In Vitro Propagation of Three Date Palm (Phoenix dactylifera L.) Varieties Using Immature Female Inflorescences
Abstract
:1. Introduction
2. Results
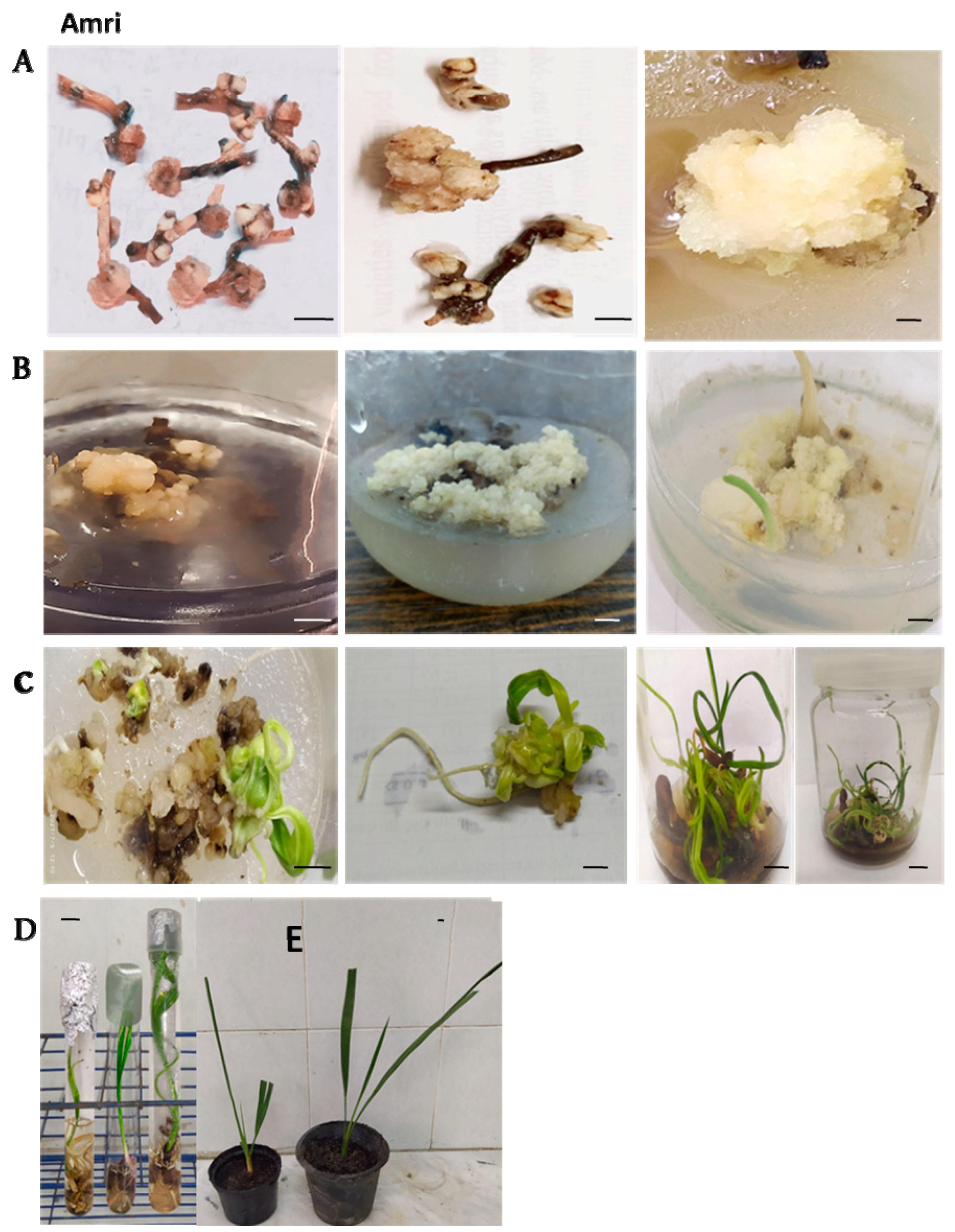
2.1. Starting Stage
2.2. Maturation Stage
2.3. Multiplication Stage
2.4. Rooting Stage
2.5. Pre-Acclimatization and Acclimatization Stages
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Sterilization and Explants Preparation
4.3. Preparation of Culture Media
4.4. Culture Conditions
4.5. Measurements
4.6. Pre-Acclimatization
4.7. Acclimatization
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| (SM) | Starting media |
| (MM) | Maturation media |
| (PM) | Multiplication media |
| (RM) | Rooting media |
| (2, 4-D) | 2,4-Dichlorophenoxyacetic acid |
| (NAA) | Naphthaleneacetic acid |
| (IAA) | Indole-3-acetic acid |
| (IBA) | Indole-3-butyric acid |
| (Kin) | Kinetin |
| (2-iP) | 2-isopentenyladenine |
| (BAP or BA) | 6-Benzylaminopurine or benzyl adenine |
| (AC) | Activated charcoal |
| (MS) | Murashige and Skoog |
References
- Vardareli, N.; Doğaroğlu, T.; Doğaç, E.; Taşkın, V.; Göçmen Taşkın, B. Genetic characterization of tertiary relict endemic Phoenix theophrasti populations in Turkey and phylogenetic relations of the species with other palm species revealed by SSR markers. Plant Syst. Evol. 2019, 305, 415–429. [Google Scholar] [CrossRef]
- Krueger, R.R. Date Palm (Phoenix dactylifera L.) Biology and Utilization. In The Date Palm Genome; Springer: New York, NY, USA, 2021; Volume 1, pp. 3–28. [Google Scholar]
- Gantait, S.; El-Dawayati, M.M.; Panigrahi, J.; Labrooy, C.; Verma, S.K. The retrospect and prospect of the applications of biotechnology in Phoenix dactylifera L. Appl. Microbiol. Biotechnol. 2018, 102, 8229–8259. [Google Scholar] [CrossRef]
- Khierallah, H.S.; Bader, S.M. Micropropagation of date palm (Phoenix dactylifera L.) var. Maktoom through direct organogenesis. In Proceedings of the III International Date Palm Conference 736, Al-Ain, United Arab Emirates, 25–27 March 2001; pp. 213–224. [Google Scholar]
- Abdalla, N.; El-Ramady, H.; Seliem, M.K.; El-Mahrouk, M.E.; Taha, N.; Bayoumi, Y.; Shalaby, T.A.; Dobránszki, J. An Academic and Technical Overview on Plant Micropropagation Challenges. Horticulturae 2022, 8, 677. [Google Scholar] [CrossRef]
- Abul-Soad, A.A.; Al-Khayri, J.M. Date palm somatic embryogenesis from inflorescence explant. In Step Wise Protocols for Somatic Embryogenesis of Important Woody Plants; Springer: New York, NY, USA, 2018; pp. 329–347. [Google Scholar]
- Hassan, M.M.; Allam, M.A.; Shams El Din, I.; Malhat, M.H.; Taha, R.A. High-frequency direct somatic embryogenesis and plantlet regeneration from date palm immature inflorescences using picloram. Genet. Eng. Biotechnol. 2021, 19, 1–11. [Google Scholar] [CrossRef]
- Magyar-Tábori, K.; Dobránszki, J.; Teixeira da Silva, J.A.; Bulley, S.M.; Hudák, I. The role of cytokinins in shoot organogenesis in apple. Plant Cell Tissue Organ Cult 2010, 101, 251–267. [Google Scholar] [CrossRef]
- Fki, L.; Masmoudi, R.; Kriaa, W.; Mahjoub, A.; Sghaier, B.; Mzid, R.; Mliki, A.; Rival, A.; Drira, N. Date palm micropropagation via somatic embryogenesis. Date Palm Biotechnol. 2011, 47–68. [Google Scholar]
- Hasan, M.; Abdullah, H.M.; Hasibuzzaman, A.S.M.; Ali, M.A. Date Palm Genetic Resources for Breeding. In Cash Crops; Springer: New York, NY, USA, 2022; pp. 479–503. [Google Scholar]
- Abul-Soad, A.A. Micropropagation of Date Palm Using Inflorescence Explants. In Date Palm Biotechnology; Springer: Dordrecht, The Netherlands, 2011. [Google Scholar]
- Abahmane, L.; Bougerfaoui, M.; Anjarne, M. Use of tissue culture techniques for date palm propagation and rehabilitation of palm groves devastated by bayoud disease. In Proceedings of the International Symposium on Date Palm, Assiut University, Assiut, Egypt, 9–11 November 1999. [Google Scholar]
- Weckx, S.; Inzé, D.; Maene, L.J.F.i.p.s. Tissue culture of oil palm: Finding the balance between mass propagation and somaclonal variation. Front. Plant Sci. 2019, 10, 722. [Google Scholar] [CrossRef]
- Al-Khayri, J. Date palm Phoenix dactylifera L. micropropagation. In Protocols for Micropropagation of Woody Trees and Fruits; Springer: New York, NY, USA, 2007; pp. 509–526. [Google Scholar]
- Kriaa, W.; Masmoudi, F.; Drira, N. In vitro culture of date palm using mature female flower explants. In Proceedings of the Fourth Symposium on Date Palm, Abu Dhabi, UAE, 15–17 March 2010; pp. 5–8. [Google Scholar]
- Pottino, B. Methods in Plant Tissue Culture; University of Maryland; College Park, MD, USA, 1981; pp. 8–29.
- Vahdati, K.; Jariteh, M.; Niknam, V.; Mirmasoumi, M.; Ebrahimzadeh, H. Somatic embryogenesis and embryo maturation in Persian walnut. In Proceedings of the V International Walnut Symposium 705. 2004, Sorrento, Italy, 30 March 2005; pp. 199–205. [Google Scholar]
- Solangi, N.; Abul-Soad, A.A.; Markhand, G.S.; Jatoi, M.A.; Jatt, T.; Mirani, A.A. Comparison among different auxins and cytokinins to induce date palm (Phoenix dactylifera L.) somatic embryogenesis from floral buds. Pak. J. Bot 2020, 52, 1243–1249. [Google Scholar] [CrossRef]
- Al-Khalifah, N.; Shanavaskhan, A. Micropropagation of date palms. Asia-Pac. Consort. Agric. Biotechnol. (APCoAB) Assoc. Agric. Res. Inst. Near East North Afr. (AARINENA) 2012, 54. [Google Scholar]
- Eshraghi, P.; Zarghami, R.; Mirabdulbaghi, M. Somatic embryogenesis in two Iranian date palm cultivars. Afr. J. Biotechnol. 2005, 4, 1309–1312. [Google Scholar]
- Fki, L.; Masmoudi, R.; Drira, N.; Rival, A. An optimised protocol for plant regeneration from embryogenic suspension cultures of date palm, Phoenix dactylifera L., cv. Deglet Nour. Plant Cell Rep. 2003, 21, 517–524. [Google Scholar] [CrossRef]
- Loutfi, K.; Chlyah, H. Vegetative multiplication of date palms from in vitro cultured inflorescences: Effect of some growth regulator combinations and organogenetic potential of various cultivars. Agronomie 2022, 18, 573–580. [Google Scholar] [CrossRef]
- Aslam, J.; Khan, S. In vitro micropropagation of’Khalas’ date palm (Phoenix dactylifera L.), an important fruit plant. J. Fruit Ornam. Plant Res. 2009, 17, 15–27. [Google Scholar]
- Taha, H.; Bekheet, S.; Saker, M. Factors affecting in vitro multiplication of date palm. Biol. Plant. 2001, 44, 431–433. [Google Scholar] [CrossRef]
- Thakur, S.; Tiwari, K.; Jadhav, S. In vitro approaches for conservation of Asparagus racemosus Willd. Vitr. Cell. Dev. Biol.-Plant 2015, 51, 619–625. [Google Scholar] [CrossRef]
- Alansi, S.; Al-Qurainy, F.; Nadeem, M.; Khan, S.; Gaafar, A.-R.Z.; Tarroum, M.; Alshameri, A. Efficient micropropagation via somatic embryogenesis of potential cultivar Sagai of Phoenix dactylifera L. Pak. J. Bot. 2018, 50, 2251–2258. [Google Scholar]
- Al Kaabi, H.; Rhiss, A.; Hassan, M. Effect of auxins and cytokinins on the in vitro production of date palm bud generative tissues and on the number of differentiated buds. In Proceedings of the Second International Conference on Date Palm, Al Ain, UAE, 25–27 March 2001; pp. 47–86. [Google Scholar]
- Metwali, E.M.; Kadasa, N.M.; Soliman, H.I.; Almaghrabi, O.A.; Fuller, M.P. In vitro propagation of date palm cultivars Magdoul and Safwai through somatic embryogenesis. Int. J. Agric. Biol. 2020, 24, 1745–1753. [Google Scholar]
- Blando, F.; Rizzello, F.; Durante, M.; De Paolis, A.; Caretto, S.; Mita, G. In Vitro Adventitious Regeneration of Artemisia annua L. Influencing Artemisinin Metabolism. Horticulturae 2021, 7, 438. [Google Scholar] [CrossRef]
- Tisserat, B. Production of free-living date palms through tissue culture. Date Palm J. 1981, 1, 43–54. [Google Scholar]
- Bekheet, S. Date palm biotechnology in Egypt. App. Sci. Report 2013, 3, 144–152. [Google Scholar]
- Abahmane, L. Date palm micropropagation via organogenesis. In Date Palm Biotechnology; Springer: New York, NY, USA, 2011; pp. 69–90. [Google Scholar]
- Ghareeb, Y.E.; Soliman, S.S.; Ismail, T.A.; Hassan, M.A.; Abdelkader, M.A.; Abdel Latef, A.A.; Al-Khayri, J.M.; Alshamrani, S.M.; Safhi, F.A.; Awad, M.F.; et al. Improvement of German Chamomile (Matricaria recutita L.) for Mechanical Harvesting, High Flower Yield and Essential Oil Content Using Physical and Chemical Mutagenesis. Plants 2022, 11, 2940. [Google Scholar] [CrossRef] [PubMed]
- Abdelnour, S.A.; Xie, L.; Hassanin, A.A.; Zuo, E.; Lu, Y. The Potential of CRISPR/Cas9 Gene Editing as a Treatment Strategy for Inherited Diseases. Front. Cell Dev. Biol. 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Raza, S.H.A.; Hassanin, A.A.; Pant, S.D.; Bing, S.; Sitohy, M.Z.; Abdelnour, S.A.; Alotaibi, M.A.; Al-Hazani, T.M.; Abd El-Aziz, A.H.; Cheng, G.; et al. Potentials, prospects and applications of genome editing technologies in livestock production. Saudi J. Biol. Sci. 2022, 29, 1928–1935. [Google Scholar] [CrossRef] [PubMed]
- Raza, S.H.A.; Hassanin, A.A.; Dhshan, A.I.M.; Abdelnour, S.A.; Khan, R.; Mei, C.; Zan, L. In silico genomic and proteomic analyses of three heat shock proteins (HSP70, HSP90-α, and HSP90-β) in even-toed ungulates. Electron. J. Biotechnol. 2021, 53, 61–70. [Google Scholar] [CrossRef]
- Eldomiaty, A.; Mahgoub, E. Morphological, Biochemical and Molecular Characterization of Rhizobia of Faba Bean Plants Grown in North Nile Delta Egypt. Pak. J. Biol. Sci. 2021, 24, 672–679. [Google Scholar]
- Al-Khayri, J.M.; Mahdy, E.M.B.; Taha, H.S.A.; Eldomiaty, A.S.; Abd-Elfattah, M.A.; Abdel Latef, A.A.; Rezk, A.A.; Shehata, W.F.; Almaghasla, M.I.; Shalaby, T.A.; et al. Genetic and Morphological Diversity Assessment of Five Kalanchoe Genotypes by SCoT, ISSR and RAPD-PCR Markers. Plants 2022, 11, 1722. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Loyola-Vargas, V.M.; Vázquez-Flota, F. Plant Cell Culture Protocols; Springer: New York, NY, USA, 2006; Volume 318. [Google Scholar]
- Singh, R.K.; Chaudhary, B.D. Biometrical methods in quantitative genetic analysis. Biom. Methods Quant. Genet. Anal. 1977; 304p. [Google Scholar]
- Rohim, F.M.; AA, A.E.-H. The effect of nanoparticles of in vitro propagation of seedling male date palm by immature inflorescences. Egypt. J. Chem. 2021, 65, 627–643. [Google Scholar]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 8th ed.; Iowa State University Press: Ames, IA, USA, 1989; p. 1191. [Google Scholar]








| SOV | Df | MS | ||
|---|---|---|---|---|
| Callus Induction Frequencies (%) | Number of Days to Initiate Callus | Callus Weight (g) | ||
| Replicates | 2 | 75.51 | 3.595 | 0.09834 |
| Media | 2 | 4,672.38 ** | 177.099 ** | 8.97575 ** |
| Variety | 2 | 5,645.85 ** | 401.173 ** | 2.86402 ** |
| Media x variety | 4 | 167.91 | 12.008 | 0.02743 |
| Error | 70 | 127.71 | 10.437 | 0.28367 |
| Total | 80 | 10,689.36 | 604.312 | 12.249 |
| h2b | 82.96% | 81.64% | 47.68% | |
| SOV | Df | MS | |
|---|---|---|---|
| Number of Somatic Embryos | Number of Shoots | ||
| Replicates | 2 | 14.848 | 12.913 |
| Media | 2 | 95.623 ** | 180.803 ** |
| Variety | 2 | 110.612 ** | 228.831 ** |
| Media x variety | 4 | 0.785 | 7.041 |
| Error | 70 | 11.281 | 18.844 |
| Total | 80 | 233.149 | 448.432 |
| h2b | 43.569% | 52.36% | |
| SOV | Df | MS | ||
|---|---|---|---|---|
| Root Number | Root Length (cm) | Shoot Length (cm) | ||
| Replicates | 2 | 0.6204 | 1.9373 | 0.1548 |
| Media | 3 | 29.4444 ** | 28.5174 ** | 81.4038 ** |
| Variety | 2 | 4.0370 * | 7.6345 ** | 15.2823 ** |
| Media x Variety | 6 | 0.1852 | 0.1444 | 1.0430 * |
| Error | 94 | 0.7291 | 0.3545 | 0.3796 |
| Total | 107 | 35.016 | 38.588 | 98.263 |
| h2b | 22.004% | 68.41% | 80.11% | |
| Medium Name | Medium Composition (1 L) |
|---|---|
| Starting media | |
| SM1 | 4.4 g MS + 30 g sucrose + 7 g agar + 9 µM 2,4-D + 5.7 µM IAA + 10 µM NAA |
| SM2 | 4.4 g MS + 30 g sucrose + 7 g agar + 4.5 µM 2,4-D + 2.85 µM I AA + 5 µM NAA |
| SM3 | 4.4 g MS + 30 g sucrose + 7 g agar + 5.7 µM IAA + 5 µM NAA |
| Maturation media | |
| MM1 | 4.4 g MS + 30 g sucrose + 7 g agar + 4.5 µM 2,4-D + 9.8 µM 2-iP + 1.5 AC |
| MM2 | 4.4 g MS + 30 g sucrose + 7 g agar +2.25 µM 2,4-D + 4.9 µM 2-iP + 1.5 AC |
| MM3 | 4.4 g MS + 30 g sucrose + 7 g agar + 1.125 µM 2,4-D + 2.45 µM 2-iP + 1.5 AC |
| Multiplication media | |
| PM1 | 4.4 g MS + 30 g sucrose + 7 g agar + 4.4 µM BA + 9.8 µM 2-iP |
| PM2 | 4.4 g MS + 30 g sucrose + 7 g agar + 2.2 µM BA + 4.6 µM kinetin |
| PM3 | 4.4 g MS + 30 g sucrose + 7 g agar + 2.2 µM BA + 4.9 µM 2-iP |
| Rooting media | |
| RM1 | 3.3 g MS + 30 g sucrose + 6 g agar + 0.5 µM NAA + 1.25 µM IBA + 3 g AC |
| RM2 | 3.3 g MS + 50 g sucrose + 6 g agar + 0.5 µM NAA + 1.25 µM IBA + 3 g AC |
| RM3 | 3.3 g MS + 30 g sucrose + 6 g agar + 0.5 µM NAA + 0.5 µM IBA + 3 g AC |
| RM4 | 3.3 g MS + 50 g sucrose + 6 g agar + 0.5 µM NAA + 0.5 µM IBA + 3 g AC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelghaffar, A.M.; Soliman, S.S.; Ismail, T.A.; Alzohairy, A.M.; Latef, A.A.H.A.; Alharbi, K.; Al-Khayri, J.M.; Aljuwayzi, N.I.M.; El-Moneim, D.A.; Hassanin, A.A. In Vitro Propagation of Three Date Palm (Phoenix dactylifera L.) Varieties Using Immature Female Inflorescences. Plants 2023, 12, 644. https://doi.org/10.3390/plants12030644
Abdelghaffar AM, Soliman SS, Ismail TA, Alzohairy AM, Latef AAHA, Alharbi K, Al-Khayri JM, Aljuwayzi NIM, El-Moneim DA, Hassanin AA. In Vitro Propagation of Three Date Palm (Phoenix dactylifera L.) Varieties Using Immature Female Inflorescences. Plants. 2023; 12(3):644. https://doi.org/10.3390/plants12030644
Chicago/Turabian StyleAbdelghaffar, Ahmed M., Said. S. Soliman, Tarek A. Ismail, Ahmed M. Alzohairy, Arafat Abdel Hamed Abdel Latef, Khadiga Alharbi, Jameel M. Al-Khayri, Nada Ibrahim M. Aljuwayzi, Diaa Abd El-Moneim, and Abdallah. A. Hassanin. 2023. "In Vitro Propagation of Three Date Palm (Phoenix dactylifera L.) Varieties Using Immature Female Inflorescences" Plants 12, no. 3: 644. https://doi.org/10.3390/plants12030644









