A Coating Based on Bioactive Compounds from Streptomyces spp. and Chitosan Oligomers to Control Botrytis cinerea Preserves the Quality and Improves the Shelf Life of Table Grapes
Abstract
:1. Introduction
2. Results
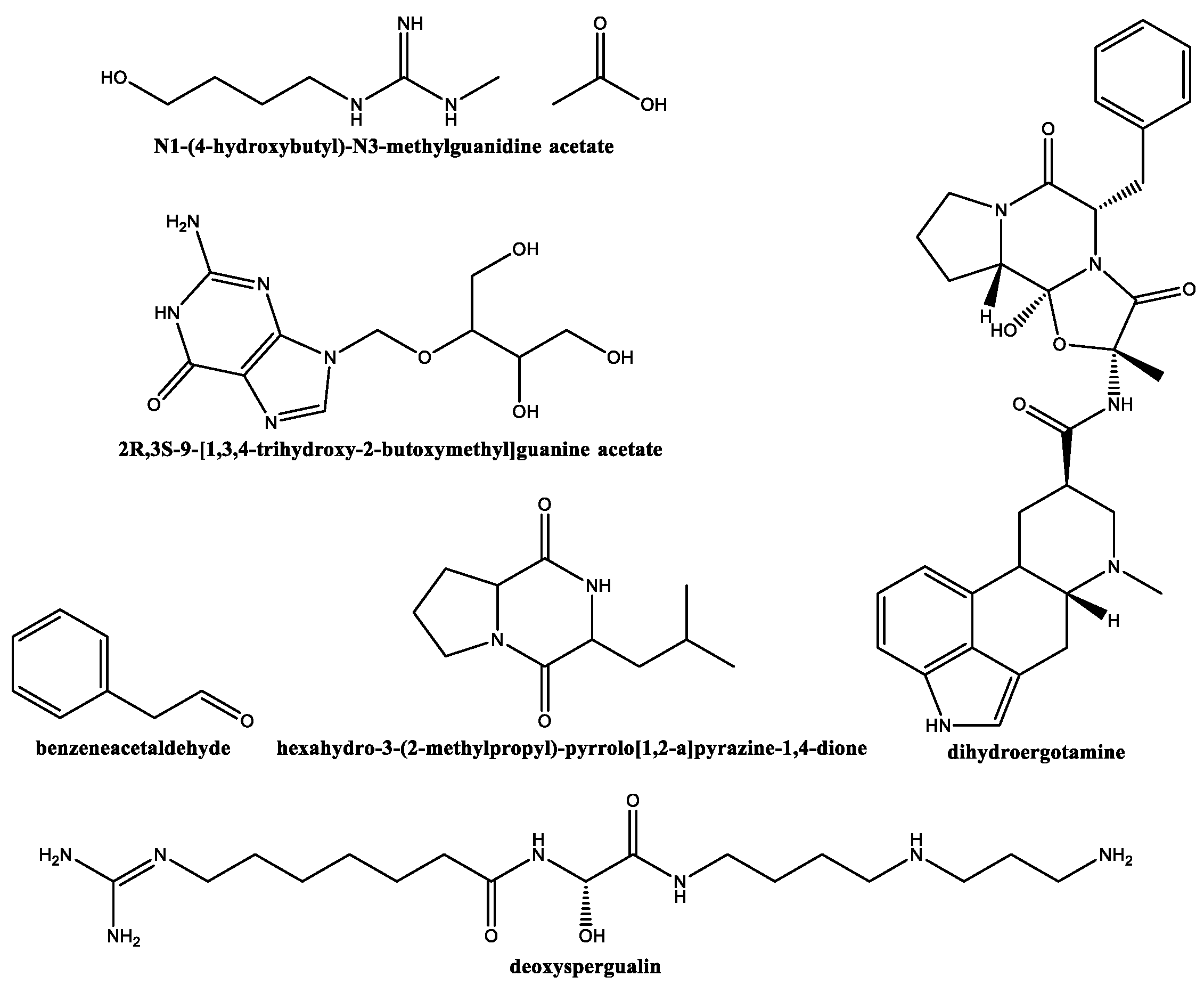
2.1. GC–MS Characterization of Secondary Metabolites of S. lavendofoliae DSM 40217 and S. rochei DSM 41729
2.2. Antifungal Activity
2.2.1. Antibiosis Assay
2.2.2. In vitro Growth Inhibition Tests
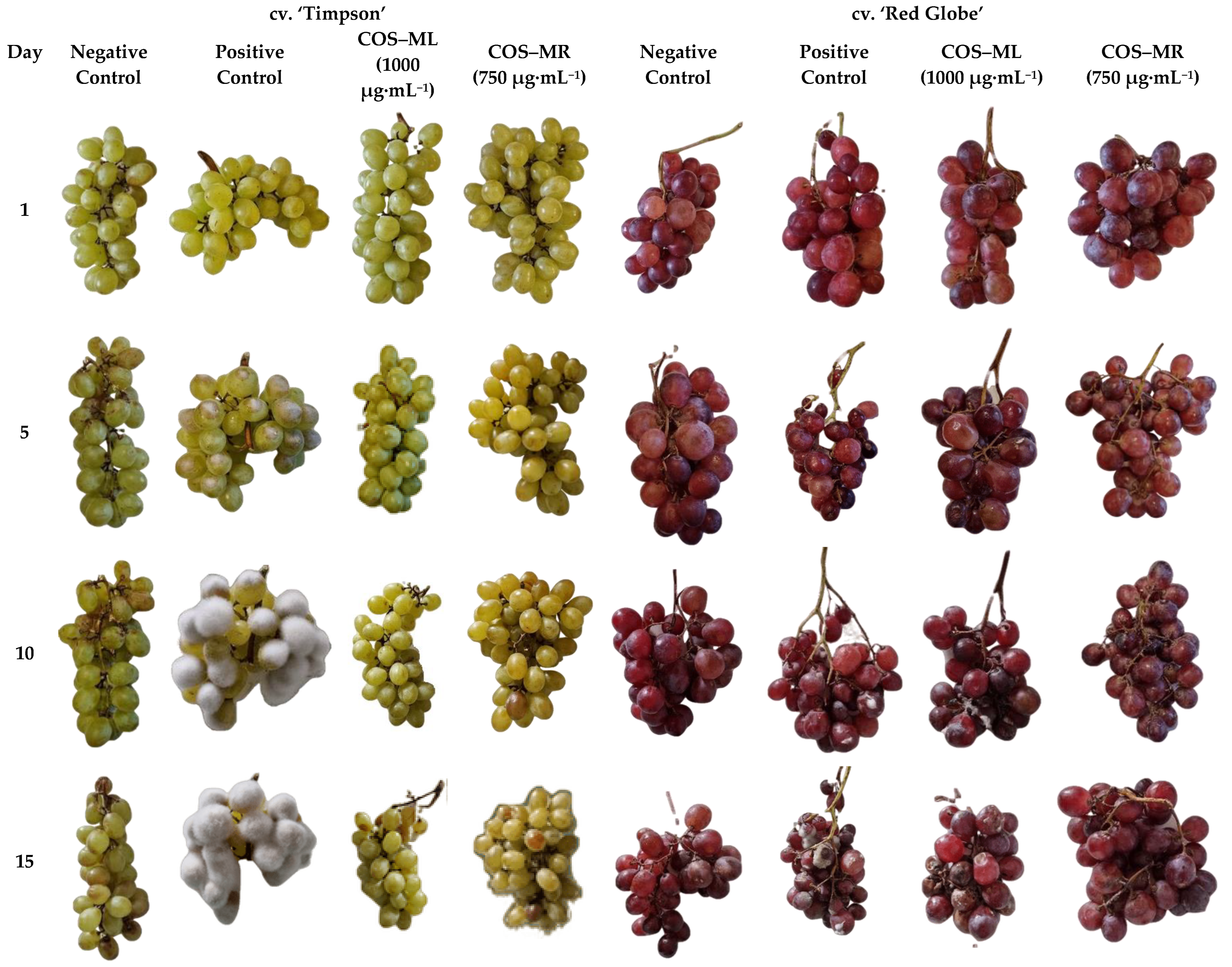
2.2.3. Ex Situ Growth Inhibition Tests
3. Discussion
3.1. On the Secondary Metabolites Profiles
3.2. Efficacy of the Treatments
3.3. Mechanism of Action
4. Materials and Methods
4.1. Bacterial and Fungal Isolates, Reagents, and Table Grapes
4.2. Preparation of Secondary Metabolites of Streptomyces spp. and Preparation of B. cinerea Conidial Suspension
4.3. Synthesis of Chitosan Oligomers and COS–Secondary Metabolites Conjugate Complexes
4.4. Antifungal Activity Assessment
4.4.1. In Vitro Tests of Mycelial Growth Inhibition
4.4.2. Ex Situ Tests of Mycelial Growth Inhibition
4.5. Gas Chromatography–Mass Spectrometry Analysis of Streptomyces spp. Secondary Metabolites
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seccia, A.; Viscecchia, R.; Nardone, G. Table grapes as functional food: Consumer preferences for health and environmental attributes. BIO Web Conf. 2019, 15, 03011. [Google Scholar] [CrossRef]
- Porat, R.; Lichter, A.; Terry, L.A.; Harker, R.; Buzby, J. Postharvest losses of fruit and vegetables during retail and in consumers’ homes: Quantifications, causes, and means of prevention. Postharvest Biol. Technol. 2018, 139, 135–149. [Google Scholar] [CrossRef] [Green Version]
- Terán Samaniego, K.; Robles Parra, M.; Preciado Rodríguez, J.M.; López López, D.C. Equidad gerencial, como una demanda intangible de mercado: Hacia organizaciones sustentables. Entre Cienc. Ing. 2022, 13, 85–93. [Google Scholar] [CrossRef]
- Mencarelli, F.; Bellincontro, A. Grape: Post-Harvest Operations; Food and Agriculture Organization of the United Nations: Rome, Italy, 2005; p. 43. [Google Scholar]
- Habib, W.; Khalil, J.; Mincuzzi, A.; Saab, C.; Gerges, E.; Tsouvalakis, H.C.; Ippolito, A.; Sanzani, S.M. Fungal pathogens associated with harvested table grapes in Lebanon, and characterization of the mycotoxigenic genera. Phytopathol. Mediterr. 2021, 60, 427–439. [Google Scholar] [CrossRef]
- Lipinski, B.; Hanson, C.; Waite, R.; Searchinger, T.; Lomax, J. Reducing Food Loss and Waste: Creating a Sustainable Food Future, Installment Two; World Resources Institute: Washington, DC, USA, 2013; p. 40. [Google Scholar]
- Kader, A. Increasing food availability by reducing postharvest losses of fresh produce. Acta Hortic. 2005, 682, 2169–2176. [Google Scholar] [CrossRef]
- Munhuweyi, K.; Opara, U.L.; Sigge, G. Postharvest losses of cabbages from retail to consumer and the socio-economic and environmental impacts. Br. Food J. 2016, 118, 286–300. [Google Scholar] [CrossRef]
- Sanzani, S.M.; Schena, L.; De Cicco, V.; Ippolito, A. Early detection of Botrytis cinerea latent infections as a tool to improve postharvest quality of table grapes. Postharvest Biol. Technol. 2012, 68, 64–71. [Google Scholar] [CrossRef]
- De Simone, N.; Pace, B.; Grieco, F.; Chimienti, M.; Tyibilika, V.; Santoro, V.; Capozzi, V.; Colelli, G.; Spano, G.; Russo, P. Botrytis cinerea and table grapes: A review of the main physical, chemical, and bio-based control treatments in post-harvest. Foods 2020, 9, 1138. [Google Scholar] [CrossRef] [PubMed]
- Avenot, H.F.; Quattrini, J.; Puckett, R.; Michailides, T.J. Different levels of resistance to cyprodinil and iprodione and lack of fludioxonil resistance in Botrytis cinerea isolates collected from pistachio, grape, and pomegranate fields in California. Crop Prot. 2018, 112, 274–281. [Google Scholar] [CrossRef]
- Liu, J.; Tian, S.; Meng, X.; Xu, Y. Effects of chitosan on control of postharvest diseases and physiological responses of tomato fruit. Postharvest Biol. Technol. 2007, 44, 300–306. [Google Scholar] [CrossRef]
- Salih, S.I.; Maaroof, M.N. Isolation and identification some species of Streptomyces bacteria producing antibiotics and molecular detection of gene 16srrna and alignment nucleotide sequences with the NCBI. Ann. For. Res 2022, 65, 4000–4013. [Google Scholar]
- Arakawa, K.; Sugino, F.; Kodama, K.; Ishii, T.; Kinashi, H. Cyclization mechanism for the synthesis of macrocyclic antibiotic Lankacidin in Streptomyces rochei. Chem. Biol. 2005, 12, 249–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anukool, U.; Gaze, W.H.; Wellington, E.M.H. In situ monitoring of Streptothricin production by Streptomyces rochei F20 in soil and rhizosphere. Appl. Environ. Microbiol. 2004, 70, 5222–5228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamashita, N.; Shin-Ya, K.; Furihata, K.; Hayakawa, Y.; Seto, H. New Ravidomycin analogues, FE35A and FE35B, apoptosis inducers produced by Streptomyces rochei. J. Antibiot. 1998, 51, 1105–1108. [Google Scholar] [CrossRef] [Green Version]
- Kanini, G.S.; Katsifas, E.A.; Savvides, A.L.; Karagouni, A.D. Streptomyces rochei ACTA1551, an indigenous Greek isolate studied as a potential biocontrol agent against Fusarium oxysporum f. sp. lycopersici. BioMed Res. Int. 2013, 2013, 387230. [Google Scholar] [CrossRef] [Green Version]
- Sarika, K.; Sampath, G.; Kaveriyappan Govindarajan, R.; Ameen, F.; Alwakeel, S.; Al Gwaiz, H.I.; Raja Komuraiah, T.; Ravi, G. Antimicrobial and antifungal activity of soil actinomycetes isolated from coal mine sites. Saudi J. Biol. Sci. 2021, 28, 3553–3558. [Google Scholar] [CrossRef]
- Ul-Rahman, A. Taificidin1 and Taificidin2, two anti-microbial agents isolated from the fermentation broth of Streptomyces roseodistaticus TA15 and Streptomyces lavendofoliae TA17. Res. J. Microbiol. 2011, 6, 328. [Google Scholar] [CrossRef] [Green Version]
- Le Goff, G.; Ouazzani, J. Natural hydrazine-containing compounds: Biosynthesis, isolation, biological activities and synthesis. Biorg. Med. Chem. 2014, 22, 6529–6544. [Google Scholar] [CrossRef]
- Murakami, S.; Harada, S.; Yamazaki, T.; Takahashi, Y.; Hamada, M.; Takeuchi, T.; Aoyagi, T. Piperastatin A, a new selective serine carboxypeptidase inhibitor produced by actinomycete. I. Taxonomy, production, isolation and biological activities. J. Enzyme Inhib. 1996, 10, 93–103. [Google Scholar] [CrossRef]
- Murakami, S.; Harada, S.; Takahashi, Y.; Naganawa, H.; Takeuchi, T.; Aoyagi, T. Piperastatin B: A new selective serine carboxypeptidase inhibitor from Streptomyces lavendofoliae MJ908-WF13. J. Enzyme Inhib. 1996, 11, 51–66. [Google Scholar] [CrossRef]
- Narayanaswamy, V.K.; Albericio, F.; Coovadia, Y.M.; Kruger, H.G.; Maguire, G.E.; Pillay, M.; Govender, T. Total synthesis of a depsidomycin analogue by convergent solid-phase peptide synthesis and macrolactonization strategy for antitubercular activity. J. Pept. Sci. 2011, 17, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, G.A.M.; Gebily, D.A.S.; Ragab, M.M.; Ali, A.M.; Soliman, N.E.-D.K.; El-Moity, T.H.A. Efficacy of antifungal substances of three Streptomyces spp. against different plant pathogenic fungi. Egypt. J. Biol. Pest Control 2022, 32, 112. [Google Scholar] [CrossRef]
- Sarkar, S.; Chandra Singh, P. Spectroscopic and simulation studies of the sequence-dependent DNA destabilization by a fungicide. ACS Omega 2021, 6, 14371–14378. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.X.; Wu, F.J.; Wu, Y.Q.; Zhang, Z.F. Study on biomedical resources of benzene/alcohol extractives of Eucalyptus leaves by GC/MS. Adv. Mater. Res. 2010, 97–101, 2231–2236. [Google Scholar] [CrossRef]
- Kiran, G.S.; Priyadharsini, S.; Sajayan, A.; Ravindran, A.; Selvin, J. An antibiotic agent pyrrolo [1, 2-a] pyrazine-1,4-dione, hexahydro isolated from a marine bacteria Bacillus tequilensis MSI45 effectively controls multi-drug resistant Staphylococcus aureus. RSC Adv. 2018, 8, 17837–17846. [Google Scholar] [CrossRef] [Green Version]
- Özdemir, A.; Turan-Zitouni, G.; Asim Kaplancikli, Z.; Demirci, F.; Iscan, G. Studies on hydrazone derivatives as antifungal agents. J. Enzym. Inhib. Med. Chem. 2008, 23, 470–475. [Google Scholar] [CrossRef]
- Pascale, G.; Sauriol, F.; Benhamou, N.; BÉLanger, R.R.; Paulitz, T.C. Novel butyrolactones with antifungal activity produced by Pseudomonas aureofaciens strain 63–28. J. Antibiot. 1997, 50, 742–749. [Google Scholar] [CrossRef] [Green Version]
- Aoyagi, K.; Abe, F.; Nemoto, K.; Abe, S.; Ishizuka, M.; Takeuchi, T.; Yamaguchi, H. The novel immunostimulant N-563, an analogue of deoxyspergualin, promotes resistance to Candida albicans infection in mice. J. Antibiot. 1994, 47, 1077–1083. [Google Scholar] [CrossRef] [Green Version]
- Starr, A.M.; Zabet-Moghaddam, M.; San Francisco, M. Identification of a novel secreted metabolite cyclo (phenylalanyl-prolyl) from Batrachochytrium dendrobatidis and its effect on Galleria mellonella. BMC Microbiol. 2022, 22, 293. [Google Scholar] [CrossRef]
- Suthar, S.K.; Chundawat, N.S.; Pal Singh, G.; Padrón, J.M.; Payghan, P.V.; Jhala, Y.K. Evaluation of anti-bacterial activity of novel 2, 3-diaminoquinoxaline derivatives: Design, synthesis, biological screening, and molecular modeling studies. Egypt. J. Basic Appl. Sci. 2022, 9, 162–179. [Google Scholar] [CrossRef]
- Yong, D.; Li, Y.; Gong, K.; Yu, Y.; Zhao, S.; Duan, Q.; Ren, C.; Li, A.; Fu, J.; Ni, J.; et al. Biocontrol of strawberry gray mold caused by Botrytis cinerea with the termite associated Streptomyces sp. sdu1201 and actinomycin D. Front. Microbiol. 2022, 13, 1051730. [Google Scholar] [CrossRef]
- Minh, N.V.; Woo, E.E.; Kim, J.-Y.; Kim, D.-W.; Hwang, B.S.; Lee, Y.-J.; Lee, I.-K.; Yun, B.-S. Antifungal substances from Streptomyces sp. A3265 antagonistic to plant pathogenic fungi. Mycobiology 2015, 43, 333–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Ning, P.; Zheng, L.; Huang, J.; Li, G.; Hsiang, T. Effects of volatile substances of Streptomyces globisporus JK-1 on control of Botrytis cinerea on tomato fruit. Biol. Control 2012, 61, 113–120. [Google Scholar] [CrossRef]
- Jiang, B.M.; Yali, H.; Jia, Z.-h.; Song, S. Streptomyces nobilis C51 suppresses gray mold caused by Botrytis cinerea in tomato. Br. Microbiol. Res. J. 2016, 16, 1–13. [Google Scholar] [CrossRef]
- Ayed, A.; Kalai-Grami, L.; Ben Slimene, I.; Chaouachi, M.; Mankai, H.; Karkouch, I.; Djebali, N.; Elkahoui, S.; Tabbene, O.; Limam, F. Antifungal activity of volatile organic compounds from Streptomyces sp. strain S97 against Botrytis cinerea. Biocontrol Sci. Technol. 2021, 31, 1330–1348. [Google Scholar] [CrossRef]
- Kim, J.D.; Park, M.Y.; Jeon, B.J.; Kim, B.S. Disease control efficacy of 32,33-didehydroroflamycoin produced by Streptomyces rectiviolaceus strain DY46 against gray mold of tomato fruit. Sci. Rep. 2019, 9, 13533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boukaew, S.; Prasertsan, P.; Troulet, C.; Bardin, M. Biological control of tomato gray mold caused by Botrytis cinerea by using Streptomyces spp. BioControl 2017, 62, 793–803. [Google Scholar] [CrossRef]
- Xu, W.-T.; Huang, K.-L.; Guo, F.; Qu, W.; Yang, J.-J.; Liang, Z.-H.; Luo, Y.-B. Postharvest grapefruit seed extract and chitosan treatments of table grapes to control Botrytis cinerea. Postharvest Biol. Technol. 2007, 46, 86–94. [Google Scholar] [CrossRef]
- Ramos-Bell, S.; Hernández-Montiel, L.G.; Velázquez-Estrada, R.M.; Herrera-González, J.A.; Gutiérrez-Martínez, P. Potential of Debaryomyces hansenii strains on the Inhibition of Botrytis cinerea in blueberry fruits (Vaccinium corymbosum L.). Horticulturae 2022, 8, 1125. [Google Scholar] [CrossRef]
- Muñoz, Z.; Moret, A. Sensitivity of Botrytis cinerea to chitosan and acibenzolar-S-methyl. Pest Manag. Sci. 2010, 66, 974–979. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, P.; Zhang, P.; Su, L.; Jia, H.; Wei, X.; Fang, J.; Jia, H. Integrative transcriptomics and metabolomics data exploring the effect of chitosan on postharvest grape resistance to Botrytis cinerea. Postharvest Biol. Technol. 2020, 167, 111248. [Google Scholar] [CrossRef]
- Celik, M.; Kalpulov, T.; Zutahy, Y.; Ish-shalom, S.; Lurie, S.; Lichter, A. Quantitative and qualitative analysis of Botrytis inoculated on table grapes by qPCR and antibodies. Postharvest Biol. Technol. 2009, 52, 235–239. [Google Scholar] [CrossRef]
- Owoyemi, A.; Lapidot, O.; Kochanek, B.; Zahavi, T.; Salzer, Y.; Porat, R.; Lichter, A. Sour rot in the vineyard is an indicator of Botrytis rot in grapes after storage. Postharvest Biol. Technol. 2022, 191, 111980. [Google Scholar] [CrossRef]
- Ma, Z.; Garrido-Maestu, A.; Jeong, K.C. Application, mode of action, and in vivo activity of chitosan and its micro- and nanoparticles as antimicrobial agents: A review. Carbohydr. Polym. 2017, 176, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.-K.; Kim, K.-Y.; Yoo, Y.-J.; Oh, S.-J.; Choi, J.-H.; Kim, C.-Y. In vitro antimicrobial activity of a chitooligosaccharide mixture against Actinobacillus actinomycetemcomitans and Streptococcus mutans. Int. J. Antimicrob. Agents 2001, 18, 553–557. [Google Scholar] [CrossRef]
- Hu, J.; Zhao, X.; Zhu, C.; Hu, F.; Hui, J. Inhibitory effect and mechanisms of sophorolipids against Staphylococcus aureus. J. Food Sci. 2012, 33, 33–36. [Google Scholar] [CrossRef]
- Ginsburg, I.; van Heerden, P.V.; Koren, E. From amino acids polymers, antimicrobial peptides, and histones, to their possible role in the pathogenesis of septic shock: A historical perspective. J. Inflamm. Res. 2017, 10, 7. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; Wang, J.; Zhang, P.; Xie, S.; Yuan, X.; Hou, X.; Yan, N.; Fang, Y.; Du, Y. In vitro and in vivo antifungal activity and preliminary mechanism of cembratrien-diols against Botrytis cinerea. Ind. Crops Prod. 2020, 154, 112745. [Google Scholar] [CrossRef]
- Ing, L.Y.; Zin, N.M.; Sarwar, A.; Katas, H. Antifungal activity of chitosan nanoparticles and correlation with their physical properties. Int. J. Biomater. 2012, 2012, 632698. [Google Scholar] [CrossRef] [Green Version]
- Buzón-Durán, L.; Martín-Gil, J.; Marcos-Robles, J.L.; Fombellida-Villafruela, Á.; Pérez-Lebeña, E.; Martín-Ramos, P. Antifungal activity of chitosan oligomers–amino acid conjugate complexes against Fusarium culmorum in spelt (Triticum spelta L.). Agronomy 2020, 10, 1427. [Google Scholar] [CrossRef]
- Kashyap, P.L.; Xiang, X.; Heiden, P. Chitosan nanoparticle based delivery systems for sustainable agriculture. Int. J. Biol. Macromol. 2015, 77, 36–51. [Google Scholar] [CrossRef]
- Cheung, R.; Ng, T.; Wong, J.; Chan, W. Chitosan: An update on potential biomedical and pharmaceutical applications. Mar. Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef]
- Gong, Y.; Liu, J.-Q.; Xu, M.-J.; Zhang, C.-M.; Gao, J.; Li, C.-G.; Xing, K.; Qin, S. Antifungal volatile organic compounds from Streptomyces setonii WY228 control black spot disease of sweet potato. Appl. Environ. Microbiol. 2022, 88, e02317-21. [Google Scholar] [CrossRef]
- Sudha, A.; Durgadevi, D.; Archana, S.; Muthukumar, A.; Suthin Raj, T.; Nakkeeran, S.; Poczai, P.; Nasif, O.; Ansari, M.J.; Sayyed, R. Unraveling the tripartite interaction of volatile compounds of Streptomyces rochei with grain mold pathogens infecting sorghum. Front. Microbiol. 2022, 13, 923360. [Google Scholar] [CrossRef]
- Ruano-Rosa, D.; Sánchez-Hernández, E.; Baquero-Foz, R.; Martín-Ramos, P.; Martín-Gil, J.; Torres-Sánchez, S.; Casanova-Gascón, J. Chitosan-based bioactive formulations for the control of powdery mildew in viticulture. Agronomy 2022, 12, 495. [Google Scholar] [CrossRef]
- Sadigh-Eteghad, S.; Dehnad, A.; Shanebandi, D.; Khalili, I.; Razmarayii, N.; Namvaran, A. Identification and characterization of a Streptomyces sp. isolate exhibiting activity against multidrug-resistant coagulase-negative Staphylococci. Vet. Res. Commun. 2011, 35, 477–486. [Google Scholar] [CrossRef]
- Pazhanimurugan, R.; Radhakrishnan, M.; Shanmugasundaram, T.; Gopikrishnan, V.; Balagurunathan, R. Terpenoid bioactive compound from Streptomyces rochei (M32): Taxonomy, fermentation and biological activities. World J. Microbiol. Biotechnol. 2016, 32, 161. [Google Scholar] [CrossRef]
- Santos-Moriano, P.; Fernandez-Arrojo, L.; Mengibar, M.; Belmonte-Reche, E.; Peñalver, P.; Acosta, F.; Ballesteros, A.; Morales, J.; Kidibule, P.; Fernandez-Lobato, M. Enzymatic production of fully deacetylated chitooligosaccharides and their neuroprotective and anti-inflammatory properties. Biocatal. Biotransform. 2018, 36, 57–67. [Google Scholar] [CrossRef]
- Yang, Y.; Shu, R.; Shao, J.; Xu, G.; Gu, X. Radical scavenging activity of chitooligosaccharide with different molecular weights. Eur. Food Res. Technol. 2005, 222, 36–40. [Google Scholar] [CrossRef]
- Maghami, G.G.; Roberts, G.A.F. Evaluation of the viscometric constants for chitosan. Makromol. Chem. 1988, 189, 195–200. [Google Scholar] [CrossRef]
- Buzón-Durán, L.; Martín-Gil, J.; Pérez-Lebeña, E.; Ruano-Rosa, D.; Revuelta, J.L.; Casanova-Gascón, J.; Ramos-Sánchez, M.C.; Martín-Ramos, P. Antifungal agents based on chitosan oligomers, ε-polylysine and Streptomyces spp. secondary metabolites against three Botryosphaeriaceae species. Antibiotics 2019, 8, 99. [Google Scholar] [CrossRef] [PubMed]
- Zambrano, E.C.; Parra, A.S.; Ortiz, Á.M.M. Biocontrol of rice sheath blight with microorganisms obtained in rice cultivated soils. Bragantia 2021, 80, e0921. [Google Scholar] [CrossRef]
- Sánchez-Hernández, E.; Andrés-Juan, C.; Buzón-Durán, L.; Correa-Guimaraes, A.; Martín-Gil, J.; Martín-Ramos, P. Antifungal activity of methylxanthines against grapevine trunk diseases. Agronomy 2022, 12, 885. [Google Scholar] [CrossRef]
- Levy, Y.; Benderly, M.; Cohen, Y.; Gisi, U.; Bassand, D. The joint action of fungicides in mixtures: Comparison of two methods for synergy calculation. EPPO Bull. 1986, 16, 651–657. [Google Scholar] [CrossRef]
- Riquelme, D.; Aravena, Z.; Valdés-Gómez, H.; Latorre, B.A.; Díaz, G.A.; Zoffoli, J.P. Characterization of Botrytis cinerea and B. prunorum from healthy floral structures and decayed ‘Hayward’ kiwifruit during post-harvest storage. Plant Dis. 2021, 105, 2129–2140. [Google Scholar] [CrossRef]

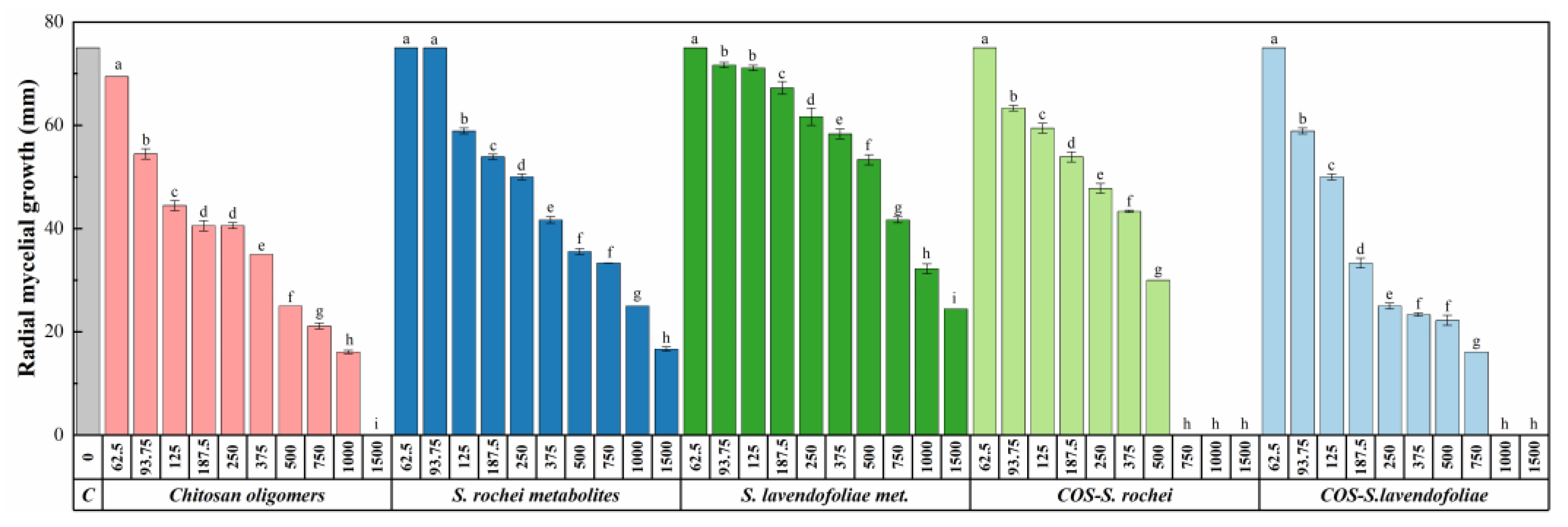
| Effective Concentration | COS | S. rochei DSM 41729 Metabolites | S. lavendofoliae DSM 40217 Metabolites | COS–S. rochei DSM 41729 Metabolites | COS–S. lavendofoliae DSM 40217 Metabolites |
|---|---|---|---|---|---|
| EC50 | 246 | 429 | 909 | 201 | 311 |
| EC90 | 1422 | 1723 | 3013 | 721 | 953 |
| Effective Concentration | Synergy Factor | |
|---|---|---|
| COS–S. rochei DSM 41729 Metabolites | COS–S. lavendofoliae DSM 40217 Metabolites | |
| EC50 | 1.55 | 1.24 |
| EC90 | 1.89 | 2.33 |
| Streptomyces ssp. | Provenance of Isolate | In Vitro Effectiveness | In Vivo Assays | Ref. | |
|---|---|---|---|---|---|
| Fruit | Effectiveness | ||||
| Streptomyces sp. sdu1201 | China | IR = 78.05% | Strawberry fruits cv. ‘Tian Bao’ | CE = 53.33%, after 2 days | [33] |
| CE = 45.44%, after 3 days | |||||
| Streptomyces sp. A3265 | n.s. | MIC = 2.5–20 µg·mL−1 | n.s. | n.s. | [34] |
| S. globisporus JK−1 | China | IR = 100% at 30,000 µg·mL−1 | Tomato fruits cv. ‘Annie’ | DI = 35.8% after 24 h, 240,000 µg·mL−1 | [35] |
| S. nobilis C51 | China | IZ = 11.07 | Tomato leaves | CE = 72.63% | [36] |
| Streptomyces sp. S97 | Tunisia | IR = 99.3% | Strawberry fruits | DI = 87% | [37] |
| S. griseus MT210913”DG5” | Egypt | IR = 70.33% | n.s. | [24] | |
| S. rochei MN700192”DG4” | IR = 70.83% | ||||
| S. sampsonii MN700191”DG1” | IR = 73.67% | ||||
| S. rectiviolaceus DY46 | n.s. | n.s. | Tomato fruits | DI = 20%, at 100,000 µg·mL−1 | [38] |
| S. philanthi RM−1−138 | Thailand | IR = 73−100% | Tomato leaves | CE = 60% | [39] |
| Tomato plants | CE = 57% | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buzón-Durán, L.; Sánchez-Hernández, E.; Sánchez-Báscones, M.; García-González, M.C.; Hernández-Navarro, S.; Correa-Guimarães, A.; Martín-Ramos, P. A Coating Based on Bioactive Compounds from Streptomyces spp. and Chitosan Oligomers to Control Botrytis cinerea Preserves the Quality and Improves the Shelf Life of Table Grapes. Plants 2023, 12, 577. https://doi.org/10.3390/plants12030577
Buzón-Durán L, Sánchez-Hernández E, Sánchez-Báscones M, García-González MC, Hernández-Navarro S, Correa-Guimarães A, Martín-Ramos P. A Coating Based on Bioactive Compounds from Streptomyces spp. and Chitosan Oligomers to Control Botrytis cinerea Preserves the Quality and Improves the Shelf Life of Table Grapes. Plants. 2023; 12(3):577. https://doi.org/10.3390/plants12030577
Chicago/Turabian StyleBuzón-Durán, Laura, Eva Sánchez-Hernández, Mercedes Sánchez-Báscones, Mari Cruz García-González, Salvador Hernández-Navarro, Adriana Correa-Guimarães, and Pablo Martín-Ramos. 2023. "A Coating Based on Bioactive Compounds from Streptomyces spp. and Chitosan Oligomers to Control Botrytis cinerea Preserves the Quality and Improves the Shelf Life of Table Grapes" Plants 12, no. 3: 577. https://doi.org/10.3390/plants12030577
APA StyleBuzón-Durán, L., Sánchez-Hernández, E., Sánchez-Báscones, M., García-González, M. C., Hernández-Navarro, S., Correa-Guimarães, A., & Martín-Ramos, P. (2023). A Coating Based on Bioactive Compounds from Streptomyces spp. and Chitosan Oligomers to Control Botrytis cinerea Preserves the Quality and Improves the Shelf Life of Table Grapes. Plants, 12(3), 577. https://doi.org/10.3390/plants12030577












