylase (RcFAH), and Lesquerella condensing enzyme gene (LfKCS3) | native promoter of camelina and seed-specific phaseolin promoter | BASTA herbicide | high levels of hydroxyl fatty acid | [172] | |
| single transgene and transgenic cassette expression | Lunaria annua Ketoacyl-CoA synthase (KCS) and the other three elongase genes from Arabidopsis | seed specific soybean glycin1 and oleosin1, cassava vein mosaic virus (CMVP) promoter | DsRed fluorescence protein | higher VLCFA production, in particular of 6-12% (C24:1Δ15) nervonic acid | [173] | |
| transgenes cassette expression | eight different acyl-carrier-thioesterase (FATB) from Caesalpinia pulcherrima, Cuphea viscosissima, Crocodylus palustris, Cladopus hookeriana and Umbellularia californica | soybean glycinin-1 promoter | DsRed fluorescence protein | medium chain FA of different lengths accumulation | [60] | |
| single transgene overexpression | Arabidopsis patatin-related phospholipase pPLAIIIδ | 35S promoter, soybean glycinin1 promoter | hygromycin B antibiotics | Increased seed oil content and decreasing cellulose content | [58] | |
| single transgene expression and RNAi | Euonymus alatus diacylglycerol-acetyltransferase (DAcT) overexpression with suppression of DGAT1 and/or PDAT1 | seed specific soybean glycin1 and oleosin1 | DsRed fluorescence protein | modification and increased level of triacylglycerol content, seed yield improvement | [174] | |
| camelina gene overexpression | camelina DGAT1B | Seed-specific Brassica napus Napin promoter | BASTA herbicide | Total seed oils were increased by ~24% | [175] | |
| single transgene expression and RNAi | Umbellularia californica 12-acyl-carrier thioesterase (FATB) expression and KASII suppression | seed specific napin promoter | mCherry fluorescence gene | higher accumulation up to 28.5% of palmitate, reduction in longer, unsaturated fatty acids in seed TAGs. | [176] | |
| overexpression and down-regulation using artificial microRNA (amiRNA) | PDAT overexpression and DGAT suppression | seed specific napin promoter | BASTA herbicide | oil modulation: a-linolenic decrease and linoleic acid increase | [177] |
| Cultivar | Type/Promoter for Cas9 | Promoter for gRNA Expression | Target Genes | sgRNA Features | Selection Marker | Mutant line Detection System | Trait/Phenotype | References |
|---|---|---|---|---|---|---|---|---|
| Suneson | Constitutive/ 35S promoter from the Cauliflower mosaic virus (CaMV35S) | Arabidopsis thaliana U6 promoter (AtU6-26) | fatty acid desaturase 2 (FAD2) genes | three independent sgRNAs on a conserved region of the 3 FAD2 genes, all designed in 5′-3′ (forward) direction | Red fluorescent protein (DsRed) | restriction enzyme screening | increased MUFA (monosaturated fatty acid) content in the seed | [186] |
| Celine | Constitutive/ Ubiquitin 4−2 promoter from Petroselinum crispum (PcUbi4-2) | Camelina sativa U6 promoter (CsU6) | fatty acid desaturase 2 (FAD2) genes | Two independent sgRNAs on a conserved region in the first 600bp of the 3 FAD2 genes, one in 5′-3′ direction, one in 3′-5′ direction | DsRed | simple allele-discriminating PCR (SAP) | increased MUFA (monosaturated fatty acid) content in the seed | [187] |
| Suneson | Constitutive/ CaMV35S | AtU6-26 | phospholipid: diacylglycerol acyltransferase 1 (PDAT1), diacylglycerol acyltransferase (DGAT1) genes | One sgRNA on a conserved region of the three PDAT1 genes and 1sgRNA for the 3DGAT1. Both sgRNA are designed in 5′-3′ direction | Hygromycin phosphotransferase | restriction enzyme screening | Reduced oil content and altered fatty acid composition | [188] |
| Suneson | Tissue-specific/ Egg cell-specific promoter (EC1.1) | AtU6-26 | fatty acid elongase 1 (FAE1) genes | One sgRNA in reverse strand (3′-5′) in a conserved region in the first 600bp of the three FAE1 genes | DsRed | sequencing of the target regions | Decreased VLCFAs C20-C24 from 22% to 2% | [189] |
| Doubled haploid line DH55 | Constitutive/Arabidopsis thaliana Elongation factor 1 (AtEF1a) | AtU6-26 | CRUCIFERIN C (CRUC) genes | 2sgRNAs in 5′-3′ and 3′-5′ direction, respectively on the first 600bp of the three target genes | Glufosinate-ammonium | droplet digital PCR (ddPCR) | increase in high value-amino acids proteins in the seed | [190] |
| Suneson, CAME | Tissue-specific/ Egg cell-specific promoter (EC1.1) | AtU6-26 | fatty acid desaturase 2 (FAD2) genes | One single sgRNA in the first 300bp in a conserved region of the three FAD2 genes designed in 5′-3′ direction | DsRed | deep sequencing of the targeted sites | increased MUFA (monounsaturated fatty acid) content in the seed | [191] |
| CAM139 | Constitutive/ PcUbi4-2 | AtU6-26 | glucosinolate transporter 1 and 2 (GTR1-GTR2); transcription factors MYB28, MYB29 | Two sgRNAs in conserved regions for each of the three homeolog genes target (7 sgRNA in total) 1sgRNA in common between GTR1 and GTR2 and 1sgRNA in common between MYB28 and MYB29 designed in both directions | DsRed | restriction enzymes screening | decrease glucosinolate content in the seed | [133] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghidoli, M.; Ponzoni, E.; Araniti, F.; Miglio, D.; Pilu, R. Genetic Improvement of Camelina sativa (L.) Crantz: Opportunities and Challenges. Plants 2023, 12, 570. https://doi.org/10.3390/plants12030570
Ghidoli M, Ponzoni E, Araniti F, Miglio D, Pilu R. Genetic Improvement of Camelina sativa (L.) Crantz: Opportunities and Challenges. Plants. 2023; 12(3):570. https://doi.org/10.3390/plants12030570
Chicago/Turabian StyleGhidoli, Martina, Elena Ponzoni, Fabrizio Araniti, Daniela Miglio, and Roberto Pilu. 2023. "Genetic Improvement of Camelina sativa (L.) Crantz: Opportunities and Challenges" Plants 12, no. 3: 570. https://doi.org/10.3390/plants12030570
APA StyleGhidoli, M., Ponzoni, E., Araniti, F., Miglio, D., & Pilu, R. (2023). Genetic Improvement of Camelina sativa (L.) Crantz: Opportunities and Challenges. Plants, 12(3), 570. https://doi.org/10.3390/plants12030570








